Abstract
Prior studies on the association between fertility treatment and childhood cancer risk have generated inconsistent results. We performed a systematic review and meta‐analysis of observation studies to summarize the evidence regarding the relation of fertility treatment with childhood cancer risk. A systematic literature search of several databases was conducted through April 2018 to identify relevant studies. The outcomes of interest included overall cancer, haematological malignancies, neural tumours, other solid tumours, and eight specific cancers. The overall risk estimates and corresponding 95% confidence intervals (CIs) were pooled using random‐effects meta‐analysis. Sixteen cohort and thirteen case–control studies were included. Results showed that children conceived by fertility treatment had significantly higher risk for developing overall cancer (relative risk [RR]: 1.16, 95% CI: 1.01, 1.32), haematological malignancies (RR: 1.39, 95% CI: 1.21, 1.60) and other solid tumours (RR: 1.57, 95% CI: 1.14, 2.16). For specific cancers, fertility treatment was associated with a significantly increased risk of leukaemia (RR: 1.31, 95% CI: 1.09, 1.57) and hepatic tumours (RR: 2.26, 95% CI: 1.32, 3.85). Sensitivity analysis validated evidence of the robustness of the findings. The results may demonstrate a possible association between fertility treatment and an increased risk of cancer among the offspring. However, the findings cannot say whether this increased risk is due to the subfertility itself or to the fertility treatment. Further research is needed to address the underlying mechanisms.
Keywords: fertility treatment, assisted reproductive technology, fertility drugs, cancer, children, meta‐analysis
Short abstract
What's new?
Fertility treatments and assisted reproduction have become increasingly common since the 1970’s. Could these treatments increase the risk of cancer among children conceived via these technologies? In this meta‐analysis, the authors found that the answer is ‘yes’: a number of positive associations were identified. They emphasize that these results should not deter potential parents from seeking treatment for infertility. However, parents should be aware that their children may have an increased risk of several childhood cancers. Further research is needed to address the underlying mechanisms.
Abbreviations
- ART
assisted reproductive technology
- CI
confidence interval
- CNS
central nervous system
- HR
hazard ratio
- NOS
Newcastle‐Ottawa Scale
- OR
odds ratio
- RR
risk ratio
- SIR
standardized incidence ratio.
Introduction
As reported, more than 7 million children worldwide have been conceived by assisted reproductive technology (ART),1 and many more children are presumably born after other types of fertility treatment. Among children born after fertility treatment, increased risk of short‐term outcomes has been well‐recognized, including birth defects, preterm birth, low birth weight and small for gestational age;2 as these children have grown up, it is very important to monitor their long‐term health effects. Since the first three studies published in the early 1980s and 1990s suggested the possibility of increased risks of cancer in children born after ART or hormonal treatment,3, 4, 5 quite a few studies have focused on the association between fertility treatment and childhood cancer. However, the findings were discrepant.
Two meta‐analyses have been performed. The meta‐analysis published in 2005 did not find an elevation of cancer risk in children conceived by fertility treatment.6 In the study, only cohort studies of ART were included, and all types of cancers were assessed together, which may lead to the potential disguise of elevated risks of certain types of cancer. In another meta‐analysis published in 2013, a statistically significantly increased risk of cancer was detected in children conceived by ART or fertility drugs, as well as the increased risk of specific cancers including leukaemia, neuroblastoma and retinoblastoma.7 However, this analysis was not exhaustive, as the specific risk of several rare but important specific cancers was not reported, such as CNS tumours, lymphoma, germ cell tumours, etc. In addition, the influence of the difference in the reference group on the overall risk was not considered. Furthermore, several large, population‐based cohort studies with longer follow‐up time have been published which showed no overall increased risk of childhood cancer in relation to fertility treatment.8, 9, 10, 11, 12
Therefore, we thought it was important to perform an updated meta‐analysis regarding the association between fertility treatment and the risk of childhood cancer. The result of our study may lead to better understanding of cancer risk in children conceived by fertility treatment, which will help to guide future management and contribute to guidelines for clinicians.
Materials and Methods
The present meta‐analysis was conducted after the Proposed Systematic Reviews and Meta‐analyses (PRISMA) and Meta‐analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines. Prior to the search, a plan regarding the study design, search strategy and study selection criteria was written and approved by all authors. The protocol was registered at PROSPERO with registration number CRD42018106192 and is available at https://www.crd.york.ac.uk/PROSPERO/#recordDetails.
Search strategy
Two authors independently identified studies published in English and Chinese prior to April 2018, and reported data on cancer risk among children born after fertility treatment. PubMed, Embase, Web of Science, China Biology Medicine disc (CBMdisc), Chinese Scientific Journals Fulltext Database (CQVIP), China National Knowledge Infrastructure (CNKI), and Wanfang Database were systematically searched. The search terms used are shown in Supporting Information File 1. Furthermore, the reviewers manually searched the reference lists of identified articles to identify any relevant studies missed in the initial search.
Exposures and outcomes
The exposure of interest was any type of fertility treatment included ART and fertility drugs. ART was defined as artificial insemination, conventional in‐vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI) and other forms of treatment. Fertility drugs included clomiphene, progesterone, gonadrotropins (human menopausal gonadotropin, follicle‐stimulating hormone), gonadotropin‐releasing hormone, human chorionic gonadotropin, and a group of other fertility drugs.
The outcomes of interest included overall cancer, haematological malignancies, neural tumours, other solid tumours, and eight specific cancers including CNS tumours, neuroblastoma, leukaemia, lymphoma, retinoblastoma, hepatic tumours, bone tumours and extraosseous sarcomas, and germ cell tumours.
Study selection
At the stage of titles and abstracts screening, we purposely broadened the inclusion criteria to obtain any relevant study. First, Chinese or English studies which centered on cancer among children born after fertility treatment were considered for inclusion. Then full texts of all selected studies were reviewed. Studies were included if they 1) were cohort or case–control in design, and 2) provided sufficient information to allow for relative risk estimates and their 95% CIs for cancer in exposed children to be calculated, including the risk ratio (RR), hazard ratio (HR), standardized incidence ratios (SIR) and odds ratio (OR). For cohort studies, the general population, children not conceived by ART, children conceived naturally, and children whose mother had a diagnosis of infertility but conceived naturally could be defined as the unexposed population. Only case–control studies that defined children without cancer as control were eligible for inclusion. Studies using a diagnosis of subfertility or infertility as the exposure variable were excluded. Reviews, conference abstracts, comments, case reports, experimental or qualitative studies, and duplicate publications were also excluded. The cases of disagreement are presented in Supporting Information File 2.
Data extraction
Two reviewers independently extracted and evaluated the data for each included article using a self‐designed data abstraction form. Disagreements were resolved through discussion or consultation with a third reviewer when consensus could not be achieved. Authors of included studies were contacted if information was unclear or missing. The following data were extracted from cohort studies: the first author and year of publication, study period, geographic region where the study was conducted, years of follow‐up, type of exposure (fertility treatment), non‐exposed population (including the general population, children not conceived by ART, children conceived naturally, and children whose mother had a diagnosis of infertility but conceived naturally), reported cancers, number of cancers (observed or expected) and study participants in exposed and unexposed children, adjustments or matches made, and risk estimates with 95% confidence intervals (CIs) (if available, the adjusted ones were extracted). For case–control studies, the following data were extracted: the first author and year of publication, study period, geographic region where the study was conducted, type of exposure (fertility treatment), reported cancers, total numbers of cancer cases and control as well as numbers of exposed children in both groups, adjustments or matches made, and risk estimates with 95% CIs (if available, the adjusted ones were extracted).
Study quality assessment
Study quality of included studies was assessed using the Newcastle‐Ottawa Scale (NOS) for cohort studies and case–control studies. This scale was composed of 8 items. It ranged from 1 to 9 stars and assessed the quality of each study based on three modules: 1) the selection of exposed and non‐exposed cohorts, or of a case and a control; 2) the comparability of cohorts, or of the case and control; and 3) the ascertainment of outcome or exposure. A study can be awarded a maximum of one star for each numbered item within the first and third module. A maximum of two stars can be given for the second module. A final score ≥ 6 (median) was regarded as high quality.
Statistical analyses
RR was used as the measure of the association between maternal fertility treatment and risk of childhood cancers among offspring. The HRs, SIRs and ORs were directly considered as RRs. For studies that reported risk estimates for different types of specific cancer under the same category separately, we combined these estimates within the same study and calculated combined RRs by a fixed‐effects model for the main analysis. For example, if a study reported the risk estimates for leukaemia and lymphoma separately, the two estimates would be combined into a single fixed‐effect estimate of haematological malignancies. In addition, for two studies regarded children born in United Kingdom (1992–2008) after non‐donor and donor ART as exposed population, we combined these two studies as a single study and calculated a combined RR by a fixed‐effect model for the data analysis phase.8, 12
Data of risk estimates and their corresponding 95% CIs were extracted from each study to calculate log‐transformed estimates and their corresponding standard errors (SEs) which were used to stabilize the variance and normalize the distribution. Cochran Q test and the I 2 statistic were used to assess the heterogeneity of RRs across studies. The Cochran Q test was used to evaluate whether the variation across studies was compatible with chance, and p < 0.1 was considered to indicate significant heterogeneity. The I 2 statistic was a quantitative indicator used to evaluate the percentage of total variance in prevalence estimates due to statistical heterogeneity rather than chance, or sampling error (I 2 > 75% indicated high heterogeneity, 51–75% indicated substantial heterogeneity, 26–50% indicated moderate heterogeneity, and ≤ 25% indicated low heterogeneity). The pooled RRs and corresponding 95% CIs were calculated using random‐effects meta‐analyses. Subgroup analyses were performed based on geographic region (e.g., European countries and non‐European countries), type of study design (e.g., cohort studies and case–control studies), type of exposure (e.g., ART and fertility drugs) and non‐exposed population (e.g., the general population, children not conceived by ART, children conceived naturally, and children whose mother had a diagnosis of infertility but conceived naturally). Given that the proportion of children conceived by fertility drugs in the background population (children conceived naturally) is negligible,13 we combined children not conceived by ART into children conceived naturally as a single group in the subgroup analysis. Since characteristic of unexposed population and adjustments or matches made were not consistent between studies, sensitivity analyses were conducted to examine the influence of different exclusion criteria on the overall RRs. Furthermore, the influence of individual studies on the overall RRs was determined by repeating the meta‐analysis after the exclusion of each included study. Concerning about the influence of small‐study effects on the results of our study, we recalculated these pooled estimates using the fixed‐effect meta‐analysis, and compared the fixed‐effect and random‐effects estimates. Publication bias was evaluated using Egger's line regression test (p < 0.05 indicated statistically significant differences). All analyses were performed using RevMan version 5.3 (Nordic Cochrane Center) and Comprehensive Meta‐Analysis Version 2.2.
Results
Identification and characteristics of studies
In total, 11,525 unique citations were identified after an initial search. Of these, 11,476 were excluded after screening titles and abstracts, mainly because they were duplicates, reviews or not related to our study (Fig. 1). Then, the full text of 47 articles were reviewed, 18 of which were excluded (details see Supporting Information Table S1). Finally, a total of 29 studies were considered to be eligible and included in the systematic review and meta‐analysis.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36
Figure 1.
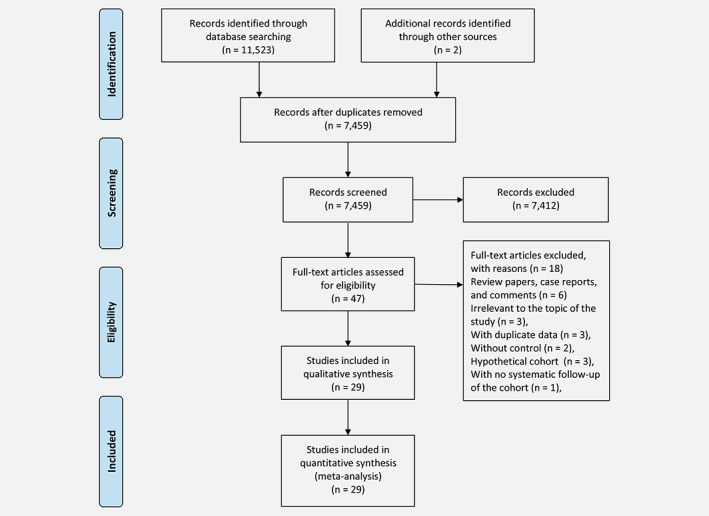
Flow chart of study selection. [Color figure can be viewed at wileyonlinelibrary.com]
Of the 29 observation studies included here, 16 were cohort in design8, 9, 10, 11, 12, 13, 14, 15, 21, 26, 27, 28, 29, 30, 31, 34 and 13 were case–control.16, 17, 18, 19, 20, 22, 23, 24, 25, 32, 33, 35, 36 The characteristics of the 16 cohort studies are shown in Table 1. A total of 327,884 children born after fertility treatment were included, in which 578 were diagnosed with cancer. The age range of these affected children was 0 to 27 years. All studies were published between 1998 and 2018. Eleven studies (68.8%; 530 cancer cases/299,458 children born after fertility treatment) were conducted in Europe,8, 10, 11, 12, 13, 15, 21, 26, 27, 28, 34 three (18.6%; 35 cancer cases/13,698 children born after fertility treatment) in Asia,9, 14, 31 and two (12.5%; 13 cancer cases/14,728 children born after fertility treatment) in Oceania.29, 30 Two studies (12.5%; 21 cancer cases/13,803 children born after fertility treatment) assessed the association between fertility treatment (ART or fertility drugs) and childhood cancer,14, 29 one (6.3%; 88 cancer cases/1,017 children born after fertility treatment) regarded children conceived after the use of fertility drugs as the exposed cohort,15 while the others (81.3%; 469 cancer cases/313,064 children born after fertility treatment) targeted on children born after ART.8, 9, 10, 11, 12, 13, 21, 26, 27, 28, 30, 31, 34 In 11 studies, cancer risk during childhood in individuals conceived by fertility treatment was compared to the risk in those not conceived by ART (in eight studies;9, 10, 11, 13, 14, 21, 27, 28 326 cancer cases/175,150 children born after fertility treatment), or those whose mother had a diagnosis of infertility but conceived naturally (in two studies;15, 29 95 cancer cases/10,496 children born after fertility treatment); in the other six studies (157 cancer cases/142,238 children born after fertility treatment), cancer rates were compared to population‐based rates in the same country over the same period.8, 12, 26, 30, 31, 34 Thirteen studies (75%; 437 cancer cases/299,999 children born after fertility treatment) stated the average follow‐up time with the range of 3.8 to 10.55 years,8, 9, 10, 11, 12, 13, 14, 26, 27, 29, 30, 31, 34 while the rest did not provide any relevant information.15, 21, 28 Only three studies (16.7%; 2 cancer cases/8,735 children born after fertility treatment) did not adjust for any confounder when estimating the risk of childhood cancer associated with maternal fertility treatment,27, 28, 34 whereas remaining studies (81.3%; 576 cancer cases/319,149 children born after fertility treatment) adjusted or matched for age, gender or other potential confounders.8, 9, 10, 11, 12, 13, 14, 15, 21, 26, 29, 30, 31
Table 1.
Selected characteristics of 16 cohort studies of fertility treatment and childhood cancers
| First author, Year | Study period | Geographic region | Follow‐up (years) | Exposure | Unexposed population | Reported cancers | No. of | Adjustments or matches made | Quality score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cancers/total exposed1 | Cancers/total unexposed1 | |||||||||
| Doyle 1998 | 1978–1991 | United Kingdom | 8.6 (mean) | ART | General population | Overall cancer | 2/2,507 | 3.5 (expected) | None | 6 |
| Bruinsma 2000 | 1979–1995 | Australia | 3.75 (median) | ART | General population | Overall cancer | 6/5,249 | 4.33 (expected) | Age | 8 |
| Lerner‐Geva 2000 | 1981–1994 | Israel | 4.3 (mean) | ART | General population | Overall cancer | 0/332 | 1.7 (expected) | Age, gender, and year of diagnosis | 7 |
| Klip 2001 | 1980–1995 | Netherlands | 6.0 (mean) | Fertility treatment | Subfertility/infertility disorder but conceived naturally | Overall cancer | 7/9,479 | 9/7,521 | Gender | 7 |
| Bradbury 2004 | 1989–2001 | United Kingdom | Not stated | ART | Spontaneous conception | Retinoblastoma | 0/176 | 24/358,094 | None | 6 |
| Kallen 2005 | 1982–2001 | Sweden | 5.5 (median) | ART | General population | Leukemia and hepatic tumours | 29/16,000 | 21.4 (expected) | Age, maternal age, parity, and smoking habits in early pregnancy | 9 |
| Lidegaard 2005 | 1995–2001 | Denmark | 4.1 (mean) for ART group, 4.5 (mean) for the non‐ART group | ART | Spontaneous conception | Leukemia, retinoblastoma and renal tumours | 0/6,052 | 72/442,349 | None | 6 |
| Kallen 2010 | 1982–2005 | Sweden | Not stated | ART | General population | Overall cancer, CNS tumours and retinoblastoma | 53/26,692 | 6405/2,417,878 | Age, maternal age, parity, smoking, and years of unwanted childlessness | 9 |
| Pinborg 2010 | 1995–2007 | Denmark | 5.4 (mean) for the ART group, 5.3 (mean) for the non‐ART group | ART | Spontaneous conception | Overall cancer | 6/11,286 | 1/4,800 | Age | 7 |
| Williams 2013 & 2018 | 1992–2008 | United Kingdom | 6.6 (mean) /7.86 (mean) | ART | General population | Overall cancer, CNS tumours, neuroblastoma, leukemia, retinoblastoma, renal tumours, hepatic tumours, bone tumours and extraosseous sarcomas, and germ‐cell tumours | 120/118,150 | 124.1 (expected) | Age and gender | 8/8 |
| Sundh 2014 | 1982–2007 | Sweden, Denmark, Finland and Norway | 9.5 (mean) | ART | Spontaneous conception | Overall cancer, CNS tumours, neuroblastoma, leukemia, lymphoma, retinoblastoma, renal tumours, hepatic tumours, bone tumours and extraosseous sarcomas, and germ‐cell tumours | 181/91,796 | 638/358,419 | Country, maternal age, parity, gender, gestational age, birth defects and chromosomal aberrations | 8 |
| Hargreave 2015 | 1964–2006 | Denmark | Not stated | Fertility drugs | Subfertility/infertility disorder but conceived naturally | Overall cancer, CNS tumours, leukemia, and lymphoma | 88/1,017 | 60/1,003 | Age | 7 |
| Reigstad 2016 | 1984–2011 | Norway | 6.9 (median) for the ART group, 13.7 (median) for the non‐ART group | ART | Spontaneous conception | Overall cancer, CNS tumours, neuroblastoma, leukemia, lymphoma, retinoblastoma, renal tumours, hepatic tumours, bone tumours and extraosseous sarcomas, and germ‐cell tumours | 51/25,782 | 4503/1,602,876 | Age, birth order, maternal age at delivery, place of birth, gender, birthweight and gestational age | 9 |
| Lerner‐Geva 2017 | 1997–2011 | Israel | 10.6 (median) for the ART group, 9.3 (median) for the non‐ART group | ART | Spontaneous conception | Overall cancer, CNS tumours, neuroblastoma, leukemia, lymphoma, retinoblastoma, renal tumours, bone tumours and extraosseous sarcomas, and germ‐cell tumours. | 21/9,042 | 361/211,763 | Maternal age, maternal education, ethnicity, plurality, gender, birthweight, congenital malformations | 9 |
| Wainstock 2017 | 1991–2013 | Israel | 10.55 (median) | Fertility treatment | Spontaneous conception | Overall cancer | 14/4,324 | 415/237,863 | Maternal age, birthweight, preterm birth, pregnancy‐related hypertensive disorders, and pregestational and gestational diabetes mellitus | 7 |
Abbreviations: ART, assisted reproductive technology; CNS, central nervous system.
When other types of cancer were reported along with overall cancer in the same study, only the data on overall cancer were shown.
The characteristics of the 13 case–control studies, which included 54,220 participants (cancer: 7,014; no cancer: 47,206) and were published between 1996 and 2013, are shown in Table 2. Six studies (46.2%; 148 individuals exposed to fertility treatment/4,045 cancer cases) were conducted in Europe16, 17, 18, 23, 33, 35 and seven (53.8%; 139 individuals exposed to fertility treatment/1,962 cancer cases) in North America.19, 20, 22, 24, 25, 32, 36 Six studies (46.2%; 148 individuals exposed to fertility treatment/3,864 cancer cases) reported the proportion of fertility drugs use in mothers whose children were diagnosed with cancer,20, 22, 32, 33, 35, 36 as two studies (15.4%; 37 individuals exposed to fertility treatment/1,300 cancer cases) reported the proportion of ART application18, 23 and five studies (38.5%; 161 individuals exposed to fertility treatment/1,607 cancer cases) reported the proportion of fertility treatment16, 17, 19, 24, 25 of which four reported the proportion of fertility drugs use and ART application separately.16, 17, 19, 24 Adjustments and matches were made in all studies.
Table 2.
Selected characteristics of 13 case–control studies of fertility treatment and cancers
| First author, Year | Study period | Geographic region | Exposure | Reported cancers | No. of | Adjustments or matches made | Quality score | |
|---|---|---|---|---|---|---|---|---|
| Treated/total cases | Treated/total control | |||||||
| Michalek 1996 | 1976–1987 | United States | Fertility drugs | Neuroblastoma | 5/183 | 1/372 | Age | 7 |
| Roman 1997 | 1962–1992 | United Kingdom | Fertility drugs | Leukaemia | 4/143 | 3/286 | Area of birth, gender, age | 7 |
| Olshan 1999 | 1992–1996 | United States | Fertility drugs | Neuroblastoma | 28/459 | 26/459 | Age of children, mother's ethnic group, mother's education, and household income | 7 |
| Schüz 1999 | 1992–1997 | Germany | Fertility drugs | CNS tumours | 18/376 | 120/2,443 | Age, gender and district of residence | 7 |
| Neuroblastoma | 8/145 | 120/2,443 | ||||||
| Leukaemia | 53/896 | 35/896 | ||||||
| Lymphoma | 8/210 | 120/2,443 | ||||||
| Renal tumours | 8/138 | 120/2,443 | ||||||
| Bone tumours and extraosseous sarcomas | 10/225 | 120/2,443 | ||||||
| McLaughlin 2006 | 1985–2001 | United States | Fertility treatment | Hepatic tumours | 5/58 | 23/6,056 | Age | 7 |
| Puumala 2007 | 1997–2002 | United States | Fertility treatment | Leukaemia | 27/158 | 28/173 | Mother's age, child's gender, ethnic group, and education | 6 |
| Mallol‐Mesnard 2008 | 2003–2004 | France | ART | CNS tumours | 11/209 | 82/1,681 | Age and gender | 7 |
| Puumala 2010 | 1996–2002 | United States | Fertility drugs | Leukaemia | 19/443 | 15/324 | Age, maternal age, maternal education, maternal ethnic group, smoking during pregnancy, household income, gestational age and birthweight. | 8 |
| Puumala 2011 | 1993–2002 | United States | Fertility drugs | Germ cell tumours | 5/278 | 11/421 | Gender and age | 7 |
| Petridou 2012 | 1996–2008 | Greece | ART | Leukaemia | 24/814 | 15/814 | Birth weight, birth order, maternal age at birth, maternal education, maternal smoking during pregnancy | 7 |
| Lymphoma | 2/277 | 1/277 | ||||||
| Foix‐L'Hélias 2012 | 2000–2006 | France | Fertility treatment | Retinoblastoma | 20/244 | 1,555/26,767 | Maternal age at birth and smoking | 6 |
| Puumala 2012 | 2000–2008 | United States | Fertility treatment | Hepatic tumours | 34/383 | 45/384 | Birthweight, age, gender, maternal age, maternal education, maternal ethnic group, plurality and gestational age | 8 |
| Rudant 2013 | 2003–2004 | France | Fertility treatment | Leukaemia | 59/764 | 82/1,681 | Age, gender, maternal age and parental professional category | 8 |
Abbreviations: ART, assisted reproductive technology; CNS, central nervous system.
After combination of the two studies from United Kingdom (1992–2008) and treatment of duplicate data (details were shown in Supporting Information File 3), among all studies included here, 12 studies reported on overall cancer, 13 studies on haematological malignancies, 10 studies on neural tumours and 13 studies on other solid tumours. For specific types of cancers, the number of studies reported on each type were as follows: 8 studies on CNS tumours, 7 studies on neuroblastoma, 13 studies on leukaemia, 6 studies on lymphoma, 8 studies on retinoblastoma, 7 studies on hepatic tumours, 6 studies on renal tumours, 5 studies on bone tumours and extraosseous sarcomas, and 5 studies on germ cell tumours.
The quality of 16 cohort studies as well as 13 case–control studies was evaluated using the NOS as shown in Supporting Information Table S2 and Supporting Information Table S3. The quality of those studies was generally good as all studies got 6 to 9 stars.
Fertility treatment and risk of overall cancer, haematological malignancies, neural tumours and other solid tumours
Figure 2 shows the result from the random‐effects model combining the RRs for overall cancer in children conceived by fertility treatment. Among the 12 cohort studies, 10 showed no statistically significant association between fertility treatment and risk of overall cancer.8, 9, 10, 11, 12, 13, 15, 26, 27, 29, 30, 31, 34, 37, 38 The RRs for the association varied from 0.23 to 2.55. Overall, children conceived by fertility treatment compared to the reference group had a significantly higher risk for developing cancer (RR: 1.16 [95% CI: 1.01, 1.32]). There was low heterogeneity across studies (I 2 = 24%, p = 0.21). No potential publication bias was found by Egger's regression test (t = 0.431, p = 0.676).
Figure 2.
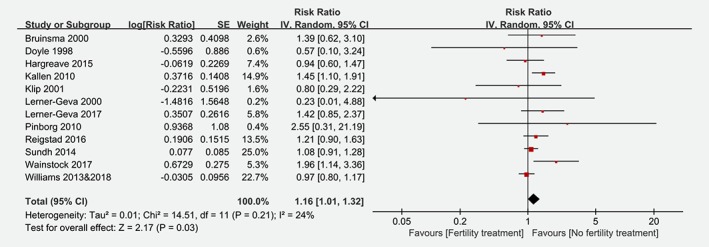
Risk of overall cancer among children conceived by fertility treatment. (CI = confidence interval; IV = inverse variance; SE = standard error). [Color figure can be viewed at wileyonlinelibrary.com]
The risk estimates of haematological malignancies, neural tumours and other solid tumours associated with fertility treatment are shown in Figure 3. Meta‐analyses showed significant increased risks for haematological malignancies (RR: 1.39 [95% CI: 1.21, 1.60]) and other solid tumours (RR: 1.57 [95% CI: 1.14, 2.16]) in children conceived by fertility treatment, but not for neural tumours (RR: 1.12 [95% CI: 0.91, 1.37]). Significant heterogeneity was only detected across studies regarding the risk estimate of other solid tumours (I 2 = 69%, p < 0.01). No potential publication bias was found by Egger's regression test (haematological malignancies: t = 0.088, p = 0.932; neural tumours: t = 1.172, p = 0.275; other solid tumours: t = 0.762, p = 0.462).
Figure 3.
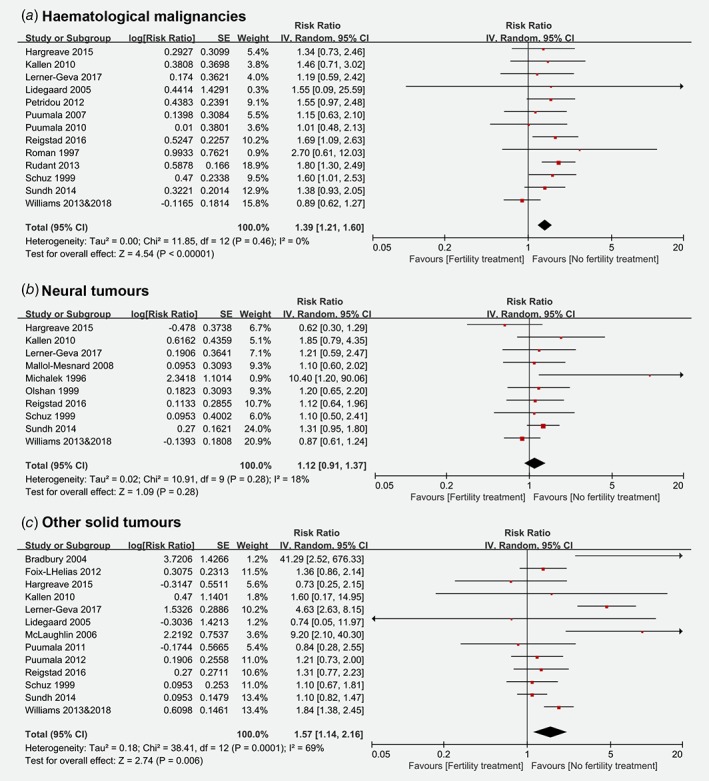
Risk of haematological malignancies, neural tumours and other solid tumours among children conceived by fertility treatment. (CI = confidence interval; IV = inverse variance; SE = standard error). [Color figure can be viewed at wileyonlinelibrary.com]
Fertility treatment and risk of specific cancers
The relationships between fertility treatment and risk of specific cancers are summarized in Table 3. The overall combined RRs in relation to fertility treatment were 1.01 (95% CI: 0.76, 1.32) for CNS tumours, 1.20 (95% CI: 0.86, 1.67) for neuroblastoma, 1.31 (95% CI: 1.09, 1.57) for leukaemia, 1.09 (95% CI: 0.74, 1.61) for lymphoma, 1.98 (95% CI: 0.85, 4.63) for retinoblastoma, 2.26 (95% CI: 1.32, 3.85) for hepatic tumours, 1.25 (95% CI: 0.68, 2.30) for renal tumours, 1.27 (95% CI: 0.73, 2.19) for bone tumours and extraosseous sarcomas, and 0.81 (95% CI: 0.37, 1.80) for germ cell tumours. Significant heterogeneity was detected across studies regarding the risk estimates of retinoblastoma, renal tumours, and bone tumours and extraosseous sarcomas (I 2 range: 60–68%; all p < 0.1), while no significant heterogeneity was found for other outcomes (I 2 range: 0–41%; all p > 0.1). No evidence of publication bias was detected by using the Egger's regression tests (t range: 0.080–1.485; all p > 0.05).
Table 3.
Meta‐analyses of risk estimates for specific cancers among children conceived by fertility treatment
| Outcome | No. of studies | RR (95% CI) | p value | Test of heterogeneity | Test of publication bias | ||
|---|---|---|---|---|---|---|---|
| I 2 (%) | p value | t | p value | ||||
| CNS tumours | 8 | 1.01 (0.76, 1.32) | 0.96 | 41 | 0.11 | 1.342 | 0.228 |
| Neuroblastoma | 7 | 1.20 (0.86, 1.67) | 0.27 | 10 | 0.35 | 0.080 | 0.938 |
| Leukemia | 13 | 1.31 (1.09, 1.57) | < 0.001 | 25 | 0.19 | 0.551 | 0.592 |
| Lymphoma | 6 | 1.09 (0.74, 1.61) | 0.65 | 0 | 0.57 | 0.169 | 0.874 |
| Retinoblastoma | 8 | 1.98 (0.85, 4.63) | 0.12 | 68 | < 0.001 | 0.795 | 0.457 |
| Hepatic tumours | 7 | 2.26 (1.32, 3.85) | < 0.001 | 35 | 0.16 | 1.485 | 0.198 |
| Renal tumours | 6 | 1.25 (0.68, 2.30) | 0.47 | 60 | 0.03 | 0.229 | 0.830 |
| Bone tumors and extraosseous sarcomas | 5 | 1.27 (0.73, 2.19) | 0.40 | 66 | 0.02 | 0.571 | 0.608 |
| Germ cell tumours | 5 | 0.81 (0.37, 1.80) | 0.61 | 19 | 0.29 | 0.643 | 0.566 |
Abbreviations: CI, confidence interval; CNS, central nervous system; RR, risk ratio.
Subgroup analyses
Subgroup analyses for risk of overall cancer, haematological malignancies, neural tumours and other solid tumours among children conceived by fertility treatment are shown in Supporting Information Table S4. When stratified by geographic region, the risk estimates of haematological malignancies (1.43, 95% CI: 1.23, 1.67) associated with fertility treatment were still significant in studies conducted in European countries, as the risk of overall cancer (1.47, 95% CI: 1.05, 2.03) and other solid tumours (2.27, 95% CI: 1.28, 4.03) further increased in studies conducted in non‐European countries. The risk estimates of overall cancer (1.16, 95% CI: 1.01, 1.32), haematological malignancies (1.25, 95% CI: 1.02, 1.51) and other solid tumours (1.73, 95% CI: 1.28, 4.03) was significantly increased when data were restricted to studies with a cohort design, as only an increased risk of haematological malignancies was found in case–control studies (1.56, 95% CI: 1.27, 1.92). Compared to the reference group, the risk estimates of overall cancer (1.16, 95% CI: 1.01, 1.34), haematological malignancies (1.30, 95% CI: 1.07, 1.58) and other solid tumours (1.75, 95% CI: 1.23, 2.48) were significant higher among children born after ART, as only risk of haematological malignancies was higher among children conceived by fertility drugs (1.61, 95% CI: 1.24, 2.11). Additionally, the risk of overall cancer (1.24, 95% CI: 1.09, 1.41; p < 0.01), haematological malignancies (1.53, 95% CI: 1.30, 1.80) and other solid tumours (1.64, 95% CI: 1.10, 2.44) among children conceived by fertility treatment was significantly higher when compared to children conceived naturally, while no increased risk was found when compared to the general population or children whose mother had a diagnosis of infertility but conceived naturally (all p > 0.05).
Subgroup analyses for the risk estimates of specific cancers associated with fertility treatment are summarized in Supporting Information Table S5. Overall, risk estimates of the following cancer outcomes increased further: leukaemia (1.37, 95% CI: 1.13, 1.66) and hepatic tumours (2.43, 95% CI: 1.11, 5.33) when data were restricted to studies conducted in European countries; retinoblastoma (7.80, 95% CI: 1.72, 35.41) and renal tumours (4.44, 95% CI: 1.78, 11.09) when data were restricted to studies conducted in non‐European countries; hepatic tumours (2.53, 95% CI: 1.22, 5.26) when data were restricted to cohort studies; leukaemia (1.58, 95% CI: 1.27, 1.96) when data were restricted to case–control studies; hepatic tumours (2.22, 95% CI: 1.42, 3.48) when data were restricted to studies having the use of ART as the exposure of interest; leukaemia (1.68, 95% CI: 1.27, 2.21) when data were restricted to studies having the use of fertility treatment as the exposure of interest; bone tumours and extraosseous sarcomas (2.27, 95% CI: 1.01, 5.08) when data were restricted to studies having the general population as the non‐exposed population; leukaemia (1.38, 95% CI: 1.14, 1.66) and hepatic tumours (2.22, 95% CI: 1.10, 4.52) when data were restricted to studies having children conceived naturally as the non‐exposed population.
Sensitivity analyses
Sensitivity analyses were performed to examine the influence of different exclusion criteria on the overall risk estimate for childhood cancers associated with fertility treatment (Supporting Information Table S6). Results showed that exclusion of three studies with children whose mother had an infertility/subfertility diagnosis but conceived naturally as control, three studies without adjustment/match, or 14 studies without any adjustment or adjusted only for age and/or gender yielded similar estimates. Further exclusion of any single study did not materially alter the risk estimates for overall cancer as well as other types of cancer (Supporting Information Table S7). In addition, to identify the influence of small‐study effects on the outcomes, fixed‐effect estimates of cancer risk were calculated and compared to the random‐effects estimates; results showed that the estimates based on the two methods were similar (Supporting Information Table S8).
Discussion
In our study, by combining the results of all available cohort and case–control studies with the conventional method of meta‐analysis, we provided evidence that fertility treatment is associated with an increased risk of overall cancer, haematological malignancies, and other solid tumours among the offspring, with a relative risk estimate of 1.16, 1.39 and 1.57, respectively. The risks increased further when data were restricted to studies with cohort design, studies having the use of ART as the exposure of interest, as well as studies having children conceived naturally as the non‐exposed population. For specific types of cancer, significantly increased risks were found only for leukaemia and hepatic tumours while no increase in risk was detected for the other subtypes of childhood cancer. Given that different types of specific cancers may have different aetiologies, it may be conceivable that the risk for developing each specific cancer is inconsistent. To the best of our knowledge, our study is the most comprehensive meta‐analysis assessing the association between fertility treatment and risk of childhood cancer, which can supply helpful information to both clinicians and couples who intend to receive fertility treatment, and help to guide further clinical management for children born after fertility treatment.
To date, there are two existing meta‐analyses of the childhood cancer risk in relation to fertility treatment. The meta‐analysis of 11 cohort studies published in 2005 found a slightly higher but non‐significant risk in children conceived by ART (RR = 1.33).6 It is worth noting that, however, given the smaller number of cancer cases (47 cases) in the study, we cannot rule out the possibility that the potential significance of the elevation in risk cannot be detected due to the limited statistical power. In contrast, the meta‐analysis published in 2013 by Hargreave et al., which based on 332 cancer cases in individuals conceived by fertility treatment, detected significant higher risks in relation to fertility treatment for overall cancer, haematological malignancies, neural tumours as well as other solid tumours, with risk estimates of 1.33, 1.59, 1.88 and 2.19, respectively.7 Furthermore, increased risks for specific cancers including leukaemia (RR = 1.65), neuroblastoma (RR = 4.04) and retinoblastoma (RR = 1.62) were also reported in the study. Consistent with the meta‐analysis published in 2013, our study found significant elevation in risks of overall cancer (RR = 1.16), haematological cancers (RR = 1.39), other solid tumours (RR = 1.57) as well as leukaemia (RR = 1.31). By comparison, our estimates were slightly lower than those in the meta‐analysis published in 2013, which may be due to the inconsistencies in included studies. On the one hand, the meta‐analysis published in 2013 included three studies using hypothetical cohorts or without systematic follow‐up of the cohort, which are not included in the present meta‐analysis due to the use of stricter selection criteria.3, 39, 40 Notably, after excluding studies with a different study design, all risk estimates in the meta‐analysis published in 2013 decreased; among them, the elevations in risk for neural tumours as well as for specific cancers including neuroblastoma and retinoblastoma become non‐significant. On the other hand, the meta‐analysis was published earlier than five large cohort studies from United Kingdom,8, 12 Denmark,15 Norway10 and four Nordic countries,11 all of which were included in our study and showed relatively lower risk estimates for overall cancer than the estimate in the meta‐analysis published in 2013, with HRs or SIRs of 0.83, 0.98, 0.94, 1.21 and 1.08, respectively. Furthermore, the risk estimates of leukaemia, neuroblastoma and retinoblastoma in most of the five studies were also lower than those in the meta‐analysis published in 2013. These five cohort studies were large with a total of 440 cancer cases identified in 236,745 children conceived by fertility treatment, and their cohort design rendered little risk of recall bias; it means they could add important data to the knowledge base and increase the power of our results.
An important limitation, which must be noted and existed in the 2013 meta‐analysis7 as well as in our study, was the inability to account for underlying parental infertility. Due to the limitation in included studies, the relative excess observed risk between fertility treatment and the development of these tumours are not evidence of causation. Both of the two meta‐analyses cannot distinguish whether the elevated cancer risk in offspring born after fertility treatment is associated with underlying parental infertility, or the procedure itself. Evidence suggested that epigenetic alterations could be an important cause of infertility, rather than an outcome of the procedure used to treat it.41, 42 Thus, infertile couples using fertility treatment could have a higher risk of epigenetic defects in their gametes, which fertility treatment is simply uncovering.43 In addition, males with fertility problems may have DNA defects in their sperm, since abnormalities such as loosely packed chromatin and DNA damage have already been reported in semen sample with poor quality.44, 45, 46 In our study, we assessed the effect of fertility treatment on childhood cancer by using subgroup analysis based on two studies regarded children whose mother had a diagnosis of infertility but conceived naturally as control, and no elevation in risk of cancer was found. Although the two studies were designed to adequately consider the underlying infertility, they involved only 95 cancer cases which might have limited statistical power to detect potential elevations in risk. Therefore, further studies are needed to clarify the relation among parental infertility, fertility treatment and cancer in offspring. For that, it is crucial to ascertain in any study design the underlying cause of infertility. If, for example, epigenetic alterations are found only among those couples with sperm maturational defects or ovarian failure but not among those with mechanical problems (e.g., tubal disease), then these alterations are unlikely to be caused by the procedure.
In addition, significant unmeasured confounding factors except for parental infertility may have contributed to the observed association between fertility treatment and childhood cancer in this meta‐analysis. Although none of them have adequately controlled all potential confounding factors, such as age, gender, maternal age at birth, socioeconomic status, maternal smoking, low birth weight, congenital malformations, and imprinting disorders,21, 25, 47, 48, 49, 50, 51, 52, 53 nearly 90% of the studies (26/29 studies) included have controlled for more than one potential confounding factors. Here, 23 studies adjusted or matched for age and/or gender of children, 13 studies for maternal age at birth, 8 studies for parental socioeconomic status, 5 studies for birth order/parity, 8 studies for birthweight and gestational age, 4 studies for maternal smoking during pregnancy, and 3 studies for maternal pregnancy complication, congenital malformations or chromosomal aberrations. Unique to our study, we conducted sensitivity analyses to consider the effects of those potential confounding factors. Results showed that exclusion of studies without any adjustment or match yielded similar results for all estimates, as exclusion of studies adjusted/matched only for age and/or gender of children did. It is worth noting that, some factors that could not be controlled within included studies including country‐specific differential medical practices, epigenetic/environmental factors, and changes in treatment of infertility over time, may also have an influence on the results of our study. If data allow, an effort could be made to present the role of those potential confounding factors in the association between fertility treatment and childhood cancer through meta‐regression or subgroup analysis processes. However, we were unable to obtain adequate data on these factors.
A further limitation of the current meta‐analysis was that about 45% of the studies (13/29 studies) were performed with a case–control design, with inherent limitations regarding inaccurate data of exposure as well as the risk of recall bias.54 However, the Motherisk Program in Toronto found that underreporting of exposure information related to clinic visits was less likely55; it means that recall bias is not a problem in studies concerning children conceived by fertility treatment, as mothers may remember the treatment they received through a clinic. Moreover, Petridou et al. pointed out that case–control studies may offer advantages over cohort studies since inappropriate cohorts might constitute the lack of evidence rather than the evidence of no effect of assisted reproduction.18
In addition, although a series of subgroup and sensitivity analyses were performed, novelty of our findings seems to be restricted by the availability of data on various types of fertility treatment, changes in treatment of infertility over time, and specification of childhood cancer due to the paucity of relevant data. Further studies with large sample size, and detailed data on types of fertility treatment and childhood cancer and changes in treatment of infertility are needed. Even though a total of 29 studies were included, part of outcomes reported relied on a limited number of studies. More relevant studies should be included in future systematic reviews to provide further support for our results. Finally, although an attempt was made to minimize the possible bias in the process of document retrieving with specific searches in major English‐Chinese databases (including master and doctoral theses), there may still be some unidentified papers. Fortunately, as the results of Egger's test showed, there was no publication bias found in all estimates.
The strength of our study is the large sample size from all available studies with a total of 578 cancer cases in individuals conceived by fertility treatment which helps to enhance statistical power to provide more reliable and precise risk estimates. Risk estimates for most of the common and rare childhood cancers were calculated, most of which were homogenous. Results of subgroup analysis could answer specific questions about study design, exposure and control, as results of the sensitivity analyses based on various exclusion criteria supported the robustness of the observed association between fertility treatment and childhood cancer. At the beginning of the study, a comprehensive search strategy was used to identify relevant studies which were later screened by a series of stricter selection criteria. Furthermore, the inclusion of more recent studies means that the findings are more likely to be related and more applicable to current practice.
Two questions that were previously raised but not resolved are again presented here. Firstly, is the association between fertility treatment and childhood cancer causal? To answer this question, several issues should be taken into account, including parental age, genetic, epigenetic, and environment confounders. If the answer to the question is yes, what is the exact mechanism behind the increase of childhood cancer risk? Epigenetic alterations may offer insights. Further well‐designed studies with large sample and longer follow‐up time, which adequately considered the potential confounding factors, are warranted to address the two questions for a better understanding of the association between fertility treatment and childhood cancer, and to provide convincing evidences for clinical management of children conceived by fertility treatment.
Finally, to better obtain meaningful data, as suggested, the national registry reporting outcomes after ART (fertility drugs as well, if available) should be cross‐linked to the cancer registry.56 A specific set of variables associated with increased cancer risk should be collected to build solid database, such as age of couples, years of unwanted childlessness, type of infertility, smoking, body mass index, genetic familiarity for cancer and others.
Conclusions
Our results may demonstrate a possible association between fertility treatment and increased risks of overall cancer, haematological malignancies, other solid tumours, leukaemia and hepatic tumours among the offspring. However, the findings cannot say whether this increased risk is due to the subfertility itself or to the fertility treatment. These findings should not prevent parents from seeking treatment of infertility, but they should be aware of the possible increased risk of cancer among their offspring. Moreover, guidelines of clinical monitoring for children conceived by fertility treatment are welcome, but should be based on valid data. Further research is needed to address the question whether fertility treatment itself or underlying parental infertility or a combination of these bring about increased risks of cancers in offspring among the pregnancies receiving fertility treatment.
Supporting information
Appendix S1 Supinfo1
Appendix S2 Supinfo2
Appendix S3 Supinfo3
Supplemental Table S1 List of excluded references and reasons for exclusion.
Supplemental Table S2 Study quality assessment overview ‐‐ cohort studies.
Supplemental Table S3 Study quality assessment overview ‐‐ case–control studies.
Supplemental Table S4 Subgroup analysis for risk of overall cancer, haematological malignancies, neural tumours and other solid tumours among children conceived by fertility treatment.
Supplemental Table S5 Subgroup analysis for specific cancer risk among children conceived by fertility treatment.
Supplemental Table S6 Sensitivity analysis for cancer risk among children conceived by fertility treatment.
Supplemental Table S7 Sensitivity analyses for the association between childhood cancer and fertility treatment.
Supplemental Table S8 Meta‐analyses for the association between childhood cancer and fertility treatment basing on fixed‐effect and random‐effects model.
Acknowledgements
The funders (Natural Science Foundation of Hunan Province, Hunan Provincial Key Research and Development Program, and National Natural Science Foundation Program of China) had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Conflict of interest: The authors declare no conflict of interest.
References
- 1. European Society of Human Reproduction and Embryology, ART fact sheet , 2018. Available from: https://www.eshre.eu/Press-Room/Resources.aspx.
- 2. Qin J, Liu X, Sheng X, et al. Assisted reproductive technology and the risk of pregnancy‐related complications and adverse pregnancy outcomes in singleton pregnancies: a meta‐analysis of cohort studies. Fertil Steril 2016;105:73–85. [DOI] [PubMed] [Google Scholar]
- 3. White L, Giri N, Vowels MR, et al. Neuroectodermal tumours in children born after assisted conception. Lancet 1990;336:1577. [DOI] [PubMed] [Google Scholar]
- 4. Toren A, Sharon N, Mandel M, et al. Two embryonal cancers after in vitro fertilization. Cancer 1995;76:2372–4. [DOI] [PubMed] [Google Scholar]
- 5. Melamed I, Bujanover Y, Hammer J, et al. Hepatoblastoma in an infant born to a mother after hormonal treatment for sterility. N Engl J Med 1982;307:820. [DOI] [PubMed] [Google Scholar]
- 6. Raimondi S, Pedotti P, Taioli E. Meta‐analysis of cancer incidence in children born after assisted reproductive technologies. Br J Cancer 2005;93:1053–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hargreave M, Jensen A, Toender A, et al. Fertility treatment and childhood cancer risk: a systematic meta‐analysis. Fertil Steril 2013;100:150–61. [DOI] [PubMed] [Google Scholar]
- 8. Williams CL, Bunch KJ, Murphy MFG, et al. Cancer risk in children born after donor ART. Hum Reprod 2018;33:140–6. [DOI] [PubMed] [Google Scholar]
- 9. Lerner‐Geva L, Boyko V, Ehrlich S, et al. Possible risk for cancer among children born following assisted reproductive technology in Israel. Pediatr Blood Cancer 2017;64:e26292. [DOI] [PubMed] [Google Scholar]
- 10. Reigstad MM, Larsen IK, Myklebust TA, et al. Risk of cancer in children conceived by assisted reproductive technology. Pediatrics 2016;137:e20152061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sundh KJ, Henningsen AK, Kallen K, et al. Cancer in children and young adults born after assisted reproductive technology: a Nordic cohort study from the Committee of Nordic ART and Safety (CoNARTaS). Hum Reprod 2014;29:2050–7. [DOI] [PubMed] [Google Scholar]
- 12. Williams CL, Bunch KJ, Stiller CA, et al. Cancer risk among children born after assisted conception. N Engl J Med 2013;369:1819–27. [DOI] [PubMed] [Google Scholar]
- 13. Pinborg A, Loft A, Aaris Henningsen AK, et al. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995‐2006. Fertil Steril 2010;94:1320–7. [DOI] [PubMed] [Google Scholar]
- 14. Wainstock T, Walfisch A, Hoham‐Vardi I, et al. Fertility treatments and pediatric neoplasms of the offspring: results of a population‐based cohort with a median follow‐up of 10 years. Am J Obstet Gynecol 2017;216:314. [DOI] [PubMed] [Google Scholar]
- 15. Hargreave M, Jensen A, Nielsen TS, et al. Maternal use of fertility drugs and risk of cancer in children‐‐a nationwide population‐based cohort study in Denmark. Int J Cancer 2015;136:1931–9. [DOI] [PubMed] [Google Scholar]
- 16. Rudant J, Amigou A, Orsi L, et al. Fertility treatments, congenital malformations, fetal loss, and childhood acute leukemia: the ESCALE study (SFCE). Pediatr Blood Cancer 2013;60:301–8. [DOI] [PubMed] [Google Scholar]
- 17. Foix‐L'Hélias L, Aerts I, Marchand L, et al. Are children born after infertility treatment at increased risk of retinoblastoma? Hum Reprod 2012;27:2186–92. [DOI] [PubMed] [Google Scholar]
- 18. Petridou ET, Sergentanis TN, Panagopoulou P, et al. In vitro fertilization and risk of childhood leukemia in Greece and Sweden. Pediatr Blood Cancer 2012;58:930–6. [DOI] [PubMed] [Google Scholar]
- 19. Puumala SE, Ross JA, Feusner JH, et al. Parental infertility, infertility treatment and hepatoblastoma: a report from the Children's oncology group. Hum Reprod 2012;27:1649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puumala SE, Ross JA, Wall MM, et al. Pediatric germ cell tumors and parental infertility and infertility treatment: a Children's oncology group report. Cancer Epidemiol 2011;35:e25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kallen B, Finnström O, Lindam A, et al. Cancer risk in children and young adults conceived by in vitro fertilization. Pediatrics 2010;126:270–6. [DOI] [PubMed] [Google Scholar]
- 22. Puumala SE, Spector LG, Wall MM, et al. Infant leukemia and parental infertility or its treatment: a Children's oncology group report. Hum Reprod 2010;25:1561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mallol‐Mesnard N, Menegaux F, Lacour B, et al. Birth characteristics and childhood malignant central nervous sytem tumors: the ESCALE study (French Society for Childhood Cancer). Cancer Detect Prev 2008;32:79–86. [DOI] [PubMed] [Google Scholar]
- 24. Puumala SE, Ross JA, Olshan AF, et al. Reproductive history, infertility treatment, and the risk of acute leukemia in children with down syndrome: a report from the Children's oncology group. Cancer 2007;110:2067–74. [DOI] [PubMed] [Google Scholar]
- 25. McLaughlin CC, Baptiste MS, Schymura MJ, et al. Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol 2006;163:818–28. [DOI] [PubMed] [Google Scholar]
- 26. Kallen B, Finnström O, Nygren KG, et al. In vitro fertilization in Sweden: child morbidity including cancer risk. Fertil Steril 2005;84:605–10. [DOI] [PubMed] [Google Scholar]
- 27. Lidegaard O, Pinborg A, Andersen AN. Imprinting diseases and IVF: Danish national IVF cohort study. Hum Reprod 2005;20:950–4. [DOI] [PubMed] [Google Scholar]
- 28. Bradbury BD. In vitro fertilization and childhood retinoblastoma. Br J Clin Pharmacol 2004;58:209–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klip H, Burger CW, de Kraker J, et al. Risk of cancer in the offspring of women who underwent ovarian stimulation for IVF. Hum Reprod 2001;16:2451–8. [DOI] [PubMed] [Google Scholar]
- 30. Bruinsma F, Venn A, Lancaster P, et al. Incidence of cancer in children born after in‐vitro fertilization. Hum Reprod 2000;15:604–7. [DOI] [PubMed] [Google Scholar]
- 31. Lerner‐Geva L, Toren A, Chetrit A, et al. The risk for cancer among children of women who underwent in vitro fertilization. Cancer 2000;88:2845–7. [DOI] [PubMed] [Google Scholar]
- 32. Olshan AF, Smith J, Cook MN, et al. Hormone and fertility drug use and the risk of neuroblastoma: a report from the Children's cancer group and the pediatric oncology group. Am J Epidemiol 1999;150:930–8. [DOI] [PubMed] [Google Scholar]
- 33. Schuz J, Kaatsch P, Kaletsch U, et al. Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol 1999;28:631–9. [DOI] [PubMed] [Google Scholar]
- 34. Doyle P, Bunch KJ, Beral V, et al. Cancer incidence in children conceived with assisted reproduction technology. Lancet 1998;352:452–3. [DOI] [PubMed] [Google Scholar]
- 35. Roman E, Ansell P, Bull D. Leukaemia and non‐Hodgkin's lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br J Cancer 1997;76:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Michalek AM, Buck GM, Nasca PC, et al. Gravid health status, medication use, and risk of neuroblastoma. Am J Epidemiol 1996;143:996–1001. [DOI] [PubMed] [Google Scholar]
- 37. Brinton LA, Krüger Kjaer S, Thomsen BL, et al. Childhood tumor risk after treatment with ovulation‐stimulating drugs. Fertil Steril 2004;81:1083–91. [DOI] [PubMed] [Google Scholar]
- 38. Ericson A, Nygren KG, Olausson PO, et al. Hospital care utilization of infants born after IVF. Hum Reprod 2002;17:929–32. [DOI] [PubMed] [Google Scholar]
- 39. Marees T, Dommering CJ, Imhof SM, et al. Incidence of retinoblastoma in Dutch children conceived by IVF: an expanded study. Hum Reprod 2009;24:3220–4. [DOI] [PubMed] [Google Scholar]
- 40. Odone‐Filho V, Cristofani LM, Bonassa EA, et al. In vitro fertilization and childhood cancer. J Pediatr Hematol Oncol 2002;24:421–2. [DOI] [PubMed] [Google Scholar]
- 41. Doornbos ME, Maas SM, McDonnell J, et al. Infertility, assisted reproduction technologies and imprinting disturbances: a Dutch study. Hum Reprod 2007;22:2476–80. [DOI] [PubMed] [Google Scholar]
- 42. Ludwig M, Katalinic A, Gross S, et al. Increased prevalence of imprinting defects in patients with Angelman syndrome born to subfertile couples. J Med Genet 2005;42:289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niemitz EL, Feinberg AP. Epigenetics and assisted reproductive technology: a call for investigation. Am J Hum Genet 2004;74:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science 1980;210:1131–3. [DOI] [PubMed] [Google Scholar]
- 45. Foresta C, Zorzi M, Rossato M, et al. Sperm nuclear instability and staining with aniline blue: abnormal persistence of histones in spermatozoa in infertile men. Int J Androl 1992;15:330–7. [DOI] [PubMed] [Google Scholar]
- 46. Sailer BL, Jost LK, Evenson DP. Mammalian sperm DNA susceptibility to in situ denaturation associated with the presence of DNA strand breaks as measured by the terminal deoxynucleotidyl transferase assay. J Androl 1995;16:80–7. [PubMed] [Google Scholar]
- 47. Inbar‐Feigenberg M, Choufani S, Butcher DT, et al. Basic concepts of epigenetics. Fertil Steril 2013;99:607–15. [DOI] [PubMed] [Google Scholar]
- 48. Schmidt LS, Schüz J, Lähteenmäki P, et al. Fetal growth, preterm birth, neonatal stress and risk for CNS tumors in children: a Nordic population‐ and register‐based case‐control study. Cancer Epidemiol Biomarkers Prev 2010;19:1042–52. [DOI] [PubMed] [Google Scholar]
- 49. Odom LN, Segars J. Imprinting disorders and assisted reproductive technology. Curr Opin Endocrinol Diabetes Obes 2010;17:517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bjørge T, Cnattingius S, Lie RT, et al. Cancer risk in children with birth defects and in their families: a population based cohort study of 5.2 million children from Norway and Sweden. Cancer Epidemiol Biomarkers Prev 2008;17:500–6. [DOI] [PubMed] [Google Scholar]
- 51. Adam M, Rebholz CE, Egger M, et al. Childhood leukaemia and socioeconomic status: what is the evidence? Radiat Prot Dosimetry 2008;132:246–54. [DOI] [PubMed] [Google Scholar]
- 52. Spector LG, Klebanoff MA, Feusner JH, et al. Childhood cancer following neonatal oxygen supplementation. J Pediatr 2005;147:27–31. [DOI] [PubMed] [Google Scholar]
- 53. Buck GM, Michalek AM, Chen CJ, et al. Perinatal factors and risk of neuroblastoma. Paediatr Perinat Epidemiol 2001;15:47–53. [DOI] [PubMed] [Google Scholar]
- 54. Reigstad MM, Oldereid NB, Omland AK, et al. Literature review on cancer risk in children born after fertility treatment suggests increased risk of haematological cancers. Acta Paediatr 2017;106:698–709. [DOI] [PubMed] [Google Scholar]
- 55. Feldman Y, Koren G, Mattice K, et al. Determinants of recall and recall bias in studying drug and chemical exposure in pregnancy. Teratology 1989;40:37–45. [DOI] [PubMed] [Google Scholar]
- 56. Levi‐Setti PE, Patrizio P. Assisted reproductive technologies (ART) and childhood cancer: is the risk real? J Assist Reprod Genet 2018;35:1773–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supinfo1
Appendix S2 Supinfo2
Appendix S3 Supinfo3
Supplemental Table S1 List of excluded references and reasons for exclusion.
Supplemental Table S2 Study quality assessment overview ‐‐ cohort studies.
Supplemental Table S3 Study quality assessment overview ‐‐ case–control studies.
Supplemental Table S4 Subgroup analysis for risk of overall cancer, haematological malignancies, neural tumours and other solid tumours among children conceived by fertility treatment.
Supplemental Table S5 Subgroup analysis for specific cancer risk among children conceived by fertility treatment.
Supplemental Table S6 Sensitivity analysis for cancer risk among children conceived by fertility treatment.
Supplemental Table S7 Sensitivity analyses for the association between childhood cancer and fertility treatment.
Supplemental Table S8 Meta‐analyses for the association between childhood cancer and fertility treatment basing on fixed‐effect and random‐effects model.


