Abstract
Objectives
The purpose of this study is to clarify the influence of social interaction on the effect of a cognitive intervention program using Go.
Methods
A single‐blind, randomized controlled trial using a classical board game “Go” was conducted. A total of 72 community‐dwelling older adults, without previous experience playing Go, were randomly assigned to three groups: (1) a face‐to‐face group (FG) in which members attended 12 Go group lessons held once a week; (2) a non‐face‐to‐face group (NFG) in which members individually underwent the same Go lessons as the FG using a tablet computer; or (3) a health education control group (CG). The main outcome variable, working memory, was assessed before and after the interventions using the Visual Memory Span Test (VMST) and the Visual Memory Span Backward (VMSB) task. Go performance and additional cognitive domains were also examined.
Results
Analysis of covariance revealed that VMST scores significantly improved after the intervention in both the FG and NFG (both P < .05). Compared with the CG, the effect size of the FG (Cohen's d = 0.89) was greater than that of the NFG (Cohen's d = 0.67). Although VMSB scores significantly improved after the intervention in the FG (P < .05), no significant changes were observed in other groups.
Conclusions
This study showed that Go game could improve visual working memory regardless of social interaction. Furthermore, findings suggested that playing board games face‐to‐face with others is more effective for cognitive function than playing alone.
Keywords: cognitive intervention, community‐dwelling older adults, Go game, leisure activity, social interaction, working memory
Key points.
Social interaction may influence the effects of cognitive intervention programs.
The intervention effect of playing Go in groups was greater than playing Go individually on a tablet.
The classical board game “Go” has significant effectiveness on visual working memory in community‐dwelling older adults.
1. INTRODUCTION
Cognitive decline is a crucial issue in aging societies. It is well known that cognitive decline is associated with cognitive reserve throughout life.1, 2, 3 Increasing cognitive reserve has attracted a great deal of attention in research regarding the prevention of dementia. To improve cognitive reserve, individuals need to participate frequently in activities over a long period without losing motivation. Cognitive leisure activities (eg, reading, playing games, etc.) are examples of such activities, many of which include social interaction in addition to intellectual stimulation. Previous studies have reported that both intellectual stimulation and social interaction reduce the risk of dementia.4, 5, 6, 7
Several intervention programs incorporating cognitive leisure activities that focus on preventing cognitive decline have been conducted. For example, “learning therapy,” consisting of reading aloud and performing simple calculations, has been suggested to improve frontal function8, 9; however, in those programs, confounding factors such as conversation and interaction with staff or other program participants were noted. Social interactions including verbal and non‐verbal behavior such as affective (ie, being thoughtful of others) and visual communication (ie, recognizing facial expressions) are regarded to influence cognitive functions.10, 11 Therefore, it is unknown whether the obtained intervention effect is influenced by reading aloud, by doing calculation, or by social interaction.
Another previous study involving cognitive intervention through a training program for reading a picture book showed improved cognitive function, especially in terms of verbal function.12 However, this program also involved close interaction with other participants. Regardless of this being an issue in numerous cognitive intervention studies that has been discussed among researchers, to our knowledge, no studies have focused on the influence of social interaction in the same intervention programs.
In the present study, we aimed to clarify this issue by conducting a cognitive intervention program using a board game. Board games require a high degree of intellectual stimulation, and it is generally easier for older adults to stay motivated to play such games over the long term. Also, since board games involve methods that must be learned both individually and in groups, it is easy to compare the intervention effects.
We focused on the game “Go” in this study. Go is the oldest and one of the most popular strategic board games in Asian countries, and it has become increasingly popular in Western countries.13 The objective of Go, which begins with a clear board, is to surround more territory with stones than the opponent. Go involves various kinds of strategies, including the need for good decision‐making and to be aware of a wide visual playing space; therefore, Go could strongly influence cognitive function (eg, visuospatial function). We previously investigated the effects of Go on cognitive function in patients with dementia and in persons with mild cognitive impairment (MCI) who were living in a nursing home.14 The results indicated that Go might improve working memory. However, confounding factors such as social interaction with others were inevitable.
Therefore, the purpose of the present study was to examine the effects of a game intervention using Go on cognitive function, focusing on the differences in social interaction which is regarded in this study as verbal and non‐verbal communication.
2. METHODS
2.1. Study design and participants
This single‐blind randomized controlled trial was conducted in Tokyo, Japan. The intervention was conducted from July through September 2017. Outcome assessments were performed at Tokyo Metropolitan Institute of Gerontology (TMIG) before and after the intervention.
We recruited participants by publishing advertisements in community newspapers, posting leaflets in housing complexes, and providing information about the program at regional health examinations. The inclusion criteria were age 65 years or older, able to perform activities of daily living independently, and not having any previous experience playing Go. Participants with medical and psychiatric disorders affecting cognitive function were excluded, as were persons with a diagnosis of dementia.
We explained the purpose, methods, and ethical considerations of the study and obtained written informed consent from each participant before enrollment. The 91 participants who provided informed consent were randomly assigned to one of the following three groups: (1) a face‐to‐face group (FG) (n = 30); (2) a non‐face‐to‐face group (NFG) (n = 30); or (3) an active control group (CG) (n = 31). After randomization, we conducted a baseline survey in June 2017 and excluded any participants who met the exclusion criteria. Finally, 81 participants who met the inclusion criteria started the program.
This study was registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN000030595). The Consolidated Standards of Reporting Trials (CONSORT)15 flow diagram is presented in Figure 1.
Figure 1.
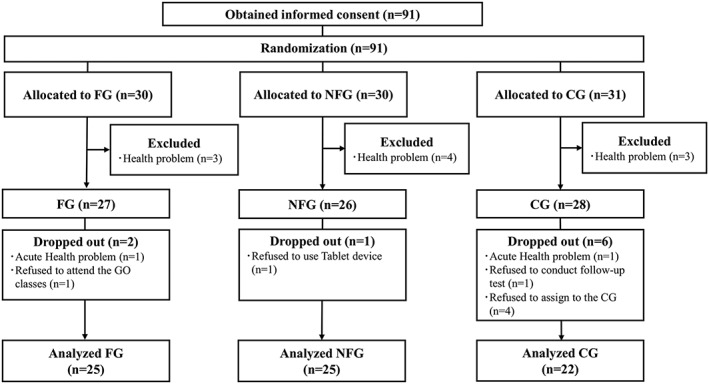
Consolidated Standards of Reporting Trials (CONSORT) flow diagram.CG: Active control group; FG: Face‐to‐face group; NFG: Non‐face‐to‐face group
2.2. Ethical approval
Ethical approval was obtained from the Institutional Review Board and Ethics Committee of the TMIG (Acceptance No. 84, 1, 2017).
2.3. Intervention
2.3.1. Face‐to‐face group (FG)
The participants attended Go group lessons, which were conducted by four Go instructors. Twelve 1‐hour Go classes were held once a week in a community center. Each class involved attending a lecture on basic Go rules and techniques (15 minutes), solving Go exercises (10 minutes), learning tactics using a model game called kifu‐narabe (10 minutes), and playing Go with others (two to four participants) (25 minutes). Participants could talk to and ask questions directly to the instructors and other participants during the Go lessons and while playing the game. Furthermore, at the end of the game, participants were allowed to share each other's impressions and feedback on the game. In the Go classes, an original textbook created by the four instructors was used. Between classes, the participants were required to complete homework assignments (one per day for 6 days) that consisted of Go exercises created by the instructors.
2.3.2. Non‐face‐to‐face group (NFG)
We provided each participant with a tablet computer during the intervention period. Using the tablet, each participant in the NFG individually attended the same Go classes as those in the FG for the same time and period and at the same frequency. Participants could not talk or ask questions directly to the instructors or other participants. A one‐time briefing session regarding the operation of the tablet was held before the intervention. We restricted the participants from operating the tablet in any way that was unrelated to the Go program. We created an original website and released videos with content identical to that learned by the FG so that the NFG could attend lectures using the tablet. For the Go exercises and kifu‐narabe, we provided the same textbooks and homework to the FG and NFG and asked them to complete it by themselves. Participants used a tablet application called “Go Quest” to play Go alone. We asked participants to return the tablet and their homework at the end of the intervention.
2.3.3. Active control group (CG)
Participants attended a 2‐hour lecture on health maintenance, including topics such as frailty, sarcopenia, and depression, once a month. All participants took part in a total of three lectures, all of which were unrelated to Go.
At the orientation session, it was explained to the participants that they were not allowed to learn anything else (including playing Go game) other than the task assigned during the intervention, and the participants complied with the rule. Therefore, the participants of the FG did not play Go using tablet during the intervention period, and the participants of the NFG did not play Go with others face‐to‐face. In the CG, we did not provide any tools for this intervention program and prohibited these participants from learning how to play Go.
2.4. Measures
We set outcome measures for Go performance and each cognitive domain. The primary outcome measure was working memory, and the secondary outcomes were verbal function, executive function, and Go performance. Assessments were performed by testers who were blinded to the participant groups.
2.5. Baseline characteristics
All participants were interviewed to obtain data on their baseline characteristics, including age, sex, education level, past medical history, medication history, functional capacity using the TMIG Index,16 and mental health using a Japanese version of the World Health Organization (WHO)‐Five Well‐Being Index (WHO‐5‐J).17
We also assessed global cognition as a baseline characteristic using the Mini‐Mental State Examination‐Japanese (MMSE‐J)18, 19 and the Japanese version of the Montreal Cognitive Assessment (MoCA‐J).20 The maximum score on both tests is 30 points. The MMSE‐J is a screening tool for dementia and has a cutoff score of 23/24. The MoCA‐J is a brief cognitive screening tool for MCI and has a clinical cutoff score of 25/26.
2.6. Cognitive assessments
2.6.1. Working memory
The Visual Memory Span Test (VMST) from the Wechsler Memory Scale‐Revised21 was used to evaluate visual working memory. The VMST is the sum of forward (VMSF) and backward (VMSB) task subscales. In the VMSF, the examiner touches random square sequences shown on a test paper, and the participants are required to repeat it. In the VMSB, the participants are required to memorize and repeat the sequences in reverse. The score ranges for the VMST, VMSF, and VMSB are 0 to 26, 0 to 14, and 0 to 12, respectively.
The Digit Span Test (DST) from the Wechsler Adult Intelligence Scale‐III22 was used to evaluate verbal working memory. The DST is the sum of forward (DSFT) and backward (DSBT) task subscales. In the DSFT, the participant is required to memorize and repeat a sequence of numbers. In the DSBT, the participants are required to memorize the sequence and repeat it in reverse. The score ranges of the DST, DSFT, and DSBT are 0 to 28, 0 to 16, and 0 to 14, respectively.
2.6.2. Verbal function
The logical memory (LM) I and II subscales of the Wechsler Memory Scale‐Revised21 were used to evaluate immediate and delayed verbal memory. In these tests, the participant is required to remember the content of two short stories. In LM I, the participant is required to repeat both stories immediately after the examiner finishes reading them, and in LM II, the participant is required to recall both stories 30 minutes after. The maximum score for both tests is 50 points.
Verbal fluency tests23 were also used to evaluate verbal function. Each participant was required to generate as many words starting with a specific Japanese syllable or belonging to the same category (eg, “animals”, “vegetables”) as possible within 1 minute.
2.6.3. Executive function
Parts A and B of the Trail Making Test (TMT‐A and TMT‐B)24, 25 were used to evaluate executive function. Both parts of the TMT consist of 25 scattered circles written on the examination paper. In TMT‐A, the circles were numbered from 1 to 25, and the participant was asked to draw lines to connect the numbers in order as quickly as possible. TMT‐A was also used to assess simple attention. In TMT‐B, the circles included either numbers 1 to 13 or the first 12 letters of the Japanese hiragana syllabary. Participants were required to connect the numbers and hiragana letters alternately (for example, participants connected “1” with “a”) as quickly as possible. Faster performance on this examination indicates higher executive function.
2.7. Assessment of Go skill
Four instructors created an original “Go test” and evaluated each participant's learning level. In accordance with the rules and techniques of Go, the Go test consists of four exercises: an early‐stage exercise, a middle‐stage exercise, and a final‐stage exercise. The maximum score is nine points. A higher score indicates better Go playing ability.
2.8. Sample size
Based on our pilot study,14 we hypothesized a medium effect size of the change of scores in the DSTs and VMSTs, which are the main outcomes in this study, among the three groups. The sample size was calculated with G*power (http://www.gpower.hhu.de) based on 95% power, a two‐sided hypothesis test, an alpha level of 5%, and an analysis of variance model between groups over time. The sample size calculation showed that we needed a total of 66 participants with consideration of a 30% drop‐out rate. The results of the G*power were described in Appendix A.
2.9. Randomization
After we received informed consent from the participants, we conducted simple randomization using a random number generation method with the RAND function in Microsoft Excel 2013. Each participant was assigned an identifier (ID number) used for randomization, and their names were deleted during the process. Then, we sent letters to the participants to inform them of their allocation.
2.10. Statistical analysis
All analyses were conducted using SPSS version 23 (IBM Inc., Chicago, IL). Baseline characteristics such as age and education were compared among the three groups using one‐way analysis of variance. The Bonferroni method was used for multiple comparisons and significant differences regarded as P < .016. Comparisons between sexes were conducted using the chi‐square test. Considering that the mean scores of TMIG Index, WHO‐5‐J, MMSE‐J, and MoCA‐J are generally not normal distributed, their mean scores were compared using the Kruskal‐Wallis test. Except for the Bonferroni method, a P value <.05 was considered statistically significant.
Mixed‐model analysis of covariance (ANCOVA) was used to assess the changes before and after the intervention in cognitive and Go test scores. The covariates were age, education level, and baseline MoCA‐J score. Group (FG, NFG, or CG) was a fixed factor, and time (pre and post) was a repeated measures factor. Considering that the Go test was originally created in this study, the normality distribution of scores is unclear. For this reason, we calculated the difference of the scores of the Go test between post‐ and pre‐tests for each group and compared the difference of scores between the intervention groups (FG and NFG) and the CG using Mann‐Whitney U tests as additional analysis.
We compared the magnitude of intervention effects among groups using Cohen's d. To calculate Cohen's d, the differences obtained by subtracting the scores from post‐ to pre‐ intervention in each group were used as indicators. We calculated Cohen's d between the FG and CG and between the NFG and CG.
3. RESULTS
3.1. Compliance with the program
Two participants in the FG, one in the NFG, and six in the CG dropped out of the intervention program for the following reasons: (1) in the FG, one participant was hospitalized with a severe medical disorder, and another refused to attend the Go classes; (2) in the NFG, one participant refused to use the tablet computer; and (3) in the CG, four participants refused to be assigned to the CG, one was hospitalized with a severe medical disorder, and one refused to participate in the follow‐up assessment. Therefore, 25, 25, and 22 participants in the FG, NFG, and CG, respectively, were included in the final analysis. The participants complied with the assigned intervention instructions during the intervention period.
3.2. Characteristics of the participants
Table 1 shows the baseline characteristics of the participants who were included in the final analysis. Significant group differences were found in the MoCA‐J (P < .05) score. Post‐hoc analysis suggested significant differences in MoCA‐J scores between the NFG and CG (P < .016). No significant differences were observed in age, sex, educational history, TMIG Index, WHO‐5‐J, or MMSE‐J.
Table 1.
Baseline characteristics of participants who were included final analysis and ANOVA results (n = 72)
| FG (n = 25) | NFG (n = 25) | CG (n = 22) | P Value | MC | ||
|---|---|---|---|---|---|---|
| Age (mean ± SD) | Years | 76.8 ± 5.4 | 76.5 ± 4.6 | 77.0 ± 3.5 | 0.926 | |
| Gender (male/female) | N | 7/18 | 5/20 | 6/16 | 0.773 | |
| Education (mean ± SD) | Years | 13.0 ± 2.2 | 11.6 ± 2.3 | 13.0 ± 2.4 | 0.444 | |
| TMIG index (mean ± SD) | Score (0‐13) | 11.7 ± 1.5 | 11.3 ± 1.3 | 11.6 ± 1.4 | 0.379 | |
| WHO‐5‐J (mean ± SD) | Score (0‐25) | 15.9 ± 5.1 | 16.9 ± 2.9 | 18.3 ± 2.8 | 0.137 | |
| MMSE‐J (mean ± SD) | Score (0‐30) | 28.2 ± 1.6 | 28.8 ± 1.0 | 28.3 ± 1.5 | 0.076 | |
| MoCA‐J (mean ± SD) | Score (0‐30) | 25.4 ± 2.7 | 25.6 ± 2.8 | 24.9 ± 3.0 | 0.036* | NFG > CG** |
Abbreviations: ANOVA, Analysis of variance; CG, Active control group; FG, Face‐to‐face group; MC, Multiple comparison; MMSE, Mini‐Mental State Examination‐Japanese; MoCA‐J, Japanese version of the Montreal Cognitive Assessment; N, Number of participants; NFG, Non‐face‐to‐face group; SD, Standard deviation; TMIG Index, Tokyo Metropolitan Institute of Gerontology Index; WHO‐5‐J, Japanese version of the World Health Organization‐Five Well‐Being Index.
Comparing the three groups:
P < .05.
Bonferroni method for multiple comparisons:
P < .016 NFG vs CG.
3.3. Effects on cognitive function
Figure 2 shows the main results of the ANCOVA comparing cognitive variables. Significant interactions were observed between groups and times in VMST (F [2, 66] = 3.5, P < .05) and VMSB (F [2, 66] = 3.3, P < .05) scores, which were the main outcome measures of this study.
Figure 2.

The mean scores and ANCOVAa results of main outcomes before and after intervention. (n = 72)ANCOVA: Analysis of covariance; CG: Active control group; FG: Face‐to‐face group; NFG: Non‐face‐to‐face group; VMST: Visual Memory Span test; VSMB: Visual Memory Span Backward task. Error bars represented standard deviation. aAge, education level and the score of MoCA‐J were set as covariates. *P < .05
For the VMST, the simple main effect showed that compared with the CG, scores in the FG were significantly higher after the intervention (P < .05) and that scores in both the FG and the NFG were significantly improved after compared with before the intervention (P < .05). No significant differences were observed in the CG. For the VMSB, the simple main effect showed that compared with the CG, scores in the FG and NFG were significantly higher after the intervention (P < .05) and that scores in the FG were significantly improved after the intervention (P < .05). No significant changes were observed in the CG.
Regarding the magnitude of the intervention effect, the change in VMST scores between the FG and CG (Cohen's d = 0.89) was greater than that between the NFG and CG (Cohen's d = 0.65). In addition, the change in VMSB scores between the FG and CG (Cohen's d = 0.67) was greater than that between the NFG and CG (Cohen's d = 0.56).
No significant differences were observed in any of the other cognitive outcomes. The scores for each cognitive test and the details of the ANCOVA including F values are shown in Appendix A.
3.4. Go playing performance
Figure 3 shows the results of the Go test. Go test scores were significantly improved in the FG and NFG (both P < .01), and the scores were significantly higher for both groups after the intervention compared with the CG (both P < .01). However, no significant differences were observed between the FG and NFG. CG did not show any significant score changes. In addition, as the results of Mann‐Whitney's U test were conducted as additional analysis, the difference of scores of the Go test was significantly large in the FG and NFG compared with that of the CG, and their scores were improved (both P < .05).
Figure 3.

The mean scores and ANCOVAa results of the GO test before and after intervention. (n = 72)ANCOVA: Analysis of covariance; CG: Active control group; FG: Face‐to‐face group; NFG: Non‐face‐to‐face group. Error bars represented standard deviation. aAge, education level and the score of MoCA‐J were set as covariates. *P < .05
4. DISCUSSION
The purpose of the present study was to examine the effects of a Go game intervention program on cognitive function, with a focus on differences in social interaction. The results indicated that visual working memory was improved in both intervention groups (FG and NFG), whereas no significant changes were seen in the CG. In addition, greater intervention effects were observed in the FG compared with the NFG although significant improvements in Go skills were seen both in the FG and NFG.
Go requires players to spatially recognize, memorize, and respond to the moves of their opponent. The results of improved visual working memory reflected the basic processes of playing Go. Moreover, the intervention effect seen in both the FG and NFG supports the hypothesis that playing Go itself improves visual working memory. Regarding the greater intervention effect seen in the FG, the results showed that playing the game face‐to‐face resulted in greater intellectual stimulation than playing the game alone. The reason for this could be affected from social interaction that the participants were attempting to recognize their opponent's facial expressions and surrounding circumstances while playing, as well as talking with their instructors or the other participants during the group lessons. Furthermore, there is a possibility that playing with others required participants to develop critical thinking and concentrate more than playing with computers. Therefore, the difference in concentration during the game may have also influenced the intervention effect in addition to social interaction.
On the other hand, the NFG was exposed to learning elements other than Go, such as tablet operation. Although this could be a potential confounding factor, we regard that the improvement observed in visual working memory was not greatly influenced by learning how to operate the tablet because a previous intervention study using a tablet computer showed no effect on visuospatial function.26
Although no significant differences were observed in the DST, which evaluated verbal working memory, a previous study showed that playing Go improved the DST in nursing home residents.14 This discrepancy may be because participants in the previous study included patients with MCI and dementia; therefore, the Go intervention may be effective to verbal working memory for such patients. However, the intervention effect may not be sufficiently large to improve outcomes in healthy older adults.
We hypothesized that verbal function would be greater in the FG than in the NFG because of the increased social interaction. However, on the verbal fluency and LM tests, which evaluate verbal function, no significant intervention effect was observed in either the FG or the NFG. Some programs that aim at improving verbal function, including the picture book reading program12 and “learning therapy,”8, 9, 27 include numerous verbal training elements, such as reading and speaking sentences. On the other hand, there is a possibility that the degree of verbal communication in the FG during the group lessons did not have enough influence on verbal function. The reason is that in this type of program, people have less opportunities to use words than programs that aim to improve verbal function. To improve verbal function, an intervention program specialized for verbal training may be necessary.
The results of the present study showed that the TMT, which was conducted to assess executive function, had no significant intervention effect in either the FG or the NFG. We hypothesized that the NFG would show greater improvements in executive function than the FG because tablet operation is a new and somewhat complex skill. However, operation of the tablet in this program was not complex enough to effect on executive function.
This study has a few limitations. Regarding the FG, the strength of the interaction and the amount of communication with others differed for each participant. Since the amount of communication could not be measured quantitatively, the exact effect is unknown. Regarding the NFG, the confirmation of progress was done by self‐report, so it was difficult to accurately measure the time the tablet was used. Not being able to control these individual differences was the limitation of this research.
In addition, the long‐term effects and the possibility of continuing to play could not be assessed in this 3‐month intervention. Therefore, follow‐up surveys to evaluate the continuation rate and changes in cognitive function should be conducted in future studies.
5. CONCLUSION
The results of the present study showed that our Go intervention program could improve visual working memory in community‐dwelling older adults. Furthermore, the intervention effects in the FG was greater than those in the NFG although the results showed that both the FG and NFG improved their Go test scores compared with the CG. These results suggest that increased social interaction via playing a board game face‐to‐face is more effective for improving cognitive function than playing the same game alone.
FUNDING
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant Number 16K13036.
DECLARATION OF CONFLICTING INTERESTS
The authors have no potential conflicts of interest to declare with respect to the research, authorship, and/or publication of this article.
AUTHORS CONTRIBUTION
Ai Iizuka was responsible for developing the study concept and design, making intervention program, recruiting participants, and draft of the manuscript. Hiroyuki Suzuki contributed making the study concept and design, analysis and interpretation of data, and drafting manuscript. Susumu Ogawa assisted acquisition of data and interpretation of data. Kimi Estela Kobayashi‐Cuya and Momoko Kobayashi assisted drafting manuscript. Awata Shuichi, Hiroki Inagaki, and Mika Sugiyama assisted recruiting participants and conducting intervention program. Toru Takebayashi contributed to the critical revision of the manuscript. Yoshinori Fujiwara supervised the development of the research question and research accomplishment. All authors approved the final manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank all the participants and our research team colleagues at Tokyo Metropolitan Institute of Gerontology for their cooperation in this study. This program was completed in cooperation with the Go instructors: Mr Fukashi Murakami, Mr Kiyoto Ishibashi, Ms Eriko Ito, and Ms Satoko Chiba; the developer of the application “Go Quest,” Mr Yasushi Tanase; and the video editing cooperator Mr Junji Masamitsu. We sincerely express our gratitude to all collaborators.
APPENDIX A.
Figure A.

Results of sample size calculation by G power. [Colour figure can be viewed at wileyonlinelibrary.com]
Table A.
Cognitive tests scores, GO test scores, and ANCOVAa results before and after intervention (n = 72)
| FG (n = 25) | NFG (n = 25) | CG (n = 22) | ANCOVAa | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Interactiontime × group | ||
| Mean ± SD | Mean ± SD | Mean ± SD | F | P value | ||||
| VMST | 15.4 ± 2.5 | 17.1 ± 2.2 | 14.9 ± 2.8 | 16.0 ± 2.4 | 15.4 ± 2.6 | 15.0 ± 3.0 | 3.56 | 0.034* |
| VMSF | 7.8 ± 1.5 | 8.8 ± 2.0 | 7.5 ± 1.9 | 8.1 ± 1.5 | 8.4 ± 1.8 | 8.5 ± 2.0 | 1.35 | 0.265 |
| VMSB | 7.6 ± 1.5 | 8.3 ± 1.1 | 7.4 ± 1.5 | 7.9 ± 1.4 | 7.0 ± 1.4 | 6.5 ± 1.5 | 3.39 | 0.040* |
| DST | 15.0 ± 3.3 | 14.8 ± 2.8 | 15.6 ± 2.6 | 15.6 ± 2.8 | 15.4 ± 4.0 | 15.5 ± 3.4 | 0.19 | 0.822 |
| DSFT | 9.5 ± 2.0 | 9.2 ± 2.0 | 9.7 ± 1.8 | 9.8 ± 1.7 | 9.8 ± 2.5 | 9.6 ± 2.1 | 0.25 | 0.776 |
| DSBT | 5.5 ± 1.7 | 5.6 ± 1.1 | 5.9 ± 1.3 | 5.8 ± 1.7 | 5.5 ± 2.1 | 5.8 ± 1.7 | 0.33 | 0.719 |
| LMI | 17.1 ± 5.5 | 19.6 ± 4.4 | 18.3 ± 5.8 | 19.9 ± 7.2 | 17.6 ± 6.9 | 20.0 ± 7.7 | 0.08 | 0.922 |
| LM IIb | 13.3 ± 6.8 | 15.8 ± 5.6 | 14.1 ± 4.4 | 17.6 ± 7.0 | 13.8 ± 6.6 | 17.0 ± 7.9 | 1.42 | 0.248 |
| LF‐Ka | 9.8 ± 3.7 | 10.2 ± 2.7 | 10.4 ± 4.2 | 11.0 ± 3.1 | 9.2 ± 3.6 | 9.8 ± 2.9 | 0.10 | 0.898 |
| LF‐Ho | 6.8 ± 2.3 | 7.5 ± 2.9 | 7.7 ± 3.2 | 7.4 ± 2.8 | 6.8 ± 2.7 | 7.0 ± 3.3 | 0.82 | 0.441 |
| CF‐A | 16.0 ± 3.6 | 16.5 ± 3.7 | 14.5 ± 4.9 | 15.4 ± 3.9 | 13.6 ± 3.8 | 15.7 ± 5.1 | 1.98 | 0.146 |
| CF‐Vc | 15.0 ± 3.6 | 14.8 ± 3.6 | 14.8 ± 4.1 | 15.5 ± 3.5 | 13.4 ± 4.3 | 13.5 ± 4.3 | 0.09 | 0.911 |
| TMT‐A | 48.4 ± 17.0 | 41.7 ± 14.3 | 45.8 ± 16.2 | 47.4 ± 13.3 | 48.7 ± 17.2 | 50.8 ± 13.7 | 3.05 | 0.054 |
| TMT‐B | 137.7 ± 66.7 | 118.1 ± 46.8 | 124.9 ± 41.7 | 126.1 ± 44.1 | 163.6 ± 104.3 | 149.8 ± 85.8 | 0.55 | 0.574 |
| GO test | 3.8 ± 1.7 | 6.5 ± 1.7 | 4.1 ± 2.1 | 6.7 ± 1.8 | 4.0 ± 1.7 | 4.1 ± 1.6 | 7.13 | 0.002** |
Abbreviations: ANCOVA, analysis of covariance; CF, Category Fluency; CG, Active control group; DST, Digit Span test; DSBT, Digit Span backward task; DSFT, Digit Span forward task; FG, Face‐to‐face group; LF; Letter Fluency; LM, Logical Memory; N, Number of participants; NFG, Non‐face‐to‐face group; SD, Standard deviation; TMT‐A, Trail Making Test Part A; TMT‐B, Trail Making Test Part B; VMST, Visual Memory Span test; VMSB, Visual Memory Span Backward task; VSMF, Visual Memory Span forward task.
Age, education level, and the score of MoCA‐J were set as covariates.
Since one participant refused to conduct LM II, the number of participant of NFG is 24.
Since one participant refused to conduct CF‐V, the number of participant of FG is 24.
P < .05.
P < .01.
Iizuka A, Suzuki H, Ogawa S, et al. Does social interaction influence the effect of cognitive intervention program? A randomized controlled trial using Go game. Int J Geriatr Psychiatry. 2019;34:324–332. 10.1002/gps.5024
REFERENCE
- 1. Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58(4):617‐622. [DOI] [PubMed] [Google Scholar]
- 3. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(03):448‐460. [PubMed] [Google Scholar]
- 4. Stern C, Munn Z. Cognitive leisure activities and their role in preventing dementia: a systematic review. Int J Evid Based Healthc. 2010;8(1):2‐17. [DOI] [PubMed] [Google Scholar]
- 5. Yates LA, Ziser S, Spector A, Orrell M. Cognitive leisure activities and future risk of cognitive impairment and dementia: systematic review and meta‐analysis. Int Psychogeriatr. 2016;28(11):1791‐1806. [DOI] [PubMed] [Google Scholar]
- 6. Krueger KR, Wilson RS, Kamenetsky JM, Barnes LL, Bienias JL, Bennett DA. Social engagement and cognitive function in old age. Exp Aging Res. 2009;35(1):45‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community‐dwelling elderly persons. Ann Intern Med. 1999;131(3):165‐173. [DOI] [PubMed] [Google Scholar]
- 8. Kawashima R, Okita K, Yamazaki R, et al. Reading aloud and arithmetic calculation improve frontal function of people with dementia. The Journals of Gerontology: Series a. 2005;60(3):380‐384. [DOI] [PubMed] [Google Scholar]
- 9. Kawashima R. Mental exercises for cognitive function: clinical evidence. J Prev Med Public Health. 2013;46(Suppl 1):S22‐S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Jaegher H, Di Paolo E, Gallagher S. Can social interaction constitute social cognition? Trends Cogn Sci. 2010;14(10):441‐447. [DOI] [PubMed] [Google Scholar]
- 11. Zuelsdorff ML, Koscik RL, Okonkwo OC, et al. Social support and verbal interaction are differentially associated with cognitive function in midlife and older age. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2017;15:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki H, Kuraoka M, Yasunaga M, et al. Cognitive intervention through a training program for picture book reading in community‐dwelling older adults: a randomized controlled trial. BMC Geriatr. 2014;14(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho C. GO A Complete Introduction to the Game. Chigasaki: Kisseido Publissing Company; 2015. [Google Scholar]
- 14. Iizuka A, Suzuki H, Ogawa S, et al. Pilot randomized controlled trial of the GO game intervention on cognitive function. Am J Alzheimers Dis Other Demen. 2018;33(3):192‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pandis N, Chung B, Scherer RW, Elbourne D, Altman DG. CONSORT 2010 statement: extension checklist for reporting within person randomized trials. BMJ. 2017;j2835:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koyano W, Shibata H, Nakazato K, Haga H, Suyama Y. Measurement of competence: reliability and validity of the TMIG index of competence. Arch Gerontol Geriatr. 1991;13(2):103‐116. [DOI] [PubMed] [Google Scholar]
- 17. Awata S, Bech P, Koizumi Y, et al. Validity and utility of the Japanese version of the WHO‐five well‐being index in the context of detecting suicidal ideation in elderly community residents. Int Psychogeriatr. 2007;19(01):77‐88. [DOI] [PubMed] [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 19. Sugishita M, Hemmi I. Validity and reliability of Mini Mental State Examination‐Japanese (MMSE‐J): a preliminary report. Japanese J Cogn Neurosci. 2010;12:186‐190. [Google Scholar]
- 20. Fujiwara Y, Suzuki H, Yasunaga M, et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal cognitive assessment. Geriatr Gerontol Int. 2010;10(3):225‐232. [DOI] [PubMed] [Google Scholar]
- 21. Wechsler D. Manual for the Wechsler Memory Scale—Revised. Texas: Psychological Corp; 1987.
- 22. Wechsler D. Wechsler Adult Scale—Third Edition. Psychological Corp: San Antonio, Texas; 1997. [Google Scholar]
- 23. Crossley M, D'Arcy C, Rawson NS. Letter and category fluency in community‐dwelling Canadian seniors: a comparison of normal participants to those with dementia of the Alzheimer or vascular type. J Clin Exp Neuropsychol. 1997;19(1):52‐62. [DOI] [PubMed] [Google Scholar]
- 24. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8(3):271‐276. [Google Scholar]
- 25. Abe M, Suzuki K, Okada K, et al. Normative data on tests for frontal lobe functions: trail making test, verbal fluency, Wisconsin card sorting test (Keio version). No To Shinkei. 2004;56(7):567‐574. [PubMed] [Google Scholar]
- 26. Chan MY, Haber S, Drew LM, Park DC. Training older adults to use tablet computers: does it enhance cognitive function? Gerontologist. 2016;56(3):475‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nouchi R, Taki Y, Takeuchi H, Nozawa T, Sekiguchi A, Kawashima R. Reading aloud and solving simple arithmetic calculation intervention (learning therapy) improves inhibition, verbal episodic memory, focus attention and processing speed in healthy elderly people: evidence from a randomized controlled trial. Front Hum Neurosci. 2016;10(21). [DOI] [PMC free article] [PubMed] [Google Scholar]


