Abstract
The safety and efficacy of sodium‐glucose cotransporter 2 inhibitors in posttransplantation diabetes mellitus is unknown. We converted stable kidney transplant patients to 10 mg empagliflozin, aiming at replacing their insulin therapy (<40 IU/d). N = 14 participants (the required sample size) completed the study visits through 4 weeks and N = 8 through 12 months. Oral glucose tolerance test (OGTT)–derived 2‐hour glucose (primary end point) increased from 232 ± 82 mg/dL (baseline) to 273 ± 116 mg/dL (4 weeks, P = .06) and to 251 ± 71 mg/dL (12 months, P = .41). Self‐monitored blood glucose and hemoglobin A1c were also clinically inferior with empagliflozin monotherapy, such that insulin was reinstituted in 3 of 8 remaining participants. Five participants (2 of them dropouts) vs nine of 24 matched reference patients developed bacterial urinary tract infections (P = .81). In empagliflozin‐treated participants, oral glucose insulin sensitivity decreased and beta‐cell glucose sensitivity increased at the 4‐week and 12‐month OGTTs. Estimated glomerular filtration rate and bioimpedance spectroscopy‐derived extracellular and total body fluid volumes decreased by 4 weeks, but recovered. All participants lost body weight. No participant developed ketoacidosis; 1 patient developed balanitis. In conclusion, although limited by sample size and therefore preliminary, these results suggest that empagliflozin can safely be used as add‐on therapy, if posttransplant diabetes patients are monitored closely (NCT03113110).
Keywords: clinical research/practice, diabetes: new onset/posttransplant, endocrinology/diabetology, kidney (allograft) function/dysfunction, kidney transplantation/nephrology, metabolism/metabolite
Short abstract
Empagliflozin appears safe, but exerts a weak antihyperglycemic effect, suggesting that the drug should be used as add‐on therapy under close medical supervision in patients with stable post–kidney transplantation diabetes mellitus.
Abbreviations
- AUC
area under the ROC curve
- BCM
body composition monitor
- eGFR
estimated glomerular filtration rate
- HbA1c
hemoglobin A1c
- IQRs
interquartile ranges
- KTRs
kidney transplant recipients
- OGTT
oral glucose tolerance test
- PTDM
posttransplantation diabetes mellitus
- SGLT2
sodium‐glucose cotransporter 2
- SMBG
self‐monitored blood glucose
1. INTRODUCTION
Posttransplantation diabetes mellitus (PTDM) is an important complication after kidney transplantation and is associated with increased cardiovascular morbidity, mortality, infections, graft failure, and healthcare cost.1 The mechanisms leading to PTDM include early postoperative stress, increased insulin demand with restoration of kidney function and insulin resistance as well as impaired insulin secretion caused by glucocorticoids and calcineurin inhibitors.2 Because there is consensus to use immunosuppression regimens with the best outcome for patient and graft survival, irrespective of PTDM risk,3 PTDM prevention4, 5 is warranted, but pharmacological therapy6 is often unavoidable.
Inhibitors of the sodium‐glucose cotransporter 2 (SGLT2) are a novel class of oral antidiabetic drugs7 that prevent the reabsorption of glucose at the brush border of the proximal tubule.8 This mechanism leads to glucosuria and to a reduction of blood glucose levels,9 thereby improving glycemic control.10 Moreover, SGLT2 inhibitors have been shown to reduce the risk of cardiovascular events in people with type 2 diabetes,11, 12 and to slow the progression of kidney disease.13, 14 Speculations about the underlying mechanisms for the cardiovascular benefit of SGLT2 inhibitors, especially on heart failure, include hemodynamic alterations15, 16 perhaps related to plasma volume contraction.17
SGLT2 inhibitors might be an important treatment option for the vulnerable, high cardiovascular risk PTDM population.18 Despite abundant information on hemoglobin A1c (HbA1c) reduction with empagliflozin monotherapy,10, 19, 20 details on the glucose metabolism under SGLT2 inhibition are scarce. For the present study, we selected stable kidney transplant recipients (KTRs) with PTDM, on long‐term exogenous insulin therapy. We primarily aimed at withdrawing insulin during an intensive examination period, firstly because the concept of using insulin against early posttransplant hyperglycemia, based on beta‐cell relief,4 might lose benefit if insulin was maintained over the longer term,21 and secondly, because there was no published evidence on the antihyperglycemic effect of SGLT2 inhibitors in PTDM patients, such that monotherapy data would be indispensable for subsequent endeavors to use SGLT2 inhibitors as add‐on therapy.
Specifically, we aimed at determining the efficacy and safety of empagliflozin in KTRs by prospectively analyzing (1) insulin sensitivity and secretion derived from an oral glucose tolerance test (OGTT) and daily glucose profiles; (2) side effects (infections, ketoacidosis, others). We also examined the influence of empagliflozin on the fluid volume status by assessing (3) bioimpedance spectroscopy‐derived markers of fluid volume and body composition. For safety reasons, we chose a short, intensive examination period from 4 weeks before to 4 weeks after insulin withdrawal, despite an overall study duration of 12 months for KTRs using empagliflozin through the initial 4‐week period. The study protocol also encompassed the possibility of reinstituting insulin onto empagliflozin therapy after evaluation of the primary end point (OGTT‐derived 2‐hour glucose) at 4 weeks.
2. METHODS
2.1. Study design, patients, and procedures
The present study was designed as a prospective, interventional, noninferiority pilot study, and conducted at the outpatient clinic of the Medical University of Vienna's Clinical Division of Nephrology (local ethics approval: EK1366/2016, trial registration: EUDRACT No. 2016‐001580‐37; NCT03113110 [http://clinicaltrials.gov]). KTRs ≥18 years, transplanted for ≥6 months, with estimated glomerular filtration rate (eGFR) ≥30 mL/min per 1.73 m2 22 and treated PTDM for ≥6 months were eligible. All patients had to be receiving exogenous insulin therapy (<40 IU per day [total units of isophane, short acting, and dual‐release insulin]). Exclusion criteria included insulin therapy ≥40 IU/d and HbA1c ≥8.5%. All patients provided written informed consent, were pseudonymized, and followed until study completion or their withdrawal from the study. An overview on the study visits and procedures is provided in Figure 1, and the details are described in the supplemental material.
Figure 1.
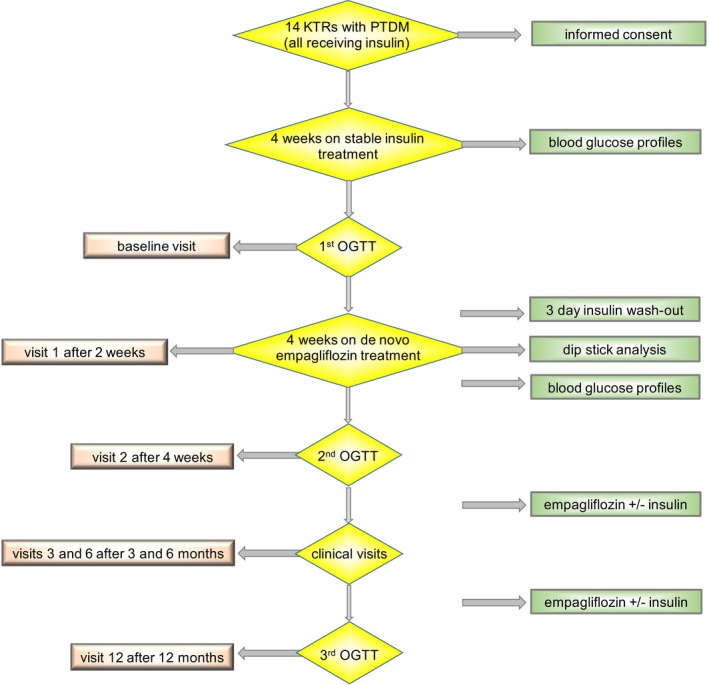
Study design. Glycemic profiles included blood glucose measurements 4 times daily by the patients themselves (blood glucose profiles). Renal function parameters were controlled at every visit. KTRs, kidney transplant recipients; PTDM, posttransplantation diabetes mellitus; OGTT, oral glucose tolerance test
2.2. Study outcomes
We chose the intra‐individual difference in the 2‐hour glucose level between the first OGTT (baseline) and the second OGTT as the primary study end point. We judged an average change of 30 mg/dL to be clinically meaningful, thereby suggesting noninferiority of the empagliflozin treatment if the 2‐hour blood glucose during the second OGTT did not show an increase of >30 mg/dL. Secondary study outcomes included laboratory parameters, anthropometric measurements, blood pressure readings, and medications. Bioimpedance spectroscopy‐based assessment of fluid volume status and body composition was performed with the body composition monitor (BCM; Fresenius Medical Care, Bad Homburg, Germany), as previously described.23 Details on the sample size calculation, OGTT‐derived parameters (including insulin sensitivity and resistance), and BCM measurements are provided in the supplemental material.
2.3. Study safety
As prespecified in the study protocol, reintroduction of exogenous insulin therapy was mandatory if self‐monitored blood glucose (SMBG) levels exceeded 300 mg/dL during the study period or if the 2‐hour glucose obtained during the second OGTT exceeded an increase of 100 mg/dL. To detect diabetic ketoacidosis,24 we performed quantitative ketone body measurements (Freestyle Precision b‐Ketone, Abbott, IL) and urinary dipstick analyses at each study visit. Renal function and other laboratory parameters were determined at each visit. There was no prespecified protocol against bacterial urinary tract infections, but according to clinical practice, empagliflozin treatment was stopped in the case of relapsing bacterial urinary tract infection after a 1‐week period of antibiotic treatment.
2.4. Reference population
Bacterial urinary tract infections occurred in 5 study patients throughout follow‐up but were not previously known to be a typical side effect of the empagliflozin treatment. We therefore amended the study protocol and obtained additional ethics approval for comparing empagliflozin‐treated KTRs with a reference group of PTDM KTRs from our outpatient clinic. Reference patients were matched 2 on 1 with the study population, using age, sex, number of grafts, time after last transplantation, and kidney function (eGFR) as matching criteria (details of the matching precision reported in Table S2). Relevant laboratory parameters (eGFR, HbA1c) and bacterial urinary tract infections (elevated urinary leukocytes plus germ‐proof through bacterial culture and administration of antibiotics) were retrospectively analyzed over the course of the study year, using the inclusion date of each study patient as the beginning of the 1‐year observational period for the matched pairs.
2.5. Statistical analysis
We summarized numerical data as means ± standard deviation or medians with interquartile ranges (IQRs), depending on their distribution. For value comparisons of ordinal and numerical data (primary and secondary outcomes), we used the Wilcoxon signed rank test for dependent samples or the paired Student t test, if data were approximately normally distributed. For nominal parameters, we used the McNemar test for paired samples. A linear mixed model served to compare the daily glucose profiles. We analyzed the occurrence of bacterial urinary tract infections in empagliflozin‐treated study patients vs reference group patients by Kaplan–Meier curves, using the log‐rank test for determination of statistical significance. A P < .05 was considered statistically significant. For calculations we used macOS Excel 2015 (Microsoft Cooperation, Redmond, WA) and macOS IBM SPSS statistics version 24 (SPSS Inc., Chicago, IL) and Stata 9.0 (Stata, College Station, TX).
3. RESULTS
3.1. Study participants
Among all 1120 KTRs monitored at our center's outpatient clinic from December 2016 through June 2017, 192 KTRs had a diabetes diagnosis recorded after transplantation, indicating that they might have developed PTDM, while 76 KTRs carried a diagnosis of “insulin‐dependent diabetes” or “type 2 diabetes” already before transplantation. Most of our screened patients did not meet the inclusion criteria (Figure 2), leaving at least 19 KTRs with stable kidney function, and adequately diagnosed PTDM who were on exogenous insulin therapy.
Figure 2.
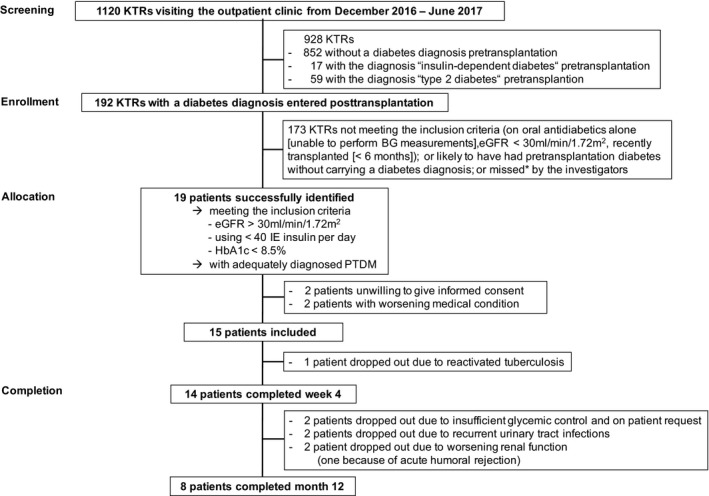
Screening, enrollment, allocation, and completion of the study. KTRs, kidney transplant recipients; BG, blood glucose; eGFR, estimated glomerular filtration rate, PTDM, posttransplantation diabetes mellitus. The primary investigators of the study were able to personally screen approximately 900 of all 1120 patients for the diagnosis of posttransplantation diabetes mellitus during the inclusion period
Fifteen KTRs were included and 14 completed the study procedures through visit 2. Mean age of the participants was 56.5 ± 7.9 years and mean time since transplantation was 69.4 ± 57.2 months (Table 1). The median time to PTDM onset was 2 weeks posttransplant; 3 participants had a late PTDM onset (from 65 to 175 months). Two participants with a second kidney allograft had developed PTDM already after having received their first transplant.
Table 1.
Patient characteristics
| Variables | Patients (N = 14) |
|---|---|
| Recipient age (y), mean (SD) | 56.5 (7.9) |
| Days between baseline and visit 2, mean (SD) | 33 (14) |
| Male, N (%) | 7 (50) |
| Immunosuppression | |
| Tacrolimus, N (%) | 11 (79) |
| Trough level, ng/mL, mean (SD) | 8.4 (3.1) |
| Cyclosporine, N (%) | 3 (21) |
| Trough level, ng/mL, mean (SD) | 64.0 (11.5) |
| Mycophenolate mofetil, N (%) | 9 (64) |
| Dose in mg per d, mean (SD) | 1,444 (391) |
| Mycophenolate sodium, N (%) | 5 (36) |
| Dose in mg per d, mean (SD) | 720 (441) |
| Glucocorticoids, N (%) | 14 (100) |
| Dose in mg Aprednislon per d, mean (SD) | 4 (1) |
| Number of grafts, median (IQR) | 1 (1‐2) |
| Time after transplantation (mo), mean (SD) | 69.4 (57.2) |
| PTDM duration (mo), mean (SD) | 68.1 (57.5) |
| PTDM onset after transplantation (mo), median (IQR) | 0.5 (0.0‐27.9) |
| Duration of insulin therapy (mo), mean (SD) | 55.4 (47.0) |
| Family history of diabetes, N (%) | 6 (43) |
| Hypertension, N (%) | 14 (100) |
| Cardiovascular comorbiditiesa, N (%) | 10 (71) |
| Antidiabetic agents | |
| Long‐acting insulin, N (%) | 8 (57) |
| Short‐acting insulin, N (%) | 4 (29) |
| Combination insulin, N (%) | 5 (43) |
| Oral antidiabetic drugs, N (%) | 3 (21) |
| Insulin, N (%) | 14 (100) |
| Dose in IU per d, mean (SD) | 27.2 (10.5) |
IQR, interquartile range; IU, international units; PTDM, posttransplantation diabetes mellitus; SD, standard deviation.
Cardiovascular comorbidities (at least 1 of the following): cerebrovascular disease or transient ischemic attack, coronary artery disease, peripheral vascular disease, cardiomyopathy or rhythm disorders, arterial or venous thrombosis.
Mean daily insulin dosage at study start was 27.2 ± 10.5 IU, mean PTDM duration was 68.1 ± 57.5 months, and mean duration of insulin therapy was 55.4 ± 47.0 months. Eleven study participants had received insulin as their first antihyperglycemic therapy, while 2 participants had initially been started on oral agents when PTDM was diagnosed, and 1 participant had received dietary advice for several years before insulin had been introduced.
3.2. Glycemic control through 4 weeks
OGTT results are shown in Figure 3 and Table S1. Fasting and 2‐hour glucose levels at baseline were 111 ± 21 mg/dL and 232 ± 82 mg/dL, respectively. Three patients had normal glucose tolerance and 1 patient had impaired glucose tolerance. Three patients were on oral antidiabetic drugs (linagliptin; sitagliptin + metformin; linagliptin, respectively), in addition to insulin. These antidiabetic drugs were discontinued along with the insulin washout (Figure 1).
Figure 3.
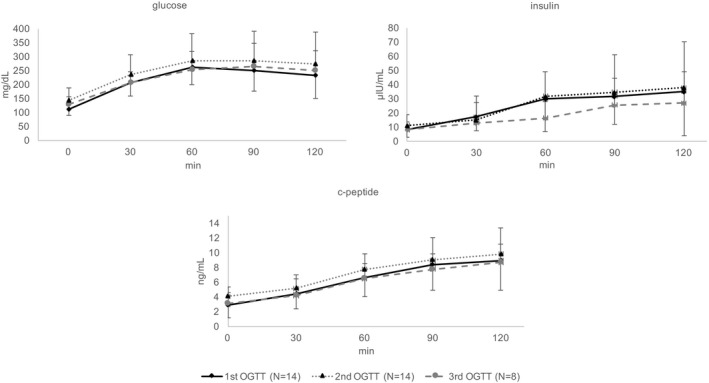
Oral glucose tolerance test (OGTT) results. Blood glucose, insulin, and c‐peptide levels determined by a 75 g oral glucose tolerance test after insulin and empagliflozin treatment. Fasting and 2‐hour glucose levels increased from 111 ± 21 mg/dL and 232 ± 82 mg/dL to 144 ± 45 mg/dL (P = .005) and 273 ± 116 mg/dL (P = .06), respectively, at 4 weeks (N = 14) and to 128 ± 27 (P = .02) and 251 ± 71 (P = .41), respectively, at 12 months (N = 8). Bold lines: baseline; dotted lines: after 4 weeks of empagliflozin treatment
From baseline to the second OGTT, fasting and 2‐hour glucose levels increased to 144 ± 45 mg/dL (P = .005) and 273 ± 116 mg/dL (P = .06), respectively (Figure 3, Table S1). SMBG profiles, shown in Figure 4, were generally higher under empagliflozin monotherapy and HbA1c levels increased from 6.5 ± 0.8 rel.% at baseline to 6.6 ± 0.7 rel.% at study visit 2 (P = .12, Table 3A).
Figure 4.
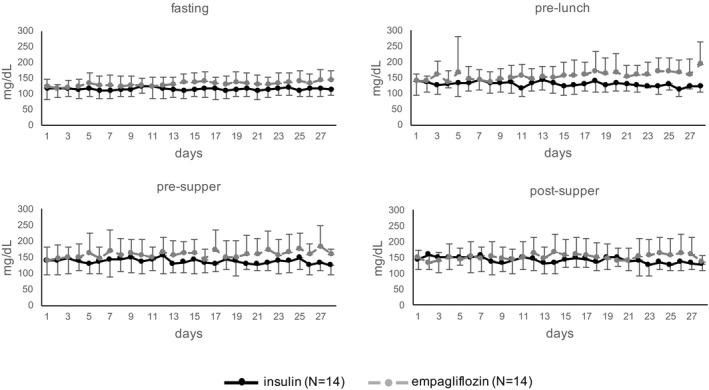
Participants' self‐monitored blood glucose profiles over 28 days. During the first 3 days of empagliflozin treatment, the insulin dosage was slowly reduced (wash‐out phase). Means and standard deviations of blood glucose levels are displayed as bold lines (blood glucose levels during the initial 4‐week period on insulin alone) and dotted lines (blood glucose levels during empagliflozin treatment). Differences in estimated means were calculated using a mixed linear model, between insulin‐ vs empagliflozin‐treated patients: Fasting: mean 114.2 ± 26.3 mg/dL vs 132.5 ± 28.8 mg/dL (95% CI −0.5 to 37 mg/dL, P = .06). Pre‐lunch: mean 131.6 ± 28.8 mg/dL vs 155.9 ± 49.7 mg/dL (95% CI −0.2 to 49 mg/dL, P = .05). Pre‐supper: mean 138 ± 37.9 mg/dL vs 158.8 ± 48.5 mg/dL (95% CI −4 to 46 mg/dL, P = .1). Post‐supper: mean 144.6 ± 43.6 mg/dL vs 152.5 ± 45.2 mg/dL (95% CI −21 to 37 mg/dL; P = .6)
3.3. OGTT‐derived insulin sensitivity and secretion/beta‐cell function through 4 weeks
OGTT‐derived indices of insulin secretion and sensitivity at baseline were comparable to our previous cohort of stable KTRs25 (Table S1). Results at baseline vs visit 2 are shown in Table 2A.
Table 2.
(A) Metabolic parameters and glycemic indices (baseline and 4 weeks, N = 14); (B) Metabolic parameters and glycemic indices (baseline through 12 months, N = 8)
| Variables | Baseline | 4 weeks | P |
|---|---|---|---|
| (A) | |||
| Descriptive parameters | |||
| Area under the curve of glucose (mmol/L)*min, mean (SD) | 1481 (310) | 1686 (574) | .14 |
| Area under the curve of insulin (pmol/L)*min, mean (SD) | 18 139 (10 572) | 19 068 (12 807) | .72 |
| Area under the curve of C‐peptide (pmol/L)*min, mean (SD) | 250 626 (98 304) | 285 949 (83 150) | .16 |
| Insulin resistance/sensitivity | |||
| Homeostatic model assessment – insulin resistance, mean (SD) | 2.23 (1.36) | 4.17 (3.46) | .03 |
| Oral glucose insulin sensitivity index mL/min per m2, mean (SD) | 390 (66) | 328 (85) | .01 |
| PREDIcted M mg kg−1 min−1, mean (SD) | 4.2 (2.0) | 3.5 (1.8) | .02 |
| Insulin secretion/beta‐cell function | |||
| Total insulin secretion nmol m−2, mean (SD) | 42.8 (18.3) | 47.2 (16.3) | .29 |
| Glucose sensitivity, pmol min−1 m−2 mM−1, mean (SD) | 28.6 (17.1) | 36.6 (23.5) | .06 |
| Insulinogenic index pmol/mmol, mean (SD) | 13.9 (11.6) | 20.6 (15.4) | .85 |
| Shape indices | |||
| Shape index of glucose mg/dL min−2, mean (IQR) | 0.06 (0.04) | 0.05 (0.02) | .12 |
| Shape index of insulin μU/mL min−2, mean (IQR) | 0.026 (0.02) | 0.023 (0.013) | .55 |
| Shape index of C‐peptide ng/mL min−2, mean (IQR) | 0.002 (0.001) | 0.002 (0.0009) | .80 |
| Variables | Baseline | 4 weeks | 12 months | P (baseline vs 4 weeks) | P (baseline vs 12 months) |
|---|---|---|---|---|---|
| (B) | |||||
| Descriptive parameters | |||||
| Area under the curve of glucose (mmol/L)*min, mean (SD) | 1 539 (286) | 1 595 (516) | 1 519 (361) | .70 | .81 |
| Area under the curve of insulin (pmol/L)*min, mean (SD) | 20 667 (12 048) | 23 136 (14 681) | 13 015 (6 046) | .56 | .05 |
| Area under the curve of C‐peptide (pmol/L)*min, mean (SD) | 250 733 (79 008) | 310 871 (92 992) | 240 617 (74 454) | .03 | .70 |
| Insulin resistance/sensitivity | |||||
| Homeostatic model assessment – insulin resistance, mean (SD) | 2.60 (1.46) | 4.18 (2.80) | 2.47 (1.70) | .12 | .88 |
| Oral glucose insulin sensitivity index mL/min per m2, mean (SD) | 382 (56) | 319 (50) | 351 (64) | .01 | .04 |
| PREDIcted M mg kg−1 min−1, mean (SD) | 3.4 (1.1) | 3.0 (1.1) | 3.7 (1.4) | .19 | .61 |
| Insulin secretion/beta‐cell function | |||||
| Total insulin secretion nmol m−2, mean (SD) | 42.0 (15.0) | 49.4 (15.3) | 40.0 (11.0) | .08 | .65 |
| Glucose sensitivity, pmol min−1 m−2 mM−1, mean (SD) | 33.6 (18.8) | 41.9 (26.3) | 40.6 (22.0) | .21 | .14 |
| Insulinogenic index pmol/mmol, mean (SD) | 16.8 (14.1) | 25.5 (17.0) | 20.1 (24.4) | .97 | .76 |
| Shape indices | |||||
| Shape index of glucose mg/dL min−2, mean (IQR) | 0.051 (0.012) | 0.046 (0.022) | 0.044 (0.018) | .56 | .34 |
| Shape index of insulin μU/mL min−2, mean (IQR) | 0.032 (0.020) | 0.030 (0.011) | 0.024 (0.0179) | .74 | .37 |
| Shape index of C‐peptide ng/mL min−2, mean (IQR) | 0.002 (0.001) | 0.002 (0.001) | 0.002 (0.001) | .63 | 1.0 |
P values in bold are <.05.
IQR, interquartile range; SD, standard deviation.
3.3.1. Descriptive parameters
The area under the ROC curve (AUC) values for glucose, insulin, and C‐peptide did not differ significantly after 4 weeks of empagliflozin treatment, but there was a trend toward higher levels: an increase of 14%, 5%, and 14% (P = .14, .72, and .16), respectively.
3.3.2. Insulin resistance/sensitivity
Oral glucose insulin sensitivity (OGIS) index decreased from 390 ± 66 to 328 ± 85 mL/min per m2 (P = .01) and similar results were found by PREDIcted M (PREDIM) (4.2 ± 2.0 to 3.5 ± 1.8 mg/kg per min, P = .02). Homeostatic model assessment for insulin resistance (HOMA‐IR), based on fasting insulin and glucose, increased from 2.23 ± 1.36 to 4.17 ± 3.46 (P = .03).
3.3.3. Insulin secretion/beta‐cell function
Insulin secretion, both total and its suprabasal component, was not different after the empagliflozin treatment. However, beta‐cell glucose sensitivity improved from 28.6 ± 17.1 to 36.6 ± 23.5 pmol·min−1·m−2·mM−1, although statistical significance was not reached (P = .06). This was also mirrored by a tendency to improve, though not significantly, of the insulinogenic index.
3.4. Anthropometric measurements, fluid volume status, and body composition through 4 weeks
Average body weight decreased by 1.6 kg from baseline to 4 weeks (P = .02), along with a decrease of 4.0 cm in waist circumference (P = .001; Table 3A). Extracellular fluid volume and total body fluid volume both decreased by 1 L (P < .001, respectively P = .008), while intracellular volume remained unchanged (P = .9). Correspondingly, fluid overload decreased from 2.7 ± 2.1 (baseline) to 1.8 ± 1.8 L (4 weeks, P = .006), which was reflected by a relative decrease in extracellular fluid volume from 13.4 ± 7.4% (baseline) to 9.7 ± 7.7% (4 weeks, P = .02).
Table 3.
(A) Secondary outcome parameters (baseline through 4 weeks, N = 14); (B) secondary outcome parameters (baseline through 12 months, N = 8)
| Variables | Baseline | 2 weeks | 4 weeks | P (baseline vs 4 weeks) |
|---|---|---|---|---|
| Creatinine mg/dL, mean (SD) | 1.3 (0.4) | 1.5 (0.4) | 1.4 (0.4) | .01 |
| eGFR mL/min per 1.73 m2, mean (SD) | 55.6 (20.3) | 47.4 (15.2) | 47.5 (15.1) | .008 |
| Glycated hemoglobin (HbA1c) %, mean (SD) | 6.5 (0.8) | — | 6.6 (0.7) | .12 |
| Hemoglobin g/dL, mean (SD) | 12.7 (1.9) | 12.7 (2.1) | 13.0 (2.1) | .05 |
| Hematocrit %, mean (SD) | 38.8 (5.4) | 39.0 (5.6) | 39.9 (5.4) | .06 |
| Magnesium mmol/L, mean (SD) | 0.70 (0.09) | 0.78 (0.06) | 0.78 (0.07) | .003 |
| Albumin g/L, mean (SD) | 43.2 (3.1) | 44.7 (2.8) | 45.5 (3.4) | .004 |
| Pro‐brain natriuretic peptide pg/mL, mean (SD) | 1131 (1381) | — | 1,076 (1444) | .58 |
| Bicarbonate mmol/L, mean (SD) | 22.4 (2.7) | 20.9 (2.1) | 22.0 (1.6) | .67 |
| Uric acid mg/dL, median (IQR) | 7.5 (6.7‐9.4) | — | 6.2 (5.9‐7.1) | .04 |
| Ketone bodies mmol/L, mean (SD) | 0.16 (0.07) | 0.22 (0.15) | 0.25 (0.10) | .08 |
| Urinary sodium mmol/L, mean (SD) | 103 (21) | — | 85 (30) | .08 |
| Albumin:creatinine ratio mg/g, median (IQR) | 87 (41‐552) | 74 (21‐379) | 62 (28‐348) | .43 |
| Protein:creatinine ratio mg/g, median (IQR) | 289 (190‐808) | 216 (137‐561) | 310 (181‐585) | .42 |
| Glucosuria mg/dL, median (IQR) | 3.0 (0.0‐6.0) | — | 1741.5 (584.0‐2255.8) | .01 |
| Urinary ketone bodies mg/dL, mean (SD) | 0 (0) | 0 (0) | 0.1 (0.5) | .34 |
| Body mass index kg/m2, mean (SD) | 27.3 (5.2) | — | 26.8 (5.7) | .14 |
| Waist circumference cm, mean (SD) | 103.1 (14.4) | — | 99.1 (14.3) | .001 |
| Weight kg, mean (SD) | 74.8 (17.2) | — | 73.2 (17.4) | .02 |
| Total body fluid volume L, mean (SD) | 36.5 (9.5) | — | 35.5 (9.1) | .008 |
| ECV, L, mean (SD) | 18.2 (5.1) | — | 17.2 (4.6) | <.001 |
| Intracellular fluid volume L, mean (SD) | 18.3 (4.6) | — | 18.3 (4.9) | .9 |
| Fluid volume overload L, mean (SD) | 2.7 (2.1) | — | 1.8 (1.8) | .006 |
| Fluid volume overload % ECV, mean (SD) | 13.4 (7.4) | — | 9.7 (7.7) | .02 |
| Muscle mass kg, mean (SD) | 36.6 (11.0) | — | 36.7 (11.9) | .95 |
| Lipid mass kg, mean (SD) | 25.7 (9.9) | — | 25.1 (11.0) | .42 |
| Adipose tissue mass kg, mean (SD) | 35.0 (13.5) | — | 34.3 (14.9) | .46 |
| Systolic blood pressure mm Hg, mean (SD) | 150.0 (25.6) | — | 147.8 (14.0) | .61 |
| Diastolic blood pressure mm Hg, mean (SD) | 86.1 (14.0) | — | 84.5 (13.5) | .67 |
| Patients taking any diuretics, N (%) | 6 (43) | — | 4 (29) | .5 |
| Antihypertensive therapy Na, mean (SD) | 3.1 (1.3) | — | 2.9 (1.4) | .08 |
| Variables | Baseline | 2 weeks | 4 weeks | 3 months | 6 months | 12 months | P (baseline vs 4 weeks) | P (baseline vs 12 months) |
|---|---|---|---|---|---|---|---|---|
| (B) | ||||||||
| Creatinine mg/dL, mean (SD) | 1.4 (0.3) | 1.5 (0.3) | 1.5 (0.4) | 1.4 (0.3) | 1.4 (0.3) | 1.4 (0.3) | .06 | .99 |
| eGFR mL/min 1.73 m2, mean (SD) | 54.0 (23.8) | 47.6 (18.1) | 45.6 (19.7) | 49.8 (16.8) | 54.1 (19.6) | 53.5 (13.3) | .01 | .93 |
| Glycated hemoglobin (HbA1c) %, mean (SD) | 6.7 (0.7) | — | 6.8 (0.6) | 6.8 (0.8) | 7.3 (1.2) | 7.1 (0.8) | .89 | .03 |
| Hemoglobin g/dL, mean (SD) | 12.8 (1.8) | 13.0 (2.2) | 13.0 (2.2) | 12.9 (2.2) | 12.9 (2.2) | 13.0 (2.4) | .4 | .66 |
| Hematocrit %, mean (SD) | 39.2 (4.8) | 39.5 (5.5) | 39.5 (5.8) | 39.7 (5.7) | 39.7 (6.0) | 38.6 (6.3) | .64 | .71 |
| Magnesium mmol/L, mean (SD) | 0.70 (0.11) | 0.76 (0.07) | 0.78 (0.07) | 0.78 (0.07) | 0.78 (0.12) | 0.77 (0.11) | .01 | .003 |
| Albumin g/L, mean (SD) | 43.1 (2.5) | 45.2 (1.8) | 44.6 (2.0) | 44.0 (3.1) | 43.5 (2.5) | 45.0 (3.0) | .11 | .05 |
| Pro‐brain natriuretic peptide pg/mL, mean (SD) | 748 (264) | — | 783 (480) | — | — | 804 (419) | .76 | .58 |
| Bicarbonate mmol/L, mean (SD) | 22.3 (2.2) | 21.2 (2.5) | 21.7 (1.4) | 22.3 (1.6) | 22.5 (1.2) | 22.1 (1.9) | .55 | .74 |
| Uric acid mg/dL, median (IQR) | 7.7 (6.3‐8.3) | — | 6.2 (5.9‐6.5) | 6.6 (4.9‐7.4) | 6.9 (5.5‐7.3) | 6.4 (5.7‐7.2) | .03 | .08 |
| Ketone bodies mmol/L, mean (SD) | 0.18 (0.10) | 0.23 (0.18) | 0.25 (0.10) | — | 0.17 (0.13) | 0.15 (0.15) | .53 | .79 |
| Urinary sodium mmol/L, mean (SD) | 109 (22) | — | 95 (30) | — | — | 92 (33) | .25 | .24 |
| Albumin:creatinine ratio mg/g, median (IQR) | 79 (9‐771) | 73 (18‐609) | 51 (30‐391) | 76 (22‐180) | 39 (7‐102) | 180 (68‐482) | .92 | .75 |
| Albuminuria mg/L, median (IQR) | 35 (7‐911) | 53 (11‐436) | 27 (8‐70) | 87 (18‐156) | 21 (5‐119) | 37 (3‐122) | .75 | .67 |
| Protein:creatinine ratio mg/g, median (IQR) | 206 (84‐901) | 216 (146‐678) | 192 (158‐464) | 200 (146‐332) | 187 (103‐210) | 348 (147‐555) | .92 | 1.0 |
| Glucosuria mg/dL, median (IQR) | 3.5 (0.0‐5.8) | — | 1741.5 (801.3‐2279.5) | — | — | — | .03 | — |
| Urinary ketone bodies mg/dL, mean (SD) | 0 (0) | 0 (0) | 0.3 (0.7) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | .35 | — |
| Body mass index kg/m2, mean (SD) | 29.3 (3.1) | — | 28.8 (3.8) | — | 28.0 (3.2) | 27.7 (3.8) | .35 | .04 |
| Waist circumference cm, mean (SD) | 109.7 (9.5) | — | 106.3 (9.2) | — | — | 102.8 (7.1) | .02 | .007 |
| Weight kg, mean (SD) | 83.7 (7.6) | — | 81.6 (7.4) | — | — | 78.7 (7.7) | .03 | .02 |
| Total body fluid volume L, mean (SD) | 40.5 (7.5) | — | 39.5 (7.2) | — | — | 38.9 (7.7) | .06 | .12 |
| ECV, L, mean (SD) | 20.3 (3.8) | — | 19.3 (3.2) | — | — | 19.5 (3.8) | .007 | .18 |
| Intracellular fluid volume, L, mean (SD) | 20.2 (4.1) | — | 20.2 (4.5) | — | — | 19.4 (4.5) | .95 | .13 |
| Fluid volume overload L, mean (SD) | 3.2 (1.9) | — | 2.3 (1.8) | — | — | 3.2 (2.8) | .04 | .92 |
| Fluid volume overload % ECV, mean (SD) | 15.0 (6.8) | — | 11.5 (7.1) | — | — | 15.2 (10.1) | .05 | .93 |
| Muscle mass kg, mean (SD) | 40.0 (11.2) | — | 40.5 (12.2) | — | — | 39.0 (12.0) | .70 | .50 |
| Lipid mass kg, mean (SD) | 29.4 (6.8) | — | 28.2 (7.6) | — | — | 26.4 (7.9) | .27 | .21 |
| Adipose tissue mass kg, mean (SD) | 39.9 (9.2) | — | 38.3 (10.4) | — | — | 35.9 (10.7) | .28 | .21 |
| Systolic blood pressure mm Hg, mean (SD) | 150 (26) | — | 149 (16) | — | 134 (20) | 145 (20) | .75 | .36 |
| Diastolic blood pressure mm Hg, mean (SD) | 86 (14) | — | 84 (16) | — | 71 (11) | 76 (11) | .59 | .02 |
| Patients taking any diuretics, N (%) | 4 (50) | — | 3 (38) | — | — | 3 (38) | 1.0 | 1.0 |
| Antihypertensive therapy Na, mean (SD) | 3.4 (1.1) | — | 3.3 (1.0) | — | — | 4.3 (1.0) | .35 | .11 |
P values in bold are <.05.
ECV, extracellular fluid volume; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SD, standard deviation.
Diuretics were not counted as antihypertensives, because diuretics were analyzed separately (1 row above).
3.5. Safety end points through 4 weeks
From baseline to 4 weeks, eGFR decreased from 55.6 ± 20.3 to 47.5 ± 15.1 mL/min per 1.73 m2 (P = .008, Table 3A). One patient had minimal urinary ketone body excretion (2 mg/dL) but no case of ketoacidosis occurred. Through the initial 4‐week empagliflozin monotherapy period, we observed bacterial urinary tract infections in 3 patients (21%) and recorded 1 hospitalization due to pneumonia in another patient. There was 1 case of mild hyponatremia (134 mEq/L) and no case of hypoglycemia or orthostatic dysregulation. One study participant had an uncomplicated balanitis, which resolved with local therapy.
3.6. Follow‐up through 12 months
After 4 weeks, exogenous insulin was re‐introduced in addition to empagliflozin therapy in 7 participants, 4 of whom later on dropped out of the study (6 dropouts altogether; details in Figure 2 and Table S5). Eight patients (3 of them with insulin add‐on therapy: insulin dose 28.0 ± 7.2 IU/d [baseline], 20 ± 2 IU/d [12 months]) completed the study through 12 months and underwent 3 subsequent study visits as well as a third OGTT (Figure 3, Table S1). Metabolic parameters and glycemic indices are shown in Table 2B and secondary outcome parameters in Table 3B. Patients who remained in the study through 12 months experienced meaningful average reductions in body weight (−5 kg), body mass index (−1.6 kg/m2), and waist circumference (−6.9 cm), but still had higher average HbA1c levels (+0.4 rel.%), compared to baseline. Fluid parameters at 12 months were comparable to baseline, but patients on average had reduced adipose tissue mass (−4 kg) and reduced muscle mass (−1 kg). Kidney function at 3, 6, and 12 months was unchanged in comparison to baseline (Table 3B), and also remained unchanged in PTDM patients from an untreated reference group (Tables S3 and S4). Bacterial urinary infections occurred in 5 empagliflozin‐treated patients, and in 9 of 24 patients from the untreated PTDM reference group (P = .81, Figure 5).
Figure 5.
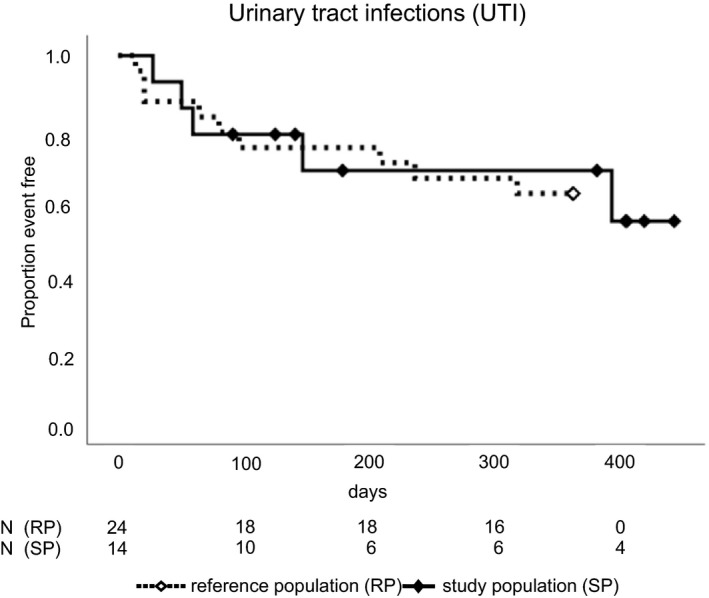
Bacterial urinary tract infections. Kaplan–Meier curves for the study population (SP) vs the matched reference population (RP). “Proportion of event free” refers to urinary tract infections, dropping out, and end of study in the SP, but to urinary tract infections alone in the RP; P = .81 by log rank test. N, number of patients
4. DISCUSSION
In this first trial on the use of an SGLT2 inhibitor in PTDM patients, we evaluated the glucose metabolism and fluid status as well as several secondary end points before and after 4 weeks on empagliflozin monotherapy, and through a subsequent follow‐up period of 1 year.
Glucose control under empagliflozin monotherapy was clinically inferior compared to the prior exogenous insulin treatment, shown by SMBG, fasting and 2‐hour glucose at the 4‐week OGTT, and HbA1c. Although this finding was clinically evident and 7 participants added insulin back to therapy through the subsequent 1‐year follow‐up, statistical significance was not reached for OGTT‐derived 2‐hour glucose at 4 weeks (primary end point). When designing the present trial, we aimed at empagliflozin monotherapy rather than add‐on therapy, at least for the intensive evaluation period, to protect OGTT‐derived 4‐week results on insulin resistance and insulin secretion from the influence of other antidiabetic drugs. In drug‐naïve type 2 diabetic patients, empagliflozin monotherapy had led to an HbA1c reduction of 0.62‐0.78 rel.%.10, 19, 20 Our study participants with PTDM had relatively high insulin doses, and their PTDM duration was likely too long for PTDM to be reversible.
OGTT‐derived indices and data on insulin resistance as well as insulin secretion have not been reported for empagliflozin‐treated patients. Glucose levels during the second OGTT were altogether higher than baseline. Beta cells seemed to react to the increased glycemic levels, because C‐peptide levels trended higher. Hepatic extraction may have accounted for the fact that the insulin AUC did not mirror C‐peptide levels. Worsening insulin sensitivity under empagliflozin treatment was consistent when considering dynamic conditions (OGIS, PREDIM). These results, however, are not surprising because the glycemic control deteriorated altogether. The observed increase in beta‐cell glucose sensitivity during empagliflozin treatment is consistent with previous clamp experiments.26
Mechanistically it has been proposed that by inhibiting SGLT2, the increased sodium delivery to the macula densa decreases glomerular hyperfiltration by enhancing the tubuloglomerular feedback.13 In the present study, eGFR transiently decreased by 8.1 mL/min per 1.73 m2 at 4 weeks (Table 3A), in line with this proposed mechanism (but rectified thereafter, Table 3B). While serum albumin increased after empagliflozin treatment, the albumin:creatinine ratio remained unchanged, unlike previous findings of a decreased progression to macroalbuminuria.13
We observed a large and statistically significant reduction in body mass index, body weight, and waist circumference by 12 months. Bioimpedance spectroscopy revealed a reduction of approximately 1 L in extracellular and total body fluid volume by 4 weeks, and a reduction in fat mass throughout the entire follow‐up. The fluid parameters, however, returned to baseline after 4 weeks. It remains to be determined whether the results of our study may be interpreted in context with a recent mediation analysis, which found that changes in markers of plasma volume (hematocrit and hemoglobin) mediated ≈50% of the effect of empagliflozin vs placebo on the risk of cardiovascular death.17
Risk of ketoacidosis24: During the 4 weeks of empagliflozin treatment, we observed a minimal ketone body excretion without any signs of ketoacidosis in 1 participant, and negative quantitative serum ketone body measurements in 11 out of 14 participants. Throughout the subsequent follow‐up of the remaining 8 participants, quantitative ketone bodies remained negative, bicarbonate stable, and no case of ketoacidosis occurred. For safety reasons, all participants were encouraged to perform urine dipstick analyses once a week, but the large majority did not provide evaluable results.
A recent meta‐analysis of randomized controlled trials showed no significant difference in urinary tract infections between SGLT inhibitors vs control.28 In KTRs, however, urinary tract infections are a major concern,29 and patients who develop urinary tract infections will likely discontinue the SGLT2 inhibitor (Figure 2), which increases urinary glucose. Concerns about urinary tract infections led to the creation of an independent PTDM reference population (Tables S2‐S4), which showed the same incidence of bacterial urinary tract infections as in empagliflozin‐treated study participants (Figure 5). The incidence of urinary tract infections among study participants and reference patients was higher than in a previous study using Medicare claims data, which was, however, not exclusively performed in PTDM patients.30 We therefore suggest careful monitoring of urinary tract infections in PTDM patients, especially when using an SGLT2 inhibitor.
The present study has several limitations, especially its small sample size and the fact that it was nonrandomized. We focused on glucose metabolism and fluid status, thereby lacking some other specific outcomes such as bone parameters. The number of statistical tests surpassed the number of study participants included, and the interpretation of the glycemic indices at 12 months is difficult, due to an even smaller number of patients and the reintroduction of exogenous insulin treatment.
However, and in conclusion, our trial represents the first study to date to assess SGLT2 inhibition in patients after solid organ transplantation (Figure 6). Among other SGLT2 inhibitor publications, our analysis is the first to present data on fluid volume, besides detailed results on OGTT‐derived indices, which are otherwise lacking for SGLT2 inhibitors. Although empagliflozin alone was not powerful enough to replace exogenous insulin in KTRs with PTDM, our results on patient safety, metabolic control, and fat loss suggest value for using and further studying empagliflozin or another SGLT2 inhibitor as add‐on therapy in immunosuppressed patients, who nevertheless have to be monitored carefully.
Figure 6.
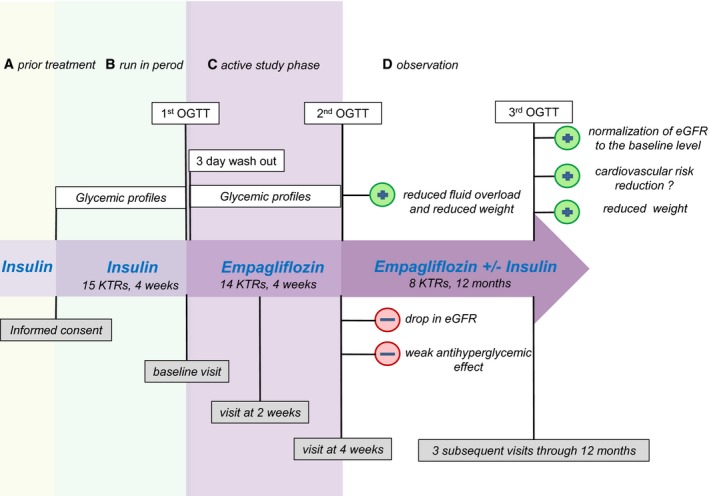
Concept. OGTT, oral glucose tolerance test; KTRs, kidney transplant recipients
AUTHOR CONTRIBUTIONS
ES, MH: Conception and design, analysis and interpretation of data; creation of tables and figures, article drafting and revision. ES, LS, LB: Acquisition of data. RR: Analysis and interpretation of data, statistics. CK, Conception and design. AT, GP, TW: Analysis and interpretation of data. MA, SS, JW, MDS: Article drafting and revision. All authors revised the paper and approved the final version of the manuscript. All authors agreed to be accountable for all aspects of the work.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
ACKNOWLEDGMENTS
The present academic study received no funding. Study medication (Jardiance®) was kindly provided as drug patterns by the First Medical Department of the Kaiser‐Franz‐Josef Hospital Vienna and the Department of Endocrinology at MUV. We thank Guntram Schernthaner for critically revising our manuscript.
Schwaiger E, Burghart L, Signorini L, et al. Empagliflozin in posttransplantation diabetes mellitus: A prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant. 2019;19:907–919. 10.1111/ajt.15223
[The copyright line for this article was changed on April 5, 2019, after original publication.]
REFERENCES
- 1. Sharif A, Baboolal K. Complications associated with new‐onset diabetes after kidney transplantation. Nat Rev Nephrol. 2011;8(1):34‐42. [DOI] [PubMed] [Google Scholar]
- 2. Hecking M, Werzowa J, Haidinger M, et al. Novel views on new‐onset diabetes after transplantation: development, prevention and treatment. Nephrol Dial Transplant. 2013;28(3):550‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharif A, Hecking M, de Vries AP, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14(9):1992‐2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hecking M, Haidinger M, Doller D, et al. Early basal insulin therapy decreases new‐onset diabetes after renal transplantation. J Am Soc Nephrol. 2012;23(4):739‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chakkera HA, Weil EJ, Pham PT, Pomeroy J, Knowler WC. Can new‐onset diabetes after kidney transplant be prevented? Diabetes Care. 2013;36(5):1406‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Werzowa J, Saemann M, Haidinger M, Krebs M, Hecking M. Antidiabetic therapy in post kidney transplantation diabetes mellitus. Transplant Rev (Orlando). 2015;29:145‐153. [DOI] [PubMed] [Google Scholar]
- 7. Akhtar N. Type 2 diabetes mellitus and Invokana: an FDA approved drug. Curr Diabetes Rev. 2013;9(6):478‐490. [DOI] [PubMed] [Google Scholar]
- 8. Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium‐glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75(12):1272‐1277. [DOI] [PubMed] [Google Scholar]
- 9. Chao EC, Henry RR. SGLT2 inhibition–a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9(7):551‐559. [DOI] [PubMed] [Google Scholar]
- 10. Liakos A, Karagiannis T, Athanasiadou E, et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta‐analysis. Diabetes Obes Metab. 2014;16(10):984‐993. [DOI] [PubMed] [Google Scholar]
- 11. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 12. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 13. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 14. Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28(1):368‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 Inhibition and cardiovascular events: why did EMPA‐REG outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59(7):1333‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rajasekeran H, Lytvyn Y, Cherney DZ. Sodium‐glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: the emerging role of natriuresis. Kidney Int. 2016;89(3):524‐526. [DOI] [PubMed] [Google Scholar]
- 17. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG Outcome trial. Diabetes Care. 2018;41(2):356‐363. [DOI] [PubMed] [Google Scholar]
- 18. Sharif A, Cohney S. Post‐transplantation diabetes‐state of the art. Lancet Diabetes Endocrinol. 2016;4(4):337‐349. [DOI] [PubMed] [Google Scholar]
- 19. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208‐219. [DOI] [PubMed] [Google Scholar]
- 20. Roden M, Merker L, Christiansen AV, et al. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug‐naive patients with type 2 diabetes: a double‐blind extension of a Phase III randomized controlled trial. Cardiovasc Diabetol. 2015;14:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lebovitz HE. Insulin: potential negative consequences of early routine use in patients with type 2 diabetes. Diabetes Care. 2011;34(Suppl 2):S225‐S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461‐470. [DOI] [PubMed] [Google Scholar]
- 23. Ernstbrunner M, Kabon B, Zotti O, et al. Intravenous fluid challenge decreases intracellular volume: a bioimpedance spectroscopy‐based crossover study in healthy volunteers. Sci Rep. 2017;7(1):9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38(9):1638‐1642. [DOI] [PubMed] [Google Scholar]
- 25. Hecking M, Kainz A, Werzowa J, et al. Glucose metabolism after renal transplantation. Diabetes Care. 2013;36(9):2763‐2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al Jobori H, Daniele G, Adams J, et al. Empagliflozin treatment is associated with improved beta cell function in T2DM. J Clin Endocrinol Metab. 2018;103:1402‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. U.S. Food and Drug Administration . https://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed April 28, 2018.
- 28. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta‐analysis. Sci Rep. 2017;7(1):2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saemann M, Horl WH. Urinary tract infection in renal transplant recipients. Eur J Clin Invest. 2008;38(suppl 2):58‐65. [DOI] [PubMed] [Google Scholar]
- 30. Abbott KC, Swanson SJ, Richter ER, et al. Late urinary tract infection after renal transplantation in the United States. Am J Kidney Dis. 2004;44(2):353‐362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


