Abstract
Background and purpose
GLORIA, a registry conducted with 375 advanced Parkinson's disease patients treated with levodopa‐carbidopa intestinal gel (LCIG) for 24 months in routine clinical care, demonstrated significant reductions from baseline in ‘off’ time and ‘on’ time with dyskinesia and improvements in the Non‐Motor Symptom Scale (NMSS) total and individual domain scores, and in Parkinson's Disease Questionnaire 8 item (PDQ‐8) total score.
Methods
Associations between baseline NMSS burden (NMSB), the multi‐domain NMSS total score and the PDQ‐8 total score were investigated for 233 patients. Baseline NMSB was assigned to five numerical categories defined by the NMSS total cutoff scores (0–20, 21–40, 41–60, 61–80 and >80). Pearson and Spearman correlations were calculated at month 24.
Results
The response of LCIG was assessed using validated criteria after 24 months. The proportion of patients decreasing ≥ 30 NMSS score points was 47% in the most affected NMSB category (NMSS total score > 80). A positive association was noted between baseline NMSB and NMSS total score (0.57, P < 0.0001), as well as between NMSS total score and PDQ‐8 total score (0.46, P < 0.0001). Associations between improvements of the NMSS domain sleep/fatigue and PDQ‐8 total score (0.32, P = 0.0001) as well as between the NMSS domain mood/cognition and PDQ‐8 total score (0.37, P < 0.0001) were also shown.
Conclusions
This analysis demonstrated positive associations between NMSS baseline burden and improvements of non‐motor symptoms. Improvements of non‐motor symptoms were associated with improved quality of life in advanced parkinsonian patients during a 2‐year treatment with LCIG and reflect the long‐term non‐motor efficacy of this treatment.
Keywords: levodopa‐carbidopa intestinal gel, non‐motor symptoms, Parkinson's disease, quality of life, routine patient care
Introduction
Parkinson's disease (PD) is characterized by a combination of motor and non‐motor symptoms (NMSs) underpinned by relevant neuropathological changes 1, 2, 3, 4, 5, 6, 7 which contribute significantly to morbidity, loss of autonomy, institutionalization and increases in healthcare costs 8, 9. The Non‐Motor Symptom Scale (NMSS) and the NMS questionnaire are validated tools 10, 11 to assess a broad range of NMSs and have been used in many recent PD studies 12, 13, 14, 15, 16, 17, 18.
Management of PD requires recognition of both motor and non‐motor disturbances as well as an understanding of the relationship between these symptoms and how they can be ameliorated by medications 9, 16. Treatment of NMSs can be challenging as these symptoms are often underestimated or unrecognized by clinicians and remain untreated 2. When identified, there is a common perception that dopaminergic drugs are not efficacious in improving these symptoms 9, 19. Furthermore, the progression of NMSs contributes importantly to the decline in quality of life (QoL) in patients with PD 9, 11, 16, 20. There are emerging clinical data that the overall burden of NMSs seems to be the major determinant of QoL, and that NMSs, as a whole, have a greater impact than motor symptoms on QoL in patients with motor fluctuations 21. A good body of evidence suggests that motor symptoms and NMSs originate from distinct pathophysiological pathways although NMSs may respond to dopaminergic treatment 22, 23.
A longitudinal multivariate analysis study showed that the total NMS burden (NMSB) significantly predicted the QoL scores whilst motor scores did not. The burden of NMSs, in particular sleep, mood and attention, had a significant impact on the QoL of PD patients 24.
The comprehensive clinical data of the GLORIA long‐term registry in 375 patients with advanced PD 25 offered the opportunity to investigate whether baseline (BL) NMSB would predict improvements of NMSs as well as improvements between NMSs and QoL following a 24‐month levodopa‐carbidopa intestinal gel (LCIG) treatment.
Patients and methods
Study design
GLORIA, a multinational, non‐interventional, observational registry, was conducted at 75 specialized movement disorder centres across 18 countries in advanced PD patients with persistent motor complications. The study protocol was approved by national and/or local independent ethics committees and health authorities at each participating institution and country. All patients provided written informed consent before enrolment in the registry. The results of the GLORIA registry, both the 12‐month interim and the final 24‐month analysis, were published 15, 26.
Patients
Male and female patients with advanced PD and severe motor complications eligible for LCIG treatment based on the approved European Commission Summary of Product Characteristics and national reimbursement criteria/local pathways were enrolled in the GLORIA study. Clinical observations of the initial 24‐month LCIG treatment phase were collected.
Efficacy and safety
The following efficacy and safety outcomes were assessed: Unified Parkinson's Disease Rating Scale (UPDRS) parts II, III, IV and V; complications of therapy [UPDRS IV items 32 and 39 modified according to the Movement Disorder Society–UPDRS (corresponding parts 4.3 and 4.1) to allow for calculation of actual hours of ‘off’ time and ‘on’ time with dyskinesias]. NMSs were assessed using the NMSS. Patient‐reported QoL was assessed using the short‐form Parkinson's Disease Questionnaire (PDQ‐8) 27 and generic EuroQoL‐5 Dimensions quality of life instrument questionnaire (EQ‐5D). All adverse drug reactions (ADR) were recorded during LCIG treatment. ADRs were defined as those adverse events that in the opinion of the investigator had at least a reasonable possibility of being causally related to the study drug, as described previously 15, 26. Clinical data were recorded at BL prior to initiation of LCIG using a temporal nasojejunal tube, at discharge from the hospital following percutaneous endoscopic gastrostomy with jejunal extension tube placement (day 1), and at months 6, 12, 18 and 24.
Post hoc analyses
Potential associations between NMSB at BL and improvements of NMSs as well as between improvements of NMSs and QoL during LCIG treatment were assessed. The following five categories of BL NMSB were numerically defined: NMSS total score 0–20, 21–40, 41–60, 61–80 and >80. Correlation analysis was performed between categories of BL NMSB and improvements of NMSS total score, improvements of NMSS total score and PDQ‐8 total score, and improvements of NMSS domain scores (sleep/fatigue and mood/cognition) and PDQ‐8 total score. The analyses are presented by Pearson and Spearman correlation coefficients (prob > |r| under H0: ρ = 0) and shown in regression plots.
Results
All patients with a BL NMSS assessment (233 out of 375 patients included in GLORIA) were included in this post hoc analysis. 12‐ and 24‐month NMSS recordings were available for 194 and 170, respectively, out of 258 patients who completed GLORIA. The mean ± SD NMSS total score, demographics and PD characteristics at BL of patients allocated to the five numerically defined NMSB categories (0–20, 21–40, 41–60, 61–80 and >80) and the total post hoc analysis population (PHP) are shown in Table 1. 12.0% of the patients were allocated to the lowest NMSB category (NMSS total score 0–20, 10.8 ± 4.77) and 38.2% to the highest NMSB category (NMSS total score >80; 112.8 ± 27.38). The mean age (66.2 ± 8.52 years in the PHP) and time since PD diagnosis (12.5 ± 5.93 years in the PHP) were similar, and time with dyskinesia (4.2 ± 3.66 h in the PHP) and ‘off’ time (5.9 ± 3.02 h in the PHP) were comparable across all five NMSB categories, whilst the PDQ‐8 total scores (46.8 ± 18.63 in the PHP) increased from 35.9 ± 17.47 in the lowest to 54.3 ± 18.81 in the highest NMSB category.
Table 1.
Demographics and baseline disease characteristics in patients allocated to one of the five baseline NMSB categories defined by the NMSS total score cut‐off scores 0–20 (N = 28), 21–40 N = 41), 41–60 (N = 39), 61–80 (N = 36) and >80 (N = 89) and in the total post hoc analysis population (N = 233)
| Baseline NMSS total score burden | ||||||
|---|---|---|---|---|---|---|
| 0–20 | 21–40 | 41–60 | 61–80 | >80 | All | |
| (N = 28) | (N = 41) | (N = 39) | (N = 36) | (N = 89) | (N = 233) | |
| Age (years) | 64.6 ± 8.98 | 64.9 ± 9.12 | 66.4 ± 7.97 | 67.5 ± 9.10 | 66.6 ± 8.13 | 66.2 ± 8.52 |
| BMI | 24.8 ± 4.17 | 25.1 ± 4.40 | 26.5 ± 5.35 | 24.7 ± 4.10 | 25.2 ± 4.23 | 25.2 ± 4.40 |
| Time since PD diagnosis (years) | 13.4 ± 5.45 | 12.7 ± 4.47 | 11.7 ± 5.96 | 12.3 ± 7.58 | 12.6 ± 5.97 | 12.5 ± 5.93 |
| UPDRS part IV modified item 32: Time with dyskinesia (h) | 3.0 ± 2.45 | 3.8 ± 2.94 | 4.8 ± 3.50 | 5.3 ± 4.71 | 4.2 ± 3.86 | 4.2 ± 3.66 |
| UPDRS part IV modified item 39: ‘Off’ time (h) | 6.0 ± 2.32 | 5.7 ± 3.47 | 5.2 ± 2.69 | 4.9 ± 3.27 | 6.6 ± 2.87 | 5.9 ± 3.02 |
| NMSS total score | 10.8 ± 4.77 | 29.6 ± 6.41 | 51.9 ± 6.32 | 70.5 ± 5.17 | 112.8 ± 27.38 | 69.18 ± 42.13 |
| Total PDQ‐8 score | 35.9 ± 17.47 | 38.6 ± 16.79 | 45.2 ± 18.98 | 46.3 ± 12.33 | 54.3 ± 18.81 | 46.8 ± 18.63 |
BMI, body mass index; NMSB, NMSS burden; NMSS, Non‐Motor Symptom Scale; PDQ‐8, Parkinson's Disease Questionnaire 8 item; UPDRS, Unified Parkinson's Disease Rating Scale.
Values are presented as mean ± SD or percentage of patients.
NMSS total scores in bold represents the key NMS data reflecting rising NMS burden as pre cut off scores in top panel.
The median NMSS and PDQ‐8 total scores for the five NMSB categories and the complete PHP at BL, 12 months and 24 months are presented in Fig. 1. The median NMSS total score changes showed a trend toward more substantial improvements from the lowest to the higher BL burden categories, at similar magnitudes at 12 months and 24 months, being highest in the >80 NMSB category. The median of the PDQ‐8 total score changes did not show a consistent trend across all five categories, and improvements showed a comparable magnitude at 12 months and 24 months. (Fig. 1).
Figure 1.
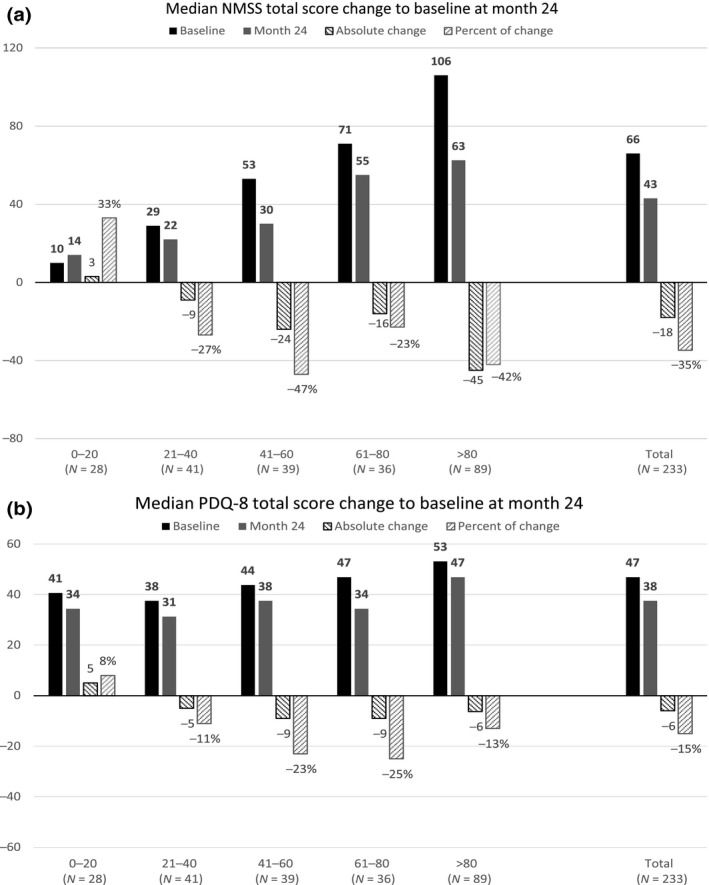
(a) Median NMSS total score and (b) median PDQ‐8 total at BL, and median change to BL scores (absolute change and percent of change) in the five BL NMSB categories defined by the BL NMSS total score ranges 0–20 (N = 28), 21–40, N = 41), 41–60 (N = 39), 61–80 (N = 36) and >80 (N = 89) at month 12 and month 24 in the total post hoc analysis population (N = 233).
The NMSS responder rates by BL NMSB categories are shown in Fig. 2. The rates for the overall response (improvement ≥5 score points) increased gradually from the lowest (0–20) to the higher NMSB categories and reached a maximum of 58% in the highest category (>80). Also, the responder rates for improvements of ≥10, ≥20 and ≥30 score points increased gradually from the lowest (0–20) to the highest NMSS total score category (>80) and reached a maximum response rate of 47% for improvements of ≥30 in NMSS scores.
Figure 2.
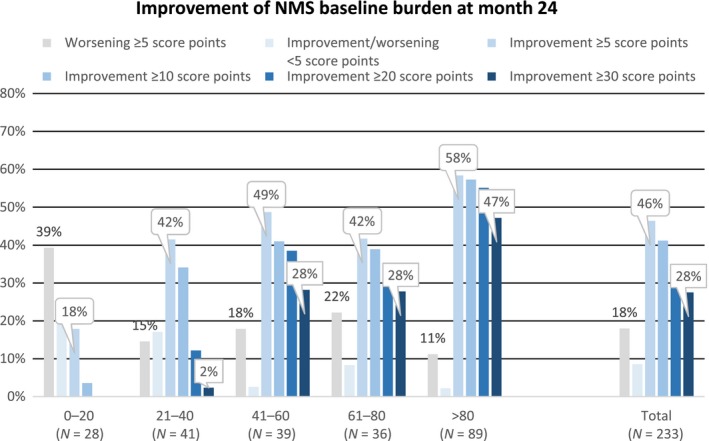
NMSS responder rates (NMSS total score improvements ≥5 score points, ≥10 score points, ≥20 score points and ≥30 score points) by the BL NMSB categories and the total post hoc analysis population (PHP).
The results of the correlation analysis are shown in Table 2. The Pearson correlation coefficient between the BL NMSS total score and the NMSS total score improvements (0.57, P < 0.0001) is strong. The Pearson correlation coefficient between the improvements of the NMSS total score and the PDQ‐8 total score improvements was moderate (0.46, P < 0.0001). The Pearson correlation coefficient between improvements of the NMSS domain sleep/fatigue and the NMSS domain mood/cognition and the PDQ‐8 total score improvements was moderate (0.32, P = 0.0001; 0.37, P < 0.0001, respectively). The corresponding regression plots are presented in Fig. 3.
Table 2.
Correlation coefficients between (a) the BL NMSS total score burden and the NMSS total score improvement, (b) NMSS total score improvements and PDQ‐8 total score improvements, (c) NMSS subdomain sleep/fatigue improvement and PDQ‐8 total score improvement and (d) NMSS subdomain mood/cognition improvement and PDQ‐8 total score improvement
| Variable | N | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| (a) Baseline NMSS total score burden and NMSS total score improvement | ||||||
| NMS total score improvement | 170 | 16.7 | 43.17 | 18 | −122 | 148 |
| NMS total score at BL | 233 | 69.2 | 42.13 | 66 | 2 | 217 |
| Pearson correlation coefficient | Spearman correlation coefficient | |||||
| Correlation coefficient | 0.56515 | Correlation coefficient | 0.56879 | |||
| P value | <0.0001 | P value | <0.0001 | |||
| N | 170 | N | 170 | |||
| (b) NMSS total score improvement and PDQ‐8 total score improvement | ||||||
| NMS total score improvement | 170 | 16.7 | 43.17 | 18 | −122 | 148 |
| PDQ‐8 total score improvement | 152 | 7.1 | 21.00 | 6.25 | −53.125 | 65.625 |
| Pearson correlation coefficient | Spearman correlation coefficient | |||||
| Correlation coefficient | 0.46392 | Correlation coefficient | 0.43782 | |||
| P value | <0.0001 | P value | <0.0001 | |||
| N | 140 | N | 140 | |||
| (c) NMSS domain sleep/fatigue improvement and PDQ‐8 total score improvement | ||||||
| NMS domain sleep/fatigue improvement | 167 | 5.3 | 11.08 | 3 | −20 | 36 |
| PDQ‐8 total score improvement | 152 | 7.1 | 21.00 | 6.25 | −53.125 | 65.625 |
| Pearson correlation coefficient | Spearman correlation coefficient | |||||
| Correlation coefficient | 0.32101 | Correlation coefficient | 0.31003 | |||
| P value | 0.0001 | P value | 0.0002 | |||
| N | 139 | N | 139 | |||
| (d) NMSS domain mood/cognition improvement and PDQ‐8 total score improvement | ||||||
| NMS domain mood/cognition improvement | 167 | 3.1 | 12.62 | 1 | −30 | 48 |
| PDQ‐8 total score improvement | 152 | 7.1 | 21.00 | 6.25 | −53.125 | 65.625 |
| Pearson correlation coefficient | Spearman correlation coefficient | |||||
| Correlation coefficient | 0.36793 | Correlation coefficient | 0.38388 | |||
| P value | <0.0001 | P value | <0.0001 | |||
| N | 139 | N | 139 | |||
Method: prob > |r| under H0: ρ = 0.
Figure 3.
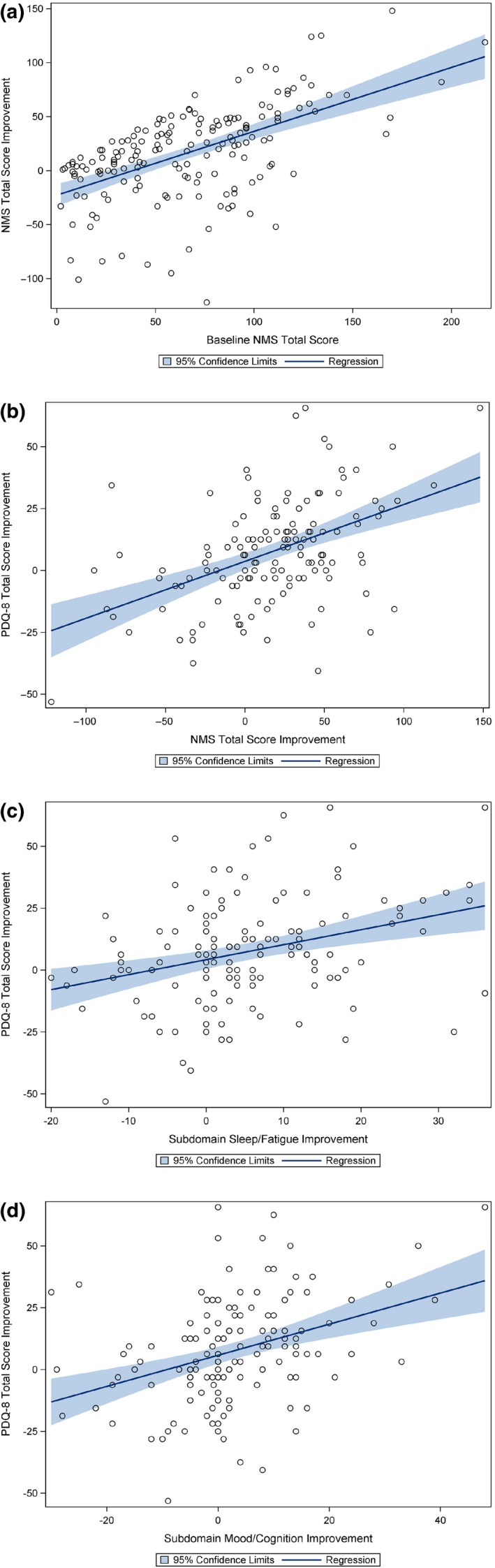
Regression plots (a) between the BL NMSS total score burden and the NMSS total score improvements; (b) between the NMSS total score improvements and the PDQ‐8 total score improvements; (c) between the improvements of the NMSS sleep/fatigue domain and the PDQ‐8 total score; (d) between the improvements of the NMSS mood/cognition domain and the PDQ‐8 total score. [Colour figure can be viewed at wileyonlinelibrary.com]
The ADRs have been described in the interim and final publication of the GLORIA registry 25, 26 and did not show any new findings compared to the known safety profile of LCIG.
Discussion
The data from this study provide us with novel insights into the possible effect of levodopa on NMSB assessed by a validated tool in PD. This post hoc analysis of the GLORIA study revealed for the first time that LCIG improved NMSs across all NMSB categories, even in those with very severe NMSs at BL, as well as QoL. Most importantly, it showed that the best predictor of improvement was BL NMS severity. These novel findings complement previous literature on the predication of QoL by NMSB 11, 24. In addition, the data are supportive of positron emission tomography imaging data suggesting a correlation of synaptic dopamine release and improvement of NMSs in PD 25.
The analysis of the GLORIA database included assessments of motor and non‐motor symptoms and QoL with results comparable to a number of recent studies 11, 15, 16, 17, 18, 20, 22, 24. The results of this first, large multinational, long‐term registry demonstrated sustained improvements with LCIG of motor and non‐motor symptoms and in NMSS subdomains (particularly sleep/fatigue, mood/cognition and gastrointestinal domains), as well as QoL in advanced PD patients at 12 and 24 months 25, 26.
These post hoc analyses provide evidence for (i) the rationale of dopaminergic treatment for NMSs of PD in conjunction with continued motor improvements as has been postulated previously 19, 28; (ii) an association between BL NMSB and treatment‐related improvements of NMSs; and (iii) a moderate correlation between improvement of NMSS total score and improvement of QoL as measured by PDQ‐8 in advanced PD patients.
Similar improvements of NMSs and QoL were demonstrated in two other open‐label studies with smaller patient collectives over a 12‐month and a mean 48‐month follow‐up period 29, 30.
Many NMSs occur early in PD and in de novo PD (some may even predate the diagnosis of PD currently) 6, 31 whilst NMSs dominate through the natural history of PD 23. The NMSB rises as the condition progresses. Given ours was an advanced PD cohort, it is not surprising that only 12% in this cohort with advanced PD showed mild to moderate NMSB (0–20). On the other hand, the largest proportion of patients (38%) were in the most severe NMSB category (>80). An association of higher NMSS total scores with worse QoL was shown 16, and the NMSB was reported to be the best predictor of QoL 11. Whilst the NMSs decreased in all subgroups carrying mild to severe NMSB, the reduction of NMSs was most pronounced in the most severe NMSB category (−43 total NMSS score points). The PDQ‐8 total score decreased in parallel with all NMSB subgroups at month 24. Similar results were published in two other studies, one cross‐sectional 11 and one longitudinal, both showing that NMSB significantly predicted QoL scores 24.
A possible threshold for a minimal clinically important difference (MCID) of the NMSS has been discussed by many authors based on a multitude of randomized, comparative and open‐label clinical trials which have used the NMSS as an outcome measure 32, 33, 34. Currently, since a minimally important difference is not fully established and not agreed upon by experts, a spectrum of numerical responder cutoffs was chosen, capturing both mild and strong responders. Even with the strictest responder definition (decrease of ≥30 NMSS score points), the proportion of responders was 47% in the most affected NMSB category (NMSS total score >80). Martinez‐Martin et al. proposed an NMSS MCID of 13.91 35. Thus, patients with a decrease of ≥20 NMSS score points in our study could be considered responders. Similarly, Horváth et al. suggested a PDQ‐8 MCID of −5.94 36, and reductions of median PDQ‐8 scores were above this threshold in the NMSB categories 41–60, 61–80 and >80.
The predictive nature of BL NMSB was confirmed by a strong correlation between the NMSS total BL score and the NMSS total score improvements (0.57, P < 0.0001). Improvement of overall NMSs may be partially due to reduction of dopamine‐related non‐motor fluctuations 20.
There is now compelling evidence (clinical and statistical) that the total NMSB may be the key determinant of QoL, especially in advanced PD 28. In our study, the Pearson correlation coefficient between NMSS total score improvement and improvement of the PDQ‐8 total score at month 24 showed a moderate correlation (0.46, P < 0.0001).
The NMSS allows for the nine subdomains to be tested individually as has been performed in previous studies 11, 18, and specifically it was of interest to explore the effect of LCIG on sleep/fatigue as well as mood/cognition domains based on evidence obtained from EuroInf 18 and other studies. In our study, a significant association existed between improvements of the two NMSS subdomain scores (sleep/fatigue and mood/cognition) and the PDQ‐8 total score improvements (<0.0001), confirming this observation from the other open‐label studies. Similar observations were reported in another study: the NMSS domains sleep/fatigue, mood/apathy and attention/memory were most significantly predictive of QoL change 24. Sleep in PD is a complex phenomenon driven by various neurotransmitter system abnormalities 26. However, a considerable element of sleep dysfunction in PD is dopaminergic and consequently in theory responsive to levodopa therapy. Our data support this observation and also form a basis for delivery of personalised medicine for PD, a key unmet need, with LCIG 37.
In conclusion, GLORIA results demonstrate that LCIG decreased NMSB, with largest benefits observed in those patients with most severe BL NMSB, and confirmed the significant association with QoL improvements.
Disclosure of conflicts of interest
Dr Chaudhuri was a study investigator and has received honorarium from UCB, AbbVie, Britannia, Mundipharma, Boehringer Ingelheim and GSK Pharmaceuticals for lecturing at symposia. He has acted as a consultant for UCB, AbbVie, Britannia, Neuronova and Mundipharma. He has received research funding from Parkinson's UK, NIHR, PDNMG, as well as educational grants from UCB, Britannia, AbbVie, GSK Pharmaceuticals, Boehringer Ingelheim and Neuronova. Dr Chaudhuri receives royalties from Oxford University Press and holds intellectual property rights for the Kings Parkinson's Pain Scale and Parkinson's Disease Sleep Scale 2. Dr Chaudhuri acknowledges independent research part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London, and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health, UK. A. Antonini was a study investigator and has received compensation for consultancy and speaker‐related activities from UCG, Boston Scientific, Boehringer Ingelheim, AbbVie and Zambon. He has received research support from Mundipharma. W. Poewe was a study investigator and has received compensation from AbbVie, AstraZeneca, Teva, Novartis, GSK, Boehringer Ingelheim, UCB, Orion Pharma, Zambon and Merz Pharmaceuticals (consultancy and lecture fees in relation to clinical drug development programmes for PD) outside the submitted work. He has received royalties from Thieme, Wiley Blackwell and Oxford University Press. W.Z. Robieson, Olga Sanchez‐Solino and L. Bergmann are employees of AbbVie and may hold AbbVie stock and/or stock options. AbbVie contributed to the design, study conduct and financial support for the study. AbbVie participated in the interpretation of data, review and approval of the publication.
Acknowledgements
Medical writing support was provided by Urs E. Gasser, ClinResearch, funded by AbbVie, and the statistical analysis was performed by Christoph Meyenberg, Koehler eClinical, funded by AbbVie.
[The copyright line for this article was changed on 18 January 2019 after original online publication]
References
- 1. Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008; 79: 368–376. [DOI] [PubMed] [Google Scholar]
- 2. Antonini A, Chaudhuri KR, Martinez‐Martin P, Odin P. Oral and infusion levodopa‐based strategies for managing motor complications in patients with Parkinson's disease. CNS Drugs 2010; 24: 119–129. [DOI] [PubMed] [Google Scholar]
- 3. Kalia LV, Lang AE. Parkinson disease. Lancet Neurol 2015; 386: 896–912. [DOI] [PubMed] [Google Scholar]
- 4. Chapuis S, Ouchchane L, Metz O, Gerbaud L, Durif F. Impact of the motor complications of Parkinson's disease on the quality of life. Mov Disord 2005; 20: 224–230. [DOI] [PubMed] [Google Scholar]
- 5. Chaudhuri KR, Odin P, Antonini A, Martinez‐Martin P. Parkinson's disease: the non‐motor issues. Parkinsonism Relat Disord 2011; 17: 717–723. [DOI] [PubMed] [Google Scholar]
- 6. Titova N, Padmakumar C, Lewis SJG, Chaudhuri KR. Parkinson's: a syndrome rather than a disease? J Neural Transm 2017; 124: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jellinger K. Neuropathobiology of non‐motor symptoms in Parkinson disease. J Neural Transm (Vienna) 2015; 122: 1429–1440. [DOI] [PubMed] [Google Scholar]
- 8. Lundqvist C, Beiske AG, Reiertsen O, Kristiansen IS. Real life cost and quality of life associated with continuous intraduodenal levodopa infusion compared with oral treatment in Parkinson patients. J Neurol 2014; 261: 2438–2445. [DOI] [PubMed] [Google Scholar]
- 9. Palhagen SE, Sydow 0, Johansson A, et al Levodopa‐carbidopa intestinal gel (LCIG) treatment in routine care of patients with advanced Parkinson's disease: an open‐label prospective observational study of effectiveness, tolerability and healthcare costs. Parkinsonism Relat Disord 2016; 29: 17–23. [DOI] [PubMed] [Google Scholar]
- 10. Chaudhuri KR, Martinez‐Martin P, Brown RG. The metric properties of a novel non‐motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord 2007; 22: 1901–1911. [DOI] [PubMed] [Google Scholar]
- 11. Martinez‐Martin P, Rodriguez‐Blazquez C, Kurtis MM, Chaudhuri KR, NMSS Validation Group . The impact of non‐motor symptoms on health‐related quality of life of patients with Parkinson's disease on behalf of the NMSS Validation Group. Mov Disord 2011; 26: 399–406. [DOI] [PubMed] [Google Scholar]
- 12. Eggert K, Schrader C, Hahn M. Continuous jejunal levodopa infusion in patients with advanced Parkinson disease: practical aspects and outcome of motor and non‐motor complications. Clin Neuropharmacol 2008; 31: 151–166. [DOI] [PubMed] [Google Scholar]
- 13. Honig H, Antonini A, Martinez‐Martin P. Intrajejunal levodopa infusion in Parkinson's disease: a pilot multicenter study of effects on non‐motor symptoms and quality of life. Mov Disord 2009; 24: 1468–1474. [DOI] [PubMed] [Google Scholar]
- 14. Reddy P, Martinez‐Martin A, Rizos P, et al Intrajejunal levodopa versus conventional therapy in Parkinson disease: motor and nonmotor effects. Clin Neuropharmacol 2012; 35: 205–207. [DOI] [PubMed] [Google Scholar]
- 15. Antonini A, Yegin A, Preda C, Bergmann L, Poewe W, on behalf of the GLORIA study investigators and coordinators . Global long‐term study on motor and non‐motor symptoms and safety of levodopa‐carbidopa intestinal gel in routine care of advanced Parkinson's disease patients; 12‐month interim outcomes. Parkinsonism Relat Disord 2015; 21: 231–235. [DOI] [PubMed] [Google Scholar]
- 16. Santos‐García D, de la Fuente‐Fernández R. Impact of non‐motor symptoms on health‐related and perceived quality of life in Parkinson's disease. J Neurol Sci 2013; 332: 136–140. [DOI] [PubMed] [Google Scholar]
- 17. Antonini A, Bauer L, Dohin E, et al Effects of rotigotine transdermal patch in patients with Parkinson's disease presenting with non‐motor symptoms – results of a double‐blind, randomized, placebo‐controlled trial. Eur J Neurol 2015; 22: 1400–1407. [DOI] [PubMed] [Google Scholar]
- 18. Martinez‐Martin P, Reddy P, Katzenschlager R, et al EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson's disease. Mov Disord 2015; 30: 510–516. [DOI] [PubMed] [Google Scholar]
- 19. Chaudhuri KR, Schapira AH. Non‐motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009; 8: 464–474. [DOI] [PubMed] [Google Scholar]
- 20. Antonini A, Barone P, Marconi R, et al The progression of non‐motor symptoms in Parkinson's disease and their contribution to motor disability and quality of life. J Neurol 2012; 259: 2621–2631. [DOI] [PubMed] [Google Scholar]
- 21. Buck P, Trautman H, Clark J. Scales for assessing nonmotor symptom severity changes in Parkinson's disease patients with symptom fluctuations. Int J Neurosci 2010; 120: 523–530. [DOI] [PubMed] [Google Scholar]
- 22. Swick TJ, Friedman JH, Chaudhuri KR, et al Associations between severity of motor function and nonmotor symptoms in Parkinson's disease: a post hoc analysis of the RECOVER study. Eur Neurol 2014; 71: 140–147. [DOI] [PubMed] [Google Scholar]
- 23. Schapira A, Chaudhuri KR, Jenner P. Non‐motor features of Parkinson disease. Nat Rev Neurosci 2017; 18: 435–450. [DOI] [PubMed] [Google Scholar]
- 24. Prakash KM, Nadkarni NV, Lye WK, Yong MH, Tan EK. The impact of non‐motor symptoms on the quality of life of Parkinson's disease patients: a longitudinal study. Eur J Neurol 2016; 23: 854–860. [DOI] [PubMed] [Google Scholar]
- 25. Politis M, Sauerbier A, Loane C, et al Sustained striatal dopamine levels following intestinal levodopa/infusions in Parkinson's disease patients. Mov Disord 2017; 32: 235–240. [DOI] [PubMed] [Google Scholar]
- 26. Antonini A, Poewe W, Chaudhuri KR, et al, on behalf of the GLORIA study co‐investigators . Levodopa‐carbidopa intestinal gel in advanced Parkinson's: final results of the GLORIA registry. Parkinsonism Relat Disord 2017; 45: 13–20. [DOI] [PubMed] [Google Scholar]
- 27. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The PDQ‐8: development and validation of a short‐form Parkinson's disease questionnaire. Psychol Health 1997; 12: 805–814. [Google Scholar]
- 28. Chaudhuri KR, Odin P, Antonini A, Martinez‐Martin P. Parkinson's disease: the non‐motor issues. Parkinsonism Relat Disord 2011; 17: 717–723. [DOI] [PubMed] [Google Scholar]
- 29. Juhász A, Aschermann Z, Ács P, et al Levodopa/carbidopa intestinal gel can improve both motor and non‐motor experiences of daily living in Parkinson's disease: an open‐label study. Parkinsonism Relat Disord 2017; 37: 79–86. [DOI] [PubMed] [Google Scholar]
- 30. Bohlega S, Abou Al‐Shaar H, Alkhairallah T, Al‐Ajlan F, Hasan N, Alkahtani K. Levodopa‐carbidopa intestinal gel infusion therapy in advanced Parkinson's disease: single Middle Eastern center experience. Eur Neurol 2015; 74: 227–236. [DOI] [PubMed] [Google Scholar]
- 31. Chaudhuri KR, Naidu Y. Early Parkinson's disease and non‐motor issues. J Neurol 2008; 255: 33–38. [DOI] [PubMed] [Google Scholar]
- 32. Martin P, Rodriguez‐Blazquez C, Abe K, et al International study on the psychometric attributes of the non‐motor symptoms scale in Parkinson disease. Neurology 2009; 73: 1584–1591. [DOI] [PubMed] [Google Scholar]
- 33. Sloan JA, Cella D, Hays RD. Clinical significance of patient‐reported questionnaire data: another step toward consensus. J Clin Epidemiol 2005; 58: 1217–1219. [DOI] [PubMed] [Google Scholar]
- 34. Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health‐related quality of life. J Clin Epidemiol 2003; 56: 395–407. [DOI] [PubMed] [Google Scholar]
- 35. Martinez‐Martin P, Rodriguez‐Blazquez C, Abe K, et al International study on the psychometric attributes of the Non‐Motor Symptoms Scale in Parkinson disease. Neurology 2009; 73: 1584–1591. [DOI] [PubMed] [Google Scholar]
- 36. Horváth K, Aschermann Z, Kovács M, et al Changes in quality of life in Parkinson's disease: how large must they be to be relevant? Neuroepidemiology 2017; 48: 1–8. (ISSN: 1423‐0208). [DOI] [PubMed] [Google Scholar]
- 37. Titova N, Chaudhuri KR. Personalized medicine and nonmotor symptoms in Parkinson's Disease. Int Rev Neurobiol 2017; 134: 1257‐1281. [DOI] [PubMed] [Google Scholar]


