Abstract
To compare the performance of a professional continuous glucose monitoring (proCGM) and a personal continuous glucose monitoring (persCGM) system worn in parallel under standardized conditions in individuals with type 1 diabetes (T1D), two CGM systems (iPro2 – proCGM; Minimed 640G – persCGM) worn in parallel using the same sensor (Enlite 2) were compared. Ten people with T1D were included in this single‐centre, open‐label study in which CGM performance was evaluated. The study consisted of a 24‐hours inpatient phase (meals, exercise, glycaemic challenges) and a 4‐day home phase. Analyses included fulfilment of ISO 15197:2013 criteria, mean absolute relative difference (MARD), Parkes Error Grid and Bland–Altman plots. During the inpatient stay, ISO 15197:2013 criteria fulfilment was 58.4% (proCGM) and 57.8% (persCGM). At home, the systems met ISO 15197:2013 criteria by 66.5% (proCGM) and 65.3% (persCGM). No difference of MARD in inpatient phase (19.1 ± 16.7% vs. 19.0 ± 19.6; P = 0.83) and home phase (18.6 ± 26.8% vs. 17.4 ± 21.3%, P = 0.87) was observed. All sensors performed less accurately during hypoglycaemia. ProCGM and persCGM showed similar performance during daytime and night‐time for the inpatient and the home phase. However, sensor performance was reduced during hypoglycaemia for both systems.
Keywords: clinical trial, continuous glucose monitoring (CGM), hypoglycaemia, type 1 diabetes
1. INTRODUCTION
In recent years, continuous glucose monitoring (CGM) has gained increasing importance in diabetes management.1 In contrast to personal CGM (persCGM), where glucose values are displayed in real time, professional CGM (proCGM) is used intermittently in a blinded mode for a short period (e.g. 6‐14 days)2 and data are retrospectively assessed by health care professionals (HCPs) to detect patterns and adjust therapy. PersCGM showed that it improved glycaemic control (HbA1c) and reduced time spent in hypoglycaemia,3, 4, 5, 6, 7 and proCGM‐supported therapy was shown to improve HbA1c in children with type 1 diabetes (T1D) and people with type 2 diabetes.8, 9
Two studies showed that glucose patterns observed during a 14‐day period of CGM are already representative of interstitial glucose patterns seen over a longer period of time (3 months).10, 11 Thus, short‐term CGM use is relevant to uncover otherwise missed glycaemic excursion when assessed via self‐monitoring of blood glucose (SMBG) and HbA1c only. Previous measures of diabetes management (HbA1c, glucometer data) are being challenged, as various CGM‐derived metrics become available (e.g. estimated HbA1c, time in target, time spent in glycaemic ranges, glycaemic variability).12 Current large‐scale cardiovascular outcome trials search for CGM data to evaluate glycaemic variability, hypoglycaemia rates and potentially better future surrogate parameters. As it is believed that the availability of a persCGM signal could have an impact on study outcomes, most studies use proCGM to evaluate glycaemia. To use CGM as a valid instrument to assess glycaemia in clinical trials, the quality of the proCGM must be assured.12
Currently, only one manufacturer provides a proCGM (iPro, Medtronic, Northridge, Los Angeles, California) and a persCGM (Medtronic Minimed 640G, Medtronic) with the same sensor technology (Enlite 2 sensor, Medtronic) that requires calibration. A few studies have compared CGM performance of different sensor systems worn in parallel, but no head‐to‐head comparison of a proCGM and persCGM system using the same sensor technology has been performed under standardized conditions.13, 14, 15 Thus, the primary objective of this study was to compare the performance of a proCGM and a persCGM system worn in parallel under standardized conditions in individuals with T1D. Our hypothesis was that the accuracy, which is influenced by the interstitial compartment and physiological lag time, is equal for both sensors when worn in parallel.
2. METHODS
2.1. Participants
The main inclusion criteria were diagnosis of T1D for over 6 months, intensified insulin treatment for at least 3 months, body mass index (BMI) <35 kg/m2 and HbA1c <86 mmol/mol (<10%). The main exclusion criteria were pregnancy, breastfeeding or females with the intention to become pregnant, the intake of any medication except insulin that significantly impacts glucose metabolism, any disease or medical condition which would interfere with the trial results, known adrenal gland disorders, pancreatic tumour or insulinoma.
2.2. Informed consent procedure
Participants gave their written informed consent prior to any trial‐related activities. The trial was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. The study protocol was approved by the local ethics committee (EK‐No. 28‐082 ex 15/16) and health authority and registered at the German Clinical Trials Register (DRKS00009604).
2.3. Study procedures
This was a single‐centre, open‐label study in people with T1D performed at the Clinical Research Centre (CRC) (Medical University of Graz, Austria). The study consisted of a 24‐hours inpatient phase at the CRC followed by a 5‐day home phase. Twenty‐four hours prior to the start of the inpatient phase, both sensors (Enlite 2), one for the iPro 2 MiniMed CGM system (proCGM) and one for the 640G MiniMed CGM system (persCGM), were inserted in parallel into the subcutaneous adipose tissue of the thigh. The persCGM system was calibrated at least twice daily by the participants using capillary blood glucose (BG) values obtained from their glucometer (Contour Next Link 2.4, Bayer Pharma AG, Berlin, Germany); the sensor of the proCGM system was calibrated retrospectively by the research team using the same calibration values as participants.
2.4. Inpatient phase
Participants arrived at 07:30 am at the CRC and received a standardized breakfast (60 g carbohydrates) at 09:15 am and an increased dose of their usual bolus insulin (180% of the calculated carbohydrate‐to‐insulin ratio) to induce mild hypoglycaemia. In the case of symptomatic hypoglycaemia, participants received a snack (15 g carbohydrates) to normalize glycaemia. At 01:00 pm, participants consumed a standardized lunch (60 g carbohydrates) with once again a 180% dose of their calculated mealtime bolus insulin dose in order to induce a second episode of mild hypoglycaemia. At 07:30 pm, participants received a standardized dinner (40 g carbohydrates) with their regular bolus insulin dose. On the following day at 07:30 am, participants performed a continuous, moderate‐intensity exercise test that included two 15‐minutes periods of exercising on a cycle ergometer at 50% of the predicted maximum oxygen consumption (VO2max),13 interspersed by one passive rest of 5 minutes. At 9:00 am, a light breakfast (40 g carbohydrates) was taken and participants injected their usual dose of bolus insulin. Participants left the CRC at 09:30 am and the home phase started. Venous plasma glucose was measured every 5 minutes from 09:00 am to 08:45 pm and every 15 minutes from 08:45 pm to 09:00 am (Super GL Glucose Analyzer, Müller GmbH, Freital, Germany). During exercise testing, venous blood samples were collected every 5 minutes.
2.5. Home phase
Participants continued using proCGM and persCGM in parallel for five consecutive days at home. Participants were asked to perform at least seven capillary glucose measurements at home using the study‐specific glucose meter. Additional measurements could be taken at any time if deemed necessary by the participant. No specific instructions on general diabetes management or hypoglycaemia management were given, and participants were asked to take care of their diabetes as usual. Data for capillary BG, meals and insulin injections were documented in a paper‐diary. The Contour Next Link 2.4 (Bayer Pharma AG) BG meter was used for capillary BG measurements.
2.6. Statistical analysis
Interstitial glucose values were compared with corresponding plasma (inpatient phase) or capillary (home‐phase) BG values. All analyses were performed separately for the inpatient and the home phase. Overall accuracy, accuracy during hypoglycaemia (<3.9 mmol/L), euglycaemia (3.9‐10.0 mmol/L) and hyperglycaemia (>10.0 mmol/L) as well as accuracy during daytime (07:00 am ‐ 09:00 pm) versus night‐time (09:00 pm ‐ 07:00 am) were assessed. Sensor accuracy was determined using ISO 15197:2013 criteria. The mean absolute relative difference (MARD) between sensor and plasma or capillary glucose measurements was calculated. CGM and plasma or capillary glucose values were compared by Bland‐Altman analysis. The clinical relevance of discrepancies between those values was illustrated by Parkes Error Grid (PEG). Results are given as mean ± standard deviation, if not indicated otherwise. The head‐to‐head comparison of the sensor performance was assessed by means of paired students t‐test and Wilcoxon matched‐pairs signed‐rank test (P < 0.05).
3. RESULTS
Ten adults with T1D (six females, age 30.5 ± 10.7 years, BMI 24.4 ± 4.6 kg/m2, diabetes duration 11.3 ± 10.7 years, HbA1c 53.3 ± 9.0 mmol/mol [7.0 ± 0.8%], 8 on insulin pump and 2 on multiple daily injections, mean daily insulin dose 39.0 ± 16.8 U) participated in the study.
3.1. Inpatient phase
A total of 606 (proCGM) and 926 (persCGM) data pairs were available during the inpatient phase. CGM systems fulfilled ISO 15197:2013 criteria by 58.4% (proCGM) and 57.8% (persCGM, P = 0.9559; Rao‐Scott adjusted chi‐square test using subjects as clusters). Both systems showed better results over night when compared with daytime with regard to ISO 15197:2013 criteria (proCGM vs. persCGM, respectively): 71.8% versus 68.7% (night‐time, P = 0.8333) and 46.9% versus 49.5% (daytime, P = 0.8318). Overall data on MARD are reported in Table 1. MARD was particularly worse during phases of highly fluctuating glucose concentrations (exercise, 2 hours postprandial): 27.0 ± 28.1% (exercise and 2 hours postprandial, n = 228) versus 16.4 ± 15.1% (remaining observation period, n = 698) for persCGM, and 23.1 ± 18.2% (exercise and 2 hours postprandial, n = 140) versus 17.9 ± 16.0% (remaining observation period, n = 466) for proCGM.
Table 1.
Sensor performance assessed by MARD during inpatient and home phase as well as separated for daytime and night‐time for overall values, hypoglycaemia (<3.9 mmol/L), euglycaemia (3.9‐10.0 mmol/L) and hyperglycaemia (>10.0 mmol/L); (n) indicates the number of sensor‐reference pairs available for proCGM and persCGM, respectively
| Inpatient phase | proCGM | persCGM | P‐value |
|---|---|---|---|
| Overall MARD (n) | 19.1 ± 16.7% (606) | 19.0 ± 19.6% (926) | 0.83 |
| MARD <3.9 mmol/L (n) | 31.9 ± 24.7% (70) | 33.8 ± 37.1% (118) | 0.97 |
| MARD 3.9‐10.0 mmol/L (n) | 17.3 ± 14.6% (495) | 17.5 ± 14.6% (730) | 0.85 |
| MARD >10.0 mmol/L (n) | 19.5 ± 14.3% (41) | 10.9 ± 9.6% (78) | 0.27 |
| Inpatient phase daytime (7:00 am‐09:00 pm) | |||
| Total MARD (n) | 23.6 ± 18.6% (326) | 23.6 ± 23.0% (527) | 0.82 |
| MARD <3.9 mmol/L (n) | 32.4 ± 25.3% (66) | 35.8 ± 38.1% (108) | 0.97 |
| MARD 3.9‐10.0 mmol/L (n) | 21.1 ± 15.6% (238) | 21.2 ± 15.9% (364) | 0.66 |
| MARD >10.0 mmol/L (n) | 24.2 ± 17.8% (22) | 11.9 ± 11.0% (55) | 0.32 |
| Inpatient phase night‐time (09:00 pm‐07:00 am) | |||
| Total MARD (n) | 13.8 ± 12.2% (280) | 13.5 ± 11.9% (399) | 0.74 |
| MARD <3.9 mmol/L (n) | 24.0 ± 12.0% (4) | 12.2 ± 11.8% (10) | 0.46 |
| MARD 3.9‐10.0 mmol/L (n) | 13.7 ± 12.5% (257) | 13.8 ± 12.2% (366) | 0.68 |
| MARD >10.0 mmol/L (n) | 14.1 ± 5.4% (19) | 8.6 ± 4.2% (23) | 0.26 |
| Home phase | proCGM | persCGM | P‐value |
| Overall MARD (n) | 18.6 ± 26.8% (281) | 17.4 ± 21.3% (383) | 0.87 |
| MARD <3.9 mmol/L (n) | 47.2 ± 61.4% (26) | 28.3 ± 34.1% (42) | 0.21 |
| MARD 3.9‐10.0 mmol/L (n) | 16.0 ± 18.4% (209) | 17.1 ± 20.3% (261) | 0.80 |
| MARD >10.0 mmol/L (n) | 14.4 ± 16.2% (46) | 12.9 ± 12.8% (80) | 0.66 |
| Home phase daytime (7:00 am‐09:00 pm) | |||
| Total MARD (n) | 19.5 ± 28.4% (207) | 17.1 ± 21.6% (274) | 0.72 |
| MARD <3.9 mmol/L (n) | 49.8 ± 64.8% (22) | 27.7 ± 36.0% (37) | 0.19 |
| MARD 3.9‐10.0 mmol/L (n) | 15.8 ± 17.3% (153) | 16.6 ± 18.7% (184) | 0.95 |
| MARD >10.0 mmol/L (n) | 16.3 ± 17.5% (32) | 11.2 ± 13.8% (53) | 0.68 |
| Home phase night‐time (09:00 pm‐07:00 am) | |||
| Total MARD (n) | 16.1 ± 21.5% (74) | 18.3 ± 20.9% (109) | 0.61 |
| MARD <3.9 mmol/L (n) | 32.8 ± 41.3% (4) | 32.2 ± 14.5% (5) | 0.76 |
| MARD 3.9‐10.0 mmol/L (n) | 16.4 ± 21.3% (56) | 18.2 ± 23.6% (77) | 0.67 |
| MARD >10.0 mmol/L (n) | 10.1 ± 12.3% (14) | 16.1 ± 10.3% (27) | 0.75 |
3.2. Home phase
During the home phase a total of 456 capillary BG measurements were performed, which is on average 8 ± 3 measurements per day. Forty‐four hypoglycaemic events (<3.9 mmol/L) were detected at home using capillary BG measurements, resulting in consumption of 41 rescue carbohydrate meals (mean carbohydrate content 23.3 ± 11.3 g). No severe hypoglycaemic event occurred. Hypoglycaemic glucose readings were overestimated in 57.7% of measurements and underestimated in 38.5% by proCGM. In 3.8%, there was no difference in hypoglycaemic glucose readings between proCGM and capillary glucose. Hypoglycaemic glucose readings were overestimated in 64.3% and underestimated in 35.7% by persCGM.
A total of 281 (proCGM) and 383 (persCGM) data pairs were collected during the home phase. The systems fulfilled ISO 15197:2013 criteria by 66.5% for proCGM versus 65.3% for persCGM (P = 0.893). At home, daytime performance for both systems was superior when compared with the inpatient phase with regard to ISO 15197:2013 criteria: 65.2% versus 67.5% (proCGM vs. persCGM, respectively; P = 0.827). During night‐time, proCGM was superior to persCGM (70.3% vs. 59.6%; P = 0.273).
During the inpatient and the home phase, no significant difference between the two systems was observed when assessed by MARD (P > 0.05). This is true across all glycaemic ranges, as well as for night‐time and daytime (Table 1). PEG analyses, separated for the inpatient and the home phase, are provided in Figure 1. During night‐time for both systems, 100% of the values were in zones A and B (benign error). During daytime, 98.5% (proCGM) versus 96.6% (persCGM) were in zones A and B. Similar results were seen during the home phase. Bland‐Altman analysis (see the supporting information, Figure 1A‐H) showed that the proCGM tended to overestimate BG during the inpatient‐phase daytime period by 11.4% and during the inpatient‐phase night‐time period by 8.5%, and during the home‐phase daytime period by 1.3% and during the home‐phase night‐time period by 2.0%. PersCGM tended to report higher values than BG at night and lower values during daytime (+5.5% vs. −5.5%) for the inpatient phase; however, during the home phase there was a less pronounced tendency for overestimation of BG for both daytime and night‐time for the persCGM.
Figure 1.
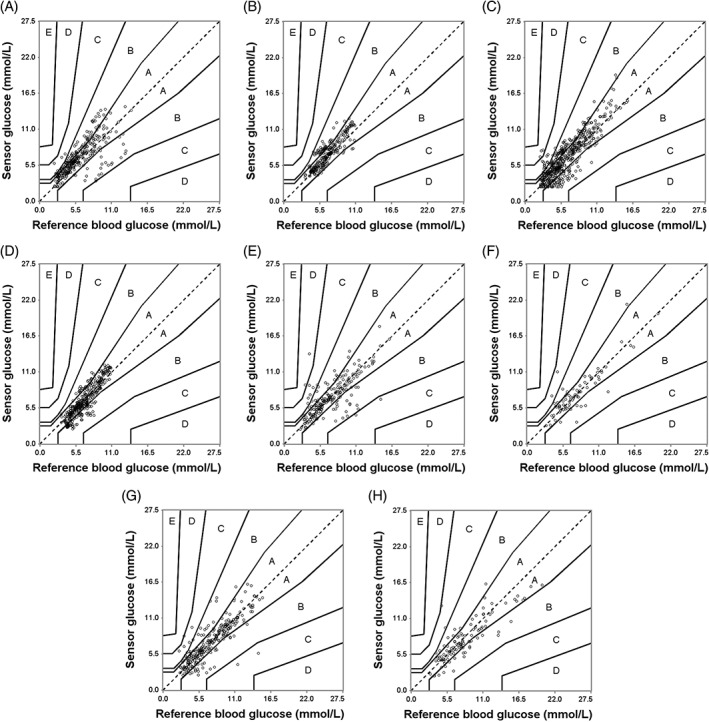
PEG analysis for proCGM during: A, inpatient phase daytime (zone A: 65.34%, zone B: 33.13%, zone C: 1.53%); B, inpatient phase night‐time (zone A: 80.36%, zone B: 19.64 %); C, persCGM during inpatient phase daytime (zone A: 65.84%, zone B: 30.74%, zone C: 2.85%, zone D: 0.57%); D, inpatient phase night‐time (zone A: 78.95%, zone B: 21.05%); E, proCGM during home phase daytime (zone A: 75.85%, zone B: 19.32%, zone C: 3.86%, zone D: 0.97%); F, home phase night‐time (zone A: 79.73%, zone B: 17.57%, zone C: 2.70%); G, persCGM during home phase daytime (zone A: 81.02%, zone B: 16.42%, zone C: 2.19%, zone D: 0.36%); and H, home phase night‐time (zone A: 71.56%, zone B: 26.61%, zone C: 1.83%)
4. DISCUSSION
Our study indicated that during the inpatient phase both systems showed similar accuracy; the performance during the home phase did not differ from the performance during the inpatient phase.
Compared with daytime, sensor performance was better for both systems at all levels of glycaemia over night, when fewer glucose fluctuations occur. Reduced sensor performance during daytime is probable because of a higher rate of change in glucose than during night‐time.16 Since real‐life conditions were mimicked by means of physical exercise and premeal bolus insulin overdosing in our study, the higher rate of glucose change might have influenced sensor accuracy for both systems.
Findings from the inpatient phase seem to translate into the home phase where sensor accuracy was higher during more stable glycaemia at night compared to day, even though data on physical exercise and meals were not documented meticulously, and sampling frequency was not high enough during the home phase to perform these analyses separately for the home phase.
As expected, both CGM systems were less accurate during hypoglycaemia than during euglycaemia and hyperglycaemia.17 The impaired accuracy during hypoglycaemia is similar to previous research investigating the performance of the persCGM system (MARD 38.8% for the lowest glucose levels).17 It is especially worrisome that both CGM systems tend to overestimate glycaemia when compared with capillary glucose. While CGM systems seem to reliably report glycaemia in euglycaemia and hyperglycaemia, accuracy during hypoglycaemia still requires improvement, as patients might make the wrong treatment decisions if true glycaemia is lower, or more often higher, than reported by persCGM. Insufficient accuracy in hypoglycaemia also affects interpretation of clinical trials that use proCGM as outcome metrics. By reporting false high glucose values by using proCGM, there might be significant under‐reporting of hypoglycaemia in clinical trials. Thus, with current sensor generations, hypoglycaemic CGM values should be confirmed by capillary glucose measurements.
Numerically, albeit not statistically significant, proCGM showed worse performance during hypoglycaemia than persCGM, especially during daytime at home (MARD: 49.8 ± 64.8% vs. 27.7 ± 36.0%). These disparities in sensor performance might be attributed to the fact that one sensor is calibrated prospectively and the other one is calibrated retrospectively, which reflects different calibration algorithms. As the prospectively calibrated sensor signal is used for immediate treatment decisions, it can be assumed that its signal should be more accurate; alternatively, the sensor signal should be shut down if it is not deemed reliable, with no glucose value being indicated.
Findings from our study add knowledge on sensor performance during different levels of glycaemia during daytime and night‐time over a period of 5 days. Systems were not only tested under standardized conditions during an inpatient phase but also at home, when participants followed their regular daily routines. Thus, it is worthwhile knowing, that especially during hypoglycaemia, CGM‐derived data need to be questioned and may require confirmation by capillary BG measurements. This is also true for proCGM when used as a diagnostic tool, both in routine care and in outcome studies where the duration and frequency of hypoglycaemia need to be interpreted with caution.2, 18
Some limitations of previous studies (short duration of experiment, lack of home phase, lack of sleep phase) were addressed. However, our study is limited by the rather small number of participants and the potential pressure artefacts (ie, on which side was the patient sleeping with regard to sensor performance). Another limitation is the unblinded persCGM signal that was available to the participants. As persCGM was shown to significantly reduce hypoglycaemia rates in clinical trials and routine care,19 potential bias might have been introduced, reducing hypoglycaemia and thus limiting the time in hypoglycaemia to use for accuracy assessment.
In conclusion, in the present analysis, proCGM and persCGM showed similar performance during daytime and night‐time at the CRC and at home, which is reassuring, in that proCGM and persCGM data gathered in clinical trials are comparable. Sensor performance for both systems was moderate during hypoglycaemia.
CONFLICTS OF INTEREST
O.M. has received lecture fees from Medtronic, and research grants from Sêr Cymru II COFUND fellowship/European Union, Novo Nordisk A/S and Dexcom Inc. T.R.P. is an advisory board member of Novo Nordisk A/S, a consultant for Roche Diabetes Care, Novo Nordisk A/S, Eli Lilly & Co, Infineon, Carnegie Bank, and has served on the speaker's bureau of Novo Nordisk A/S and Astra Zeneca. F.A. received speaker honoraria from Astra Zeneca and Boehringer Ingelheim. H.S. is an advisory board member and is on the speaker's bureau of Amgen, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk and Sanofi, and received unrestricted research grants from Astra Zeneca, Boehringer Ingelheim, MSD, Novo Nordisk and Sanofi. J.K.M. is a member of the advisory board of Becton‐Dickinson, Boehringer Ingelheim, Eli Lilly, Medtronic, and Sanofi, and received speaker honoraria from Abbott Diabetes Care, Astra Zeneca, Eli Lilly, Nintamed, Novo Nordisk, Roche Diabetes Care, Sanofi, Servier, and Takeda. The remaining authors declare no conflict of interest.
Author contributions
O.M. and M.P. drafted the manuscript. T.R.P., H.S. and J.K.M. designed and performed the study, interpreted data and contributed to discussions. T.A. performed statistical analyses and reviewed the manuscript. F.A., H.K., D.H., P.K. and M. M. performed the study. All authors critically revised the article and approved the final version of the manuscript. J.K.M. is the guarantor of this work.
Supporting information
Figure S1. PEG analysis for proCGM during inpatient phase day‐time (zone A: 65.34 %, zone B: 33.13 %, zone C: 1.53 %) (A), inpatient phase night‐time (zone A: 80.36 %, zone B: 19.64 %) (B), persCGM during inpatient phase day‐time (zone A: 65.84 %, zone B: 30.74 %, zone C: 2.85 %, zone D: 0.57 %) (C), inpatient phase night‐time (zone A: 78.95 %, zone B: 21.05 %) (D), proCGM during home‐phase day‐time (zone A: 75.85 %, zone B: 19.32 %, zone C: 3.86 %, zone D: 0.97 %) (E), home‐phase night‐time (zone A: 79.73 %, zone B: 17.57 %, zone C: 2.70 %) (F), persCGM during home‐phase day‐time (zone A: 81.02 %, zone B: 16.42 %, zone C: 2.19 %, zone D: 0.36 %) (G) and home‐phase night‐time (zone A: 71.56 %, zone B: 26.61 %, zone C: 1.83 %) (H).
ACKNOWLEDGMENTS
The authors thank Bernd Tschapeller (Joanneum Research GmbH) for data management, Hans‐Peter Schadler (Joanneum Research GmbH) for statistical assistance and Sarah Bischof (Medical University of Graz) for data monitoring. We want to thank the volunteers for their participation in the study.
Moser O, Pandis M, Aberer F, et al. A head‐to‐head comparison of personal and professional continuous glucose monitoring systems in people with type 1 diabetes: Hypoglycaemia remains the weak spot. Diabetes Obes Metab. 2019;21:1043–1048. 10.1111/dom.13598
Funding information This study was performed within the SPIDIMAN project which was funded by the European Commission under the 7th Framework Program (grant agreement no. 305343).
“Othmar Moser and Marlene Pandis contributed equally to this work.”
REFERENCES
- 1. DeSalvo DJ, Miller KM, Hermann JM, et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D exchange and DPV initiative. Pediatr Diabetes. 2018;19:1271‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fonseca VA, Grunberger G, Anhalt H, et al. Continuouse glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of endocrinology. Endocr Pract. 2016;22:1008‐1021. [DOI] [PubMed] [Google Scholar]
- 3. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, Kröger J, Weitgasser R. Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet. 2016;388:2254‐2263. [DOI] [PubMed] [Google Scholar]
- 4. Oskarsson P, Antuna R, Geelhoed‐Duijvestijn P, Kröger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre‐specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018;61:539‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J‐P, Rayman G. Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the Management of Insulin‐Treated Type 2 diabetes: a multicenter, open‐label randomized controlled trial. Diabetes Ther. 2017;8:55‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections. JAMA. 2017;317:371. [DOI] [PubMed] [Google Scholar]
- 7. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Ann Intern Med. 2017;167:365. [DOI] [PubMed] [Google Scholar]
- 8. Sperling MA. Continuous subcutaneous insulin infusion and continuous subcutaneous glucose monitoring in children with type 1 diabetes mellitus: boon or bane? Pediatr Diabetes. 2001;2:49‐50. [DOI] [PubMed] [Google Scholar]
- 9. Kim SK, Kim HJ, Kim T, et al. Effectiveness of 3‐day continuous glucose monitoring for improving glucose control in type 2 diabetic patients in clinical practice. Diabetes Metab J. 2014;38:449‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xing D, Kollman C, Beck RW, et al. Optimal sampling intervals to assess long‐term glycemic control using continuous glucose monitoring. Diabetes Technol Ther. 2011;13:351‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long‐term glycemic control. Diabetes Technol Ther. 2018;20:314‐316. [DOI] [PubMed] [Google Scholar]
- 12. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taleb N, Emami A, Suppere C, et al. Comparison of two continuous glucose monitoring systems, Dexcom G4 platinum and Medtronic paradigm Veo Enlite system, at rest and during exercise. Diabetes Technol Ther. 2016;18:561‐567. [DOI] [PubMed] [Google Scholar]
- 14. Kropff J, Bruttomesso D, Doll W, et al. Accuracy of two continuous glucose monitoring systems: a head‐ to‐head comparison under clinical research centre and daily life conditions. Diabetes Obes Metab. 2015;17:343‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luijf YM, Mader JK, Doll W, et al. Accuracy and reliability of continuous glucose monitoring systems: a head‐to‐head comparison. Diabetes Technol Ther. 2013;15:721‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taub MB, Peyser TA, Erik Rosenquist J. Numerical simulation of the effect of rate of change of glucose on measurement error of continuous glucose monitors. J Diabetes Sci Technol. 2007;1:685‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zijlstra E, Heise T, Nosek L, Heinemann L, Heckermann S. Continuous glucose monitoring: quality of hypoglycaemia detection. Diabetes Obes Metab. 2013;15:130‐135. [DOI] [PubMed] [Google Scholar]
- 18. Fitzpatrick C, Chatterjee S, Seidu S, et al. Association of hypoglycaemia and risk of cardiac arrhythmia in patients with diabetes mellitus: a systematic review and meta‐analysis. Diabetes Obes Metab. 2018;20:2169‐2178. [DOI] [PubMed] [Google Scholar]
- 19. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317:379‐387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PEG analysis for proCGM during inpatient phase day‐time (zone A: 65.34 %, zone B: 33.13 %, zone C: 1.53 %) (A), inpatient phase night‐time (zone A: 80.36 %, zone B: 19.64 %) (B), persCGM during inpatient phase day‐time (zone A: 65.84 %, zone B: 30.74 %, zone C: 2.85 %, zone D: 0.57 %) (C), inpatient phase night‐time (zone A: 78.95 %, zone B: 21.05 %) (D), proCGM during home‐phase day‐time (zone A: 75.85 %, zone B: 19.32 %, zone C: 3.86 %, zone D: 0.97 %) (E), home‐phase night‐time (zone A: 79.73 %, zone B: 17.57 %, zone C: 2.70 %) (F), persCGM during home‐phase day‐time (zone A: 81.02 %, zone B: 16.42 %, zone C: 2.19 %, zone D: 0.36 %) (G) and home‐phase night‐time (zone A: 71.56 %, zone B: 26.61 %, zone C: 1.83 %) (H).


