Abstract
Background
Transcutaneous electrical nerve stimulation has proven to be effective in alleviating chronic pain from facial myalgias. We evaluated the efficacy of a novel handheld microcurrent‐emitting device in short‐term, office‐based treatment of patients with sinus pain. This device, which is U.S. Food and Drug Administration (FDA)‐cleared, detects and treats regions corresponding to nerve fibers.
Methods
Randomized, double‐blinded, placebo‐controlled trial. Seventy‐one participants with facial pain attributed to self‐reported nasal/sinus disease were recruited from a tertiary rhinologic practice and the surrounding community and randomly assigned to either office‐based use of an active (n = 38) or placebo (n = 33) microcurrent emitter. The study device was repetitively applied by each patient to the bilateral periorbital areas for 5 minutes. A visual analogue scale (VAS) for pain severity was administered before, and 10 minutes after, treatment.
Results
Active microcurrent‐treated patients had a reduction in mean pain score from 5.63 pretreatment to 3.97 posttreatment (mean difference, 1.66; 95% confidence interval [CI], 1.20 to 2.12). Patients using the sham device also reported sinus pain reductions (mean difference, 0.91; 95% CI, 0.61 to 1.21). However, the active device demonstrated a significantly greater reduction in pain compared to sham (0.75‐point difference, p = 0.007). Notably, 23.7% of patients using the active device had a reduction of 3 or more points by VAS compared to 0% of sham device patients (p = 0.003). One minor occurrence of transient facial skin erythema was noted.
Conclusion
This trial suggests that treatment of rhinologic facial pain using this noninvasive microcurrent device is safe and effective in providing rapid relief of nasal/sinus pain. Additional studies with longer term follow‐up are warranted.
Keywords: transcutaneous electrical nerve stimulation, microcurrent, facial pain, sinus pain, rhinologic facial pain, chronic rhinosinusitis, CRS
The prevalence of sinus pain in the U.S. population is estimated to be 2.1%, or 2.37 million people, using a representative sampling of 113.5 million adults from the U.S. population.1 Although the impact of sinus pain has not been specifically studied, a large‐scale European investigation assessing the impact of chronic pain determined that 60% of patients who suffer from chronic pain, defined as pain persisting for greater than 6 months, visited their physician 2 to 9 times during that 6‐month period. Of these patients, 55% were treating their pain with nonsteroidal anti‐inflammatory medications, 43% with paracetamol, and 13% with weak opioids.2
Various treatment methods have been used for nonrhinologic facial pain. Patients with persistent idiopathic facial pain (PIFP), for example, are often treated with low‐dose tricyclic antidepressants.3 Additional interventions, such as botulinum toxin injections, have been successful in treating PIFP, as well as various types of neuralgia.3, 4 Nonpharmacologic approaches, including acupuncture, biofeedback, and dental splinting, have been considered, but evidence for their efficacy is limited.3 Effective treatments for rhinologic facial pain specifically, however, are lacking. Pain and pressure attributed to the sinuses often prompts patients suffering from allergic rhinitis to seek medical attention, and these symptoms can be significantly reduced with appropriate medical treatments.5, 6 Additionally, facial pain in patients with a diagnosis of chronic rhinosinusitis (CRS) is associated with significantly worse quality of life outcomes.7 Thus, there is a pressing need for the management of facial pain symptoms to both improve quality of life measures and potentially reduce, or mediate, the number of physician visits and medication prescriptions. Currently, the treatment of facial pain from sinus disease is limited to the use of analgesics and anti‐inflammatory agents, including ibuprofen, acetaminophen, and steroids.8 To date, no other noninvasive technology has been utilized as part of a multimodal treatment strategy for the management of rhinologic facial pain.
The notion that sensory nerve stimulation can provide pain relief was described as early as 1965 by Melzack and Wall9 in their report on the gate‐control theory of pain. Since that time, transcutaneous electrical nerve stimulation (TENS) has been successfully utilized in the management of several types of chronic pain. A large‐scale, observational study found that fixed‐site, high‐frequency TENS was effective in treating multisite chronic pain in a dose‐response pattern.10 In a separate study evaluating a cohort of women diagnosed with facial myalgia, patients undergoing therapy with conventional TENS had a significant reduction in pain when compared to a control group using a visual analogue scale (VAS) readout.11
The purpose of this study was to evaluate the safety, efficacy, and feasibility of using a novel, handheld, microcurrent‐emitting device in the short‐term, office‐based treatment of patients with complaints of sinus pain.
Patients and methods
Following institutional review board (IRB) approval, a randomized, placebo‐controlled, double‐blinded clinical trial was conducted in patients with facial pain related to either diagnosed or self‐reported sinonasal disease or symptoms. Participants with an initial pain score of at least 4 out of 10 using a VAS (range 0 to 10 with 10 being most severe) were included. Exclusion criteria included the presence of a dental infection, pregnancy or planned pregnancy, identifiable cranial neuropathy, focal neurological finding or neurologic symptom that required medical workup, or the presence of an implanted electric stimulation device, such as a Pacemaker or cochlear implant. Patients were recruited from a tertiary rhinology clinical practice as well as the surrounding community via advertisements in local newspapers and on Internet‐based social media platforms.
Using a random number generator, we assigned each subject to 1 of the 2 study groups in the order that they entered the study. Recruited patients were thus randomly assigned to either the active, handheld microcurrent emitter device or the placebo/sham device. Both devices had the same appearance, vibrations, and characteristics, with the exception that only the active device emitted a microcurrent with its vibrations. After reading the instructions for use, patients self‐administered the treatment unaided by the study staff. The assigned device was applied by all study participants to the facial skin in a confined, repetitive “H” pattern above and below the bilateral orbits for 5 minutes to span between the bilateral supraorbital and infraorbital nerve regions and across the nasal dorsum. The active device, which recently received U.S. Food and Drug Administration (FDA) clearance, detects areas of low impedance, which correspond to the presence of nerve fibers, and emits microampere alternating current in these regions. When a low impedance region is detected, the device emits haptic vibration to let the user know to hold the device in place temporarily while the stimulation is applied (Supporting Video 1). The placebo device included all features of the active device, including vibration, except that it did not emit pulsed alternating current. After completion of the treatment, each patient waited 10 minutes and again used the VAS to score their perceived posttreatment pain. Investigators were blinded to the assigned treatment because both devices were the identical in appearance and simply labeled either “A” or “B.” The study coordinators were not blinded to VAS results, but they were unaware of which device corresponded to respective patient scores. The independent statistician was blinded to device/placebo identity, and study principal investigator (PI, JVN) was blinded to both device and all scoring results until statistically analyzed. All participants completed the study in the same manner, with 5 minutes allotted for treatment, and 10 minutes allotted after exposure to active/placebo device use before rescoring of pain.
Statistical analysis
One‐sample and 2‐sample t tests were used to analyze continuous variables. Fisher's exact test was used to analyze categorical variables. Statistical analysis was performed using JMP Pro 14.0.0 (SAS Institute, Cary, NC). Prior to study initiation, a statistical simulation was used to assess the proposed study sample size to allow for the detection of a point difference of 1.4 in pain scores at the 0.05 significance level with 90% power, assuming a t test as appropriate.
Results
Seventy‐one patients were enrolled, with 20 recruited from a tertiary care rhinology practice (28.1%) and 51 from the community. Of these patients, 38 were randomized to receive therapy using the active handheld microcurrent device, whereas 33 were assigned to the placebo device.
Enrolled patients largely reported facial pain that they attributed to sinonasal diseases, such as allergic rhinitis (69%), CRS (38%), and active upper respiratory infection (4.2%). In 5.6% of the patients, the diagnosis was unknown/unassigned, and/or the patient attributed the facial pain to nasal congestion. Patients’ demographics and clinical characteristics within each group are shown in Table 1.
Table 1.
Patient demographics and clinical characteristics by treatment group*
| Active (n = 38) | Placebo (n = 33) | p a | |
|---|---|---|---|
| Age (years), mean ± SD | 44.6 ± 14.9 | 43.6 ± 14.8 | 0.7666 |
| Female (%) | 63.2 | 63.6 | 0.8072 |
| CRS (%) | 36.8 | 39.4 | 1.00 |
| Allergic rhinitis (%) | 68.4 | 69.7 | 1.00 |
| Current cold/URI (%) | 5.3 | 3.0 | ND |
| Barometric pressure (%) | 2.6 | 3.0 | ND |
| Acute sinusitis (%) | 2.6 | 9.1 | ND |
| Unknown sino/nasal disease (%) | 5.3 | 6.0 | ND |
| Pain location (%) | |||
| Cheeks | 71.1 | 96.9 | 0.0032 |
| Forehead | 71.1 | 36.4 | 0.0044 |
| Between the eyes | 31.6 | 30.3 | 1.00 |
| Pain quality (%) | |||
| Dull | 42.1 | 60.6 | 0.1554 |
| Pressure | 73.7 | 54.5 | 0.1350 |
| Sharp | 18.4 | 12.1 | 0.5270 |
| Burning | 6.1 | 0.0 | ND |
| Pretreatment VAS score, mean ± SD | 5.6 ± 1.4 | 6.0 ± 1.3 | 0.2096 |
*Of the 71 patients enrolled in the study, 20 derived from the tertiary sinus clinic with documented sinonasal diagnoses. The remaining 51 patients were enrolled through the community and provided self‐reported sinonasal (and other) disease.
aND indicates that fewer than 5 subjects fell in any 1 category and no test was performed.
CRS = chronic rhinosinusitis; ND = not determined; SD = standard deviation; URI = upper respiratory infection; VAS = visual analogue scale.
The primary endpoint was the change in pain score using the VAS. No subjects experienced an increase in pain score following treatment. The mean VAS pain score reduction for patients treated with the placebo device was 0.91 points between pretreatment and posttreatments, whereas patients given the active microcurrent emitter had an average reduction of 1.66 points, both statistically significant with p < 0.0001 (Table 2). When comparing the difference in pain score reduction between the devices, there was a 0.75‐point (range, 1.66 to 0.91 points) greater reduction in patients who used the active microcurrent device (p = 0.0074; Table 3 and Fig. 1A). The subjects treated with the active device also had a greater percent decrease in pain levels than those treated with the placebo device (mean percent change pretreatment‐to‐posttreatment: active, 29.6% vs placebo, 15.9%; p = 0.005, Table 3 and Fig. 1B). Notably, 23.7% of patients treated with the active microcurrent device achieved a ≥3‐point reduction in pain scores, whereas none of the patients in the placebo group experienced the same degree of reduced pain (p = 0.0027) (Table 3 and Fig. 1D).
Table 2.
Pretreatment and posttreatment pain scores for each group
| Group | Pretreatment | Posttreatment | Δ | p |
|---|---|---|---|---|
| Active | ||||
| Mean ± SD | 5.6 ± 1.4 | 4.0 ± 1.7 | −1.6 | <0.0001 |
| 95% CI | (5.2, 6.1) | (3.4, 4.5) | ||
| Placebo | ||||
| Mean ± SD | 6.0 (1.3) | 5.1 (1.6) | −0.9 | <0.0001 |
| 95% CI | (5.6, 6.5) | (4.6, 5.7) |
Δ = (posttreatment mean score – pretreatment mean score); CI = confidence interval; SD = standard deviation.
Table 3.
Pain score reduction by group
| Pain reduction | Active (n = 38) | Placebo (n = 33) | Δ | p |
|---|---|---|---|---|
| Absolute pain reduction | ||||
| Mean ± SD | –1.7 ± 1.4 | –0.9 ± 0.8 | 0.8 | 0.0074 |
| 95% CI | (–1.2, –2.1) | (–0.6, –1.2) | ||
| Percent pain reduction (%) | ||||
| Mean ± SD | –29.6 ± 24 | –15.9 ± 15.6 | 13.8 | 0.0051 |
| 95% CI | (–21.7, –37.5) | (–10.3, –21.4) | ||
| Improvement of 3 or more points (%) | 23.7 | 0.0 | 23.7 | 0.0027 |
Δ = (active device − placebo device) scores or percentages; CI = confidence interval; SD = standard deviation.
Figure 1.
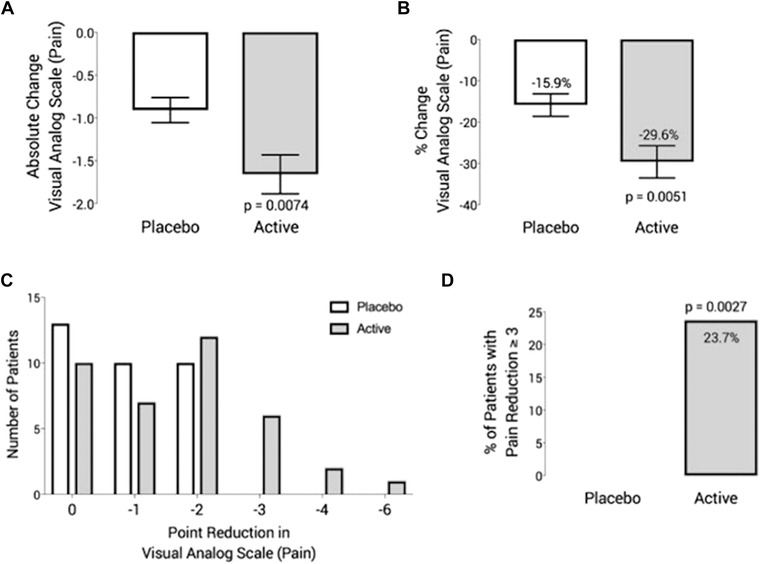
Effect of device use on self‐reported pain, as assessed by VAS. (A) Mean absolute change in VAS. (B) Mean percent change in VAS. (C) Number of participants reporting decreases in pain from 0 to –6 points. (D) Percent of patients with pain reduction greater than or equal to 3 points. VAS = visual analogue scale.
Patients were given a posttreatment questionnaire to assess their experience with the treatment. Almost 75% (74.6%) of patients strongly agreed that performing the treatment was easy, and 54.9% strongly agreed that performing the treatment was rapid. Additionally, greater than 80% of the patients given the active device reported that they found microcurrent treatment to be appropriate for treating their sinus pain and preferred the device to their existing forms of treatment (Table 4).
Table 4.
Treatment preference by group
| Treatment preference | Active n (%) | Placebo n (%) | p |
|---|---|---|---|
| Find device appropriate for treatment | 32 (84.2) | 25 (75.8) | 0.3719 |
| Prefer device to current treatment | 31 (81.6) | 29 (87.9) | 0.4643 |
There were no major complications and 1 minor (2.6%) development of transient reddening of the area stimulated by the active device. This finding dissipated 15 minutes after use, and caused no discomfort or short‐term/long‐term sequelae.
Discussion
In this prospective, randomized, double‐blinded clinical trial, the use of a transcutaneous, noninvasive microcurrent emitter for treatment of self‐reported sinonasal facial pain was statistically and clinically effective in rapidly alleviating pain when compared to a placebo device. Neither device resulted in complete resolution of pain levels in study subjects, but decreased the severity of pain. Patients enrolled in the study were referred for participation from a clinic in 28.1% of the cases, whereas the majority of participants (71.9%) were recruited through generic advertising methods. Patients recruited through the latter means had self‐reported diagnoses or referenced past medical encounters, but for a small subset of patients (n = 4) there was no known/attributed diagnosis. However, no statistical difference was noted in the reduction of VAS pain scores between the participants enrolled from the clinic vs advertising (data not shown). Additionally, any biases imposed from self‐reported diagnoses would be expected to have overlapping effects on scoring for both the placebo and active device participants, given the randomized assignments utilized.
For the pain relief evaluation, we anticipated and observed a marked placebo effect. Given that sensory nerves can be activated by both electrical and mechanical stimulation, the haptic vibration present in the placebo device may also have contributed to the alleviation of pain, or perception of pain relief, when applied to the periorbital region. Despite this component contributing to a positive response to the placebo arm, recorded pain scores were still significantly and reliably reduced by comparison following use of the active device. Given the prevalence of sinus pain and its impact on several dimensions of health care, novel mechanisms to address this common symptom are needed.
An important limitation of this study was the short follow‐up time of 10 minutes following device use, which circumscribes the ability to draw conclusions about facial pain relief beyond the immediate posttreatment period. An additional limitation is the use of VAS subjective measurement for pain, lacking objective metrics to assess for changes in pain. However, as a pilot investigation for this technology, this trial opens opportunities for future research to assess the efficacy of this transcutaneous microcurrent application for longer‐term, sinus‐related pain relief.
Conclusion
This randomized, double‐blinded, placebo‐controlled trial suggests that treatment of rhinologic facial pain with the use of a noninvasive microcurrent device is safe and effective in providing a statistically significant, rapid reduction of nasal/sinus pain complaints. Patient self‐administration of the device was feasible and the treatment was considered to be easy and fast. With this foundation, incremental studies can be envisioned to assess the value of this device for reliable and durable administration of sinus pain relief.
Supporting information
Supporting Video 1. User self‐administration of microcurrent therapy device. The user places the device in contact with the facial skin and glides it until haptic vibration is emitted. This indicates that a treatment point has been identified. The user holds the device in place for approximately 7 seconds while alternating current is emitted until the vibration stops. The user continues gliding the device until the next treatment point is located. The user applies the device repetitively for 5 minutes following a “figure H” path above and below each orbit and across the nasal dorsum.
Acknowledgments
We acknowledge Karen Copeland, PhD, from Boulder Statistics, LLC, for expert statistical analysis.
How to Cite this Article: Maul XA, Borchard NA, Hwang PH, Nayak JV. Microcurrent technology for rapid relief of sinus pain: a randomized, placebo‐controlled, double‐blinded clinical trial. Int Forum Allergy Rhinol. 2019;9:352–356.
[Correction added on 2/14/2019, after first online publication: A correction was made to the abstract.]
X.A.M. and N.A.B. contributed equally to this work.
Funding sources for the study: Tivic Health Systems, Inc.
Potential conflict of interest: P.H.H. is a member of the scientific advisory board of Tivic Health Systems, Inc.
Presented orally at the Annual ARS Meeting on October 5‐6, 2018, in Atlanta, GA. The presentation received the Runner‐Up Award for Best Clinical Paper of the meeting.
References
- 1. Bhattacharyya N, Gilani S. Prevalence of potential adult chronic rhinosinusitis symptoms in the United States. Otolaryngol Head Neck Surg. 2018;159:522‐525. [DOI] [PubMed] [Google Scholar]
- 2. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287‐333. [DOI] [PubMed] [Google Scholar]
- 3. Weiss AL, Ehrhardt KP, Tolba R. Atypical facial pain: a comprehensive, evidence‐based review. Curr Pain Headache Rep. 2017;21:8. [DOI] [PubMed] [Google Scholar]
- 4. Meng F, Peng K, Yang JP, Ji FH, Xia F, Meng XW. Botulinum toxin‐A for the treatment of neuralgia: a systematic review and meta‐analysis. J Pain Res. 2018;11:2343‐2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agius AM, Sama A. Rhinogenic and nonrhinogenic headaches. Curr Opin Otolaryngol Head Neck Surg. 2015;23:15‐20. [DOI] [PubMed] [Google Scholar]
- 6. Ratner PH, Howland WC, 3rd , Jacobs RL, et al. Relief of sinus pain and pressure with fluticasone propionate aqueous nasal spray: a placebo‐controlled trial in patients with allergic rhinitis. Allergy Asthma Proc. 2002;23:259‐263. [PubMed] [Google Scholar]
- 7. DeConde AS, Mace JC, Ashby S, Smith TL, Orlandi RR, Alt JA. Characterization of facial pain associated with chronic rhinosinusitis using validated pain evaluation instruments. Int Forum Allergy Rhinol. 2015;5:682‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1‐S39. [DOI] [PubMed] [Google Scholar]
- 9. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;3699:971‐979. [DOI] [PubMed] [Google Scholar]
- 10. Kong X, Gozani SN. Effectiveness of fixed‐site high‐frequency transcutaneous electrical nerve stimulation in chronic pain: a large‐scale, observational study. J Pain Res. 2018;11:703‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Giorgi I, Castroflorio T, Sartoris B, Deregibus A. The use of conventional transcutaneous electrical nerve stimulation in chronic facial myalgia patients. Clin Oral Investig. 2017;21:275‐280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Video 1. User self‐administration of microcurrent therapy device. The user places the device in contact with the facial skin and glides it until haptic vibration is emitted. This indicates that a treatment point has been identified. The user holds the device in place for approximately 7 seconds while alternating current is emitted until the vibration stops. The user continues gliding the device until the next treatment point is located. The user applies the device repetitively for 5 minutes following a “figure H” path above and below each orbit and across the nasal dorsum.


