Abstract
Secukinumab, a fully human monoclonal antibody neutralizing interleukin‐17A, has been shown to have significant efficacy in the treatment of moderate to severe psoriasis. Long‐term (3‐year) efficacy and safety of secukinumab in Japanese patients with moderate to severe psoriasis were evaluated in an extension study of a large phase 3 global study (SCULPTURE). In the core study, 52 Japanese patients with 75% improvement of Psoriasis Area and Severity Index (PASI‐75) response at week 12 were re‐randomized to a fixed interval (FI; every 4 weeks) schedule and retreatment as needed (RAN), in which patients received placebo until start of relapse, at which time secukinumab was reinitiated. Fifty Japanese patients completed the 52‐week core study, and 47 patients entered the extension study with the same double‐blind regimens up to week 152. All patients in the secukinumab 300 mg FI and seven patients in 150 mg FI groups completed 3 years of treatment. PASI‐90 and ‐100 at the end of year 3 were achieved in 69.2% and 53.8%, respectively, in 300 mg FI and 42.9% and 42.9%, respectively, in 150 mg FI, indicating high sustained response in 300 mg FI. Mean absolute PASI was continually low in 300 mg FI and numerically higher in 150 mg FI. Dermatology Life Quality Index of 0/1 was maintained by approximately two‐thirds of 300 mg FI patients, and all EuroQoL 5‐Dimension Health Questionnaire domain measures were also improved. FI dosing was consistently more efficacious than RAN. The safety profile of secukinumab remained favorable, with no new safety concerns identified.
Keywords: Dermatology Life Quality Index, EuroQol 5‐Dimension, psoriasis, randomized controlled trial, secukinumab
Introduction
Secukinumab, a fully human monoclonal antibody that selectively neutralizes interleukin (IL)‐17A, has been shown to have significant efficacy in the treatment of moderate to severe psoriasis, demonstrating a rapid onset of action and sustained response with a favorable safety profile.1, 2, 3, 4, 5, 6 Given the chronic and potentially lifetime course of moderate to severe plaque psoriasis,7 it is critical to assess the long‐term safety and optimal maintenance regimen of efficacy.
The SCULPTURE core study (NCT01406938) was a randomized, double‐blind, parallel‐group, 52‐week trial designed to compare different secukinumab maintenance treatment regimens, a fixed interval (FI; every 4 weeks) schedule and retreatment as needed (RAN), in patients with moderate to severe plaque psoriasis.3 An extension study of the SCULPTURE core study (NCT01640951) was conducted to collect long‐term double‐blind data of secukinumab. In the extension study, patients continued the same double‐blind regimens as the core study up to week 152.8 The study was open‐label from year 4. Efficacy, safety and health‐related quality of life (HRQoL) data from a total of 3 years of double‐blind extension treatment in the overall study population, approximately 75% of whom were Caucasian, has been reported earlier, and recently, efficacy and safety of secukinumab through 5 years of treatment was reported.9 However, there is no report on the impact of maintenance treatment regimens, FI versus RAN, on HRQoL, and for long‐term data of secukinumab in the Japanese population. Moreover, previous reports focused on 300 mg arms, and 150 mg data was not included. Unlike Western countries, a 150 mg dose was approved for psoriatic patients with bodyweight of 60 kg or less in Japan, in addition to the usual adult dose of 300 mg. It is therefore essential to report the 150 mg data of Japanese patients in this study. The information would be also useful for the patients in other Asian countries who have relatively low bodyweights. Here, we present the data, including details of HRQoL and 150 mg dose, from Japanese patients participating in the SCULPTURE and extension studies, covering 3 years of double‐blind treatment from core study baseline.
Methods
Study design
SCULPTURE was a randomized, double‐blind, parallel‐group, 52‐week trial designed to compare different secukinumab maintenance treatment regimens in patients with moderate to severe plaque psoriasis.3 Eligible patients were randomized 1:1 to s.c. secukinumab 300 or 150 mg groups (Fig. S1). Secukinumab was administrated at baseline and weeks 1, 2, 3, 4 and 8. At week 12, 75% improvement in baseline Psoriasis Area and Severity Index (PASI‐75) responders were re‐randomized 1:1 to maintenance therapy on FI or RAN regimens at the same dose received in the first 12 weeks. For the FI regimen, patients received secukinumab every 4 weeks. For the RAN regimen, patients received secukinumab at week 12, followed by placebo until start of relapse, defined as loss of 20% or more of maximum PASI score improvement versus baseline, plus loss of PASI‐75 response. When start of relapse criteria were fulfilled, the patients received active secukinumab at the originally randomized dose at scheduled study visits (i.e. every 4 weeks) until regain of PASI‐75 response; at this point, the patient started receiving placebo again.
Patients who completed the 52‐week SCULPTURE core study entered an extension study if the patient and investigator expected benefits from the extension. Patients were not required to achieve PASI‐75 at the end of the core study to enroll for the extension phase. Patients who received s.c. secukinumab 300 or 150 mg in the FI or RAN regimen in the SCULPTURE maintenance period continued the same dose and treatment regimen and returned for visits up until the last blinded dose at week 152.
The study protocol was approved by the institutional review board of each participating site, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Eligible patients provided written informed consent in the core study.
Patients
Eligibility criteria for the SCULPTURE core study included age of 18 years or more and a diagnosis (≥6 months prior to randomization) of chronic moderate to severe plaque psoriasis, defined as all of the following: PASI score of 12 or more; investigator's global assessment, 2011 modified version score of 3 or 4; and body surface area affected of 10% or more. Additionally, patients were required to have a history of inadequate psoriasis control with any of the following: topical treatments, phototherapy, systemic therapy or a combination of these. Use of concomitant psoriasis therapy during the study was prohibited, with the exception of topical corticosteroids applied to the face, scalp, and/or genitoanal area for 14 or less consecutive days.
Outcomes
The primary objective of the extension study was to assess the long‐term safety and tolerability of secukinumab in patients with moderate to severe chronic plaque‐type psoriasis who completed treatment in the core study. The secondary objectives included the long‐term efficacy of secukinumab administrated at RAN versus FI regimens in patients with moderate to severe chronic plaque‐type psoriasis who were PASI‐75 responders at week 12 in the core study. HRQoL assessments such as Dermatology Life Quality Index (DLQI) and EuroQoL 5‐Dimension Health Questionnaire (EQ‐5D) were also evaluated.10, 11 The EQ‐5D is a questionnaire with five questions in five dimensions (mobility, self‐care, usual activities, pain/discomfort and anxiety/depression) each with three levels of health state description (“no problems”, “moderate problems”, “severe problems”).10 Subsequently 243 patterns of health quality of life (QoL) are converted to a utility value on a scale ranging from 0 (death) to 1 (completely healthy) using the associated conversion table. Health state was also assessed with visual analog scale score from 0 (worst possible health state) to 100 (best possible health state). The EQ‐5D results were analyzed up to week 76, as the number of patients to have answered the questionnaire decreased after this assessment point. Safety assessments consisted of adverse event (AE) assessment, physical examination, monitoring of vital signs and laboratory values, and electrocardiograms.
Statistical analysis
For all efficacy assessments, summary statistics were calculated by visit and treatment group. The proportion of patients with PASI‐75, ‐90 or ‐100 response, DLQI 0/1 response (in DLQI total score), the proportion of patients reporting each category on EQ‐5D domains, and absolute mean change from baseline in EQ‐5D visual analog scale score were all analyzed using observed values without imputation of any missing values. EQ‐5D data were only included up to week 76, as fewer patients provided data afterward. AE were reported as exposure‐adjusted incidence rates (incidence per 100 patient‐years).
Results
Patients
The SCULPTURE core study randomized 966 patients 1:1 to secukinumab at 300 or 150 mg. At week 12, PASI‐75 responders (n = 843) were re‐randomized 1:1 to FI or RAN maintenance regimens at their respective doses. After the completion of the 52‐week core study, a total of 642 patients provided written consent to enter the extension study, of whom 168 and 172 patients were randomized to the secukinumab 300 mg FI arm and RAN arm, respectively, and 152 and 150 patients were randomized to the secukinumab 150 mg FI arm and RAN arm, respectively.
The core study included 62 Japanese patients with secukinumab 300 mg (n = 31) or 150 mg (n = 31), of whom 59 patients completed the induction phase. PASI‐75 response was achieved by 52 patients at week 12, who were re‐randomized to FI or RAN at each dose. Fifty Japanese patients completed the core study, and a total of 47 patients entered the extension study with 13 and 14 patients in the secukinumab 300 mg FI arm and RAN arm, respectively, and 10 patients each in the secukinumab 150 mg FI arm and RAN arm. All of the 13 patients in the secukinumab 300 mg FI arm continued the study up to week 152, while 12 patients continued in the secukinumab 300 mg RAN arm (reasons of discontinuation were physician decision and AE). In the 150 mg FI arm and RAN arm, seven and eight patients, respectively, continued up to week 152. Three patients in the 150 mg FI arm, one female with bodyweight of 60 kg or less (48.6 kg) and two males of more than 60 kg (77.6, 81.5 kg), discontinued due to lack of efficacy, and two patients in the 150 mg RAN arm, one female with bodyweight of more than 60 kg (69.0 kg) and one male of 60 kg or less (52.8 kg), discontinued due to subject/guardian decision. The treatment arms included patients previously treated with biologics: two (infliximab, adalimumab) in the 300 mg FI arm, four (two each of ustekinumab and infliximab) in the 300 mg RAN arm, and one each (infliximab and adalimumab) in the 150 mg FI and RAN arms, respectively.
Baseline demographics and background characteristics for the extension treatment period were representative of the psoriatic population, and no clinically meaningful differences were noted between the secukinumab 300 mg FI and RAN treatment arms (Table 1). The baseline demographics for 150 mg FI and RAN treatment arms are presented in Table S1.
Table 1.
Characteristics of extension study patients at core study baseline
| Secukinumab 300 mg FI (n = 13) | Secukinumab 300 mg RAN (n = 14) | |
|---|---|---|
| Age, years | ||
| Mean ± SD | 52.2 ± 12.70 | 47.9 ± 9.31 |
| Sex, n (%) | ||
| Male | 9 (69.2) | 13 (92.9) |
| Race, n (%) | ||
| Asian | 13 (100) | 14 (100) |
| BMI, kg/m2 | ||
| Mean ± SD | 24.89 ± 2.91 | 25.79 ± 5.58 |
| Bodyweight | ||
| ≤60 kg, n (%) | 5 (38.5) | 0 (0) |
| PASI score | ||
| Absolute mean PASI ± SD | 27.1 ± 10.21 | 23.54 ± 10.44 |
| Previous systemic treatment, n (%) | ||
| Any | 8 (61.5) | 11 (78.6) |
| Biologic | 2 (15.4) | 4 (28.6) |
BMI, body mass index; FI, fixed interval; PASI, Psoriasis Area and Severity Index; RAN, retreatment as needed; SD, standard deviation.
Efficacy
Overall, Japanese patients in the secukinumab 300 mg FI arm sustained PASI‐75, ‐90 and ‐100 response levels from week 12 to week 152 (Figs 1,2). In contrast, the proportion of PASI‐75, ‐90 and ‐100 responders in the RAN arms started to decline from week 20 and were lower compared with the FI arms. At week 152, the proportion of patients with a PASI‐75 response was notably higher in the 300 mg FI (100.0%) compared with the 300 mg RAN (58.3%) arm. Similarly, the proportion of patients with a PASI‐90/100 response at week 152 was notably higher in the 300 mg FI arm (69.2%/53.8%) compared with the 300 mg RAN (33.3%/8.3%) arm.
Figure 1.
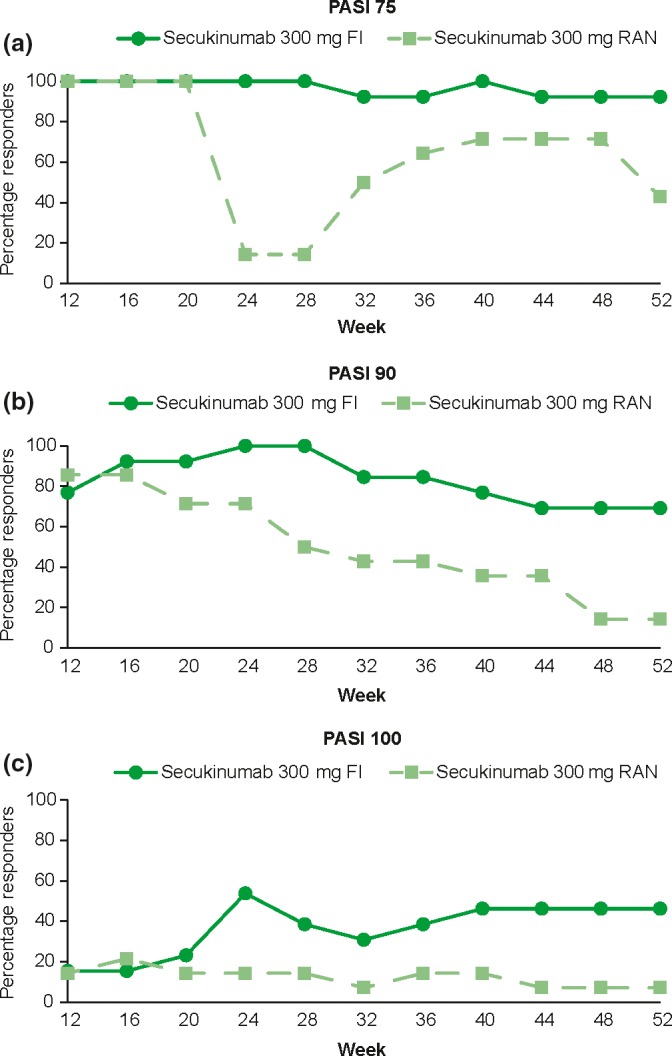
Percentage of patients with PASI response from week 12 through week 52 in the secukinumab 300 mg arm. The data was analyzed using observed values without imputation of any missing values. FI, fixed interval; PASI, Psoriasis Area and Severity Index; RAN, retreatment as needed.
Figure 2.
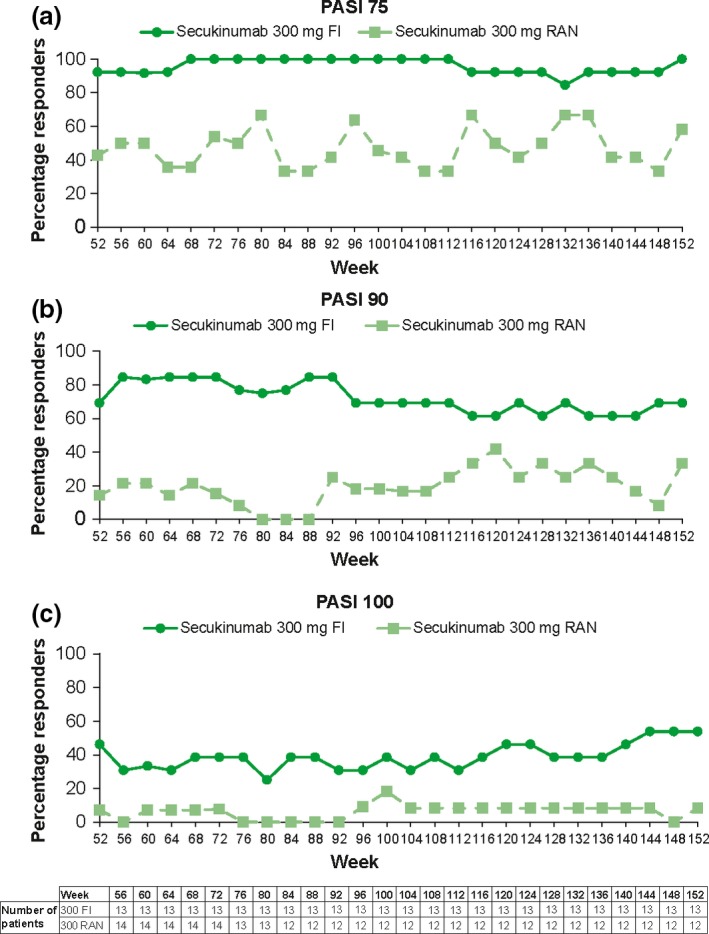
Percentage of patients with PASI response from week 52 through week 152 in secukinumab 300 mg arm. The data was analyzed using observed values without imputation of any missing values. FI, fixed interval; PASI, Psoriasis Area and Severity Index; RAN, retreatment as needed.
PASI‐75/90/100 responses at the lower dose of 150 mg FI and RAN regimens in Japanese patients (71.4%/42.9%/42.9% and 37.5%/0%/0%, respectively) were numerically less compared with 300 mg FI (100%/69.2%/53.8%) and RAN (58.3%/33.3%/8.3%; Figs S2,S3).
Two patients previously treated with biologics in the 300 mg FI arm were a PASI‐75–89 responder and a PASI‐90–100 responder at week 152, while two of four patients with previous biologics in the 300 mg RAN arm did not achieve PASI‐50; the rest of the patients were a PASI‐75–89 and a PASI‐90–100 responder at week 152.
Patients in the 300 mg FI arm achieved a high level of psoriasis control, with the mean absolute PASI continually less than 3 after week 12 (Fig. 3). Mean PASI of the 300 mg RAN arm increased after the switch to placebo at week 12, but remained in the range of 5–9 that was one‐third of the baseline mean PASI score, with the retreatment of secukinumab at the start of relapse until regain of PASI‐75 response. The mean absolute PASI of the patients in the 150 mg FI and RAN arms was numerically higher compared with corresponding 300 mg arm (Fig. S4).
Figure 3.
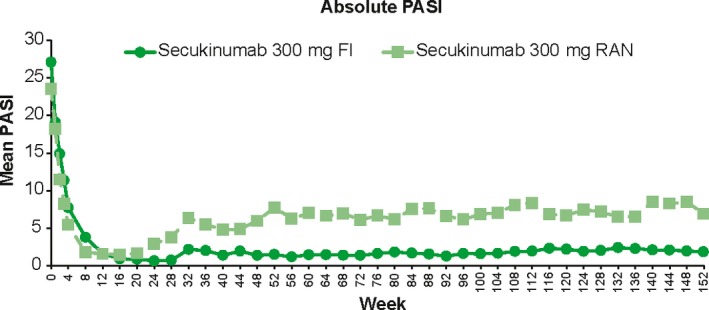
Mean absolute PASI from week 0 through week 152 in secukinumab 300 mg arm. The data was analyzed using observed values without imputation of any missing values. FI, fixed interval; PASI, Psoriasis Area and Severity Index; RAN, retreatment as needed.
A DLQI total score of 0 or 1 was quickly achieved and maintained by approximately two‐thirds (61.5–76.9%) of patients with 300 mg FI from week 12 until week 156, the DLQI assessment point of last blinded dose at week 152 (Fig. 4). Consistently, the patients with DLQI 0/1 in the 300 mg FI arm were mostly PASI‐90 responders (≥seven patients) from week 12 to week 156. At week 156, seven of eight patients with DLQI 0/1 were PASI‐90 responders, who were 87.5% of total PASI‐90 responders (7/8 patients) in the FI arm, and the remainder was a PASI‐75–89 responder (1/5 patients).
Figure 4.
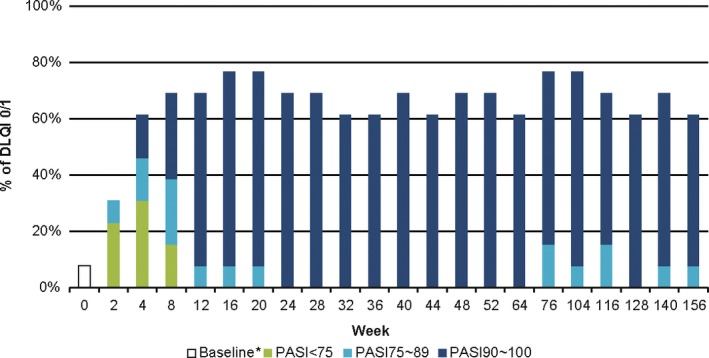
Proportion of DLQI total score 0/1 with the PASI response from week 0 through week 156 in the secukinumab 300 mg FI arm. *Week 0 (baseline): one patient had DLQI 0/1 at baseline. The data was analyzed using observed values without imputation of any missing values. DLQI, Dermatology Life Quality Index; FI, fixed interval; PASI, Psoriasis Area and Severity Index.
The proportion of DLQI 0/1 response was slightly lower in the 300 mg RAN arm compared with the 300 mg FI arm, with a variation ranging 35–66% of patients (Fig. S5). A drop in DLQI 0/1 was observed at week 32 in the RAN arm, which was expected because five patients began the start of relapse for the first time at this time point (20 weeks after week 12). PASI‐75 response was regained by one administration of secukinumab (week 32) in two patients and by two administrations (week 32 and 36) in three patients, resulting in a quick recovery in the DLQI 0/1 response. The patients with DLQI 0/1 in the RAN arm were a mixture of different PASI responses; for example, three PASI‐90 responders out of total seven patients with DLQI 0/1 at week 156 (Fig. S5). To evaluate the effect of relapse on the correlation of PASI and DLQI, absolute PASI and DLQI scores at each patient's baseline and first relapse were plotted individually (Fig. S6). The calculated slope of the trendline for the relapse was smaller than that of the baseline (0.510 vs 0.982), implying an elevation of DLQI score at the relapse.
Mean EQ‐5D utility score increased after secukinumab treatment toward healthy state in both 300 mg FI and RAN arms with a larger deviation observed in RAN arm later in the maintenance period (0.732, 0.935, 0.946 and 0.950 vs 0.734, 0.932, 0.871 and 0.924 at week 0, 16, 52 and 76; Fig. S7). The proportion of patients in each of the answer categories of the EQ‐5D by question and visit (based on observed values) showed that patients treated with secukinumab 300 mg FI experienced high rates of improvement in all of the EQ‐5D domain measures (Fig. 5). At week 52 and 76, all patients of the 300 mg FI arm (n = 13) were in the category of no problems in mobility and anxiety/depression. In the 300 mg RAN arm, an improvement was observed before the randomization at week 12, but the patients with no problems decreased during the maintenance period with the RAN regimen (Fig. S8). Consistently, EQ‐5D visual analog scale score (i.e. improvement) was constantly high with 300 mg FI treatment, but slightly declined with 300 mg RAN after an increase from baseline (Fig. 6).
Figure 5.
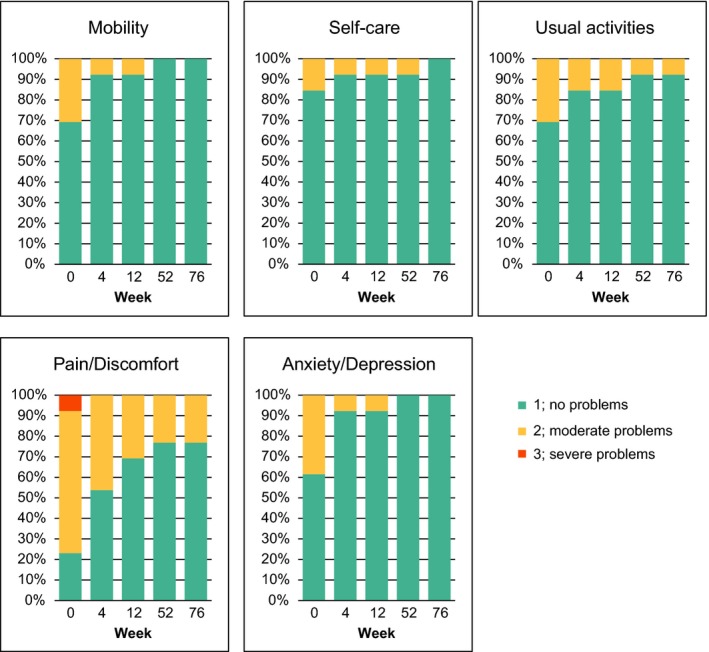
EQ‐5D domains from week 0 through week 76 in the secukinumab 300 mg FI arm. The data was analyzed using observed values without imputation of any missing values. EQ‐5D, EuroQoL 5‐Dimension Health Questionnaire; FI, fixed interval.
Figure 6.
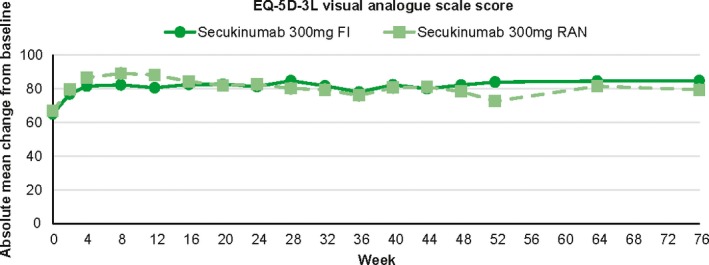
Health states of VAS over time change of secukinumab 300 mg FI arm and 300 mg RAN arm from week 0 through week 76. The data was analyzed using observed values without imputation of any missing values. EQ‐5D, EuroQoL 5‐Dimension Health Questionnaire; FI, fixed interval; RAN, retreatment as needed; VAS, visual analog scale.
Safety
Non‐fatal serious AE were reported in two patients in the 300 mg FI arm (diabetes mellitus and cervical dysplasia) and three patients in the 300 mg RAN arm (one patient with liver injury and femur fracture due to a car accident, one patient each of altered state of consciousness and bacterial tonsillitis). The most common AE observed from week 0 to week 152 in the Japanese population was nasopharyngitis in both the 300 mg FI and RAN arms. The incidences of folliculitis and hypertension were higher in the 300 mg FI than 300 mg RAN arm (Table 2). With regard to AE of special interest, one case of moderate Candida infection, which was completely resolved in 14 days with concomitant medication without discontinuation of secukinumab therapy, was reported in the 300 mg FI arm.
Table 2.
Treatment‐emergent adverse events, entire treatment period (week 0 to week 156)
| n (%) | Secukinumab 300 mg FI 13 patients n (IR) | Secukinumab 300 mg RAN 14 patients n (IR) |
|---|---|---|
| Any AE | 13 (256.5) | 14 (294.1) |
| Death | 0 | 0 |
| Non‐fatal SAE | 2 (5.55) | 3 (8.92) |
| Most common AE by preferred term† | ||
| Nasopharyngitis | 9 (45.8) | 8 (35.5) |
| Folliculitis | 5 (16.5) | 1 (2.8) |
| Hypertension | 4 (12.8) | 2 (5.5) |
| Eczema | 3 (9.4) | 2 (5.8) |
| Pruritus | 3 (9.3) | 1 (2.8) |
| Eosinophilia | 3 (9.1) | 0 (0.0) |
| Dermatitis contact | 3 (8.5) | 0 (0.0) |
| AE of special interest | ||
| Candida infections | 1 (2.5) | 0 (0.0) |
| Serious infections | 0 (0.0) | 1 (2.1) |
| Neutropenia | 0 (0.0) | 0 (0.0) |
| Malignancy‡ | 0 (0.0) | 0 (0.0) |
| MACE§ | 0 (0.0) | 0 (0.0) |
| Depression | 0 (0.0) | 1 (7.1) |
| Inflammatory bowel disease | ||
| Ulcerative colitis | 0 (0.0) | 0 (0.0) |
| Crohn's disease | 0 (0.0) | 0 (0.0) |
†≥Three patients in the 300 mg FI group. ‡Defined as malignant or unspecified tumors (excluding basal cell carcinoma and squamous cell carcinoma). §Defined as myocardial infarction, stroke or cardiovascular death. All patients with MACE had two or more pre‐existing risk factors. None of the events was considered to be related to study drug by the investigators and none led to study discontinuation. Only patients who completed the SCULPTURE core study and continued into the extension are included in this analysis. A patient with multiple occurrences of an AE under one treatment is counted only once in the AE category for that treatment. AE, adverse event; FI, fixed interval; IR, incidence rate per 100 patient‐years; MACE, major adverse cardiovascular event; RAN, retreatment as needed; SAE, serious AE.
Discussion
Psoriasis is a chronic and potentially lifetime skin disease. Evaluation of efficacy and safety profiles of psoriasis therapies over multiple years, ideally under double‐blind conditions, is needed to understand the potential of long‐term disease control. Moreover, it is clinically important to determine the optimal regimen for a long‐term maintenance treatment.
Secukinumab is a recombinant, high‐affinity, fully human monoclonal antibody, that selectively binds to and neutralizes IL‐17A. SCULPTURE was a phase 3 study designed to compare FI and RAN maintenance regimens at two doses (300 mg and 150 mg s.c. secukinumab) in patients with moderate to severe plaque psoriasis up to week 52). The double‐blind phase was extended up to week 152 to enable a fair comparison of the maintenance regimens. Here, we report the results of Japanese patients included in the SCULPTURE core and extension studies.
In this long‐term study, all 13 patients re‐randomized to the 300 mg FI arm at week 12 completed the 3‐year double‐blind phase, indicating high durability of the FI treatment with secukinumab 300 mg. Moreover, a high degree of efficacy was maintained through 3 years of treatment in the secukinumab 300 mg FI arm, with nine of 13 patients (69%) exhibiting clear (PASI‐100) or almost clear skin (PASI‐90) at week 152. The PASI‐90 responders included one of two patients previously treated with biologics in the arm, suggesting the possibility of high responses in the patients who failed previous biologics. Long‐term psoriasis control was achieved with the mean absolute PASI in the 300 mg FI arm remaining under 3 during years 2 and 3 of treatment.
Secukinumab 300 mg FI demonstrated consistently high and sustained responses with improvement in QoL over time, as assessed by DLQI and EQ‐5D. Notably, approximately two‐thirds of the 300 mg FI arm reported no effect of skin disease on their lives (DLQI 0/1) up to week 152. Moreover, DLQI 0/1 was achieved mainly by PASI‐90 responders in the 300 mg FI arm (7/8 patients) and was infrequent among PASI‐75–89 responders (1/5 patients), suggesting that the high improvement goal, namely PASI‐90 response, is critical to achieve DLQI 0/1. Improvement in HRQoL was also demonstrated in all EQ‐5D domains, with all patients reporting no problem in the EQ‐5D domain of mobility and anxiety/depression at week 52 and 76.
Secukinumab administrated in the 300 mg RAN arm was less efficacious over time than the 300 mg FI arm, consistent with the overall results of the study. Although no statistics were conducted for the Japanese patients due to lack of power, the superiority of the FI arm over the RAN arm was demonstrated in the entire populations.8 Analysis of correlation of PASI and DLQI score at relapse suggested an impaired QoL at relapse compared with baseline, supporting use of continuous treatment, namely 300 mg FI, to prevent deterioration of patients’ QoL caused by relapse.
Efficacy of the lower dose of 150 mg was less compared with the same regimens of 300 mg. Administration of 150 mg as a single dose was approved in Japan for patients with bodyweight of 60 kg or less. Of the three patients (one female, two males) with bodyweight of 60 kg or less in the 150 mg FI arm, two male patients achieved PASI‐100, which may support the approved label in Japan. However, the remaining female patient of low weight discontinued the study due to lack of efficacy. It is noteworthy that the two more discontinued patients in the 150 mg FI arm had relatively high bodyweight (77.6, 81.5 kg), which may explain the insufficient response of the patients. Besides, compared with the 150 mg FI arm, efficacy further reduced in the 150 mg RAN arm with none achieving PASI‐90, indicating the necessity of continuous treatment especially in the case of 150 mg dose.
Recently published comprehensive analysis of pooled safety data from 10 studies supported the favorable safety profile of secukinumab in patients with moderate to severe psoriasis.5 This analysis of Japanese patients, as well as the overall population in the SCULPTURE core and extension study, demonstrated a consistent safety profile with that of the pooled data and provides supportive evidence on the long‐term favorable safety profile.
A limitation of this report was that the sample size of Japanese patients was too small to permit statistical analysis of the results. Although there was no obvious difference from the overall population in the study, the results must be interpreted with caution.
This extension study reported the sustained efficacy and favorable safety profile of a secukinumab 300 mg FI regimen in Japanese patients with moderate to severe plaque psoriasis for up to 3 years. It is of note that all 13 Japanese patients in the secukinumab 300 mg FI arm completed the 3‐year double‐blind phase of the study, with a high level of efficacy in PASI responses and mean PASI score.
Supporting information
Figure S1. (a) Core study design and (b) extension study design.
Figure S2. Percentage of patients with Psoriasis Area and Severity Index (PASI) response from week 12 through week 52 in secukinumab 150 mg arm.
Figure S3. Percentage of patients with Psoriasis Area and Severity Index (PASI) response from week 52 through week 152 in secukinumab 150 mg arm.
Figure S4. Mean absolute Psoriasis Area and Severity Index (PASI) from week 0 through week 152 in secukinumab 150 mg arm.
Figure S5. Proportion of Dermatological Life Quality Index (DLQI) total score 0/1 with Psoriasis Area and Severity Index (PASI) response from week 0 through week 156 in secukinumab 300 mg RAN arm.
Figure S6. Effect of relapse on correlation of absolute Psoriasis Area and Severity Index (PASI) and Dermatological Life Quality Index (DLQI) scores in secukinumab 300 mg retreatment as needed (RAN) arm.
Figure S7. EuroQoL 5‐Dimension Health Questionnaire (EQ‐5D) over time in the secukinumab (a) 300 mg fixed interval (FI) and (b) retreatment as needed (RAN) arms from week 0 through week 76.
Figure S8. EuroQoL 5‐Dimension Health Questionnaire (EQ‐5D) domains from week 0 to week 76 in the secukinumab 300 mg retreatment as needed (RAN) arm.
Table S1. Characteristics of extension study patients at core study baseline
Acknowledgments
The following principle investigators in the Japanese subgroup are acknowledged for their support: Dr Kei Ito (JR Sapporo Hospital), Dr Hidehisa Saeki (The Jikei University School of Medicine), Dr Tadashi Terui (Nihon University Itabashi Hospital), Dr Hajime Iizuka (Asahikawa Medical University), Dr Takafumi Etoh (Tokyo Teishin Hospital), Dr Masatoshi Abe (Gunma University Graduate School of Medicine), Dr Kazuhiro Inafuku (Kimitsu Chuo Hospital) and Dr Yujiro Takae (Keio University Hospital). The authors would like to thank Kentaro Ishii (Novartis Pharma KK) and Sumeet Sood (Novartis Healthcare Pvt Ltd) for providing editorial and medical writing support. The sponsor (Novartis) has performed the statistical analysis, and Dr Okubo takes responsibility for the accuracy of the results. All authors reviewed and provided feedback on subsequent versions and agreed on the final version and to submit the manuscript for publication. The study was sponsored by Novartis Pharma AG.
Conflict of Interest
M. Y., T. S. and Y. T. are employees of Novartis Pharmaceuticals. Y. O., M. O. and A. M. received research grants and/or speaker fees from Novartis Pharmaceuticals. H. N. received consultancy fee from Novartis Pharmaceuticals.
References
- 1. Blauvelt A, Prinz JC, Gottlieb AB et al Secukinumab administration by pre‐filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol 2015; 172(2): 484–93. [DOI] [PubMed] [Google Scholar]
- 2. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis‐results of two phase 3 trials. N Engl J Med 2014; 371(4): 326–38. [DOI] [PubMed] [Google Scholar]
- 3. Mrowietz U, Leonardi CL, Girolomoni G et al Secukinumab retreatment‐as‐needed versus fixed‐interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double‐blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol 2015; 73(1): 27–36. e1 [DOI] [PubMed] [Google Scholar]
- 4. Paul C, Lacour JP, Tedremets L et al Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol 2015; 29(6): 1082–90. [DOI] [PubMed] [Google Scholar]
- 5. van de Kerkhof PC, Griffiths CE, Reich K et al Secukinumab long‐term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol 2016; 75(1): 83–98. e4 [DOI] [PubMed] [Google Scholar]
- 6. Ohtsuki M, Morita A, Abe M et al Secukinumab efficacy and safety in Japanese patients with moderate‐to‐severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo‐controlled, phase 3 study. J Dermatol 2014; 41(12): 1039–46. [DOI] [PubMed] [Google Scholar]
- 7. Gisondi P, Del Giglio M, Girolomoni G. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci 2017; 18(11): 2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bissonnette R, Luger T, Thaci D et al Secukinumab sustains good efficacy and favourable safety in moderate‐to‐severe psoriasis after up to 3 years of treatment: results from a double‐blind extension study. Br J Dermatol 2017; 177(4): 1033–42. [DOI] [PubMed] [Google Scholar]
- 9. Bissonnette R, Luger T, Thaci D et al Secukinumab demonstrates high sustained efficacy and a favorable safety profile in patients with moderate to severe psoriasis through 5 years of treatment (SCULPTURE extension study). J Eur Acad Dermatol Venereol 2018; 32(9): 1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The EuroQol group . EuroQol‐a new facility for the measurement of health‐related quality of life. Health Policy 1990; 16(3): 199–208. [DOI] [PubMed] [Google Scholar]
- 11. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19(3): 210–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (a) Core study design and (b) extension study design.
Figure S2. Percentage of patients with Psoriasis Area and Severity Index (PASI) response from week 12 through week 52 in secukinumab 150 mg arm.
Figure S3. Percentage of patients with Psoriasis Area and Severity Index (PASI) response from week 52 through week 152 in secukinumab 150 mg arm.
Figure S4. Mean absolute Psoriasis Area and Severity Index (PASI) from week 0 through week 152 in secukinumab 150 mg arm.
Figure S5. Proportion of Dermatological Life Quality Index (DLQI) total score 0/1 with Psoriasis Area and Severity Index (PASI) response from week 0 through week 156 in secukinumab 300 mg RAN arm.
Figure S6. Effect of relapse on correlation of absolute Psoriasis Area and Severity Index (PASI) and Dermatological Life Quality Index (DLQI) scores in secukinumab 300 mg retreatment as needed (RAN) arm.
Figure S7. EuroQoL 5‐Dimension Health Questionnaire (EQ‐5D) over time in the secukinumab (a) 300 mg fixed interval (FI) and (b) retreatment as needed (RAN) arms from week 0 through week 76.
Figure S8. EuroQoL 5‐Dimension Health Questionnaire (EQ‐5D) domains from week 0 to week 76 in the secukinumab 300 mg retreatment as needed (RAN) arm.
Table S1. Characteristics of extension study patients at core study baseline


