Abstract
Background
Osimertinib is recommended for non‐small cell lung cancer (NSCLC) patients with EGFR mutation; however, it is unclear whether body size variables affect the efficacy of osimertinib in such patients. This study assessed the potential effect of body surface area (BSA) and body mass index (BMI) on osimertinib chemotherapy in patients with T790M‐positive advanced NSCLC who progress on prior EGFR‐tyrosine kinase inhibitors (TKIs).
Methods
We conducted a prospective observational cohort study. Median BSA and BMI were used as cut‐off values to evaluate the impact of body size variables on osimertinib chemotherapy.
Results
The median BSA and BMI of 47 patients were 1.50 m2 and 21.5 kg/m2, respectively. Clinical outcomes did not significantly differ between the high and low BSA groups, with response rates of 59.1% and 56.0% (P = 0.83) and progression‐free survival (PFS) of 7.6 and 9.1 months (P = 0.69), respectively. Similarly, there were no significant differences between the high and low BMI groups relative to response rates, which were 60.8% and 54.1% (P = 0.64), respectively, and PFS, which was 7.6 months in both groups (P = 0.38). No significant differences were observed among toxicity profiles in relation to BSA or BMI. Multivariate analysis identified better performance status, young age, and EGFR exon 19 deletion as independent favorable predictors of PFS.
Conclusion
The efficacy of osimertinib does not significantly vary relative to body size variables of patients with T790M‐positive NSCLC who progress on prior EGFR‐TKIs.
Keywords: Body mass index, body surface area, non‐small cell lung carcinoma, osimertinib, progression‐free survival
Introduction
Lung cancer is a major cause of cancer death. Non‐small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers.1 Targeted therapies are currently being developed to improve efficacy in driver‐oncogene positive NSCLC patient populations. Small‐molecule tyrosine kinase inhibitors (TKIs) that target EGFR have been introduced clinically for the treatment of NSCLC. Meta‐analyses have clearly indicated improved progression‐free survival (PFS) and response rates in patients with EGFR mutations administered EGFR‐TKI therapy including gefitinib, erlotinib, and afatinib, compared to patients administered chemotherapy with cytotoxic drugs.2, 3, 4, 5 Based on these results, EGFR‐TKIs have become the standard regimen for patients with advanced NSCLC harboring an EGFR mutation. In addition, EGFR‐TKIs combined with chemotherapy in NSCLC patients with EGFR mutations is reported achieve longer survival and tolerable side effects.6, 7, 8 However, despite initial responses to EGFR‐TKI, the majority of patients will experience disease progression within two years as a result of acquired resistance.9, 10, 11, 12, 13, 14, 15, 16, 17 In approximately 60% of patients, the mechanism of acquired resistance is the development of an additional EGFR mutation, EGFR T790M.16 Osimertinib is a mono‐anilino‐pyrimidine compound that irreversibly and selectively targets EGFR‐TKI‐sensitizing and T790M resistant mutant forms of EGFR, while sparing wild‐type EGFR.18, 19, 20 A Phase I/II AURA trial was conducted to determine the safety and efficacy of osimertinib in patients with advanced NSCLC who experience disease progression after previous treatment with EGFR‐TKIs.21 Osimertinib showed high efficacy in patients with T790M mutation, with an objective response rate (ORR) of 61% and median PFS of 9.6 months. To confirm results of the single‐arm, Phase I/II AURA trial, a randomized, Phase III trial (AURA3) was conducted that demonstrated the superiority of osimertinib treatment over standard chemotherapy with platinum and pemetrexed in patients with EGFR‐mutated and centrally confirmed T790M‐positive advanced NSCLC who experienced disease progression after first‐line EGFR‐TKI therapy.22 Analysis of the primary endpoint indicated a significantly longer PFS in patients administered osimertinib compared to those treated with platinum chemotherapy. This result established the role of osimertinib as the standard‐of‐care for patients harboring the T790M resistance mutation who progress on first‐line EGFR‐TKIs. Because the standard dose of osimertinib was determined as 80 mg/day, a uniform dosage of 80 mg/day is prescribed, regardless of body size. Although dose adjustments based on body surface area (BSA) have been made in chemotherapy with cytotoxic agents, it is unknown whether body size variables, such as BSA or body mass index (BMI), affect the efficacy of osimertinib therapy in NSCLC patients who carry an EGFR mutation.
The objective of this study was to determine whether BSA and BMI affect the efficacy of osimertinib in patients with advanced NSCLC harboring a T790M mutation.
Methods
Patient selection
We conducted a prospective observational cohort study at Kitasato University Hospital between January 2017 and April 2018 to evaluate the efficacy and safety of osimertinib in patients with T790M‐positive advanced NSCLC who experienced disease progression after first‐line EGFR‐TKI therapy including gefitinib, erlotinib, and afatinib. The eligibility criterion of this study was histologically or cytologically confirmed NSCLC and stage IIIB/IV disease or recurrence according to the new Union for International Cancer Control criteria, version 8. We excluded patients who did not have at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.23 Patient characteristics, including age at diagnosis, gender, Eastern Cooperative Oncology Group performance status (PS) at the start of the osimertinib treatment, smoking status, clinical stage, tumor histology, BSA, BMI, brain metastasis status, number of metastatic lesions, and number of previous chemotherapy regimens, were identified by chart review. Patients were classified according to smoking status as current smokers, former light smokers (having smoked a total of ≤ 10 pack‐years plus smoking cessation at least 15 years previously), and never smokers (a lifetime history of having smoked < 100 cigarettes). We used the following formula to calculate BSA: BSA (m2) = (body weight [kg])0.425 × (height [cm])0.725 × 0.007184. The BMI kg/m2 at the start of treatment was defined as the weight (kg) divided by the height (m) squared.
All patients provided written informed consent. The Kitasato University Hospital institutional ethics review board approved the study. After obtaining written consent, the patients were treated with 80 mg of osimertinib per subject until disease progression or unacceptable adverse events occurred.
Analysis of EGFR mutations
A sample of the primary tumor, a metastatic lesion, or pleural effusion fluid was used as a specimen to test for EGFR mutation via the peptide nucleic acid‐locked nucleic acid PCR clamp method and the Cobas EGFR Mutation Test. Tumor biopsy cytology specimens, along with plasma specimens recovered by liquid biopsy, were tested for EGFR T790M status using the Cobas EGFR Mutation Test.
Response and toxicity assessment
After the initiation of osimertinib treatment, a computed tomography (CT) scan of the chest and abdomen was carried out every two to three months or at more frequent intervals. Positron emission tomography (PET) or bone scintigraphy and CT or magnetic resonance imaging (MRI) of the cranium were performed when patients exhibited significant symptoms associated with tumor lesions or at six‐month intervals. Response to treatment was re‐evaluated by two investigators according to RECIST 1.1.23 Medical records were reviewed to evaluate the toxicities experienced by all patients. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria version 4 grading system.
Statistical analysis
The Fisher's exact test was used to assess the distributions of categorical characteristics according to whether the patients’ BSA was ≥ 1.50 m2 (high‐BSA group) or < 1.50 m2 (low‐BSA group), as well as according to whether the patients’ BMI was ≥ 21.5 (high‐BMI group) or < 21.5 kg (low‐BMI group). The toxicities were also compared according to the median BSA and BMI by Fisher's exact test. PFS was measured from the start of gefitinib therapy to treatment failure (death, documentation of disease progression, or appearance of unacceptable toxicity) or the date the final follow‐up examination was censored. Overall survival (OS) was defined as the interval between the start of gefitinib therapy to death from any cause or the date of censoring. The survival curves were plotted using the Kaplan–Meier method and differences according to BSA and BMI were analyzed using the log‐rank test. Cox's proportional hazard models of variables including age, gender, smoking status, PS, stage, brain metastasis status, type of EGFR mutation, number of prior regimens, BSA, and BMI were used to predict the hazard rates for PFS. The differences in response rates according to BSA and BMI were compared by Fisher's exact test. P < 0.05 was used as the criterion for statistical significance. All statistical analyses were performed using SPSS version 17.0.
Results
Patient characteristics
A total of 47 NSCLC patients treated with osimertinib between May 2016 and April 2018 were included in the final analysis. The basic characteristics of the patients were: 66% female, median age 73 years, and 66% had a good PS (0 or 1) (Table 1). The patients suffered from adenocarcinoma (47 patients, 100%) and stage IV disease or postoperative recurrence (47 patients, 100%). The median BSA was 1.50 m2 (range: 1.16–1.79 m2) and the median BMI was 21.5 kg/m2 (range: 14.0–28.2 kg/m2). There were significantly higher percentages of men (87% vs. 35%, P < 0.001), non‐elderly (e.g. <75) patients (72% vs. 44%, P < 0.001), patients with good PS (62% vs. 34%, P = 0.03), smokers (79% vs. 42%, P = 0.024), and patients with L858R point mutation (66% vs. 29%, P = 0.015) in the high‐BSA (BSA ≥ 1.5 m2) group than in the low‐BSA (BSA < 1.5 m2) group (Table 2). Regarding the BMI, there were significantly higher percentages of patients with a good PS (62% vs. 27%, P = 0.01) and L858R point mutation (61% vs. 29%, P = 0.048) in the high‐BMI (BMI ≥ 21.5 kg/m2) group than in the low‐BMI (BMI < 21.5 kg/m2) group (Table 3).
Table 1.
Patient characteristics
| Characteristics | N = 47 (%) |
|---|---|
| Age (years), median, range | 73 (42–91) |
| Gender | |
| Male/Female | 16 (34)/31 (66) |
| Performance status | |
| 0–1/2–4 | 31 (66)/16 (34) |
| EGFR genotype | |
| Exon 19 deletion/L858R | 30 (64)/17 (36) |
| Histology | |
| Adenocarcinoma | 47 (100) |
| Stage | |
| IV or recurrence | 6 (13)/41 (87) |
| Smoking status | |
| Current smoker | 16 (34) |
| Never or former light smoker | 31 (66) |
| Type of EGFR‐TKI | |
| Gefitinib/Erlotinib/Afatinib | 33 (70)/9 (19)/5 (11) |
| BSA (m2) | |
| ≥ 1.5 | 25 (53) |
| < 1.5 | 22 (47) |
| BMI (kg/m2) | |
| ≥ 21.5 | 24 (51) |
| < 21.5 | 23 (49) |
| Brain metastasis | |
| Positive/Negative | 16 (34)/31 (66) |
| Number of metastatic lesions | |
| 1 | 18 (38) |
| ≥ 2 | 29 (62) |
| Number of prior regimens (median, range) | 2 (1–6) |
| 1 | 20 (42) |
| ≥ 2 | 27 (58) |
BMI, body mass index; BSA, body surface area; TKI, tyrosine kinase inhibitor.
Table 2.
Patient characteristics in the high‐BSA (BSA ≥ 1.50 m2) group
| N (%) | P | ||
|---|---|---|---|
| Characteristics | BSA < 1.5 | BSA ≥ 1.5 | |
| Gender | < 0.001 | ||
| Male | 2 (13) | 14 (87) | |
| Female | 20 (65) | 11 (35) | |
| Age (years) | < 0.001 | ||
| < 75 | 8 (28) | 21 (72) | |
| ≥ 75 | 14 (78) | 4 (22) | |
| Performance status | 0.03 | ||
| 0–1 | 12 (38) | 20 (62) | |
| 2–4 | 10 (67) | 5 (34) | |
| Smoking status | 0.024 | ||
| Current smoker | 3 (21) | 11 (79) | |
| Never or former light smoker | 19 (58) | 14 (42) | |
| Stage | 0.60 | ||
| Postoperative recurrence | 3 (50) | 3 (50) | |
| Stage IV | 19 (46) | 22 (54) | |
| Brain metastasis | 0.39 | ||
| Positive | 6 (38) | 10 (62) | |
| Negative | 16 (52) | 15 (48) | |
| EGFR genotype | 0.015 | ||
| Exon 19 deletion | 10 (34) | 20 (66) | |
| L858R | 12 (71) | 5 (29) | |
| Prior regimens | 0.25 | ||
| 1 | 11 (55) | 9 (45) | |
| ≥ 2 | 11 (41) | 16 (59) | |
BSA, body surface area.
Table 3.
Patient characteristics in the high‐BMI (BMI ≥ 21.5 kg/m2) group
| N (%) | P | ||
|---|---|---|---|
| Characteristics | BMI < 21.5 | BMI ≥ 21.5 | |
| Gender | 0.21 | ||
| Male | 6 (34) | 10 (66) | |
| Female | 17 (55) | 14 (45) | |
| Age (years) | 0.16 | ||
| < 75 | 12 (41) | 17 (59) | |
| ≥ 75 | 11 (61) | 7 (39) | |
| Performance status | 0.01 | ||
| 0–1 | 11 (38) | 20 (62) | |
| 2–4 | 12 (73) | 4 (27) | |
| Smoking status | 0.59 | ||
| Current smoker | 7 (50) | 7 (50) | |
| Never or mild former light smoker | 16 (48) | 17 (52) | |
| Stage | 0.65 | ||
| Postoperative recurrence | 3 (50) | 3 (50) | |
| Stage IV | 20 (48) | 21 (52) | |
| Brain metastasis | 0.46 | ||
| Positive | 9 (53) | 8 (47) | |
| Negative | 14 (47) | 16 (53) | |
| EGFR genotype | 0.048 | ||
| Exon 19 deletion | 10 (33) | 20 (67) | |
| L858R | 13 (76) | 4 (24) | |
| Prior regimens | 0.57 | ||
| 0 | 10 (50) | 10 (50) | |
| ≥ 1 | 13 (48) | 14 (52) | |
BMI, body mass index.
Response to osimertinib according to body surface area (BSA) and body mass index (BMI)
An objective response was obtained in 27 of the 47 patients, indicating an objective response rate (ORR) of 57.4% (95% confidence interval [CI] 43.3–71.5%) (Table 4). We used the median BSA and BMI values as the cutoff values to evaluate the impact of body size on the efficacy of osimertinib monotherapy. The response rate was 59.1% (95% CI 38.6–79.6%) in the low‐BSA group and 56% (95% CI 36.5–75.5%) in the high‐BSA group, indicating no statistically significant difference (P = 0.83). The response rate was 60.8% (95% CI 40.8–80.8%) in the low‐BMI group and 54.1% (95% CI 34.2–74.0%) in the high‐BMI group, also indicating no statistically significant difference (P = 0.64).
Table 4.
Responses to osimertinib therapy
| Response | All patients (n = 47) |
BSA < 1.5 (n = 22) |
BSA ≥ 1.5 (n = 25) |
BMI < 21.5 (n = 23) |
BMI ≥ 21.5 (n = 24) |
|---|---|---|---|---|---|
| Complete response | 0 | 0 | 0 | 0 | 0 |
| Partial response | 27 | 13 | 14 | 14 | 13 |
| Stable disease | 12 | 3 | 9 | 3 | 9 |
| Progressive disease | 7 | 5 | 2 | 5 | 2 |
| Not evaluable | 1 | 1 | 0 | 1 | 0 |
| Response rate | 57.4% | 59.1% | 56.0% | 60.8% | 54.1% |
| P = 0.83 | P = 0.64 | ||||
BMI, body mass index; BSA, body surface area.
Toxicities
The most common non‐hematologic toxicities of any grade were diarrhea (18 patients, 38.3%), skin rash (15 patients, 31.9%), and fatigue (10 patients, 21.3%). Grade 3 diarrhea occurred in two patients. Regarding hematologic toxicities of any grade, thrombocytopenia (10 patients, 21.3%), anemia (7 patients, 14.9%) and leukopenia (6 patients, 12.8%) were observed. Grade 3 thrombocytopenia occurred in one patient. A comparison of toxicities in relation to BSA and BMI is shown in Tables 5 and 6, respectively. There were no significant differences in the frequencies of any of the toxicities relative to BSA or BMI.
Table 5.
Toxicities in the low‐BSA (BSA < 1.50 m2) and high‐BSA (BSA ≥ 1.50 m2) groups
| All grades | ≥ Grade 3 | |||||
|---|---|---|---|---|---|---|
| BSA ≥ 1.5 | BSA < 1.5 | BSA ≥ 1.5 | BSA < 1.5 | |||
| Toxicity | N (%) | N (%) | P | N (%) | N (%) | P |
| Leukopenia | 5 (25%) | 1(4.5%) | 0.13 | 0 (0%) | 0 (0%) | |
| Neutropenia | 2 (8%) | 1(4.5%) | 0.55 | 0 (0%) | 0 (0%) | |
| Anemia | 4 (16%) | 3(13.6%) | 0.57 | 0 (0%) | 0 (0%) | |
| Thrombocytopenia | 7 (28%) | 3(13.6%) | 0.20 | 1 (4.0%) | 0 (0%) | 0.54 |
| Skin rash | 9 (36%) | 6(27.2%) | 0.37 | 0 (0%) | 0 (0%) | |
| Liver dysfunction | 3 (12%) | 1(4.5%) | 0.35 | 0 (0%) | 0 (0%) | |
| Diarrhea | 9 (36%) | 9(40.9%) | 0.48 | 0(0%) | 2 (9.1%) | 0.21 |
| Nausea | 3 (12%) | 3(13.6%) | 0.79 | 0 (0%) | 0 (0%) | |
| Anorexia | 3 (12%) | 4(18.2%) | 0.43 | 0 (0%) | 1 (4.5%) | 0.95 |
| Constipation | 2 (8%) | 1(4.5%) | 0.55 | 0 (0%) | 0 (0%) | |
| Paronychia | 1 (4%) | 4(18.2%) | 0.14 | 0 (0%) | 0 (0%) | |
| Fatigue | 8 (32%) | 2(9.1%) | 0.07 | 0 (0%) | 0 (0%) | |
| Dry skin | 1 (4%) | 1(4.5%) | 0.53 | 0 (0%) | 0 (0%) | |
| Mucositis oral | 3 (12%) | 1(4.5%) | 0.35 | 0 (0%) | 0 (0%) | |
| Neuropathy | 0 (0%) | 1(4.5%) | 0.95 | 0 (0%) | 0 (0%) | |
| Conjunctivitis | 1 (4%) | 2(9.1%) | 0.45 | 0 (0%) | 0 (0%) | |
| Pneumonitis | 3 (12%) | 2(9.1%) | 0.56 | 0 (0%) | 0 (0%) | |
| Ileus | 0 (0%) | 1(4.5%) | 0.95 | 0 (0%) | 0 (0%) | |
| Edema | 0 (0%) | 1 (4.5%) | 0.95 | 0 (0%) | 0 (0%) | |
| Cellulitis | 0 (0%) | 1 (4.5%) | 0.95 | 0 (0%) | 0 (0%) | |
| Renal dysfunction | 3 (12.0%) | 0 (0%) | 0.28 | 0 (0%) | 0 (0%) | |
| Fever | 1 (4.0%) | 0 (0%) | 0.54 | 0 (0%) | 0 (0%) | |
BSA, body surface area.
Table 6.
Toxicities in the low‐BMI (BMI < 21.5 kg/m2) and high‐BMI groups (BMI ≥ 21.5 kg/m2)
| All grades | ≥ Grade 3 | |||||
|---|---|---|---|---|---|---|
| BMI ≥ 21.5 | BMI < 21.5 | BMI ≥ 21.5 | BMI < 21.5 | |||
| Variable | N (%) | N (%) | P | N (%) | N (%) | P |
| Leukopenia | 5 (20.8%) | 1 (4.3%) | 0.10 | 0 (0%) | 0 (0%) | |
| Neutropenia | 2 (8.3%) | 1 (4.3%) | 0.52 | 0 (0%) | 0 (0%) | |
| Anemia | 3 (12.5%) | 4 (17.4%) | 0.48 | 0 (0%) | 0 (0%) | |
| Thrombocytopenia | 6 (25.0%) | 4 (17.4%) | 0.39 | 1 (4.2%) | 0 (0%) | 0.51 |
| Skin rash | 10(41.2%) | 5 (21.7%) | 0.12 | 0 (0%) | 0 (0%) | |
| Liver dysfunction | 3 (12.5%) | 1 (4.3%) | 0.32 | 0 (0%) | 0 (0%) | |
| Diarrhea | 10(41.2%) | 8 (34.8%) | 0.43 | 0 (0%) | 2 (8.7%) | 0.23 |
| Nausea | 2 (8.3%) | 4 (17.4%) | 0.31 | 0 (0%) | 0 (0%) | |
| Anorexia | 2 (8.3%) | 5 (21.7%) | 0.19 | 0 (0%) | 1 (4.3%) | 0.49 |
| Constipation | 1 (4.2%) | 2 (8.7%) | 0.48 | 0 (0%) | 0 (0%) | |
| Paronychia | 4 (16.7%) | 1 (4.3%) | 0.52 | 0 (0%) | 0 (0%) | |
| Fatigue | 6 (25.0%) | 4 (17.4%) | 0.39 | 0 (0%) | 0 (0%) | |
| Dry skin | 1 (4.2%) | 1 (4.3%) | 0.74 | 0 (0%) | 0 (0%) | |
| Mucositis oral | 3 (12.5%) | 1 (4.3%) | 0.32 | 0 (0%) | 0 (0%) | |
| Neuropathy | 1 (4.2%) | 0 (0%) | 0.51 | 0 (0%) | 0 (0%) | |
| Conjunctivitis | 1 (4.2%) | 2 (8.7%) | 0.48 | 0 (0%) | 0 (0%) | |
| Pneumonitis | 2 (8.3%) | 3 (13.0%) | 0.48 | 0 (0%) | 0 (0%) | |
| Ileus | 0 (0%) | 1 (4.3%) | 0.49 | 0 (0%) | 0 (0%) | |
| Edema | 0 (0%) | 1 (4.3%) | 0.49 | 0 (0%) | 0 (0%) | |
| Cellulitis | 0 (0%) | 1 (4.3%) | 0.49 | 0 (0%) | 0 (0%) | |
| Renal dysfunction | 3 (12.5%) | 0 (0%) | 0.12 | 0 (0%) | 0 (0%) | |
| Fever | 1 (4.3%) | 1 (4.3%) | 0.74 | 0 (0%) | 0 (0%) | |
BMI, body mass index.
Survival
The cutoff date of the survival data update was the end of November 2018. The median follow‐up period at the time of survival analysis was 10.6 months. The median PFS and survival of the entire patient population was 7.6 (95% CI 6.4–8.8) and 14.7 (95% CI 9.1–20.5) months, respectively (Fig 1). The median PFS rates in the low‐BSA and high‐BSA groups were 9.1 (95% CI 3.7–14.5) and 7.6 (95% CI 6.7–8.5) months, respectively, indicating statistically non‐significant differences (P = 0.69) (Fig 2a). The median PFS rates in the low‐BMI and high‐BMI groups were 7.6 (95% CI 2.0–13.2) and 7.6 (95% CI 6.6–8.6) months, respectively, indicating statistically non‐significant differences (P = 0.38) (Fig 2b). Univariate analysis identified PS, brain metastasis status, and the number of prior regimens as significantly predictive of PFS, while multivariate analysis identified patient age, PS, and EGFR genotype as independent predictors of PFS (Table 7). We evaluated 1.40 and 1.60 m2 as alternative BSA cutoff values in the univariate analysis, but none of the differences in PFS were significant (BSA < 1.40 m2: hazard ratio [HR] 0.83, P = 0.58; BSA < 1.60 m2: HR 0.076, P = 0.43). We also evaluated 20.0 and 23.0 kg/m2 as alternative BMI cutoff values in the univariate analysis and did not observe any significant differences in PFS (BMI < 20 kg/m2: HR 0.84, P = 0.63; BMI < 23 kg/m2: HR 1.13, P = 0.71). In patients with good PS scores, the median PFS values in the low‐BSA and high‐BSA groups were 10.2 (95% CI 1.9–18.5) and 7.8 (95% CI 6.1–9.5) months, respectively, without any significant differences (P = 0.64). The median PFS rates in the low‐BMI and high‐BMI groups were 10.2 (95% CI 0.1–22.9) and 7.8 (95% CI 6.3–9.3) months, respectively, indicating statistically non‐significant differences (P = 0.63) (Fig 3).
Figure 1.
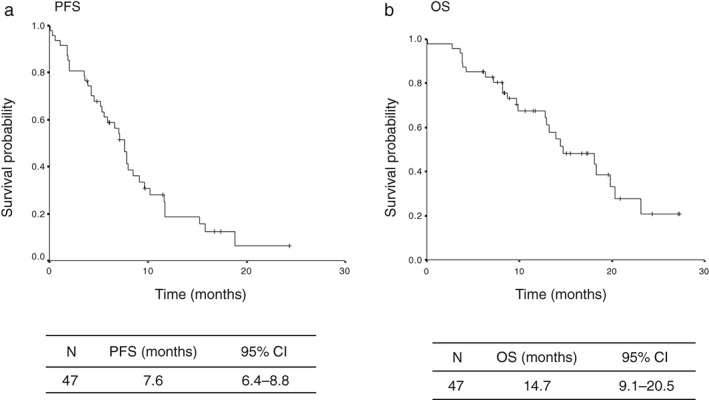
Kaplan–Meier plots of (a) progression‐free survival (PFS) and (b) overall survival (OS). CI, confidence interval.
Figure 2.
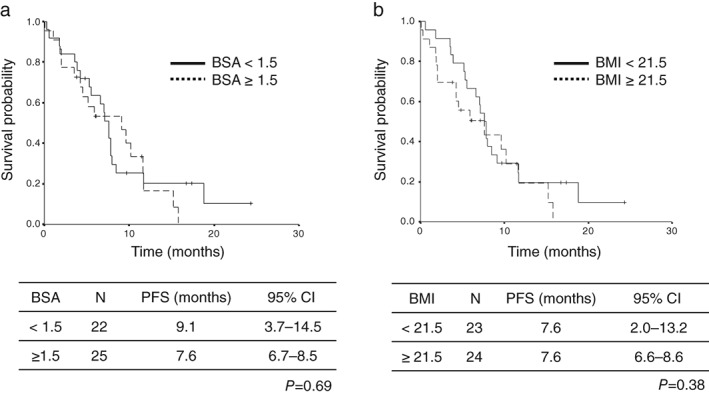
Kaplan–Meier plots of progression free survival (PFS) in relation to body size: (a) body surface area (BSA) ( ) BSA < 1.5, and (
) BSA < 1.5, and ( ) BSA ≥ 1.5; and (b) body mass index (BMI) (
) BSA ≥ 1.5; and (b) body mass index (BMI) ( ) BMI < 21.5, and (
) BMI < 21.5, and ( ) BMI ≥ 21.5. CI, confidence interval.
) BMI ≥ 21.5. CI, confidence interval.
Table 7.
Cox regression analysis of PFS
| PFS | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Variable | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P |
| Gender Male vs. Female |
0.95 (0.47–1.88) | 0.87 | ||
| Age (years), median, range < 75 vs. ≥ 75 | 0.59 (0.29–1.17) | 0.13 | 0.33 (0.15–0.72) | 0.005 |
| Performance status 0–1 vs. 2–3 | 2.09 (1.01–4.30) | 0.046 | 2.49 (1.14–5.43) | 0.022 |
| EGFR genotype | 1.88 (0.95–3.72) | 0.07 | 2.83 (1.32–6.06) | 0.007 |
| Exon 19 deletion | ||||
| L858R point mutation | ||||
| Smoking status | 1.24 (0.62–2.48) | 0.54 | ||
| Current smoker | ||||
| Never or former light smoker | ||||
| Stage | 1.66 (0.64–4.33) | 0.30 | ||
| Postoperative recurrence | ||||
| Stage IV | ||||
| Brain metastasis | 2.06 (1.05–4.05) | 0.036 | Excluded | |
| Positive vs. Negative | ||||
| Number of prior regimens | 2.03 (1.03–4.00) | 0.041 | Excluded | |
| 0 vs. ≥ 1 | ||||
| BSA | 0.88 (0.45–1.70) | 0.70 | ||
| < 1.51 vs. ≥ 1.51 | ||||
| BMI | 0.75 (0.39–1.45) | 0.39 | ||
| < 21.5 vs. ≥ 21.5 | ||||
BMI, body mass index; BSA, body surface area; CI, confidence interval; PFS, progression‐free survival.
Figure 3.
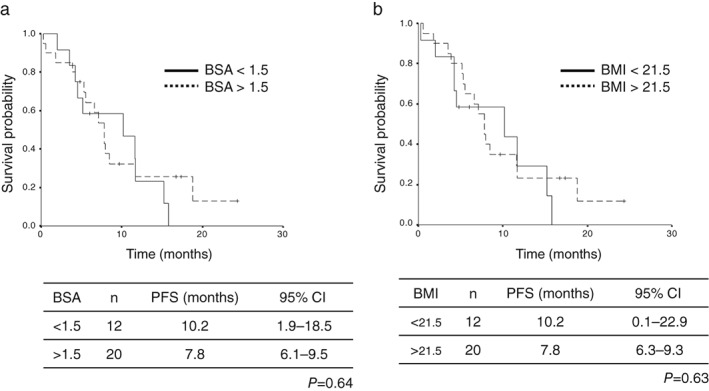
Kaplan–Meier plots of progression free survival (PFS) in relation to body size of patients with good performance status: (a) body surface area (BSA) ( ) BSA < 1.5, and (
) BSA < 1.5, and ( ) BSA ≥ 1.5; and (b) body mass index (BMI) (
) BSA ≥ 1.5; and (b) body mass index (BMI) ( ) BMI < 21.5, and (
) BMI < 21.5, and ( ) BMI ≥ 21.5. CI, confidence interval.
) BMI ≥ 21.5. CI, confidence interval.
Discussion
In approximately 60% of patients, the mechanism of acquired resistance is the development of an additional EGFR mutation, T790M.13 While osimertinib treatment is recognized as the standard‐of‐care for patients harboring the T790M resistance mutation who progress on first‐line EGFR‐TKIs, the standard dose of osimertinib is 80 mg/day, a uniform dosage regardless of patients’ body size. The results of this prospective observational study show that BSA and BMI have no statistically significant effect on the clinical outcomes of osimertinib monotherapy, including the response rate and PFS, in patients with T790M‐positive advanced NSCLC who experience disease progression after first‐line EGFR‐TKI therapy. To our knowledge, this is the first report to evaluate the relationship between osimertinib treatment and body size in this type of patient. The degree of toxicities, including diarrhea, thrombocytopenia, and skin, was also assessed. The toxicity profiles did not vary significantly among the patients in relation to the BSA or BMI. Because the results for the categorical variables indicated a significantly higher proportion of patients with good PS in the high‐BSA and high‐BMI groups than in the low‐BSA and low‐BMI groups, it is reasonable to ascribe this significant difference to the body weight loss caused by disease progression. Moreover, re‐evaluation of the PFS results in patients with good PS did not indicate any statistically significant differences in the median PFS relative to BSA or BMI. In addition, we also changed the cutoff values of BSA and BMI but failed to detect any statistically significant differences in PFS.
A previous phase III (AURA III study) showed an ORR to osimertinib of 71% (95% CI 65–76) in patients with T790M‐positive advanced NSCLC who experienced disease progression after first‐line EGFR‐TKI therapy,22 which is higher than the 57.4% ORR observed in our study. The number of patients in our study with poor PS and the number of chemotherapy regimens prior to osimertinib therapy per patient were 16 (34%) and 2, respectively. However, in the AURA III study, all patients had good PS and 96% had received only one prior regimen, explaining the differences in ORR and PFS of osimertinib therapy between our study and the AURA III trial.
We previously found that the efficacy of gefitinib in patients with NSCLC harboring an EGFR mutation did not differ in relation to their BSA.24 Similarly, Imai et al. reported that gefitinib efficacy in patients with NSCLC harboring sensitive EGFR mutations did not differ relative to BSA, body weight, and BMI.25 Another study showed that the efficacy of gefitinib in NSCLC patients did not significantly differ between doses of 250 or 500 mg/day.26, 27 According to results of these studies, although most cytotoxic anticancer agent regimens are based on BSA‐adjusted doses, we conclude that the dosage of gefitinib could not be adjusted based on a body size variable, such as BSA. Furthermore, a Phase I/II AURA trial was conducted to determine the safety and efficacy of osimertinib in patients with advanced NSCLC who experienced disease progression after previous treatment with EGFR‐TKIs.21 Among the patients with the T790M mutation, osimertinib had ORRs of 83%, 79%, and 77% at daily doses of 80, 160, and 240 mg, respectively. Thus, it is reasonable to conclude that the tumor responses of osimertinib therapy were not dose‐dependent, and the observations of our study appear to support the lack of a correlation between tumor response and osimertinib dose.
There were several limitations to our study. Firstly, the sample size may not have been sufficient. Secondly, there was no pharmacokinetic validation accompanying the observations on the efficacy of osimertinib in relation to BSA and BMI.
In conclusion, the efficacy of osimertinib in patients with T790M‐positive advanced NSCLC who experienced disease progression after prior EGFR‐TKI therapy did not significantly vary relative to BSA or BMI. Based on our findings, we propose that the next step in the development of effective osimertinib regimens could be a study examining the relationship between body size variables and the efficacy of osimertinib monotherapy in the first‐line setting for NSCLC patients with sensitive EGFR mutations.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank the staff members of the Department of Respiratory Medicine, Kitasato University School of Medicine for their suggestions and assistance.
References
- 1. Siegel R, DeSantis C, Virgo K et al. Cancer treatment and survivorship statistics, 2012. Cancer J Clin 2012; 62: 220–41. [DOI] [PubMed] [Google Scholar]
- 2. Bria E, Milella M, Cuppone F et al. Outcome of advanced NSCLC patients harboring sensitizing EGFR mutations randomized to EGFR tyrosine kinase inhibitors or chemotherapy as first‐line treatment: A meta analysis. Ann Oncol 2011; 22: 2277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petrelli F, Borgonovo K, Cabiddu M, Barni S. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR‐mutated non‐small‐cell lung cancer: A meta‐analysis of 13 randomized trials. Clin Lung Cancer 2012; 13: 107–14. [DOI] [PubMed] [Google Scholar]
- 4. Paez JG, Jänne PA, Lee JC et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–500. [DOI] [PubMed] [Google Scholar]
- 5. Hsia TC, Liang JA, Li CC, Chien CR. Comparative effectiveness of concurrent chemoradiotherapy versus EGFR‐tyrosine kinase inhibitors for the treatment of clinical stage IIIb lung adenocarcinoma patients with mutant EGFR . Thorac Cancer. 2018; 9: 1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang A, Li R, Zhao J et al. Epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors combined with chemotherapy in first‐line treatment in an advanced non‐small cell lung cancer patient with EGFR sensitive mutation. Thorac Cancer 2016; 7: 614–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui J, Zhang Y, Su D, Li T, Li Y. Efficacy of combined icotinib and pemetrexed in EGFR mutant lung adenocarcinoma cell line xenografts. Thorac Cancer 2018; 9: 1156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang A, Li R, Zhao J et al. Combination TS‐1 plus EGFR‐tyrosine kinase inhibitors (TKIs) for the treatment of non‐small cell lung cancer after progression on first‐line or further EGFR‐TKIs: A phase II, single‐arm trial. Thorac Cancer. 2016; 7: 614–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitsudomi T, Morita S, Yatabe Y et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8. [DOI] [PubMed] [Google Scholar]
- 10. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 1: 239–46. [DOI] [PubMed] [Google Scholar]
- 11. Han JY, Park K, Kim SW et al. First‐SIGNAL: First‐line single‐agent iressa versus gemcitabine and cisplatin trial in never‐smokers with adenocarcinoma of the lung. J Clin Oncol 2012; 30: 1122–8. [DOI] [PubMed] [Google Scholar]
- 12. Inoue A, Kobayashi K, Maemondo M et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin‐paclitaxel for chemo‐naïve non‐small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013; 24: 54–9. [DOI] [PubMed] [Google Scholar]
- 13. Fukuoka M, Wu YL, Thongprasert S et al. Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in Asia (IPASS). J Clin Oncol 2011; 29: 2866–74. [DOI] [PubMed] [Google Scholar]
- 14. Jänne PA, Ou SH, Kim DW et al. Dacomitinib as first‐line treatment in patients with clinically or molecularly selected advanced non‐small‐cell lung cancer: A multicentre, open‐label, phase 2 trial. Lancet Oncol 2014; 15: 1433–41. [DOI] [PubMed] [Google Scholar]
- 15. Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–22. [DOI] [PubMed] [Google Scholar]
- 16. Sequist LV, Yang JC, Yamamoto N, O'Byrne K et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–34. [DOI] [PubMed] [Google Scholar]
- 17. Park K, Tan EH, O'Byrne K et al. Afatinib versus gefitinib as first‐line treatment of patients with EGFR mutation‐positive non‐small‐cell lung cancer (LUX‐lung 7): A phase 2B, open‐label, randomised controlled trial. Lancet Oncol 2016; 17: 577–89. [DOI] [PubMed] [Google Scholar]
- 18. Cross DA, Ashton SE, Ghiorghiu S et al. AZD9291, an irreversible EGFR TKI, overcomes T790M‐mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014; 4: 1046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirano T, Yasuda H, Tani T et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non‐small‐cell lung cancer. Oncotarget 2015; 6: 38789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masuzawa K, Yasuda H, Hamamoto J et al. Characterization of the efficacies of osimertinib and nazartinib against cells expressing clinically relevant epidermal growth factor receptor mutations. Oncotarget 2017; 8: 105479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jänne PA, Yang JC, Kim DW et al. AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med 2015; 372: 1689–99. [DOI] [PubMed] [Google Scholar]
- 22. Mok TS, Wu Y‐L, Ahn M‐J et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017; 376: 629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eisenhauer EA, Therasse P, Bogaerts J et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 24. Igawa S, Kasajima M, Ishihara M et al. Evaluation of gefitinib efficacy according to body surface area in patients with non‐small cell lung cancer harboring an EGFR mutation. Cancer Chemother Pharmacol 2014; 74: 939–46. [DOI] [PubMed] [Google Scholar]
- 25. Imai H, Kuwako T, Kaira K et al. Evaluation of gefitinib efficacy according to body mass index, body surface area, and body weight in patients with EGFR‐mutated advanced non‐small cell lung cancer. Cancer Chemother Pharmacol 2017; 79: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fukuoka M, Yano S, Giaccone G et al. Multi‐institutional randomized phase II trial of gefitinib for previously treated patients with advanced non‐small‐cell lung cancer (the IDEAL 1 trial). (Published erratum appears in J Clin Oncol 2004;22:4863). J Clin Oncol 2003; 21: 2237–46. [DOI] [PubMed] [Google Scholar]
- 27. Kris MG, Natale RB, Herbst RS et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non‐small lung cancer: A randomized trial. JAMA 2003; 290: 2149–58. [DOI] [PubMed] [Google Scholar]


