Abstract
Chronic itch with an itch–scratch vicious circle is a significant problem in a large amount of diseases. Some of these diseases, such as psoriasis, atopic dermatitis, prurigo nodularis, Sézary syndrome, uremic pruritus, diabetes and jaundice, are common. For a very long time, chronic itch has been a thorny problem with few effective treatments. Because of this, itch researchers and dermatologists seek to find the mechanisms among chronic itch, inflammatory cytokines and neurons. As an immediate area of research focus, we are going to find the peripheral cross‐talk between neurons and skin cells. Two receptors, named transient receptor potential channel vanilloid 1 and transient receptor potential channel ankyrin transmembrane protein 1, have been shown to play important roles in chronic itch. Many advances have been made so far this decade. This review talks about the updated mechanism of itch‐related inflammatory cytokines via transient receptor potential channels in cutaneous chronic itch and corresponding diseases. The search for itch‐related inflammatory mediators and the structure of transient receptor potential channels this decade could deepen our understanding of the mechanism of itch and help us find more treatments of chronic itch in the future.
Keywords: chronic cutaneous itch, dorsal root ganglion neurons, inflammatory mediators, transient receptor potential channel ankyrin transmembrane protein 1, transient receptor potential channel vanilloid 1
Introduction
Chronic itch is defined as a more than 6 weeks’ (1.5 months’) progression which causes an uncomfortable feeling or scratch. A validated postal questionnaire was sent to 4500 individuals from the German general population. The point prevalence of chronic pruritus was 13.5%, 12‐month prevalence 16.4% and lifetime prevalence 22.0%.1 Chronic itch seriously affects people's physical and mental health and it is also a common feature of diseases such as psoriasis, atopic dermatitis (AD), prurigo nodularis (PN), Sézary syndrome, uremic pruritus, diabetes and jaundice. Eighty‐seven to 100% of the AD patients were found having chronic itch.2 Until now, there are still many aspects of itch signal transmission that are unexplained, and treatments for chronic itch are lacking. As such, the relationship between inflammatory mediators and itch must be explored.3 Many itch‐related diseases cause the release of itch mediators which could act on their own receptors expressed in dorsal root ganglion (DRG) neurons. Subsequently, these signals activate the phospholipase C (PLC)/protein kinase C (PKC)/transient receptor potential channel vanilloid 1 (TRPV1)/transient receptor potential channel ankyrin transmembrane protein 1 (TRPA1) pathway. TRPV1 and TRPA1 are associated with chronic pruritus. With TRPV1 and TRPA1 phosphorylated, the ion channel open, calcium enters the cytoplasm. Finally, the itch signal is transmitted through the spinal cord to the brain.4 Here, we review recent advances in understanding how itch is transmitted from the periphery to the center of neurons as well as the roles of TRPV1/TRPA1 channels in recognizing itch‐related stimuli in DRG. In addition to these, we reviewed the cutaneous chronic itch‐related diseases which caused by many pruritic mediators via TRPV1/TRPA1 channels.
Neurons
Dorsal root ganglion neurons branch out to the skin with the expression of TRPV1/TRPA1 and other receptors. Some of the itch‐related inflammatory mediators act on receptor tyrosine kinase (RTK) such as cytokines and nerve growth factor (NGF). The others act on G protein‐coupled receptors (GPCR) such as proteases, histamine, serotonin bradykinin and prostaglandin (PG)E2. Following the activation of their own receptors, intracellular pathways can lead to the sensitization of TRPV1 or TRPA1. The pathway known as the GPCR/RTK‐TRPV1/TRPA1 axis plays an important role in detecting and responding to diverse nociception.5, 6 GPCR/RTK expresses mostly in the neurons of nociception. The activation of GPCR/RTK further activates PLC which plays an important role in connecting GPCR/RTK and intracellular signaling. The activation of PLC hydrolyzes phosphatidylinositol‐4,5‐bisphosphate (PIP2) into inositol‐1,4,5‐triphosphate and diacylglycerol (DAG). Subsequently, PIP2 and DAG activate PKC which lead to the activation of TRPV1 and TRPA1. Finally, the opening of these two channels increases the concentration of cellular calcium (Fig. 1).7 The perception of itch is transmitted from the afferent fibers (C fiber) endings in the epidermis and dermis to neuron body in DRG (the first‐order neurons). Then, the signal needs to be transmitted to the spinal cord (the second‐order neurons). Subsequently, it is transmitted to the thalamus (the third‐order neurons) through the spinothalamic tract, and eventually the signal radiates to the cortical neurons (Fig. 2).8 It was also found that the parabrachial nucleus (PBN), one of the brain stem structures, acted as a central relay for both acute and chronic itch. In the spinal cord, gastrin‐releasing peptide receptor‐positive neurons are required for itch and could activate PBN neurons through synaptic contact. It means that the spinoparabrachial pathway also plays a key role in itch, in addition to the classical spinothalamic tract pathway.9
Figure 1.
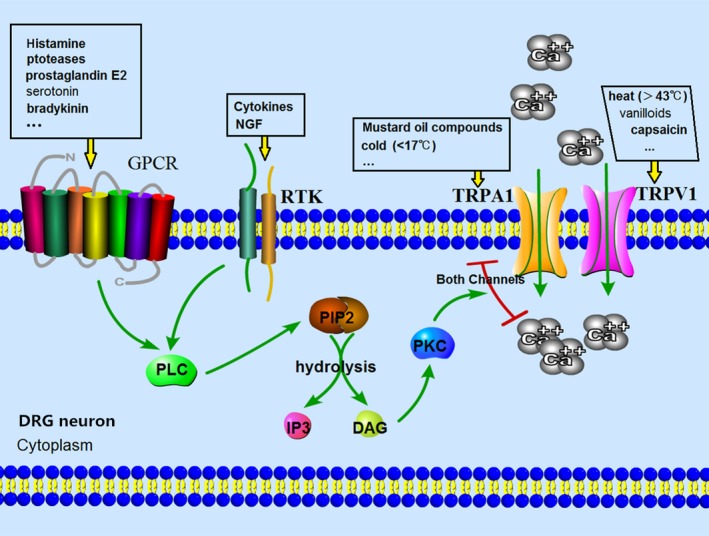
Dorsal root ganglion (DRG) neurons branch out to the skin with transient receptor potential channel vanilloid 1 (TRPV1)/transient receptor potential channel ankyrin transmembrane protein 1 (TRPA1) and other receptors expressing. Some of the itch‐related inflammatory mediators act on receptor tyrosine kinase (RTK) such as cytokines and nerve growth factor (NGF). The others act on G protein‐coupled receptors (GPCR) such as proteases, histamine, serotonin, bradykinin and prostaglandin E2. Following the activation of their own receptors, the intracellular pathway can lead to the sensitization of TRPV1 or TRPA1. GPCR/RTK expressed mostly in the neurons of nociception, phospholipase C (PLC), plays an important role in connecting GPCR/RTK and intracellular signaling. The activation of PLC hydrolyze phosphatidylinositol‐4,5‐bisphosphate (PIP2) into inositol‐1,4,5‐triphosphate (IP3) and diacylglycerol (DAG). Subsequently, PIP2 and DAG activate PKC which lead to the activation of TRPV1 and TRPA1. Finally, the opening of these two channels increases the concentration of cellular calcium.
Figure 2.
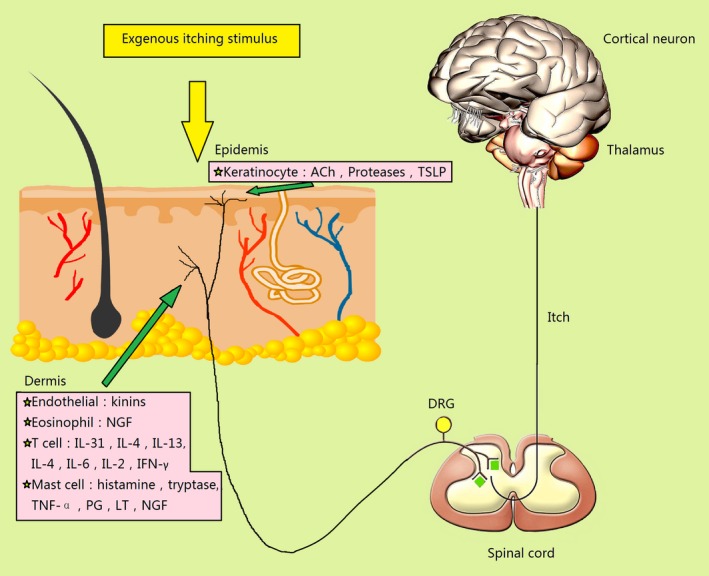
Dorsal root ganglion (DRG) neurons branch out to the skin with transient receptor potential channel vanilloid 1 (TRPV1)/transient receptor potential channel ankyrin transmembrane protein 1 (TRPA1) and other receptors expressing which receive external or internal stimuli. The perception of itch is transmitted from the afferent fiber (C fiber) endings in the epidermis and dermis to neuron body in DRG (the first‐order neurons). Then, the signal needs to be transmitted to the spinal cord (the second‐order neurons). Subsequently, it is transmitted to the thalamus (the third‐order neurons), and eventually the signal radiates to the cortical neurons. IFN, interferon; IL, interleukin; LT, leukotrienes; NGF, nerve growth factor; PG, prostaglandin; TNF, tumor necrosis factor; TSLP, thymic stromal lymphopoietin.
Transient Receptor Potential Channel Vanilloid 1
Transient receptor potential channel vanilloid 1 is a member of the transient receptor potential family. It is a cation channel (mainly Ca2+) triggered by vanilloids, capsaicin, heat (temperature, >43°C), protons (pH < 5.9), inflammatory mediators and so forth.10 The structure of TRPV1 was exploited by electron cryomicroscopy in 2013, which provided a structural blueprint for understanding the function of TRPV1.11 Recently, scientists have found that there are three significant amino acid residue phosphorylation sites: S800, S502 and T704. T704 and S800 contribute to PKC‐induced hypersensitivity to heat. S502 and S800 contribute to PKC‐induced hypersensitivity to capsaicin. S800 contributes to PKC‐induced hypersensitivity to acid (Fig. 3). These phosphorylation sites may serve as the targets for the development of antipruritic drugs in the future.12, 13 As TRPV1 is activated, calcium influx forms electrical signals on nerve fibers. The receptor transduces nociceptive signals such as pain, itch and heat in the peripheral nervous system. As is known, the TRPV1 nociceptor is a subset of primary sensory afferent neurons residing in cranial and spinal sensory ganglia such as DRG and TG.10 Recently, TRPV1 has been found to be expressed not only in neurons but also in other cells or tissues such as keratinocytes, mast cells, dendritic cells, fibroblasts, vascular endothelial cells, monocytes, hair follicles, sweat glands, smooth muscle, pneumocytes, urothelium, gut epithelium, thymocytes and so forth.14
Figure 3.
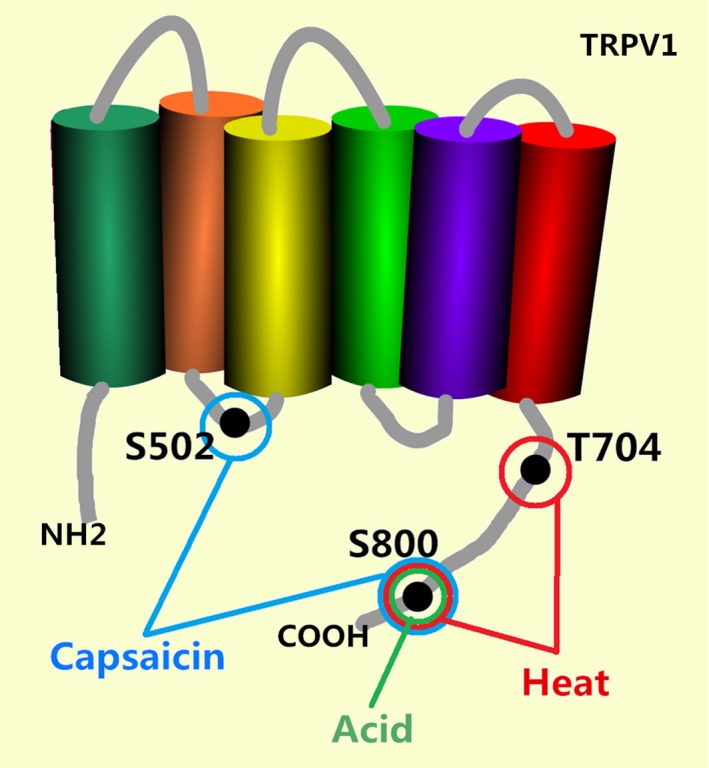
There are three significant amino acid residue phosphorylation sites: S800, S502 and S704. T704 and S800 contribute to protein kinase C (PKC)‐induced hypersensitivity to heat. S502 and S800 contribute to PKC‐induced hypersensitivity to capsaicin. S800 contributes to PKC‐induced hypersensitivity to acid. These phosphorylation sites may serve as the targets for the development of antipruritic drugs in the future. TRPV1, transient receptor potential channel vanilloid 1.
Transient Receptor Potential Channel Ankyrin Transmembrane Protein 1
Transient receptor potential channel ankyrin transmembrane protein 1 is another member of the transient receptor potential family and is also a nociceptive cationic (mainly Ca2+) thermoresponsive channel. In contrast to TRPV1, TRPA1 is implicated in cold thermal sensation (temperatures <17°C). The structure of TRPA1 had been exploited by single‐particle electron cryomicroscopy in 2015, which provided a structural blueprint for understanding the function of TRPA1.15 Besides cold stimuli, a variety of exogenous and endogenous activators or sensitizers of TRPA1 have been identified, such as mustard oil compounds (allyl isothiocyanate), garlic compounds (allicin), cinnamon compounds (cinnamaldehyde), environment (acrolein) and so forth.5 These agonists directly modify C619, C639 and C663 cystein residue sites in the TRPA1 molecule (Fig. 4). These cystein residue sites may serve as the targets for the development of antipruritic drugs in the future.16 The antagonists of TRPA1 hold potential for treating neurogenic inflammatory conditions provoked or exacerbated by irritant exposure.15
Figure 4.
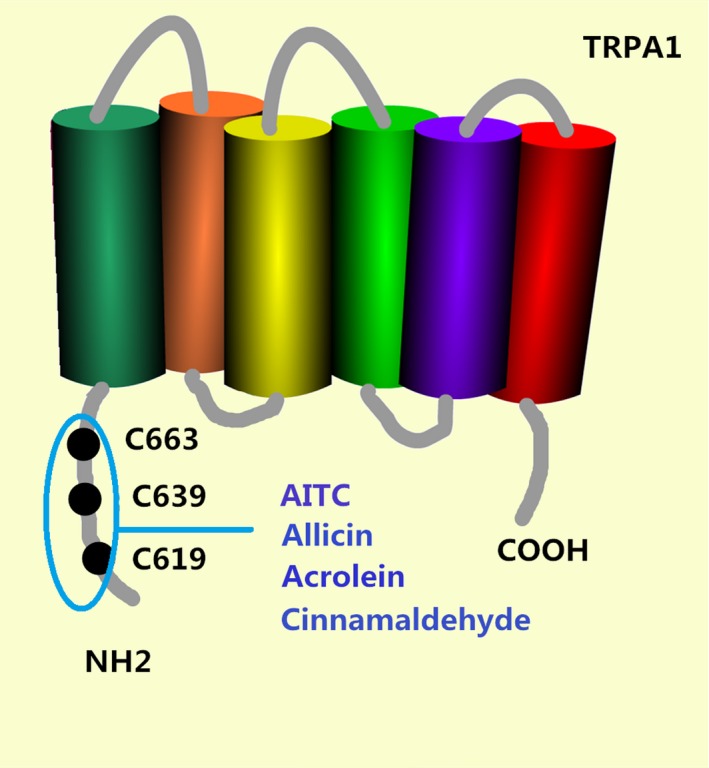
A variety of exogenous and endogenous activators or sensitizers of transient receptor potential channel ankyrin transmembrane protein 1 (TRPA1) have been identified, such as mustard oil compounds (allyl isothiocyanate, AITC), garlic compounds (allicin), cinnamon compounds (cinnamaldehyde) and environment (acrolein). These agonists directly modify C619, C639 and C663 cystein residue targets.
Differences and Similarities between TRPV1 and TRPA1
Transient receptor potential channel vanilloid 1 and TRPA1 are co‐expressed in a large amount of neurons, such as DRG and trigeminal ganglion (TG), where they integrate numerous nociceptors. TRPV1 and TRPA1 also act as nociceptive sensors and potentiate the inflammatory process.5 Many stimuli could sensitize TRPV1 and TRPA1. There are many similarities but also many different points between TRPV1 and TRPA1. TRPV1 is responsible for histamine‐dependent itch while TRPA1 is not. Scientists demonstrated that capsazepine (TRPV1 antagonist) significantly receded scratching behavior caused by histamine. However, AP18 (TRPA1 antagonist) showed no behavioral effects on histamine‐induced itch.17 In addition to histamine, it was been found that TRPV1 took part in imiquimod and chloroquine‐induced itch.17, 18 TRPA1‐deficient mice showed attenuated behavioral responses to bovine adrenal medulla (BAM) and chloroquine‐induced itch.19, 20 However, TRPV1 was found to be unnecessary for BAM‐induced itch.17 Thymic stromal lymphopoietin (TSLP) also activates sensory neurons through TRPA1 rather than TRPV1, evoking severe itch in mice.21 The similarity is that some cytokines including NGF, interleukin (IL)‐4/IL‐13 and IL‐31‐related itch acted through both TRPV1 and TRPA1.6, 12, 22, 23, 24, 25 Generally speaking, TRPA1 acts as an important role for histamine‐independent itch while TRPV1 are responsible for both histamine‐dependent and ‐independent itch.6, 17
Chronic Itch‐Related Inflammatory Mediators and Corresponding Diseases
It has been known for a long time that skin inflammation lowers the threshold of pruritic stimuli. Some of the inflammatory cells release mediators which could bind to their own receptors expressed on sensory neurons. For example, mast cells release histamine, tryptase, tumor necrosis factor‐α, PG and leukotrienes; T cells release IL‐2, IL‐6, IL‐4, IL‐13 and IL‐31; keratinocytes release acetylcholine and proteases; and endothelial cells release kinins and protease‐IV.5, 6 Like TRPV1 and TRPA1, the corresponding receptors of the above‐mentioned inflammatory mediators are largely co‐expressed in a subset of sensory neurons, namely DRG and TG, which mediate the interaction between inflammatory mediators and responses in sensory neurons.6 For instance, an analysis of the systemic distribution of IL‐31 receptor (IL‐31R) in 63 different human tissues found that IL‐31R transcripts are most abundantly expressed in DRG. Both human and mouse DRG neurons express IL‐31R and these neurons largely co‐express TRPV1. Of small‐diameter DRG neurons (diameter, <30 μm; TRPV1+ and TRPA1+), 50.6% were IL‐31R+.6, 26 In addition, histamine receptor (HR) has been found co‐expressed with TRPV1 in DRG. There was a HR+‐neuron response of 77.8% to capsaicin (TRPV1 agonist).27 For another example, a scientist demonstrated that all TSLP receptor (TSLP)R+ neurons are also peripherin‐positive by co‐staining of TSLPR and peripherin (a marker of small‐diameter DRG neurons.21 This implied that TRP channels could be bridges which connect pruritus‐related inflammatory factors with itch signals on the nerve fibers in many diseases. The inflammatory mediators and their corresponding diseases with chronic itch can be seen in Table 1.
Table 1.
Itch‐related inflammatory mediators and corresponding diseases with chronic cutaneous itch
| Inflammatory mediators | Corresponding diseases |
|---|---|
| IL‐31 | AD,23, 26, 28, 29, 30, 31, 32 CTCL,26, 29, 30 chronic urticaria,29, 30 PN,29, 30, 31 CSU23, 32 |
| IL‐4 and IL‐13 | AD12, 33, 34, 35, 36 |
| NGF | CTCL,41 PN,30, 31 AD,25 psoriasis42 |
| Histamine | AD,29 eczema,6, 7 urticaria,6, 7 |
| TSLP | AD10 |
| IL‐2 and IFN‐γ | Uremic pruritus,50 AD10 |
Many itch‐related inflammatory mediators such as IL‐2, IL‐4, IL‐13, IL‐31, NGF, histamine, TSLP and IFN‐γ are related to a variety of diseases which could cause cutaneous chronic itch. AD, atopic dermatitis; CSU, chronic spontaneous urticaria; CTCL, cutaneous T‐cell lymphoma; IFN, interferon; IL, interleukin; NGF, nerve growth factor; PN, prurigo nodularis; TSLP, thymic stromal lymphopoietin.
Interleukin‐31
Interleukin‐31 is secreted mainly by T‐helper (Th)2 cells, which activates a heterodimeric receptor composed of IL‐31RA and oncostatin M receptor in keratinocytes and nerve fiber endings. The expression of IL‐31R in peripheral nerves of mice and humans has been demonstrated. Thus, it suggests that IL‐31 secreted by Th2 cells may act on peripheral nerves, directly causing the pruritus. Furthermore, clinical trials have demonstrated that blockade of IL‐31 signals by a specific antibody effectively alleviates AD‐associated pruritus. s.c. injection of IL‐31 can cause severe itch in rats. IL‐31 binds to a heterodimeric receptor at TRPV1+/TRPA1+ C‐fibers, keratinocytes, macrophages and so forth.26, 28 The DRG neurons co‐express TRPV1 and IL‐31R. As such, IL‐31‐induced itch requires the participation of TRPV1 and TRPA1.6, 23 Interestingly, in vitro, the culture of DRG accompanied by IL‐31 for 3 days resulted in growth of neurons and nerve elongation which is closely related to itch. However, this phenomena was independent of TRPV1.28 IL‐31 is involved in transmission of pruritus and promotion of inflammation. The blood level of IL‐31 is increased in many pruritic skin diseases including AD, cutaneous T‐cell lymphoma (CTCL), chronic urticaria and PN.29, 30 Interestingly, skin biopsies from PN patients with an atopic background, in comparison with healthy skin from healthy individuals, showed a 50‐fold upregulation of IL‐31 mRNA.31 It has been demonstrated that IL‐31 plays a key role in the itch of AD as well as its pathogenesis. The cytokine leads to itch through the activation of IL‐31R on sensory neurons.23 Inhibiting the activity of IL‐31 could reduce the severity of itch in patients with AD. CIM331, a kind of IL‐31Rα antibody, has already completed a phase II clinical trial in curing AD‐induced itch.32 BMS‐981164, the antibody against IL‐31, has been on trail in clinical phase I.29 Targeting therapy of IL‐31Rα may control the Th2 caused itch, including AD and CTCL.26 Furthermore, IL‐31 is involved in the itch and pathogenesis of chronic spontaneous urticaria (CSU), and the level in serum could be one of the biomarkers of disease severity or treatment response in CSU.23, 32
Interleukin‐4 and ‐13
Interleukin‐4 and ‐13 are the cytokines secreted by Th2 cells. Their receptors share a common subunit. The transgenic overexpression of IL‐4 or IL‐13 in mice cause a severe itch. In some skin diseases like AD, the blood levels of these two cytokines are increased. A study in mice showed that the pruritus induced by IL‐13 in AD was at least in part through the activation of TRPA1.12 TRPV1 knockdown weakened airway hyperresponsiveness and airway inflammation, induced by IL‐13 in BALB/c mice. IL‐13 increased the levels of TRPV1 in lungs of BALB/c mice.24 A recent study shows that in the background of AD, IL‐4 and IL‐13 are important to disease pathogenesis. In addition to this, they eventually progress to chronic itch. As is demonstrated, the activation of IL‐4R modulates the neurons of both humans and mice through the IL‐4Rα/Janus kinase (JAK)1/signal transducer and activator of transcription pathway which is indispensable in the development of chronic itch. As such, in chronic itch patients, the itch was relieved when treated with a JAK inhibitor which represents a promising target for treatment of chronic itch.33 IL‐4 and IL‐13 play important roles in the pathogenesis and development of itch in AD patients. Dupilumab, a human IL‐4 monoclonal antibody directed against the IL‐4Rα of IL‐4 and IL‐13 receptors, was permitted by the US Food and Drug Administration for the treatment of moderate to severe AD in 2017.34 Lebrikizumab, a monoclonal antibody against IL‐13, has been the subject of a phase II trial in patients who have moderate to severe AD. The trial was completed in 2017 but the results had not been published.35, 36
Nerve Growth Factor
Nerve growth factor directly correlates with scratching behavior in mice, which has been demonstrated. The higher the blood level of NGF is, the greater the degree of scratching in animal models. NGF upregulates neuropeptides, such as substance P (SP) and calcitonin gene‐related peptide (CGRP). CGRP and SP are involved in neurogenic inflammation and hypersensitization of pruriceptors.6 NGF can be released by many cells, such as mast cells and eosinophils. In a study of localization of NGF and its receptors in the human nasal mucosa, Wu et al.37 observed positive staining for NGF in 2% of mast cells, 0.2% of T cells and 62.2% of activated eosinophils by double immunofluorescence staining technology, and demonstrated that eosinophils, submucosal glands and nasal epithelium constitute the main source of NGF in nasal mucosa. NGF can cause the skin nerve sensitization and sprout.31 In human skin, i.d. injection of NGF sensitizes receptors that cause non‐histaminergic itch.38 The intracellular pathway of NGF is associated with tropomyosin‐receptor kinase A (TrkA) activation that increases phosphoinositide (PI)3 kinases. Then, PI3 kinases increase TRPV1 expression and intracellular calcium, which in turn release SP and CGRP.6, 39 Nerve fibers within the epidermis and the dermis play important roles in PN which could arise from various origins with chronic itch and scratch, such as AD, chronic kidney failure and nervous system diseases. The reasons for PN are complex, and unknown in most circumstances. The hyperplasia of nerves in the dermis and hypoplasia in the epidermis are interesting findings for PN and may be related to the ongoing generation of pruritus in PN. Neurotrophins, especially NGF and its receptor, increase in nerve fibers of PN.31 Lesional keratinocytes of subjects with pruritus demonstrated significantly increased expression of the NGF high‐affinity receptor, TrkA.40 Anti‐NGF therapy can be used for the treatment of itch in NC/Nga AD mice, which could reduce the increased density of nerve fibers and the instances of scratching. NGF and its receptor may be one of the treatment targets for itch.25 Many patients with CTCL have severe itch. Although the serum level of brain‐derived neurotrophic factor in patients with CTCL is not higher than normal people, the serum level of NGF is higher than healthy people. In Sézary syndrome patients, NGF expression increases in keratinocytes, and nerve fiber density increases in the area of skin lesions.41 Interestingly, however, a recent find showed that none of the tested molecular markers including the neuron‐distracting SEMA3A and neuron‐attracting NGF altered in lesional and non‐lesional skin in PN subjects.30 In addition, the NGF/TrkA/TRPV1 pathway is considered as an important mechanism of psoriasis. In human keratinocytes of psoriatic plaques, the level of NGF synthesis is high compared with normal people. In respect of pruritus, the skin lesions in psoriatic patients are more richly innervated in both dermis and epidermis. Significantly, in psoriatic plaques there exists a large amount of NGF‐immunoreactive keratinocytes as well as the high expression of TrkA in nerve fibers compared with non‐lesional skin or non‐pruritic lesions.42 In imiquimod‐induced mouse psoriasis‐like models, TRPA1 was required for the activation of sensory neurons and partially responsible for itch. Imiquimod was a direct but weak TRPA1 agonist and imiquimod‐responsive TRPA1+ neurons were more sensitive than other TRPA1+ neurons in response to noxious stimuli.43
Histamine
Histamine can cause histamine receptor 1 (H1R) activation and then itch, which has known for a very long time.44 It was until 2007, however, that a scientist found that the activation of H1R finally opens TRPV1 on DRG neurons, which could cause the influx of calcium and conduction of itch.45 Histamine receptors belong to GPCR. As demonstrated, H1R and histamine receptor 4 (H4R) are associated with itch. These two receptors are also involved in the activation of the PKC/TRPV1 pathway.27 In recent years, H4R has been gaining increasing attention. H4R has been found expressed mainly on mast cells, dendritic cells and eosinophils. These cells are important in the pathophysiology of AD. Some scientists found that in H4R‐deficient mice, histamine‐induced itch is reduced, which indicates that the H4R may be important in itch perception in AD. A combination of H1R and H4R antagonists also attenuate itch and inflammation, similar to prednisolone. In a phase II trial, JNJ39758979, a compound that inhibits H4R, has been shown to be effective in treating AD patients with pruritus in Japan.29 Scientists found that the first and second generations of antihistamines which act on H1R could reduce itch in a trail including 61 patients; however, the effect was rather moderate. Thus, H4R may play more important roles in itch than H1R. These observations need to be confirmed in larger patient groups.46 Furthermore, many allergic skin diseases such as a variety of dermatitis, eczema, urticaria, drug rash and contact dermatitis are associated with histamine.6, 7
Thymic Stromal Lymphopoietin
Thymic stromal lymphopoietin, another cytokine produced by keratinocytes which triggers dendritic cell‐mediated Th‐cell inflammatory responses plays an important role in itch. Keratinocyte‐derived TSLP directly activates cutaneous sensory neurons to promote itch perception. It then activates TRPA1 and thereby induces itch. In AD patients, the expression of TSLP is increased.6 In mice, overexpression of TSLP induces AD‐like skin.10 AMG157 is a monoclonal antibody against TSLP. The clinical phase I study of AMG157 was finished in 2011; however, the clinical data on the efficacy for this drug are not yet known. There is some early evidence that AMG157 attenuates most measures of allergen‐induced asthmatic responses, but its ability to treat atopic dermatitis has not been reported.47 MK8226 is another inhibitor against TSLPR, a clinical phase I study in participants with moderate‐to‐severe AD patients was started in 2012 but terminated in 2016 due to financial reasons.29, 48
Interleukin‐2 and γ‐Interferon
Interleukin‐2 and interferon (IFN)‐γ are the cytokines secreted by Th1 cells. Th1 cells mediate the type IV hypersensitivity (delayed‐type hypersensitivity) which means that the excessive activation of Th1 cells will lead to many diseases. Th1 cells and their cytokines play important roles in the chronic inflammatory diseases. Many scientists have supposed that IL‐2 and IFN‐γ are related to TRP channels; however, it has not been demonstrated until now. As commonly known, IL‐2 is a strong itch mediator whether in a healthy person or patient. Injection of IL‐2 into healthy controls or into patients with AD induces a 48–72‐h itch that appears after some delay.6, 49 However, the exact mechanism remains unknown.47 Recently, it has been found that the cytokines secreted by Th1 cells, especially IL‐2 and IFN‐γ, are increased in uremic pruritus.50 Interestingly, as a systemic treatment of metastatic melanoma, IL‐2 could cause severe itch.29 As we all know, the acute phase of AD is caused by Th2 and Th22 infiltration. In the chronic phase of AD, in addition to the rise of Th2 and Th22 cells in the first 48 h, Th1 cells gradually appear in skin lesions. Regarding the pathological mechanism of chronic AD, it is suspected that IFN‐γ is the cause of the thickening in skin.51 Regarding IL‐2, it is not only an immune regulatory cytokine, but also a neuromodulation molecule of the central nervous system. Some evidences indicate that systemic changes of IL‐2 can lead to neurological and psychiatric symptoms such as headache, memory impairment, cognitive impairment, anxiety and pruritus. It is still unknown whether IL‐2 leads to increased central cognition to the itch or the activation of some receptors. More research is required. Whether these two cytokines are associated with TRPV1 or TRPA1 needs to be explored.26 Cyclosporin, which inhibits T‐lymphocyte activation mediated by the IL‐2 autocrine pathway, reduces inflammation and pruritus in AD.10
Prospect of Treatment
In acute itch, the first‐line treatment is drugs acting on histamine receptors or opioid receptors and glucocorticoid. However, the treatment of chronic itch has always been a big problem. There is no standard or general treatment for chronic itch.52 We could pay more attention to the methods of curing chronic itch. Drugs acting on cellular components, including keratinocytes, eosinophils and soluble factors, such as IL‐31, IL‐4, IL‐13 and TSLP, need to be explored.10, 11 The monoclonal antibodies of IL‐31, IL‐4, IL‐13 and TSLP are being clinically researched. A number of TRPV1 antagonists have been developed53 and some TRPV1 channel inhibitors also trialed at the clinical stage.3, 4, 54 Until now, at least 15 small molecular compounds have entered phase 1 clinical trials and five of these antagonists have progressed to phase II clinical trials. However, due to some side‐effects, there still have been no advances beyond that.55 AMG‐517 is the first TRPV1 antagonist that has progressed to a phase I clinical trial. Its termination was due to the side‐effect of hyperthermia.56 To reduce the systemic side‐effects, topical forms of these agents are being developed. Long‐term treatment with a topical form of AMG‐9810, a TRPV1 antagonist, was shown to promote tumors in preclinical mouse models.57 Most recently, this risk has been discharged by experiments in vitro and in vivo on AMG‐9810, SB‐705498, PAC‐14028 and capsazepine. The correlation between tumor development and the topical use of TRPV1 antagonists is still in debate.58 Because of these, a large amount of studies need to be done and targeted therapies are gradually appearing on the horizon.
Conclusion
At present, the mechanism of itch is still little known to us worldwide. Pruritus can be caused by a variety of pathogeneses.59 The mechanisms of chronic itch, inflammatory cytokines and neurons have been explored this decade. However, the researches are still in a primary stage.60 This review describes the itch mechanism of various inflammatory mediators in the chronic itch‐related diseases, as well as their role in pruritus and the relationships with the TRP receptors on the nerve fibers and the molecular mechanism of their interaction. We have attempted to summarize some of the mechanisms of chronic pruritus.
As far as we are concerned, TRPV1 and TRPA1 are bridges associated with pruritus‐related inflammatory factors and itch signals on the nerve fibers. Itch‐related inflammatory factors repeatedly stimulate TRPV1 and TRPA1 for a long time in a pathological state, which would decrease the threshold of itch sensation. Thus, it sends the body into a state of chronic itch.
There has been no standard criterion of treatment for itch until now. In particular, chronic itch and non‐histamine pruritus lack effective antipruritic treatments. This review summarizes many of the experimental drugs targeting itch‐related inflammatory mediators, which may be of clinical value in the future. In addition, we believe that drugs acting on different amino acid residue sites of TRPV1 and TRPA1 may be explored in the future and the treatment of chronic itch may be developed in this direction. All this requires the unremitting efforts of scientists.
Acknowledgment
This work was supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CAMS‐2017‐I2M‐1‐011).
Conflict of Interest
None declared.
References
- 1. Matterne U, Apfelbacher C, Loerbroks A. Prevalence, correlates and characteristics of chronic pruritus: a population‐based cross‐sectional study. Acta Derm Venereol 2011; 91(6): 674–679. [DOI] [PubMed] [Google Scholar]
- 2. Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis getting the itch out. Clin Rev Allergy Immunol 2016; 51(3): 263–292. [DOI] [PubMed] [Google Scholar]
- 3. Sanders KM, Nattkemper LA, Yosipovitch G et al Advances in understanding itching and scratching: a new era of targeted treatments. F1000 Res 2016;5:2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keun YJ, Hwan MK, Ji HK. Investigation of the functional difference between the pathological itching and neuropathic pain‐induced rat brain using manganese‐enhanced MRI. Acta Radiol 2016; 57(7): 861–868. [DOI] [PubMed] [Google Scholar]
- 5. Veldhuis NA, Poole DP, Grace M, McIntyre P, Bunnett NW. The G protein‐coupled receptor‐transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacol Rev 2015; 67(1): 36–73. [DOI] [PubMed] [Google Scholar]
- 6. Olivier G, L'Herondelle K, Lebonvallet N et al TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro‐inflammatory response induced by their activation and their sensitization. Protein Cell 2017; 8(9): 644–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sang KH, Valeria M, Melvin S. Phospholipase Cβ3 mediates the scratching response activated by the histamine H1 Receptor on C‐fiber nociceptive neurons. Neuron 2006; 52: 691–703. [DOI] [PubMed] [Google Scholar]
- 8. Chih HL. Immune regulation in pathophysiology and targeted therapy for itch in atopic dermatitis. Dermatol Sin 2016; 34: 1–5. [Google Scholar]
- 9. Mu D, Deng J, Liu KF et al A central neural circuit for itch sensation. Science 2017; 357(6352): 695–699. [DOI] [PubMed] [Google Scholar]
- 10. Boillat A, Alijevic O, Kellenberger S. Calcium entry via TRPV1 but not ASICs induces neuropeptide release from sensory neurons. Mol Cell Neurosci 2014; 61(C): 13–22. [DOI] [PubMed] [Google Scholar]
- 11. Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo‐microscopy. Nature 2013; 504(7478): 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang S, Joseph J, Ro JY et al Modality‐specific mechanisms of protein kinase C‐induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain 2015; 156(5): 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ufret‐Vincenty CA, Klein RM, Collins MD, Rosasco MG, Martinez GQ, Gordon SE. Mechanism for phosphoinositide selectivity and activation of TRPV1 ion channels. J Gen Physiol 2015; 145(5): 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao Y, Bao L, Chan LS, DiPietro LA, Chen L. Aberrant wound healing in an epidermal interleukin‐4 transgenic mouse model of atopic dermatitis. PLoS ONE 2016; 11(1): e0146451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015; 520(7548): 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 2006; 103(51): 19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lucaciu OC, Connell GP. Itch sensation through transient receptor potential channels: a systematic review and relevance to manual therapy. J Manipulative Physiol Ther 2013; 36(6): 385–393. [DOI] [PubMed] [Google Scholar]
- 18. Kim SJ, Park GH, Kim D et al Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)‐expressing neurons. Proc Natl Acad Sci USA 2011; 108(8): 3371–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson SR, Gerhold KA, Bifolck‐Fisher A et al TRPA1 is required for histamine‐independent, Mas‐related G protein‐coupled receptor‐mediated itch. Nat Neurosci 2011; 14(5): 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson SR, Nelson AM, Batia L et al The ion channel TRPA1 is required for chronic itch. J Neurosci 2013; 33(22): 9283–9294.23719797 [Google Scholar]
- 21. Moran TP, Vickery BP. The epithelial cell‐derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Pediatrics 2014; 134(Suppl 3): S160–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malin S, Molliver D, Christianson JA et al TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci 2011; 31(29): 10516–10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin W, Zhou Q, Liu C, et al Increased plasma IL‐17, IL‐31, and IL‐33 levels in chronic spontaneous urticaria. Sci Rep 2017; 7(1): 17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rehman R, Bhat YA, Panda L, Mabalirajan U. TRPV1 inhibition attenuates IL‐13 mediated asthma features in mice by reducing airway epithelial injury. Int Immunopharmacol 2013; 15(3): 597–605. [DOI] [PubMed] [Google Scholar]
- 25. Mitsutoshi T, Kenji T. Itch and nerve fibers with special reference to atopic dermatitis: therapeutic implications. J Dermatol 2014; 41: 205–212. [DOI] [PubMed] [Google Scholar]
- 26. Cevikbas F, Wang X, Akiyama T et al A sensory neuron‐expressed interleukin‐31 receptor mediates T helper cell‐dependent itch involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 2014; 133(2): 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tunyu J, Niuniu Y, Yan Y et al TRPV1 and PLC participate in histamine H4 receptor‐induced itch. Neural Plast 2016;2016:1682972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakashima C, Otsuka A, Kabashima K. Interleukin‐31 and interleukin‐31 receptor‐new therapeutic targets for atopic dermatitis. Exp Dermatol 2018; 27(4): 327–331. [DOI] [PubMed] [Google Scholar]
- 29. Wong LS, Wu T, Lee CH. Inflammatory and noninflammatory itch: implications in pathophysiology‐directed treatments. Int J Mol Sci 2017; 18(7): E1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pereira MP, Pogatzki ZE, Snels C et al There is no functional small‐fibre neuropathy in prurigo nodularis despite neuroanatomical alterations. Exp Dermatol 2017; 26(10): 969–971. [DOI] [PubMed] [Google Scholar]
- 31. Zeidler C, Stander S. The pathogenesis of Prurigo nodularis‐’Super Itch’ in exploration. Eur J Pain 2016; 20(1): 37–40. [DOI] [PubMed] [Google Scholar]
- 32. Hamann CR, Thyssen JP. Monoclonal antibodies against interleukin 13 and interleukin 31RA in development for atopic dermatitis. J Am Acad Dermatol 2018; 78(3S1): S37–S42. [DOI] [PubMed] [Google Scholar]
- 33. Oetjen LK, Mack MR, Feng J et al Sensory neurons co‐opt classical immune signaling pathways to mediate chronic itch. Cell 2017; 171(1): 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paton DM. Dupilumab: human monoclonal antibody against IL‐4Rαfor moderate to severe atopic dermatitis. Drugs Today (Barc) 2017; 53(9): 477–487. [DOI] [PubMed] [Google Scholar]
- 35. Wittmann M, McGonagle D, Werfel T. Cytokines as therapeutic targets in skin inflammation. Cytokine Growth Factor Rev 2014; 25: 443. e51. [DOI] [PubMed] [Google Scholar]
- 36. Clinicaltrials.gov [https://www.clinicaltrials.gov/]. A Study of Lebrikizumab in Participants With Persistent Moderate to Severe Atopic Dermatitis. [Cited 2018 June 14]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02340234?term=Lebrikizumab&rank=9.
- 37. Wu X, Myers AC, Goldstone AC, Togias A, Sanico AM. Localization of nerve growth factor and its receptors in the human nasal mucosa. J Allergy Clin Immunol 2006; 118(2): 428–433. [DOI] [PubMed] [Google Scholar]
- 38. Rukwied RR, Main M, Weinkauf B, Schmelz M. NGF sensitizes nociceptors for cowhage‐ but not histamine‐induced itch in human skin. J Invest Dermatol 2013; 133: 268–270. [DOI] [PubMed] [Google Scholar]
- 39. Xuming Z, Jiehong HP, McNaughton A. NGF rapidly increases membrane expression of TRPV1 heat‐gated ion channels. EMBO J 2005; 24: 4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szepietowski JC, Reich A. Pruritus in psoriasis: an update. Eur J Pain 2016; 20(1): 41–46. [DOI] [PubMed] [Google Scholar]
- 41. Wang FP, Tang XJ, Wei CQ et al Dupilumab treatment in moderate‐to‐severe atopic dermatitis: a systematic review and meta‐analysis. J Dermatol Sci 2018; 90(2): 190–198. [DOI] [PubMed] [Google Scholar]
- 42. Roblin D, Yosipovitch G, Boyce B et al Topical TrkA kinase inhibitor CT327 is an effective, novel therapy for the treatment of pruritus due to psoriasis: results from experimental studies, and efficacy and safety of CT327 in a phase 2b clinical trial in patients with psoriasis. Acta Derm Venereol 2015; 95(5): 542–548. [DOI] [PubMed] [Google Scholar]
- 43. Esancy K, Condon L, Feng J, Kimball C, Curtright A, Dhaka A. A zebrafish and mouse model for selective pruritus via direct activation of TRPA1. Elife 2018; 7:e32036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xuming Z. Targeting TRP ion channels for itch relief. Naunyn‐Schmiedeberg's Arch Pharmacol 2015; 388: 389–399. [DOI] [PubMed] [Google Scholar]
- 45. Shim WS, Tak MH, Lee MH et al TRPV1 mediates histamine‐induced itching via the activation of phospholipase A2 and 12‐lipoxygenase. J Neurosci 2007; 27(9): 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Domagała A, Szepietowski J, Reich A. Antihistamines in the treatment of pruritus in psoriasis. Postepy Dermatol Alergol 2017; 34(5): 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tidwell WJ, Fowler JF. T‐cell inhibitors for atopic dermatitis. J Am Acad Dermatol 2018; 78(3S1): S67–S70. [DOI] [PubMed] [Google Scholar]
- 48. Clinicaltrials.gov [https://www.clinicaltrials.gov/]. A Study of Intravenous MK‐8226 in Participants With Moderate‐to‐Severe Atopic Dermatitis (MK‐8226‐003). [Cited 2018 June 14]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01732510?term=MK8226&draw=1&rank=1.
- 49. Kremer AE, Feramisco J, Reeh PW, Beuers U, Oude Elferink RP. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochim Biophys Acta 2014; 1842(7): 869–892. [DOI] [PubMed] [Google Scholar]
- 50. Azim AA, Farag AS, El‐Maleek Hassan DA, Abdu SM, Lashin SM, Abdelaziz NM. Role of interleukin‐2 uremic pruritus among attendants of Al‐Zahraa Hospital dialysis unit. Indian J Dermatol 2015; 60(2): 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Biedermann T, Skabytska Y, Kaesler S, Volz T. Regulation of T cell immunity in atopic dermatitis by microbes: the Yin and Yang of cutaneous inflammation. Front Immunol 2015; 13(6): 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leslie TA, Greaves MW, Yosipovitch G. Current topical and systemic therapies for itch. Handb Exp Pharmacol 2015; 226: 337–356. [DOI] [PubMed] [Google Scholar]
- 53. Serafini M, Griglio A, Aprile S et al Targeting transient receptor potential vanilloid 1 (TRPV1) channel softly: the discovery of Passerini adducts as a topical treatment for inflammatory skin disorders. J Med Chem 2018; 61(10): 4436–4455. [DOI] [PubMed] [Google Scholar]
- 54. Bonchak JG, Swerlick RA. Emerging therapies for atopic dermatitis: TRPV1 antagonists. J Am Acad Dermatol 2018; 78(3S1): S63–S66. [DOI] [PubMed] [Google Scholar]
- 55. Lee Y, Hong S, Cui M, Sharma PK, Lee J, Choi S. Transient receptor potential vanilloid type 1 antagonists: a patent review (2011‐2014). Expert Opin Ther Pat 2015; 25(3): 291–318. [DOI] [PubMed] [Google Scholar]
- 56. Gavva NR, Treanor JJ, Garami A et al Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 2008; 136(1–2): 202–210. [DOI] [PubMed] [Google Scholar]
- 57. Li S1, Bode AM, Zhu F et al TRPV1‐antagonist AMG9810 promotes mouse skin tumorigenesis through EGFR/Akt signaling. Carcinogenesis 2011; 32(5): 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park M, Naidoo AA, Burns A et al Do TRPV1 antagonists increase the risk for skin tumourigenesis? A collaborative in vitro and in vivo assessment. Cell Biol Toxicol 2018; 34(2): 143–162. [DOI] [PubMed] [Google Scholar]
- 59. Weisshaar E, Szepietowski JC, Darsow U et al European guideline on chronic pruritus. Acta Derm Venereol 2012; 92(5): 563–581. [DOI] [PubMed] [Google Scholar]
- 60. Azimi E, Xia J, Lerner EA. Peripheral mechanisms of itch. Curr Probl Dermatol 2016; 50: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


