Abstract
Purpose
The aim of the present study was to investigate age‐specific normative retinal oxygen saturation values and explore the associated factors in healthy Chinese school‐aged children with different refractive statuses.
Design
Population‐based observational cross‐sectional study.
Methods
Children aged 7–19 years were enrolled. Each participant underwent a series of comprehensive ocular examinations, including axial length (AL), cycloplegic refraction and Oxymap T1 imagery following cycloplegia. The acquired oximetry images were measured, and the values of the retinal oxygen saturation parameters were calculated. The independent factors of the retinal oxygen saturation were analysed using multiple linear regression. The oxygen saturation of retinal arteries (SaO2) and veins (SvO2) as well as the differences between the arteries and veins (AVD) were measured as the main outcomes.
Results
In total, 1461 participants were included in the study. The mean age of the participants was 12.1 ± 3.2 years, and 53.0% were boys. The mean SaO2, SvO2 and AVD values were 83.7 ± 6.4%, 50.1 ± 5.4% and 33.6 ± 5.4%, respectively, and the values increased with age. Girls had higher SvO2 and lower AVD than boys (p < 0.05). The Pearson correlation coefficients among spherical equivalent (SE) and SaO2, SvO2 and AVD were −0.372, −0.203 and −0.240, respectively (all p < 0.001), while the correlations between AL and SaO2, SvO2 and AVD were 0.276, 0.106 and 0.221, respectively (all p < 0.001). The myopia group had significantly higher SaO2, SvO2 and AVD than the emmetropia and hyperopia groups (p < 0.001), but the high myopia group had lower SaO2 and SvO2 than the moderate myopia group. When age, gender, body mass index (BMI), intraocular pressure (IOP) and axial length (AL) were included as factors in the multiple regression, older age was associated with higher SaO2, SvO2 and AVD, while longer AL was associated with higher SaO2 and AVD. Gender was an independent factor predicting SvO2, while gender and BMI were the independent factors predicting AVD. Age explained more variance than AL in SaO2, SvO2 and AVD.
Conclusions
Our population‐based study provides age‐specific profiles of retinal oxygen saturation in Chinese children and adolescents. Older age and longer AL were important independent factors of increased retinal oxygen saturation.
Keywords: children, myopia, oxygen saturation, retinal vessel
Introduction
Oxygen supply and consumption play crucial roles in the normal functioning of human tissues and organs. The retina, which consists of some the most metabolically active tissue in the human body (Kaur et al. 2008), also relies on normal oxygen metabolism to maintain good vision. Retinal arteries supply nutrition and oxygen to the inner retina, while the outer retina is supplied by choroidal vessels. Therefore, obtaining information about retinal oxygen saturation can help us to improve our understanding about ocular diseases. One approach is the use of recently invented novel equipment (Oxymap T1 Retinal Oximeter), which can detect human retinal oxygen saturation in vivo non‐invasively.
Several research studies have shown the relationship between retinal oxygen saturation changes and some ocular diseases. For example, severe glaucomatous damage was associated with increased oxygen saturation in retinal venules (SvO2) and a decreased arteriovenous difference (AVD), indicating reduced retinal oxygen consumption consistent with tissue loss (Vandewalle et al. 2014). In exudative age‐related macular degeneration, the AVD was smaller than in a healthy group, consistent with reduced oxygen extraction by retinal vessels in AMD patients (Geirsdottir et al. 2014). In high myopia, the oxygen saturation in retinal arteries (SaO2) and AVD was lower than in normal eyes (Zheng et al. 2015). Likewise, in other ophthalmic conditions such as diabetic retinopathy (Jorgensen et al. 2014; Man et al. 2015), retinitis pigmentosa (Eysteinsson et al. 2014; Battu et al. 2015), ranibizumab treatment in CRVO (Traustason et al. 2014) and vitrectomy (Lim et al. 2014), significant changes were also observed in retinal oxygen saturation.
However, the relationship between retinal oxygen saturation and age remains controversial. Nakano et al. (2016) found increased SaO2, stable SvO2 and increased AVD with age in 20–93‐year‐old healthy Japanese individuals, while Geirsdottir et al. (2012) reported stable SaO2, decreased SvO2 and increased AVD with age in 18–80‐year‐old healthy Caucasians. In a normal population under 18 years of age, Liu et al. (2017)) found increased SaO2, stable SvO2 and increased AVD with age. The cause of this discrepancy might be the differences in the ethnicities and ages of the enrolled participants. To our knowledge, there have been few studies focusing on the distribution of retinal oxygen saturation in the normal paediatric population and limited research exploring its relation to refractive status. There have been some research studies that attempted to establish a normal value for retinal oxygen saturation, but mostly in adults and Caucasians (Palsson et al. 2012; Paul et al. 2013; O'Connell et al. 2014; Turksever et al. 2015; Nakano et al. 2016). Liu et al. (2017) recorded the normal retinal oxygen saturation values for 122 healthy Chinese children and adolescents 5–18 years of age and reported a preliminary relation to refractive error, but they did not provide age‐specific values or a comparison between genders.
Therefore, the present study investigated retinal oxygen saturation in a large sample of healthy, school‐aged Chinese children and adolescents with different refractive statuses, aiming to describe the distribution of retinal oxygen saturation, provide age‐specific normal values and explore the associated factors.
Materials and Methods
Participants
In the present study, students aged 7–19 years from six schools in the Jiading District in Shanghai were enrolled with cluster sampling. All participants and their parents understood the study protocol and signed informed consent forms. Participants were excluded based on the following criteria: amblyopia (best corrected visual acuity [BCVA] <20/25) (Negrel et al. 2000), severe eye diseases (strabismus, keratoconus, morning glory disc anomaly), previous ocular surgery, inability to follow the Oxymap examination process and low image quality. This study was approved by the Institutional Review Board of Shanghai General Hospital, Shanghai Jiaotong University and was conducted under the tenets of the Declaration of Helsinki.
Ophthalmic examinations
The field investigation team included one ophthalmologist, five optometrists and one nurse. Uncorrected and BCVA were assessed with a retro‐illuminated Early Treatment of Diabetic Retinopathy Study (ETDRS) chart with tumbling optotypes. The ophthalmologist checked for ocular abnormalities using slit‐lamp biomicroscopy and a direct ophthalmoscopic. Obvious strabismus, cataracts, nystagmus and ptosis were recorded. IOP was measured using a noncontact tonometer (T‐1000, Nidek, Japan). AL was measured using an IOL Master (version 5.02, Carl Zeiss Meditec, Oberkochen, Germany). Autorefraction was performed after complete cycloplegia using a Desktop Autorefractor (Model No.: KR‐8800; Topcon Corporation, Tokyo, Japan). A mean value was provided based on three reliable readings in each eye. The procedure for cycloplegia was as follows: one drop of anaesthetic agent (Alcaine, Alcon) was placed in the eyes, and 15 seconds later, two drops of 1% cyclopentolate (Alcon) were placed in the eyes at five‐minute intervals. Cycloplegia was achieved adequately when the pupil dilated to 6 mm or greater and the light reflex was absent.
Oxymap image acquisition
Oxymap T1 is a non‐invasive instrument used for measuring in vivo SaO2, SvO2 and the AVD. The theoretical basis of Oxymap T1 is the difference in light absorbance of deoxyhaemoglobin and oxyhaemoglobin at specific wavelengths (570 and 600 nm). After collecting fundus images at these two wavelengths, the relative oxygen saturation values in retinal vessels were calculated using a computerized algorithm.
Oxymap examination was conducted after the participants reached complete cycloplegia and followed standard procedures (version from November 21, 2013). The examination room was completely dark, with the only two light sources being the computer screen and the Oxymap T1 illuminating lamp. Participants were seated comfortably for at least 15 min before the Oxymap examination. The standard procedure for operating the machine was as follows: First, the machine was set to the standard status, with a flash power of 50 W, the aim light set to the lowest level, the small aperture set to ON, the small pupil set to ON and the acquisition angle set to 50°. Second, images were taken with the optic disc in the centre. Next, the second step was repeated. If no satisfactory images were acquired in the two captures, a third (and final) capture was performed. Participants were required to close their eyes and rest for at least 30 seconds between the two captures. Only images of the children's right eyes were collected. All the images were taken by the same experienced operators.
Analysis and measurement of acquired Oxymap images
The analysis and measurement of the acquired images were conducted following the manufacturer's protocol. Only the optic disc‐centred images with a quality above 6.0/10.0 and without eyelash interference were included in the analyses. During measurement, vessels wider than 8.0 pixels and longer than 50 pixels in the measurement zone were selected. The measurement zone (Fig. 1) was between the second circle (twice the diameter of the optic‐centred circle) and the third circle (three times the diameter of the optic‐centred circle). All the branching points and vessel crossings were excluded. When there was a vessel branch, we chose the parent vessel if it was longer than 50 pixels. If the parent vessel was shorter than 50 pixels, we chose both branch vessels. As for vessel crossings, we chose proximal and distal segments together, as if they were from a continuous, undivided vessel (Liu et al. 2017).
Figure 1.
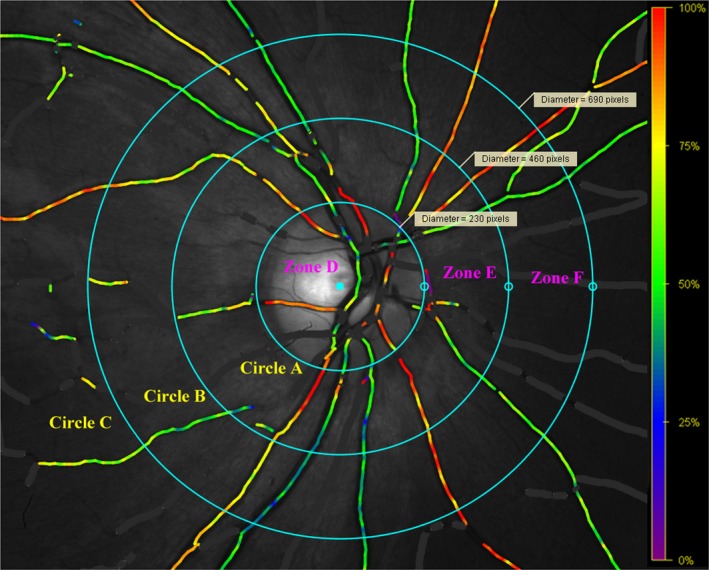
Measurement zone. Circle A: the optic disc circle; Circle B: twice the diameter of Circle A; Circle C: three times the diameter of Circle A. Measurement zone is between Circle B and Circle C (Zone F), and all vessels were selected within this area. [Colour figure can be viewed at wileyonlinelibrary.com]
After vessel selection, the software automatically calculated and presented the data, including SaO2, SvO2, vessel width and vessel length. The overall mean saturations of the arteries and veins of the retina were then calculated with two methods, the unweighted arithmetic mean and the weighted mean (weighted by the fourth power of the vessel diameter) (Hardarson 2013; Liu et al. 2017), respectively. For example, if there were five selected arteries in an eye, each had a measured saturation S and diameter D. The weighted mean saturation was then calculated as follows:
Then, the difference between SaO2 and SvO2 (AVD) was calculated as SaO2 minus SvO2.
The unweighted arithmetic mean and weighted mean (by the fourth power of vessel width) were obtained using two different calculation methods to determine the overall saturation of the retina. A paired t‐test showed that the difference between the weighted and unweighted saturation values was statistically significant (p < 0.001 for SaO2, SvO2 and AVD). The vessel‐width‐weighted saturation value takes the amount of blood flow into account, so it may be closer to the actual mean saturation of the retina. Therefore, we used the weighted value in our study.
Oxymap image analysis consistency
In the pilot study, to evaluate the consistency of the image analysis, twenty images were measured and analysed by two trained graders back‐to‐back. The inter‐grader ICCs for SaO2 and SvO2 were 0.960 (95% CI: 0.901–0.984) and 0.952 (95% CI: 0.882–0.981), respectively. The results of the Bland–Altman analysis are shown in Fig. 2. The inter‐grader 95% LoA were −1.4% to 5.2% (SaO2) and −3.8% to 3.3% (SvO2). The inter‐grader consistency was high and satisfactory.
Figure 2.
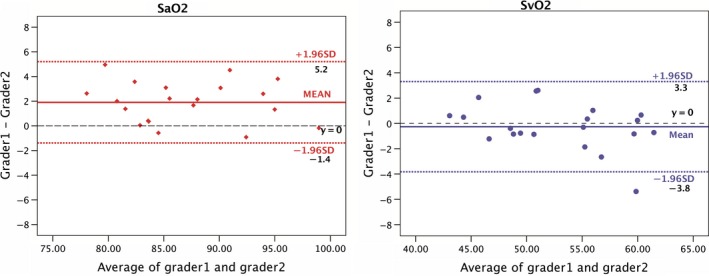
The Bland–Altman plot represents the images analysing consistency between two graders. [Colour figure can be viewed at wileyonlinelibrary.com]
Statistical analysis
An online database system for real‐time examination of data with automatic logic checking was used for data input during the fieldwork. Two staff members independently input retinal oxygen saturation data into an Epidata 3.1 database with automatic logical verification. The holistic dataset that was output for analysis was adjudicated to ensure that there were no discrepancies. Statistical software (IBM SPSS Statistics Inc., version 21.0, Chicago, IL, USA) was used to conduct the statistical analyses. Only data from the right eyes of the participants were included in the final analyses.
Quantitative variables are presented as the mean ± standard deviation (SD). Spherical equivalent (SE) was calculated (SE = sphere + 0.5 × cylinder) (Negrel et al. 2000) and used to categorize refractive status. Myopia, emmetropia and hyperopia were defined as SE ≤ −0.5 dioptres (D), −0.5 D < SE < +0.5 D, and SE ≥ +0.5 D, respectively. Myopia was further divided into three groups: mild (−3.0 D < SE ≤ −0.5), moderate (−5.0 D < SE ≤ −3.0) and high (SE ≤ −5.0). The data distribution was tested with a Kolmogorov–Smirnov test. ICCs were used to evaluate the Oxymap imaging repeatability and image analysis reliability. Pearson's correlation coefficients were calculated to assess the relationship between oxygen saturation and age, refractive error and AL. Parameter comparisons between the different refractive groups were performed with chi‐square tests for categorical variables or an analysis of variance (anova) for continuous variables. Paired t‐tests were used to compare the differences in the unweighted and weighted means. A one‐way anova was utilized to investigate the variance of the retinal oxygen saturation in the myopia groups. Multiple linear regression analysis was performed to explore independent factors associated with oxygen saturation. A p‐value < 0.05 (two‐tailed) was considered to be statistically significant.
Results
General characteristics of the participants
Of the 1693 children and adolescents initially enrolled, 232 were excluded, including 45 with amblyopia, one with morning glory disc anomaly, two with coloboma of the choroid, 141 who failed to co‐operate and 43 with poor image quality. Consequently, a total of 1461 participants aged 7–19 years were included in the analysis, with a mean age of 12.1 ± 3.2 years (53.0% boys). A comparison of the participants that were included (1461) and excluded due to failed co‐operation and low image quality (184) showed that the excluded participants were younger (mean age 10.3 ± 2.4 versus 12.1 ± 3.2 years, p < 0.001), but there was no significant difference in gender (boys 56.5%, girls 53.0%). The mean AL of the 1461 included participants was 24.00 ± 1.24 mm (range from 20.65 to 29.42 mm), and the mean SE was −1.02 ± 2.31D (range from −11.63 to +6.00 D). Girls had a significantly shorter AL and lower BMI. In all, there were 726 myopes, 252 emmetropes and 483 hyperopes. The mean diameter of the selected arteries was 12.8 ± 1.1 pixels while that of the veins was 15.4 ± 1.2 pixels. There was no significant difference in mean diameter between boys and girls (p = 0.112 for arteries, p = 0.501 for veins). Details of the general characteristics are presented in Table 1.
Table 1.
General characteristics of the 1461 participants
| Parameters | Total | Boys, N = 774, 53.0% | Girls, N = 687,47.0% | p | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | ||
| Age, year | 12.1 ± 3.2 | 7 to 19 | 12.0 ± 3.2 | 7 to 19 | 12.1 ± 3.3 | 7 to 19 | 0.592 |
| AL, mm | 24.00 ± 1.24 | 20.65 to 29.42 | 24.23 ± 1.21 | 20.91 to 29.42 | 23.73 ± 1.21 | 20.65 to 28.09 | <0.001 |
| SE, D | −1.02 ± 2.31 | −11.625 to 6.00 | −0.93 ± 2.24 | −11.375 to 5.00 | −1.11 ± 2.38 | −11.625 to 6.00 | 0.150 |
| IOP, mmHg | 16.33 ± 3.13 | 7.0 to 29.0 | 16.2 ± 2.99 | 7.0 to 26.0 | 16.47 ± 3.27 | 7.0 to 29.0 | 0.107 |
| BMI, kg/m2 | 18.92 ± 4.00 | 11.24 to 40.09 | 19.36 ± 4.20 | 11.35 to 40.09 | 18.42 ± 3.67 | 11.24 to 38.3 | <0.001 |
| SaO2, % | 83.7 ± 6.4 | 62.9 to 118.1 | 83.5 ± 6.6 | 62.9 to 118.1 | 83.9 ± 6.2 | 67.5 to 106.3 | 0.245 |
| SvO2, % | 50.1 ± 5.3 | 26.8 to 69.8 | 49.4 ± 5.1 | 27.4 to 69.8 | 50.7 ± 5.5 | 26.8 to 69.7 | <0.001 |
| AVD, % | 33.6 ± 5.4 | 16.0 to 58.4 | 34.0 ± 5.8 | 16.0 to 58.4 | 33.1 ± 5.0 | 16.4 to 52.0 | 0.001 |
| D‐a, pixels | 12.8 ± 1.1 | 9.9 to 16.7 | 12.7 ± 1.1 | 9.9 to 16.7 | 12.8 ± 1.0 | 10.5 to 16.6 | 0.112 |
| D‐v, pixels | 15.4 ± 1.2 | 11.9 to 19.8 | 15.4 ± 1.2 | 12.2 to 19.5 | 15.4 ± 1.2 | 11.9 to 19.8 | 0.501 |
Significance was tested using t‐tests.
AL = axial length; AVD = arteriovenous difference of retinal oxygen saturation; BMI = body mass index; D = dioptre; D‐a = average diameter of selected arteries; D‐v = average diameter of selected veins; IOP = intraocular pressure; SaO2 = mean oxygen saturation of retinal arteries; SE = spherical equivalent; SvO2 = mean oxygen saturation of retinal veins.
Retinal oxygen saturation and its association with age and gender
The SaO2, SvO2 and AVD were normally distributed (SaO2: kurtosis = 0.78, skewness = 0.38, p = 0.159; SvO2: kurtosis = 0.41, skewness = −0.13, p = 0.640; AVD: kurtosis = 0.41, skewness = 0.25, p = 0.236). The mean SaO2, SvO2 and AVD values were 83.7 ± 6.4 (range: 62.9–118.1), 50.1 ± 5.3 (range: 26.8–69.8) and 33.6 ± 5.4 (range: 16.0–58.4), respectively. There were no significant differences between boys and girls in SaO2 (p = 0.245), but the girls had higher SvO2 (p < 0.001) and lower AVD than boys (p = 0.001). The age‐specific oxygen saturation is shown in Table 2. The three parameters of retinal oxygen saturation showed an increasing trend with increases in age (Table 2 and Fig. 3), increasing from 80.7 ± 4.3 in 7‐year‐olds to 87.5 ± 6.9 in 18‐year‐olds for SaO2. SvO2 increased from 47.9 ± 5.7 in 7‐year‐olds to 52.0 ± 5.3 in 18‐year‐olds, and AVD increased from 32.8 ± 5.5 in 7‐year‐olds to 35.5 ± 5.8 in 18‐year‐olds.
Table 2.
Oxygen saturation by age
| Age, years | N | Mean ± SD, % | Range, % | |
|---|---|---|---|---|
| 7 | 51 | SaO2 | 80.7 ± 4.3 | 70.3 to 88.6 |
| SvO2 | 47.9 ± 5.7 | 37.1 to 63.8 | ||
| AVD | 32.8 ± 5.5 | 18.9 to 43.6 | ||
| 8 | 119 | SaO2 | 79.3 ± 4.8 | 63.0 to 95.7 |
| SvO2 | 47.7 ± 4.8 | 36.1 to 60.5 | ||
| AVD | 31.6 ± 5.1 | 21.0 to 46.8 | ||
| 9 | 181 | SaO2 | 80.9 ± 5.4 | 62.9 to 93.9 |
| SvO2 | 49.1 ± 4.7 | 36.2 to 63.4 | ||
| AVD | 31.8 ± 5.5 | 16.0 to 46.3 | ||
| 10 | 232 | SaO2 | 82.2 ± 5.5 | 67.5 to 102.1 |
| SvO2 | 49.6 ± 5.0 | 27.4 to 61.3 | ||
| AVD | 32.6 ± 5.4 | 19.4 to 49.6 | ||
| 11 | 206 | SaO2 | 82.9 ± 5.9 | 68.1 to 102.7 |
| SvO2 | 48.8 ± 5.4 | 26.8 to 60.6 | ||
| AVD | 34.1 ± 5.1 | 16.4 to 49.5 | ||
| 12 | 140 | SaO2 | 82.9 ± 6.3 | 69.9 to 97.8 |
| SvO2 | 49.4 ± 5.5 | 37.2 to 60.2 | ||
| AVD | 33.4 ± 5.6 | 20.4 to 47.6 | ||
| 13 | 73 | SaO2 | 84.9 ± 6.0 | 72.3 to 102.5 |
| SvO2 | 51.4 ± 4.3 | 42.9 to 60.6 | ||
| AVD | 33.5 ± 5.0 | 21.2 to 50.4 | ||
| 14 | 75 | SaO2 | 85.3 ± 5.2 | 73.5 to 100.0 |
| SvO2 | 51.2 ± 4.5 | 38.5 to 62.9 | ||
| AVD | 34.1 ± 5.0 | 24.1 to 50.1 | ||
| 15 | 62 | SaO2 | 85.2 ± 5.3 | 71.3 to 101.2 |
| SvO2 | 49.6 ± 4.7 | 39.4 to 61.4 | ||
| AVD | 35.5 ± 4.4 | 24.9 to 43.8 | ||
| 16 | 98 | SaO2 | 88.3 ± 5.9 | 67.2 to 99.8 |
| SvO2 | 53.3 ± 4.9 | 38.2 to 69.7 | ||
| AVD | 35.0 ± 4.9 | 26.5 to 46.3 | ||
| 17 | 125 | SaO2 | 88.7 ± 6.5 | 73.7 to 118.1 |
| SvO2 | 52.8 ± 5.7 | 39.2 to 69.8 | ||
| AVD | 35.9 ± 5.4 | 23.5 to 58.4 | ||
| 18–19 | 99 | SaO2 | 87.6 ± 6.9 | 73.6 to 106.7 |
| SvO2 | 52.0 ± 5.3 | 36.6 to 67.1 | ||
| AVD | 35.5 ± 5.8 | 25.3 to 54.4 | ||
| Total | 1461 | SaO2 | 83.7 ± 6.4 | 62.9 to 118.1 |
| SvO2 | 50.1 ± 5.3 | 26.8 to 69.8 | ||
| AVD | 33.6 ± 5.4 | 16.0 to 58.4 |
Figure 3.
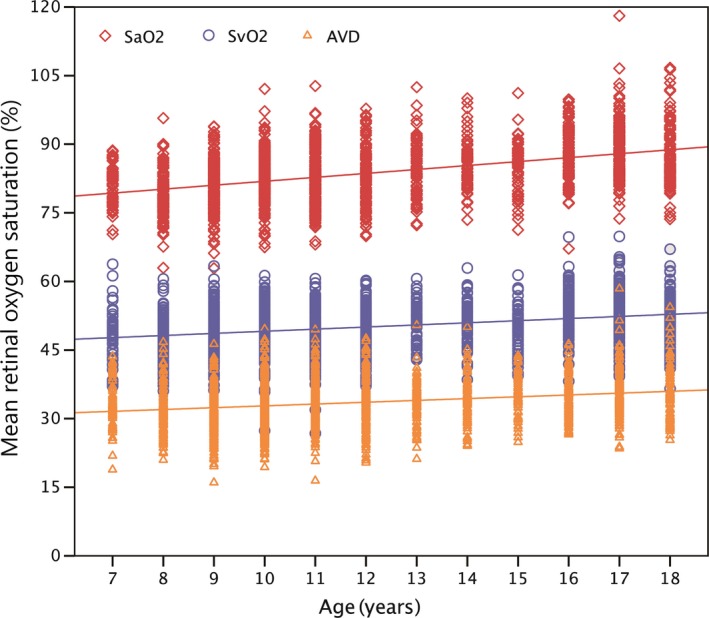
The association between retinal oxygen saturation and age in children aged 7–19 years. SaO2: Mean oxygen saturation in major retinal arteries (p < 0.001, r = 0.430); SvO2: Mean oxygen saturation in major retinal veins (p < 0.001, r = 0.278); AVD: Arteriovenous difference in mean oxygen saturation (p < 0.001, r = 0.234). Thirteen 19‐year‐old children were added to the 18‐year‐old group. [Colour figure can be viewed at wileyonlinelibrary.com]
Relation between retinal oxygen saturation and refractive error and AL
In the simple linear regression analysis, the three parameters of retinal oxygen saturation, SaO2, SvO2 and AVD, had negative relationships with refractive error and were positively correlated with AL (Fig. 4). The correlation coefficients of refractive error and retinal oxygen saturation were r = −0.372 in SaO2, r = −0.203 in SvO2 and r = −0.240 in AVD (all p < 0.001). The correlation coefficients for AL and retinal oxygen saturation were r = 0.276 in SaO2, r = 0.106 in SvO2 and r = 0.221 in AVD (all p < 0.001).
Figure 4.
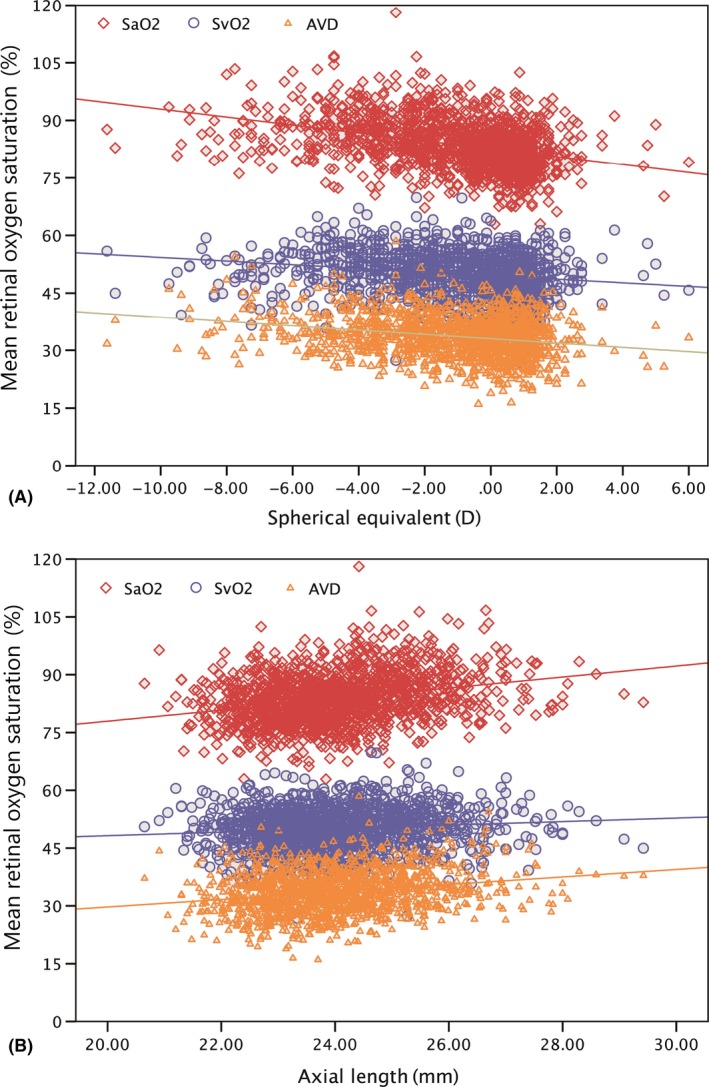
The association between retinal oxygen saturation and refractive error and axial length in children aged 7–19 years. SaO2: Mean oxygen saturation in major retinal arteries; SvO2: Mean oxygen saturation in major retinal veins; AVD: Arteriovenous difference in mean oxygen saturation. A: Oxygen saturation and refractive error. SaO2 (p < 0.001, r = −0.372), SvO2 (p < 0.001, r = −0.203) and AVD (p < 0.001, r = −0.240). B: Oxygen saturation and axial length. SaO2 (p < 0.001, r = 0.276), SvO2 (p < 0.001, r = 0.106) and AVD (p < 0.001, r = 0.221). [Colour figure can be viewed at wileyonlinelibrary.com]
In the myopia groups, the SaO2, SvO2 and AVD were 86.0 ± 6.5%, 51.4 ± 5.3% and 34.6 ± 5.3%, respectively, which were significantly higher than in the hyperopic and emmetropic groups (p < 0.001, one‐way anova). The AVD in the high myopia group (36.5 ± 5.7%) was significantly higher than that in the moderate myopia group (35.4 ± 5.1%), which was significantly higher than in the mild myopia group (33.9 ± 5.3) (p < 0.001, one‐way anova). However, the high myopia group had lower SaO2 and SvO2 than the moderate myopia group. Given that the oxygen saturation increased with more myopic refractive errors, it is worth noting that the increasing trends of SaO2 and SvO2 with refractive error were alleviated and even reversed in the high myopia group. The SaO2 levels of the mild, moderate and high myopia groups were 84.9 ± 6.4%, 87.7 ± 6.3% and 87.6 ± 6.3%, respectively. The SvO2 levels of the mild, moderate and high myopia groups were 51.0 ± 5.3%, 52.3 ± 5.2% and 51.2 ± 5.8%, respectively (Table 3).
Table 3.
Retinal oxygen saturation in different refractive statuses (Mean ± SD)
| Refractive status | n | SaO2 (%) | SvO2 (%) | AVD (%) |
|---|---|---|---|---|
| Hyperopes | 483 | 81.0 ± 5.5 | 48.5 ± 5.0 | 32.4 ± 5.3 |
| Emmetropes | 726 | 82.2 ± 5.5 | 49.2 ± 5.0 | 33.0 ± 5.3 |
| Myopes | 252 | 86.0 ± 6.5 | 51.4 ± 5.3 | 34.6 ± 5.3 |
| F | 108.579 | 47.059 | 25.207 | |
| p | <0.001 | <0.001 | <0.001 | |
| Mild myopes | 449 | 84.9 ± 6.4 | 51.0 ± 5.3 | 33.9 ± 5.3 |
| Moderate myopes | 179 | 87.7 ± 6.3 | 52.3 ± 5.2 | 35.4 ± 5.1 |
| High myopes | 98 | 87.6 ± 6.3 | 51.2 ± 5.8 | 36.5 ± 5.7 |
| F | 16.212 | 3.646 | 12.093 | |
| p | <0.001 | 0.027 | <0.001 |
The oxygen saturation of different refractive groups. The classification criteria are defined as follows: hyperopia: SE ≥ +0.5 D; emmetropia: −0.5 D < SE < +0.5 D; Myopia: SE ≤ −0.5 D. mild myopia: −3.0 D < SE ≤ −0.5; moderate myopia: −5.0 D < SE ≤ −3.0; and high myopia: SE ≤ −5.0. F: F value in one‐way anova. p: p value in one‐way anova.
Independent factors associated with retinal oxygen saturation
Age, gender, BMI, IOP and AL were included in a multivariate analysis of SaO2, SvO2 and AVD using multiple linear regression (Table 4). Since SE was strongly collinear with AL, it was not included in the establishment of the model, considering the fact that SE can relate to a number of parameters other than AL (particularly in children).
Table 4.
Multiple regression analysis of associations with retinal oxygen saturation
| Independent Variables | Unstandardized coefficients | Standardized coefficients | VIF | Variable's p value | R 2 |
|---|---|---|---|---|---|
| SaO2 | |||||
| Intercept | 63.966 | 0.000 | 0 | <0.001‡ | 0.19 |
| Age | 0.737 | 0.372 | 1.779 | <0.001‡ | |
| Gender | 0.558 | 0.043 | 1.091 | 0.078 | |
| BMI | 0.072 | 0.045 | 1.325 | 0.098 | |
| IOP | 0.020 | 0.010 | 1.019 | 0.688 | |
| AL | 0.346 | 0.067 | 1.543 | <0.023* | |
| SvO2 | |||||
| Intercept | 46.523 | 0 | 0 | <0.001‡ | 0.092 |
| Age | 0.506 | 0.306 | 1.779 | <0.001‡ | |
| Gender | 1.163 | 0.109 | 1.091 | <0.001‡ | |
| BMI | −0.037 | −0.028 | 1.325 | 0.336 | |
| IOP | −0.017 | −0.010 | 1.019 | 0.689 | |
| AL | −0.137 | −0.032 | 1.543 | 0.306 | |
| AVD | |||||
| Intercept | 17.443 | 0.000 | 0 | <0.001‡ | 0.076 |
| Age | 0.231 | 0.138 | 1.779 | <0.001‡ | |
| Gender | −0.604 | −0.055 | 1.091 | 0.035* | |
| BMI | 0.110 | 0.080 | 1.325 | 0.005† | |
| IOP | 0.037 | 0.021 | 1.019 | 0.403 | |
| AL | 0.483 | 0.110 | 1.543 | <0.001‡ | |
AL = axial length; BMI = body mass index; IOP = intraocular pressure; R 2 = R‐square of the regression equation; VIF = Variance inflation factor.
p < 0.05.
p < 0.01.
p < 0.001.
According to the regression models, the SaO2 significantly increased with older age (β = 0.737, p < 0.001) and longer AL (β = 0.346, p < 0.05). Moreover, SvO2 significantly increased with older age (β = 0.506, p < 0.001) and female gender (β = 1.163, p < 0.001). The independent factors associated with AVD were age (β = 0.231, p < 0.001), gender (β = −0.604, p = 0.035), BMI (β = 0.110, p = 0.005) and AL (β = 0.483, p < 0.001). According to the standardized coefficients, age explained more of the variance in SaO2, SvO2 and AVD than did AL.
Discussion
To our knowledge, the present study contained the largest school‐aged population ever considered to provide age‐specific normative values of retinal oxygen saturation and to explore the associated factors, especially age, gender and AL. The results demonstrated that the three parameters of retinal oxygen saturation (arterial: SaO2; venous: SvO2; and the arteriovenous difference: AVD) increased with age, and SaO2 and AVD were positively associated with a longer AL.
Repeatability and reliability of Oxymap T1
Researchers have confirmed the reliability of Oxymap using a standardized procedure (Palsson et al. 2012; Goharian et al. 2015), although the reliability may be affected by factors such as the angle of gaze. Palsson et al. (2012) found that the angle of gaze had a substantial effect on the saturation value, as the measured saturation values were lower in the inferior part of the fundus image than in the superior part. To reduce the effect of this bias, we restricted the direction of gaze while capturing the images and chose images with the optic disc in the centre for measurement.
For each eye, the mean oxygen saturation of all main vessels was calculated as a weighted rather than a simple average (weighted by the fourth power of the vessel diameter). Hence, larger vessels with higher blood flow had more influence on the mean value than smaller vessels.
Normal values
Our study provided age‐specific normal values of retinal oxygen saturation in 7‐ to 19‐year‐old children and adolescents. The mean values of SaO2, SvO2 and AVD were 83.7 ± 6.4%, 50.1 ± 5.4% and 33.6 ± 5.4%, respectively, and the SaO2 and SvO2 values were lower than those reported in adults (Geirsdottir et al. 2012; Yip et al. 2014; Mohan et al. 2015; Nakano et al. 2016). Based on a study of 98 healthy Indian adults aged 18–63 years, Mohan et al. (2015) reported that the normal values of SaO2 and SvO2 were 90.3 ± 6.6% and 56.9 ± 6.3%, respectively, and the AVD value of 33.2 ± 5.2% was similar to the value that we obtained. In a study examining 122 children and adolescents aged five to 18 years, Liu et al. (2017) found that the normal values of SaO2, SvO2 and AVD were 85.5 ± 7.1%, 48.2 ± 5.5% and 37.3 ± 6.5%, respectively, which were similar to the values in our study, except for AVD, for which we obtained a much lower value. This difference could be due to the different age distributions of the study samples. As our study demonstrated, retinal oxygen saturation increases with age. Thus, the oxygen saturation values might continue increasing in adolescence until reaching adult levels, which may be attributed to local and systemic growth and physiological development during adolescence, for example, the increasing thickness of some retinal layers with age during childhood (Read et al. 2015, 2017). In addition, the pigment of the eye fundus has a significant influence on the measurement of oxygen saturation (Hammer et al. 2008), which partly explained the difference in the normal retinal oxygen saturation values. Fundus diseases might also lead to different levels of retinal oxygen saturation. Attention should be paid to these factors in the clinical use of the retinal oxygen saturation index.
Age
Between the ages of 7 and 19 years, our study showed that the three oxygen saturation values all increased with age. Furthermore, age explained more variance than SE in SaO2 and SvO2, while for AVD, SE explained the most variance, followed by age.
Liu et al. (2017) reported that SaO2 and AVD values increased with age in 122 children and adolescents aged 5–18 years. However, SvO2 was not found to be associated with age in single and multiple regression analyses. The reason for this could have been the small sample size and the varied distribution. In adults over 18 years of age, the relationship between retinal oxygen saturation and age seemed to be different. With increasing age, Geirsdottir et al. (2012) observed unchanged SaO2, decreased SvO2 and increased AVD levels, whereas Nakano et al. (2016) observed increased SaO2, unchanged SvO2 and increased AVD levels. In the present study, we did not include data from healthy adults, but based on the large sample of adolescents, we found that the oxygen saturation in retinal arteries and veins increased with age during adolescence, which might be attributed to the local and systemic physiological growth that occurs at this stage.
Gender and body mass index
The present study is the first study to report a gender difference in retinal oxygen saturation in normal children and adolescents. Girls had significantly higher SvO2 and lower AVD levels than boys, according to both t‐tests and multiple linear regression analysis. The difference in AVD partly reflects the oxygen consumption of the retinal tissues. The higher SvO2 and lower AVD levels indicated that girls’ retinas tend to consume less oxygen than boys’ retinas. This finding was consistent with the fact that females have a lower basal metabolic rate than males, as reported by Lazzer et al. (2010) in a study of obese children and adolescents. Therefore, we inferred that the lower metabolic rate and lower oxygen consumption in the female retina is a reflection of the lower systematic metabolic status of the whole female body.
The present study also showed through multiple linear regression that AVD increased as BMI increased. This finding could be attributed to the influence of the basal metabolic rate. A higher BMI may cause a higher basal metabolic rate, which could partly explain the increase in AVD in children who had higher BMIs.
Previously, we had doubted that the difference in oxygen saturation between males and females might be caused by the difference in vessel diameter (i.e. larger size correlates with larger vessels, resulting in greater blood and oxygen delivery). However, in the present study, there was no statistical difference in vessel diameter between boys and girls (Table 1).
AL and refractive error
Increased myopic refractive errors and AL are main features of myopic eyes. In the present study, longer ALs and more myopic refractive errors were associated with increased SaO2, SvO2 and AVD between the ages of 7 and 19 years. Liu et al. (2017) also found a significant correlation between retinal oxygen saturation and SE in a normal adolescent sample. However, they did not observe a change in AVD as myopic refractive error increased, which could be attributed to an insufficient number of participants. In adults with high myopia, Zheng et al. (2015) reported that both SaO2 and AVD values significantly decreased compared to emmetropic eyes—a totally different trend from that seen in children. This could be due to the decreased oxygen requirement of the atrophic and degenerated retina in high myopia adults, whereas in young people with myopia, there is usually no atrophy or degeneration of the retina.
It is still unknown whether the change in the retinal oxygen saturation of children is a cause or a result of myopia. The compensation theory may provide an explanation. As we know, nutrition and oxygen are supplied to the retina by both retinal arteries and choroidal vessels. Both are critical to normal retinal function. In early stages of myopia, the choroid is thinner than normal but retinal thickness remains stable (Jin et al. 2016). The thinning of the choroid might decrease the blood and oxygen supply from choroidal sources, and as a result, require more oxygen from retinal sources. Then, the retina may adjust to this circumstance by increasing retinal oxygen saturation. In our opinion, myopia increases oxygen consumption and oxygen demand from the retina. This increased oxygen consumption results in higher AVD, and the increased oxygen demand requires more oxygen supply from retinal arteries.
However, it is worth noting that the trends of SaO2 and SvO2 change with refractive errors were inconsistent between the mild/moderate myopia and high myopia groups. In other words, when the refractive error was higher than −5.0 D (mild/moderate myopia), the retinal oxygen saturation increased with more myopic refractive errors, but when the refractive error was lower than −5.0 D (high myopia), the SaO2 remained stable while SvO2 started to decrease (Table 3). Hence, the increasing trends of SaO2 and SvO2 with refractive error were alleviated and even reversed when the refractive error was lower than −5.0 D (high myopia group). This change in the trend indicates that something different occurs in highly myopic eyes.
One possible explanation is decompensation. The retinal arteries are able to adjust to adapt to the slight increase in myopic refractive errors and the subsequent increase in oxygen demand, as we inferred above. However, this adjustment and compensation are not infinite. When the refractive error increased to a certain extent (such as −5.0 D), exceeding the compensation ability of the retina, the arterial oxygen saturation no longer increased with SE. At the same time, the retina continued to consume more oxygen, resulting in a decrease in venous oxygen saturation and an increase in AVD. We believe that this decompensation contributes to the progression of high myopia‐related retinopathy.
There is possibility that the changing saturation values with associated factors is due to artefacts. It has been reported that the application of a mydriatic agent can influence the blood flow of the retina (Tsui et al. 2013). To minimize any such influence, we excluded children who had poor pupil dilation and subsequent poor image quality to make the results comparable and reduce bias caused by mydriasis. Another factor that could interfere with saturation values is the density of the retinal pigmented epithelial (RPE) (Hammer et al. 2008). Currently, there seem to be no methods for measuring the density of RPE, so we tried to control that factor through participant selection: all the participants were from the same ethnic group (Chinese Han) and tended to have similar levels of retinal pigmentation.
There were several limitations in the present study. First, we did not measure ocular perfusion pressure or take it into account. Geirsdottir et al. (2012) reported that there was a slight but significant increase in retinal arterial and venous oxygen saturation with increased ocular perfusion pressure. The missing ocular perfusion pressure could influence the accuracy of the multivariate analysis to some extent, but the main trend of the related factors was not altered. Second, the average normal arterial oxygen saturation value in the present study was 83.7%, which was lower than those reported in most previous studies. The younger study participants, slightly adjusted vessel selection procedures or ethnic factors could be responsible for this difference. However, the relative correlation between retinal oxygen saturation and age, gender, refractive error, AL and other variables remained. Third, the R‐square of the regression equation was not high, which means that some the associated factors were not included in the regression analysis, for example, the density of RPE or the choroid thickness, which thus might be considered in future research. Finally, the present study used a cross‐sectional design. Further follow‐up studies are required to elucidate the causal relationship between retinal oxygen saturation and associated factors.
In conclusion, this large sample size allowed us to determine age‐specific normal retinal oxygen saturation values for Chinese children and adolescents, which were lower than those of adults found in previous research. SaO2, SvO2 and AVD values increased with age. Meanwhile, SaO2, SvO2 and AVD increased with myopic refractive errors and eye axials. Males had lower SvO2 and higher AVD than females. In multiple regression models, age explained more variance than SE in terms of SaO2 and SvO2, whereas for AVD, SE explained the most variance, followed by age.
This study was funded by the Excellent Young Talent Training Project of Shanghai Health and Family Planning Commission (Grant No. 2017YQ019), the Key Discipline of Public Health–Eye Health in Shanghai (Grant No. 15GWZK0601), the National Natural Science Foundation of China for Young Staff (Grant No. 81402695), the National Natural Science Foundation of China (Grant No. 81570851), the Overseas High‐end Research Team–Eye Health in Shanghai (GWTD2015S08) and the Three‐year Action Program of Shanghai Municipality for Strengthening the Construction of the Public Health System (2015–2017) (Grant No. GWIV‐13.2).
Contributor Information
Xiangui He, Email: xianhezi@163.com.
Xun Xu, Email: drxuxun@sjtu.edu.cn.
References
- Battu R, Mohan A, Khanna A, Kumar A & Shetty R (2015): Retinal oxygen saturation in retinitis pigmentosa and macular dystrophies in asian‐Indian eyes. Invest Ophthalmol Vis Sci 56: 2798–2802. [DOI] [PubMed] [Google Scholar]
- Eysteinsson T, Hardarson SH, Bragason D & Stefansson E (2014): Retinal vessel oxygen saturation and vessel diameter in retinitis pigmentosa. Acta Ophthalmol 92: 449–453. [DOI] [PubMed] [Google Scholar]
- Geirsdottir A, Palsson O, Hardarson SH, Olafsdottir OB, Kristjansdottir JV & Stefansson E (2012): Retinal vessel oxygen saturation in healthy individuals. Invest Ophthalmol Vis Sci 53: 5433–5442. [DOI] [PubMed] [Google Scholar]
- Geirsdottir A, Hardarson SH, Olafsdottir OB & Stefansson E (2014): Retinal oxygen metabolism in exudative age‐related macular degeneration. Acta Ophthalmol 92: 27–33. [DOI] [PubMed] [Google Scholar]
- Goharian I, Iverson SM, Ruiz RC, Kishor K, Greenfield DS & Sehi M (2015): Reproducibility of retinal oxygen saturation in normal and treated glaucomatous eyes. Br J Ophthalmol 99: 318–322. [DOI] [PubMed] [Google Scholar]
- Hammer M, Vilser W, Riemer T & Schweitzer D (2008): Retinal vessel oximetry‐calibration, compensation for vessel diameter and fundus pigmentation, and reproducibility. J Biomed Optics 13: 054015. [DOI] [PubMed] [Google Scholar]
- Hardarson S. (2013): Protocol for Acquisition and Analysis of Oxymap T1 Oximetry Images. Reykjavik, Iceland: Oxymap. [Google Scholar]
- Jin P, Zou H, Zhu J et al. (2016): Choroidal and retinal thickness in children with different refractive status measured by swept‐source optical coherence tomography. Am J Ophthalmol 168: 164–176. [DOI] [PubMed] [Google Scholar]
- Jorgensen CM, Hardarson SH & Bek T (2014): The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision‐threatening retinopathy. Acta Ophthalmol 92: 34–39. [DOI] [PubMed] [Google Scholar]
- Kaur C, Foulds WS & Ling EA (2008): Hypoxia‐ischemia and retinal ganglion cell damage. Clin Ophthalmol (Auckland, N.Z.) 2: 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzer S, Bedogni G, Lafortuna CL et al. (2010): Relationship between basal metabolic rate, gender, age, and body composition in 8,780 white obese subjects. Obesity (Silver Spring, Md.) 18: 71–78. [DOI] [PubMed] [Google Scholar]
- Lim LS, Tan L & Perera S (2014): Retinal vessel oxygen saturation increases after vitrectomy. Invest Ophthalmol Vis Sci 55: 3851–3856. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang S, Liu Y, Liu LJ, Lv YY, Tang P, Jonas JB & Xu L (2017): Retinal oxygen saturation in Chinese adolescents. Acta Ophthalmol 95: e54–e61. [DOI] [PubMed] [Google Scholar]
- Man RE, Sasongko MB, Xie J et al. (2015): Associations of retinal oximetry in persons with diabetes. Clin Exper Ophthalmol 43: 124–131. [DOI] [PubMed] [Google Scholar]
- Mohan A, Dabir S, Yadav NK, Kummelil M, Kumar RS & Shetty R (2015): Normative database of retinal oximetry in Asian Indian eyes. PLoS ONE 10: e0126179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Shimazaki T, Kobayashi N et al. (2016): Retinal oximetry in a healthy Japanese population. PLoS ONE 11: e0159650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrel AD, Maul E, Pokharel GP, Zhao J & Ellwein LB (2000): Refractive Error Study in Children: sampling and measurement methods for a multi‐country survey. Am J Ophthalmol 129: 421–426. [DOI] [PubMed] [Google Scholar]
- O'Connell RA, Anderson AJ, Hosking SL, Batcha AH & Bui BV (2014): Test‐retest reliability of retinal oxygen saturation measurement. Optom Vis Sci 91: 608–614. [DOI] [PubMed] [Google Scholar]
- Palsson O, Geirsdottir A, Hardarson SH, Olafsdottir OB, Kristjansdottir JV & Stefansson E (2012): Retinal oximetry images must be standardized: a methodological analysis. Invest Ophthalmol Vis Sci 53: 1729–1733. [DOI] [PubMed] [Google Scholar]
- Paul JP, O'Connell RA, Hosking SL, Anderson AJ & Bui BV (2013): Retinal oxygen saturation: novel analysis method for the oxymap. Optom Vis Sci 90: 1104–1110. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ, Vincent SJ & Alonso‐Caneiro D (2015): Macular retinal layer thickness in childhood. Retina (Philadelphia, Pa.) 35: 1223–1233. [DOI] [PubMed] [Google Scholar]
- Read SA, Alonso‐Caneiro D & Vincent SJ (2017): Longitudinal changes in macular retinal layer thickness in pediatric populations: myopic vs non‐myopic eyes. PLoS ONE 12: e0180462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traustason S, la Cour M & Larsen M (2014): Retinal vascular oximetry during ranibizumab treatment of central retinal vein occlusion. Br J Ophthalmol 98: 1208–1211. [DOI] [PubMed] [Google Scholar]
- Tsui E, Sehi M, Cheng RW, Wan J, Wong T, Dorner S, Fisher JA & Hudson C (2013): The impact of topical mydriatic ophthalmic solutions on retinal vascular reactivity and blood flow. Exp Eye Res 112: 134–138. [DOI] [PubMed] [Google Scholar]
- Turksever C, Orgul S & Todorova MG (2015): Reproducibility of retinal oximetry measurements in healthy and diseased retinas. Acta Ophthalmol 93: e439–e445. [DOI] [PubMed] [Google Scholar]
- Vandewalle E, Abegao Pinto L, Olafsdottir OB et al. (2014): Oximetry in glaucoma: correlation of metabolic change with structural and functional damage. Acta Ophthalmol 92: 105–110. [DOI] [PubMed] [Google Scholar]
- Yip W, Siantar R, Perera SA, Milastuti N, Ho KK, Tan B, Wong TY & Cheung CY (2014): Reliability and determinants of retinal vessel oximetry measurements in healthy eyes. Invest Ophthalmol Vis Sci 55: 7104–7110. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Zong Y, Li L et al. (2015): Retinal vessel oxygen saturation and vessel diameter in high myopia. Ophthalmic Physiol Opt 35: 562–569. [DOI] [PubMed] [Google Scholar]


