Abstract
Background
Histaminolytic activity mediated by diamine oxidase (DAO) is present in plasma after induction of severe anaphylaxis in rats, guinea pigs, and rabbits. Heparin released during mast cell degranulation in the gastrointestinal tract might liberate DAO from heparin‐sensitive storage sites. DAO release during anaphylaxis has not been demonstrated in humans.
Methods
Plasma DAO, tryptase, and histamine concentrations of four severe anaphylaxis events were determined at multiple serial time points in two patients with systemic mastocytosis. The histamine degradation rates were measured in anaphylaxis samples and in pregnancy sera and plasma with comparable DAO concentrations.
Results
Mean DAO (132 ng/mL) and tryptase (304 ng/mL) concentrations increased 187‐ and 4.0‐fold, respectively, over baseline values (DAO 0.7 ng/mL, tryptase 76 ng/mL) during severe anaphylaxis. Under non‐anaphylaxis conditions, DAO concentrations were not elevated in 29 mastocytosis patients compared to healthy volunteers and there was no correlation between DAO and tryptase levels in mastocytosis patients. The histamine degradation rate of DAO in plasma from mastocytosis patients during anaphylaxis is severely compromised compared to DAO from pregnancy samples.
Conclusion
During severe anaphylaxis in mastocytosis patients, DAO is likely released from heparin‐sensitive gastrointestinal storage sites. The measured concentrations can degrade histamine, but DAO activity is compromised compared to pregnancy samples. For accurate histamine measurements during anaphylaxis, DAO inhibition is essential to inhibit further histamine degradation after blood withdrawal. Determination of DAO antigen levels might be of clinical value to improve the diagnosis of mast cell activation.
Keywords: anaphylaxis, diamine oxidase, heparin, histamine degradation, mastocytosis
Abbreviations
- (e)LOQ
(estimated) limit of quantification
- ANA
anaphylaxis
- CHO
Chinese hamster ovary
- CV
coefficient of variation
- DAO
diamine oxidase
- DIMAZ
diminazene aceturate
- DM
DAOMAST study
- HIS
histamine
- HV
healthy volunteer
- IQR
interquartile range
- IU
international unit
- LC‐MS/MS
liquid chromatography‐tandem mass spectrometry
- LOB
limit of blank
- LOD
limit of detection
- MAP
mean arterial pressure
- MCAS
mast cell activation syndrome
- MC
mast cell
- PUT
putrescine
- rh
recombinant human
- SD
standard deviation
1. INTRODUCTION
Diamine oxidase (DAO) was first described almost 90 years ago because of its histamine degradation activity.1, 2 In humans, high DAO mRNA and activity are found in the gastrointestinal tract, kidney, and placenta.3 Expression in the placenta is restricted to extravillous trophoblast cells and therefore of fetal and not as assumed for decades of maternal origin.4 Plasma DAO concentrations increase at least 100‐fold during pregnancy,5 but the physiological function of this rise is not clear.
The high DAO expression in the gastrointestinal tract might protect from histamine present in contaminated food or generated by bacteria within the gut microbiome. Inactivation of DAO using aminoguanidine in pigs and sheep followed by exogenous histamine challenge strongly supports this protection mechanism.6, 7 Does DAO have any protective function against endogenously released histamine after mast cell (MC) or basophil activation?
Mastocytosis is characterized by an increased number of MCs in various organ systems.8, 9 Consequently, tryptase and histamine concentrations are elevated and tryptase levels correlate with MC burden.10, 11, 12 Histamine, its metabolites, and tryptase concentrations rapidly increase during anaphylaxis and are used in the differential diagnosis of MC activation and mast cell activation syndromes (MCAS).13, 14, 15, 16, 17, 18 Increased serum/plasma tryptase concentrations are caused by liberation from MCs and not basophils, which contain equal amounts of histamine but more than 100‐fold less tryptase.19 Nevertheless, in more than a third of subjects during anaphylaxis, tryptase concentrations measured within 1‐2 hours after onset of symptoms were not increased defined as levels > 11.4 ng/mL. Tryptase levels were increased in 76% of severe anaphylaxis patients.20 Nevertheless, absolute values above >11.4 ng/mL tryptase and not a relative increase above baseline were used to calculate the percentage of patients with increased tryptase concentrations. Basal serum tryptase levels are also elevated in familial hypertryptasemia with symptomatic or asymptomatic course and in a small percentage of non‐anaphylaxis and non‐mastocytosis subjects.21, 22, 23, 24 Histamine is also used as a biomarker of MC degranulation, but the plasma concentrations decline rapidly limiting the usefulness of histamine as indicator of MC activation.14, 16 Additional markers to measure MC activation might be clinically helpful for differential diagnosis of histamine‐like symptoms or anaphylaxis. If MC mediator release can be firmly established, secondary prevention strategies like trigger avoidance or desensitization might be implemented.25
Heparin is released during MC degranulation. Bleeding complications during anaphylaxis and in patients with systemic mastocytosis have been assigned to released heparin or heparin‐like substances.26, 27, 28, 29 Liberation of DAO by exogenous high molecular weight heparin into blood plasma has been shown in many vertebrates including humans.30, 31, 32, 33, 34, 35
The rational hypothesis, how MC activation could lead to DAO release, is supported by animal studies after induction of severe anaphylaxis. Mast cell degranulation leads to release of heparin, which is liberating DAO from the storage sites in the gastrointestinal tract in rats and rabbits and in the liver in guinea pigs.36, 37, 38, 39, 40, 41 Most of these animal studies induced severe anaphylaxis with high mortality questioning the relevance for the majority of human non‐fatal hypersensitivity reactions. We did not find any publication showing increased DAO concentrations during anaphylaxis in humans.
In this study, we wanted to test whether DAO is released during severe anaphylaxis in humans and to demonstrate that the resulting concentrations of DAO are able to degrade histamine possibly mitigating the potential life‐threatening effects of high circulatory histamine concentrations. If the measured DAO levels are able to degrade histamine, published histamine concentrations measured during the course of anaphylaxis might be underestimated, because DAO will degrade histamine ex vivo after blood withdrawal, plasma preparation, and downstream activities. Immediate inactivation of DAO after blood withdrawal was considered mandatory for reliably measuring histamine concentrations after induction of anaphylactic shock in animal experiments.42
2. MATERIALS AND METHODS
2.1. Human diamine oxidase ELISA
The development and characterization of the human DAO ELISA was recently published in detail.4, 43 We tested two commercially available DAO ELISA kits but they were not reliable to measure human DAO.43
2.2. Tryptase ELISA
Total serum tryptase concentrations were measured by the commercial fluoroimmunoenzyme assay (Thermo Scientific, Uppsala, Sweden) in the clinical chemistry laboratory of the Vienna General Hospital using an accredited procedure. The detection limit of the assay is 1 ng/mL, and the coefficient of variation (CV) is 4.5% at 9 ng/mL.
2.3. Measurement of histamine concentrations using a histamine ELISA
Histamine concentrations were measured using a histamine ELISA (Immunotech IM2562; Beckman Coulter, Vienna, Austria) and an in‐house development LC‐MS/MS method. The LC‐MS/MS method was developed, because the histamine ELISA can demonstrate considerable variability at histamine concentrations above 10 ng/mL.
2.4. Measurement of histamine concentrations using LC‐MS/MS
A detailed description of this method has been recently published.4
2.5. Histamine degradation assay
Histamine degradation rates were determined by incubating serum or plasma samples with 100 ng/mL (900 nmol/L) histamine base for various durations at 37°C. There is no obvious difference using plasma or serum. The reactions were stopped at different time points by adding a final concentration of 50 μmol/L diminazene aceturate (Sigma, D7770, Vienna, Austria). Diminazene is a potent and selective DAO inhibitor with an inhibition constant K i of 13 nmol/L against rhDAO.44 The samples were then immediately snap‐frozen in liquid nitrogen and stored at −30°C until measurement of histamine concentration using LC‐MS/MS and ELISA.
2.6. Mastocytosis study DAOMAST00101
A total of 138 mastocytosis patients were contacted. Forty‐four were not reachable and 51 not interested to participate. Twenty‐nine of the 43 included patients (67%) were diagnosed with indolent systemic mastocytosis (ISM). The diagnosis of all included subjects is listed in Table S1. The diagnosis of mastocytosis was established according to the criteria provided by the World Health Organization.45 Inclusion criteria included at least 18 years of age at the time of study entry and presence of mastocytosis‐related symptoms during the last 12 months. A detailed description of the anaphylaxis events in Subjects A and B is provided in the Supplementary Information. Both suffer from systemic mastocytosis and are positive for the KIT D816V mutation. A short description of the four severe events is provided in the results section.
2.7. Ethics
The local ethics committee of the Medical University of Vienna approved the study of patients with mastocytosis (DAOMAST00101 study; ethics committee number: 1012/2013), recurrent abortions (source of control pregnancy plasma and serum samples; DAOPROM study; ethics committee number: 1666/2012), and blood withdrawals from healthy volunteers. All patients and healthy individuals provided informed consent before collection of blood samples.
2.8. Statistical analysis
Only standard statistical procedures have been used.
3. RESULTS
In the DAOMAST study, a total of 43 patients with mastocytosis were included and plasma samples were obtained at baseline, after 24 and 48 weeks. After enrollment, two patients suffered from four severe, life‐threatening anaphylaxis events. A detailed description of the four events in the two patients is described in the Supplementary Information. Briefly, in Patient A, both severe events rapidly progressed similarly. Anaphylaxis was accompanied by flush in the face, stuffy nose, vomiting, and diarrhea and an extension of the flush to the arms and chest. At this time, the EpiPen was injected by the spouse. The typical time window from onset of symptoms before the patient went into shock and became unconscious was only 15 minutes. In both severe events, no blood pressure recordings were possible by the emergency physicians and in the first minutes after arriving at the hospital. This corresponds to Grade IIIB according to Niggemann and Beyer46 or Grade 5 according to Cox et al47 and is therefore considered a severe systemic reaction.
Both severe events of Patient B were triggered by an upper respiratory tract infection. The patient had symptoms for many hours before drop in blood pressure and compensatory increase in heart rate. In both events, the mean arterial blood pressure (MAP) decreased to 60 mm Hg but fluctuated from 51 to 68 mm Hg with increased pulse amplitude because of diastolic blood pressure recordings of as low as 37 mm Hg. The pulse rate was constantly elevated to 110‐125 per minute and the respiratory rate to more than 20 per minute over the next hours. Intense flushing and vomiting accompanied both events triggering admission to the emergency department. The normal mean (SD) MAP measured 24 times over 16 days without symptoms was 91 (5.9) mm Hg with a pulse rate of 68 (7.8) per minute. In both severe events of Patient B, the MAP decreased from a mean of 91 to 60 with diastolic blood pressure readings partially below 40 with MAPs of about 50 mm Hg. This is a decrease of at least 34% (60/91) or a maximum of 45% (50/91) in the MAP readings and therefore this decrease fulfilled the hypotension criteria and the anaphylaxis events might be classified as Grade IIIA according to Niggemann and Beyer46 or Grade 5 according to Cox et al47 and also considered severe systemic reactions.
The DAO and tryptase concentrations at baseline and of four severe and one mild anaphylaxis events are shown in Figure 1A,B. We measured on average an 187‐fold increase in DAO concentrations compared to baseline values (Figure 1A,C; Table 1). Total tryptase concentrations increased 4.3‐fold during anaphylaxis (Figure 1B,D; Table 1). In the two subjects, the concentrations of both antigens were stable at three measurements during the DAOMAST study period of 48 weeks (Figure 1A,B). The mild anaphylaxis event induced only a minor but clearly measurable increase in both enzymes.
Figure 1.
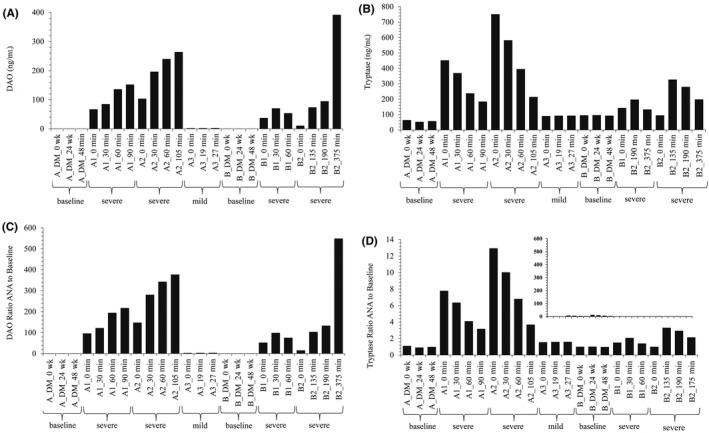
Diamine oxidase (DAO) and tryptase concentrations increase during anaphylaxis in mastocytosis patients. DAO (A) and tryptase (B) concentrations of citrate plasma samples from four severe and one mild anaphylaxis event were determined in duplicates and the means are presented. A1, A2 and B1, B2 were four severe anaphylaxis events in Patients A and B, respectively, whereas A3 was a mild event in Patient A; baseline values were determined during the DAOMAST mastocytosis study at 0, 24, and 48 wk. C,D, shows the ratios of the enzyme concentrations during the anaphylaxis events to the mean baseline values. The scales are not identical. The inset in (D) presents the data in a comparable scale as in (C). Minutes are calculated from the first blood withdrawal time point. This is not equal with the onset of anaphylaxis symptoms. In Patient A, the time window from onset to severe symptoms is <60 min. For severe anaphylaxis events in Patient B, arrival at the emergency department might be considered start of severe symptoms and then the time from onset of severe symptoms and blood withdrawal is <45 min. Table 1 summarizes the data from Figure 1. DM, DAOMAST study; min, minutes; wk, weeks
Table 1.
Diamine oxidase (DAO) and tryptase levels increase significantly during anaphylaxis
| Mean (median; SD baseline; ng/mL) | Mean (median) ANA (ng/mL) | Range ANA (ng/mL)c | Mean ratio ANA over baseline | Ratio DAO over tryptase ratio | |
|---|---|---|---|---|---|
| Severe events DAOa | 0.71 (0.7; 0.02) | 132 (95) | 11; 392 | 187 | 47 |
| Severe events Tryptasea | 76 (79; 20.6) | 304 (238) | 95; 751 | 4.0 | |
| Mild event DAO | 0.70 (0.7; 0.0) | 2.4 (2.4) | 3.4 | 2.2 | |
| Mild event Tryptase | 58 (57; 5.8) | 92 (92) | 1.6 | ||
| Mean DAO ratio b | Mean tryptase ratio | ||||
| Severe eventsa | 187 | 4.6 | 40 |
ANA, anaphylaxis; SD, standard deviation.
Includes four events in two patients with in total 15 measurements; mean baseline values for severe or mild anaphylaxis events were calculated based on six (two patients) or three measurements, respectively, during the DAOMAST study.
Mean of 15 DAO or tryptase ratios calculated by dividing levels during anaphylaxis by the mean baseline values.
Minimum and maximum are listed.
During the anaphylaxis events, DAO and tryptase concentrations increased significantly over baseline values. Do these two antigens also positively correlate under non‐anaphylaxis conditions? DAO and tryptase concentrations from 36 systemic mastocytosis patients were not correlated (Figure 2A; R = 0.077; P = 0.66) suggesting that elevated tryptase concentrations do not lead to increased DAO concentrations. The mean DAO concentration (1.5 ng/mL) was 20‐fold lower compared to the mean tryptase value (30.3 ng/mL; Figure 2A).
Figure 2.
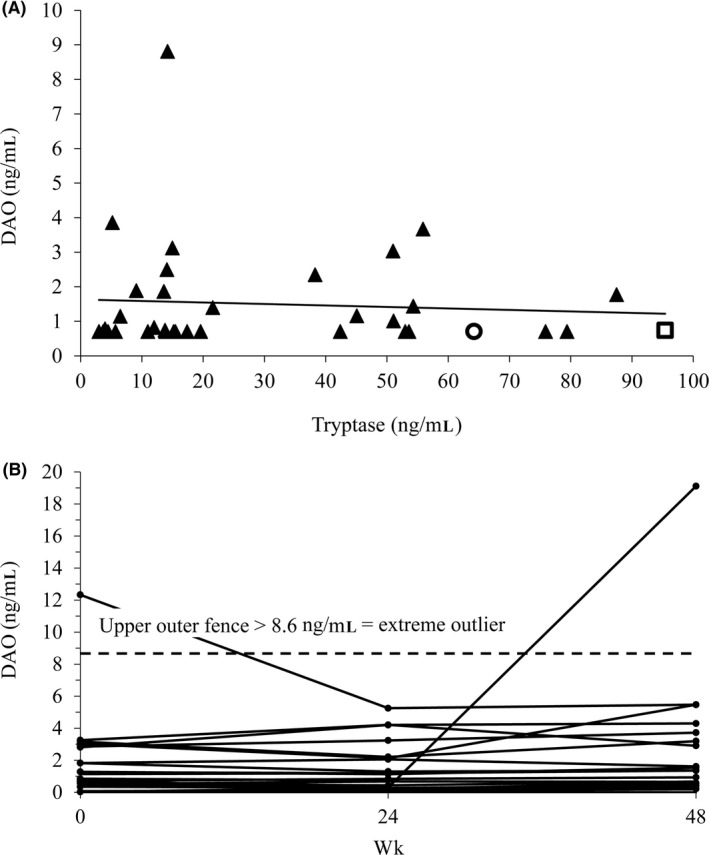
No correlation between diamine oxidase (DAO) and tryptase concentrations in 36 mastocytosis patients at baseline under stable non‐anaphylaxis conditions. A, Tryptase and DAO plasma concentrations were measured at baseline of the DAOMAST study. The two patients with the four severe anaphylaxis events are shown as circle and square. DAO concentrations below the estimated limit of quantification (eLOQ) of 0.7 ng/mL were set to the eLOQ. The mean (SD; median) concentrations for DAO and tryptase are 1.5 (1.6; 0.74) and 30.3 (27.0; 15.4) ng/mL, respectively. The sample at 8.8 ng/mL DAO concentration is an extreme outlier using the concentration at Quartile 3 + 3 × the interquartile range (IQR) as outlier definition. The upper outer fence for an extreme outlier is 6.5 ng/mL; B, DAO concentrations in 80 serum samples from 29 patients included in the DAOMAST study were measured in duplicates at baseline (0 wk) and 24 and 48 wk after inclusion. Only patients with at least two available samples were included. Visits were scheduled only under stable disease symptoms. The upper outer fence for extreme outlier calculations (Quartile 3 + 3 × IQR) among the 80 samples is 8.6 ng/mL; wk, weeks
Are DAO concentrations stable in mastocytosis patients or do they significantly fluctuate possibly explaining the increased DAO concentrations during the anaphylaxis events? In Figure 2B, the DAO concentrations from 29 mastocytosis patients are shown at baseline, after 24 and 48 weeks. The mean (SD, median) DAO concentration of all 80 samples is 1.8 (2.7, 1.03) ng/mL with a strong contribution of the two extreme outliers to the mean with 20% and to the SD with 48%. Nevertheless, the two outliers are 7‐ to 11‐fold below the mean DAO concentration during the four severe anaphylaxis events. The mean DAO concentration of these 80 samples is 73‐fold lower compared to the mean of 132 ng/mL measured during the four severe anaphylaxis events. The 99% quantile of the DAO concentration in the 29 mastocytosis patients from Figure 2B is 12.5 ng/mL. In the Table S4, the best available DAO concentration measurements are compared to the data from this study. The 99% quantile DAO concentrations are only above 20 ng/mL during pregnancy and after heparin administration.
These data imply that DAO antigen levels are stable in indolent systemic mastocytosis and show only minor variations, which highly unlikely can explain the high DAO antigen levels during the severe anaphylaxis events.
Histamine concentrations at the three baseline visits and during the anaphylaxis events are shown in Figure 3A for Subject A and Figure 3B for Subject B. In the second severe event of Subject B, we collected blood in citrate vacutainer blood collection tubes with and without DIMAZ at a final concentration of 50 μmol/L. DIMAZ is a highly potent DAO inhibitor with a K i of 13 nmol/L.44 Both types of tubes were immediately placed on ice. Histamine concentrations were similar with and without DIMAZ (Figure 3B). The relatively rapid drop of histamine in both severe events of Subject A within 30‐60 minutes is somewhat unusual. Did we withdraw “exactly” at the time, when the degranulation of MCs and therefore histamine release stopped? The histamine value at 30 minutes in the second severe anaphylaxis event (A2_30 minutes) is even below the three baseline histamine concentrations and the three values from the mild anaphylaxis event, although the clinical symptoms were still severe (Figure 3A).
Figure 3.
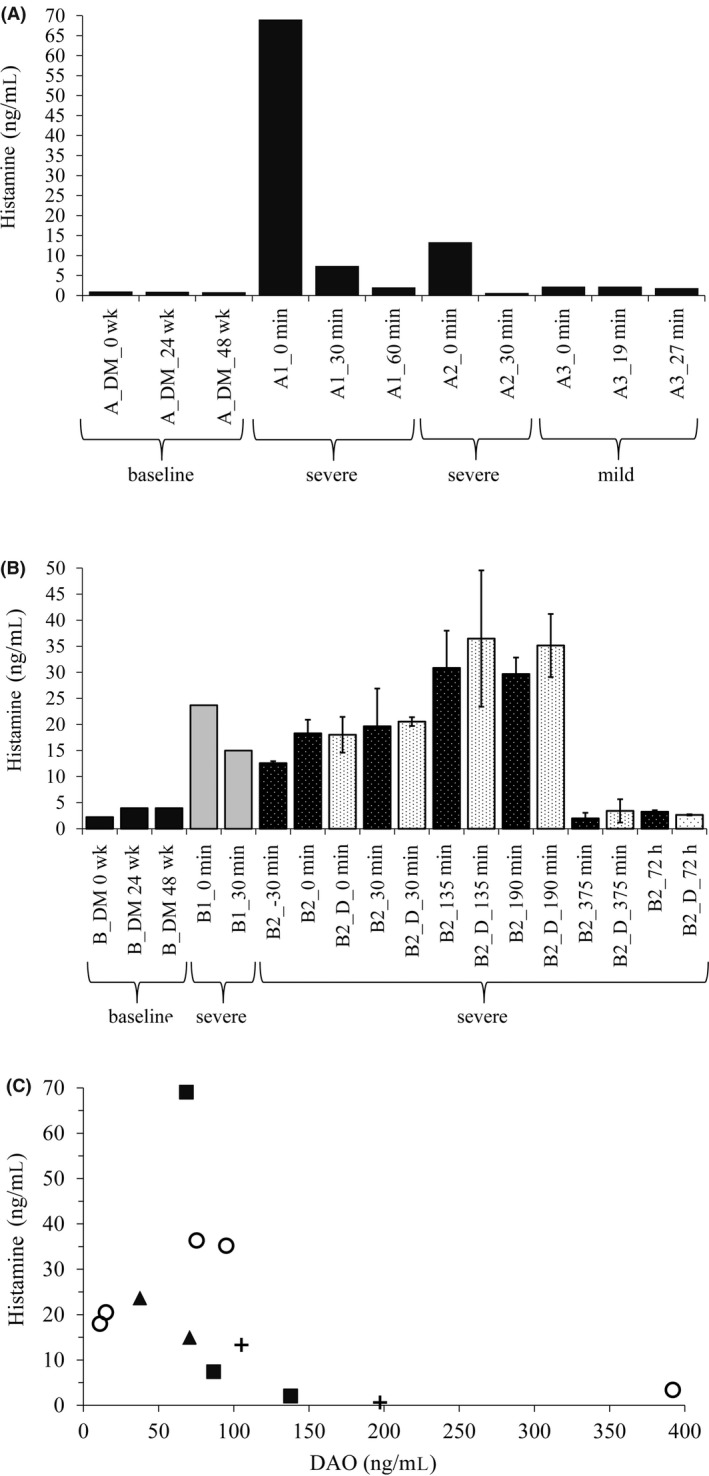
Rapid decline of life‐threatening histamine levels during severe anaphylaxis might be partially caused by high diamine oxidase (DAO) concentrations. A,B, Histamine concentrations are shown at 0, 24, and 48 wk during the DAOMAST study and during the four severe and one mild anaphylactic event at multiple time points. They were measured in duplicates using the Immunotech histamine ELISA. The mean is shown. For event B2, the samples were also measured twice in singlicates using the LC‐MS/MS method. The mean (±SEM; n = 2) of the histamine ELISA and LC‐MS/MS measurements is shown. In the second severe anaphylaxis event of Patient B, we also withdrew blood into citrate vacutainer tubes containing the potent DAO inhibitor diminazene aceturate. B2 ‐30 min is a sample not shown in Figure 1 withdrawn by an emergency physician 30 min before the 0 time point presented in Figure 1. At the 72‐h time point, the patient was symptom‐free; minutes are calculated from the first blood withdrawal time point. This is not equal with the onset of anaphylaxis symptoms (see Figure 1). C, Histamine concentrations inversely correlate with DAO levels. The figure shows 12 values from the four independent severe anaphylaxis events with the same symbols corresponding to the same event; D, diminazene aceturate; DM, DAOMAST study; min, minutes; wk, weeks; h, hours
There was also a strong decrease of histamine during the second severe event of Subject B between 190 and 375 minutes (Figure 3B). At the same time, clinical symptoms, especially the high pulse rate, markedly improved. In Subject A, plasma samples were not cooled allowing continuous histamine degradation. Exogenous enzymatic histamine degradation was excluded in event B2 because samples were immediately placed on ice and spiked with a potent DAO inhibitor. Is it possible that released DAO is degrading histamine in vivo but also in vitro (ex vivo) after blood withdrawal? In Figure 3C, DAO concentrations seem to negatively correlate with histamine levels. An exact P‐value cannot be calculated with the limited data. Samples were immediately placed on ice only for events B1 and B2. The samples from A1 and A2 were withdrawn at a distant hospital and left for a few hours at room temperature, leaving enough time for DAO to degrade histamine ex vivo. Nevertheless, the data in Figure 3C also imply that DAO at 50‐100 ng/mL is not able to efficiently degrade histamine, because histamine concentrations of 10‐70 ng/mL were measured.
Histamine and putrescine are the preferred natural substrates for human DAO. Using the substrate concentration with half‐maximal velocity K m as indicator for substrate affinity, DAO shows the highest affinity to histamine with a K m of 2.8 μmol/L followed by putrescine with 20 μmol/L.3 There is a wide range of published K m values with putrescine and histamine as substrates for human DAO. For putrescine and histamine, the range (median) is from 3.4 to 94 (26) and 2.5 to 124 (4.6) μmol/L, respectively (see Table S2). There is no real consensus for appropriate K m values using human DAO with either substrate. Nevertheless, the Elmore et al3 data are probably the best available.
During the severe anaphylaxis events, the highest measured histamine concentration of 70 ng/mL corresponds to 629 nmol/L histamine, which is already 4.5‐fold below the lowest published K m of 2.8 μmol/L for histamine. Other histamine concentrations in Figure 3A,B are at least 10‐fold below the K m. Therefore, the important question, whether histamine can be degraded by DAO concentrations reached during severe anaphylaxis, cannot be properly addressed using published activity or velocity data. Based on the published K m data, DAO should be very slow in degrading histamine more than 10‐fold below the K m. Nevertheless, the slope or shape of the histamine degradation velocity curve between low histamine concentrations and the lowest published K m of 2.8 μmol/L is not known. Therefore, we determined the histamine degradation rates of anaphylaxis samples and pregnancy samples as positive controls.
Figure 4A shows the time‐dependent histamine degradation of 4 serum and 12 plasma samples from late second‐/third‐trimester pregnancies after adding 100 ng/mL (900 nmol/L) histamine. We used 100 ng/mL because histamine concentrations during severe anaphylaxis can readily reach this level.14, 26 The inset of Figure 4A shows the histamine degradation rate derived from the 1st derivative of the histamine degradation curve plotted against the histamine concentration. In the 16 pregnancy samples, the mean (SD) DAO concentration was 125 (45) ng/mL. The mean half‐life of histamine in Figure 4A is 3.4 minutes calculated from the degradation rate of 0.2059. DAO was able to degrade histamine below the limit of detection of 1 ng/mL or 9 nmol/L. Histamine concentrations after 10 minutes showed a statistically highly significant correlation with DAO antigen concentrations in the 16 pregnancy samples (P = 0.0011; correlation coefficient = 0.74). Two sera from healthy volunteers with DAO concentrations below 2 ng/mL and two pregnancy sera with inhibited DAO (mean DAO concentration 136 ng/mL) using diminazene aceturate at 10 μmol/L were not able to degrade histamine (Figure 4A).
Figure 4.
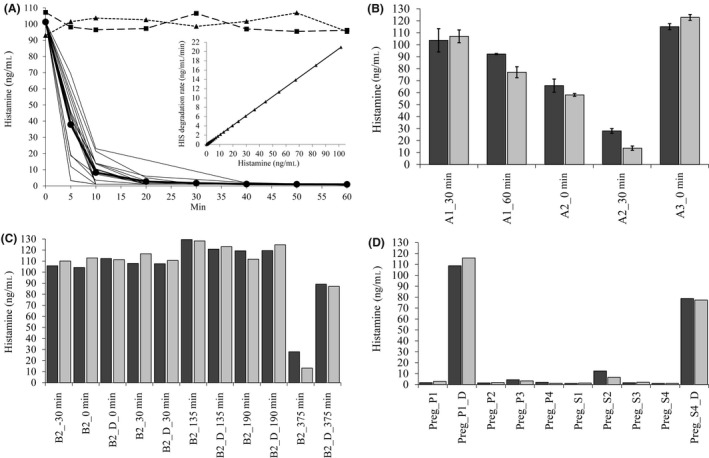
Diamine oxidase (DAO) in pregnancy serum or plasma rapidly degrades 100 ng/mL (900 nmol/L) exogenously added histamine but DAO activity from anaphylaxis events is severely compromised. A, Sixteen plasma and serum samples (second and third trimester) with a mean (SD) DAO concentration of 125 (45) ng/mL were spiked with 100 ng/mL histamine, and histamine was measured at multiple time points. The mean degradation curve is shown in bold and filled circles ([HIS] ng/mL = 101.5 * exp(−0.2059 * Min)). The filled triangles represent the mean histamine concentrations of two pregnancy sera in the presence of 10 μmol/L diminazene aceturate, a potent human DAO inhibitor. The filled squares show the absence of any histamine degradation using plasma from two healthy volunteers (DAO < 2 ng/mL). Inset, The degradation rate was calculated using the 1st derivative of the above equation (histamine degradation rate (ng/mL/min) = 101.5 * −0.2059 * exp(−0.2059 * Min)) and plotted against the histamine concentration used for this experiment. The regression line equals histamine degradation rate = 0.2059 * histamine concentration. B, Plasma samples from A1, A2 (both severe anaphylaxis), and A3 (mild) events were spiked with 100 ng/mL histamine and incubated for 30 and 60 min at 37°C (dark and light gray bars, respectively). Histamine was measured using the LC‐MS/MS method in duplicates, and the mean with SEM is shown. C, Plasma samples from severe anaphylaxis event B2 were spiked with 100 ng/mL histamine base and incubated for 30 and 60 min (dark and light gray bars, respectively). Histamine was measured in singlicates using the LC‐MS/MS method; D, in the same experiment, four pregnancy samples were tested for histamine degradation activity as controls; dark and light gray bars represent 30‐ and 60‐min incubation, respectively; D, diminazene aceturate; P, plasma; Preg, pregnancy; S, serum; min, minutes
Diamine oxidase at 125 ng/mL in pregnancy serum or plasma is rapidly degrading exogenous histamine. Is DAO in anaphylaxis samples also able to rapidly degrade histamine? Because of limited plasma volume, we measured histamine degradation only after 30 and 60 minutes. DAO activity in anaphylaxis samples is severely compromised compared to pregnancy samples (Figure 4B,C). DAO concentrations between 74 and 138 ng/mL were not able to degrade histamine below 50 ng/mL after 30 or 60 minutes corresponding to 10‐20 ng/mL pregnancy‐equivalent DAO concentrations. The method to calculate pregnancy‐equivalent DAO concentrations is described in the Data S1. It is based on the mean histamine degradation curve from Figure 4A and calculates the percent activity of the anaphylaxis compared to the pregnancy samples. The pregnancy‐equivalent DAO concentrations and activities of the tested anaphylaxis samples are presented in Tables S5‐S7. Only the DAO levels of 196 and 392 ng/mL were able to relevantly degrade histamine after 30 and 60 minutes, although the pregnancy‐equivalent activity was only 16% and 17%, respectively (Figure 4B,C; Tables S5, S6). In the same experiment, the eight pregnancy control samples with comparable DAO concentrations degraded histamine to a mean of 3.2 and 2.5 ng/mL after 30 and 60 minutes, respectively (Figure 4D; Table S7).
These two mastocytosis patients were treated during the severe anaphylaxis events with different medications at high doses but these compounds do not inhibit DAO activity (Figure S3).
4. DISCUSSION
The massive release of DAO into the circulation during severe anaphylaxis in mastocytosis patients confirms and extends decades‐old published animal data. The DAO concentrations measured in the four severe anaphylaxis events are highly unlikely a result of natural enzyme fluctuations. Table S8 summarizes published DAO activity data after conversion to recombinant human DAO equivalent concentrations from anaphylaxis studies in rats, guinea pigs, and rabbits. After 3‐5 minutes, DAO concentrations already reached 80‐200 ng/mL, which is comparable to levels found during pregnancy or anaphylaxis in mastocytosis patients. The animal data are in remarkable agreement with human DAO concentrations during pregnancy, after heparin administration, and in non‐pregnant subjects (see Table S4 and Ref. 43). Schmutzler et al39 published a 30‐fold increase in plasma DAO activity in guinea pigs after induction of severe anaphylaxis. The same group showed increased histaminolytic activity reflected in shortened histamine half‐lives in plasma of guinea pigs after induction of anaphylaxis or heparin infusion.41 Aminoguanidine, a relatively specific, potent irreversible DAO inhibitor, completely blocked histamine breakdown in plasma after induction of anaphylaxis in several animal studies.37, 38, 40, 41
It is intriguing to speculate that DAO is actually liberated by the heparin/tryptase complex released from MCs in the gastrointestinal tract.48 A putative heparin‐binding site was described for DAO.44 The heparin component of the heparin/tryptase complex might compete with heparan sulfate glycosaminoglycans of the basement membrane.49 Because DAO is only expressed at high concentrations in the gastrointestinal tract and the kidneys, the DAO storage sites in the former are more likely the source of liberated DAO, because the number of MCs in human kidneys is very low.50 Mast cell density values of renal tissue in mastocytosis patients have not been published.
Are DAO concentrations also elevated during severe anaphylaxis in non‐mastocytosis patients? Considering that tryptase is a clinically used biomarker especially for severe anaphylaxis with hypotension in non‐mastocytosis patients20, 51 and a 47‐fold ratio of DAO increase over tryptase shown in this study, DAO concentrations might be also increased during anaphylaxis in non‐mastocytosis patients or in subjects with MCAS. The 4‐ to 5‐fold increase of tryptase concentrations during severe anaphylaxis over baseline in this study is consistent with tryptase elevations during anaphylaxis in non‐mastocytosis subjects. Borer‐Reinhold et al52 measured a 2.6‐ to 3.8‐fold increase during severe anaphylaxis two to five hours after allergen exposure in non‐mastocytosis patients. A 4‐ to 5‐fold increase in tryptase concentrations between controls and anaphylaxis cases in the emergency department with shock and hypoxemia was published recently.51 Patients with severe allergic shock or cardiac arrest during anesthesia showed more than 10‐fold elevated tryptase concentrations.53 Nevertheless, the starting values of tryptase are in the majority of cases in non‐mastocytosis patients below 11 ng/mL and therefore about 1‐ to 10‐fold lower compared to tryptase levels in mastocytosis patients. Only multiple measurements of DAO during anaphylaxis in non‐mastocytosis patients will clarify, whether DAO is increased similar to mastocytosis patients. If the likelihood for the detection of MC activation can be improved, DAO measurements could be of clinical benefit. The correct identification of individuals with MCAS might be increased. Except during pregnancy and after heparin administration, DAO concentrations are undetectable or if detectable on average only about 1‐2 ng/mL in healthy volunteers43 and mastocytosis patients (this study).
The half‐life of 90‐150 minutes for tryptase and 20‐30 hours for human DAO post‐parturition might explain the delayed increase of DAO with already decreasing tryptase concentrations in the severe anaphylaxis events.15, 54, 55 While DAO concentrations were still increasing 90 minutes after withdrawing the first sample, tryptase levels decreased following a half‐life of about 60‐120 minutes. Assuming that the half‐life of DAO is also 20‐30 hours in non‐pregnant individuals, no data are available, and DAO concentrations might be elevated for a few days. An extended DAO half‐life might improve the probability to detect MC degranulation.
What are the consequences of highly elevated DAO concentrations during severe anaphylaxis in vivo and in vitro? If blood is withdrawn from subjects with high DAO concentrations, plasma histamine will likely be degraded and the measured values artificially lower, if DAO is not immediately inactivated. Most published histamine measurements during anaphylaxis did not explicitly inactivate DAO and some of the low measured histamine concentrations might be partially an artifact, if high DAO levels were present. In the clinical environment, it is certainly not uncommon to leave blood for 30 minutes or even longer at room temperature before preparing plasma and analyzing or freezing the samples. Blood will slowly cool from 37°C to the ambient temperature possibly leaving sufficient time for DAO to degrade histamine. DAO will also degrade histamine during thawing and rewarming of frozen samples necessary for downstream assays like histamine measurements. Morel et al56 showed rapid and complete degradation of 4.4 ng/mL histamine using plasma from a pregnant woman with a half‐life of 2.5 minutes. Almeida wrote that the half‐life of histamine was <3 minutes in human plasma obtained from a third‐trimester pregnancy.57 They used 75 nmol/L or 8.3 ng/mL histamine to determine the degradation rate. We are not aware of any other publication measuring the histamine degradation rate of human DAO at both reasonable DAO and histamine concentrations. If DAO is present in the circulation at 50 ng/mL or more, histamine measurements without the use of a potent DAO inhibitor are likely artificially lower.
Animal studies could not unequivocally answer the question, whether DAO per se or liberated endogenous DAO is mitigating the severity of anaphylaxis by degrading histamine. In most animal anaphylaxis studies, induction of shock was performed with high antigen concentrations and was extremely severe with massive activation of MCs, possibly basophils and high mortality. Released DAO did not have enough time to degrade histamine. Other mediators are certainly also involved in the development of severe symptoms during anaphylaxis, and histamine sensitivity varies significantly between animal species. Therefore, it is impossible to assign mortality or severe morbidity in animal studies to a single mediator.
We obviously cannot answer the same question for human anaphylaxis events. The measured histamine concentrations, especially the large drop of histamine in the B2 event combined with the increase of DAO, argue that DAO was degrading histamine in vivo. Hemodynamic improvement reflected in a normalization of the heart rate coincided with increased DAO levels.
The cause behind the greatly reduced DAO activities during anaphylaxis compared to pregnancy samples must be addressed. The medications used for the treatment of the severe anaphylaxis cases do not inhibit DAO activity. The lack of appropriate human material is severely limiting these investigations. Nevertheless, recombinant DAO might be administered at high concentrations overcoming the presence of a possible DAO inhibitor or inactivation mechanism.
In conclusion, DAO increases more than 100‐fold during severe anaphylaxis in mastocytosis patients and is likely involved in the in vivo and ex vivo degradation of histamine. Measurements of DAO with tryptase during suspected MC activation might increase the rate of correct MCAS diagnosis. Correct determination of histamine levels in samples with high DAO concentrations requires immediate inactivation of DAO after blood withdrawal. Otherwise, DAO degrades histamine and artifactually low histamine concentrations will be measured. The mechanism causing significantly reduced DAO activity in relation to antigen levels must be investigated. This putative DAO inhibitor might interfere with histamine degradation using recombinant human DAO to treat life‐threatening histamine effects during MC activation or severe anaphylaxis.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
TB designed the experiments, performed data analysis, and interpretation and wrote the manuscript. BR and TS developed and characterized the LC‐MS/MS histamine quantification method and analyzed the data. RR performed statistical analysis. KP performed DAO ELISA and DAO activity assays and analysis of data. WS and PV were investigators in the DAOMAST study. PV critically read and revised the manuscript. BJ was the principal investigator in the DAOMAST study and contributed to the writing of the manuscript. All authors read, revised, and approved the final version of the manuscript for publication.
Supporting information
ACKNOWLEDGMENTS
We would like to thank all mastocytosis patients for participation in the DAOMAST study. The allowance to withdraw extra blood samples under severe anaphylaxis conditions in the two subjects and therefore under extreme stress is highly appreciated. This study would not have been possible without this exceptional attitude. Saijo Joseph, Christa Firbas, and Sabine Schranz are greatly acknowledged for their contributions to the DAOMAST study. The pregnancy samples were provided by Sophie Pils collected during a recurrent abortion study. We would like to thank all women for participation in this study and also the healthy volunteers for donating blood samples.
Boehm T, Reiter B, Ristl R, et al. Massive release of the histamine‐degrading enzyme diamine oxidase during severe anaphylaxis in mastocytosis patients. Allergy. 2019;74:583–593. 10.1111/all.13663
REFERENCES
- 1. Best CH. The disappearance of histamine from autolysing lung tissue. J Physiol. 1929;67:256‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Best CH, McHenry EW. The inactivation of histamine. J Physiol. 1930;70:349‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elmore BO, Bollinger JA, Dooley DM. Human kidney diamine oxidase: heterologous expression, purification, and characterization. J Biol Inorg Chem. 2002;7:565‐579. [DOI] [PubMed] [Google Scholar]
- 4. Velicky P, Windsperger K, Petroczi K, et al. Pregnancy‐associated diamine oxidase originates from extravillous trophoblasts and is decreased in early‐onset preeclampsia. Sci Rep. 2018;8:6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Southren AL, Kobayashi Y, Sherman DH, Levine L, Gordon G, Weingold AB. Diamine oxidase in human pregnancy: plasma diamine oxidase in nonpregnant and normal pregnant patients. Am J Obstet Gynecol. 1964;89:199‐203. [DOI] [PubMed] [Google Scholar]
- 6. Sjaastad ÖV. Potentiation by aminoguanidine of the sensitivity of sheep to histamine given by mouth. Effect of aminoguanidine on the urinary excretion of endogenous histamine. Q J Exp Physiol Cogn Med Sci. 1967;52:319‐330. [DOI] [PubMed] [Google Scholar]
- 7. Sattler J, Häfner D, Klotter HJ, Lorenz W, Wagner PK. Food‐induced histaminosis as an epidemiological problem: plasma histamine elevation and haemodynamic alterations after oral histamine administration and blockade of diamine oxidase (DAO). Agents Actions. 1988;23:361‐365. [DOI] [PubMed] [Google Scholar]
- 8. Valent P, Sperr WR, Schwartz LB, Horny HP. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non‐mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114:3‐11. [DOI] [PubMed] [Google Scholar]
- 9. Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garriga MM, Friedman MM, Metcalfe DD. A survey of the number and distribution of mast cells in the skin of patients with mast cell disorders. J Allergy Clin Immunol. 1988;82:425‐432. [DOI] [PubMed] [Google Scholar]
- 11. Sperr WR, Jordan JH, Fiegl M, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136‐141. [DOI] [PubMed] [Google Scholar]
- 12. Doyle LA, Sepehr GJ, Hamilton MJ, Akin C, Castells MC, Hornick JL. A clinicopathologic study of 24 cases of systemic mastocytosis involving the gastrointestinal tract and assessment of mucosal mast cell density in irritable bowel syndrome and asymptomatic patients. Am J Surg Pathol. 2014;38:832‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast‐cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;25(316):1622‐1626. [DOI] [PubMed] [Google Scholar]
- 14. Van der Linden PW, Hack CE, Poortman J, Vivie‐Kipp YC, Struyvenberg A, van der Zwan JK. Insect‐sting challenge in 138 patients: relation between clinical severity of anaphylaxis and mast cell activation. J Allergy Clin Immunol. 1992;90:110‐118. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26:451‐463. [DOI] [PubMed] [Google Scholar]
- 16. Stone SF, Cotterell C, Isbister GK, Holdgate A, Brown SGA. Elevated serum cytokines during human anaphylaxis: identification of potential mediators of acute allergic reactions. J Allergy Clin Immunol. 2009;124:786‐792. [DOI] [PubMed] [Google Scholar]
- 17. Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126:1099‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valent P. Mast cell activation syndromes: definition and classification. Allergy. 2013;68:417‐424. [DOI] [PubMed] [Google Scholar]
- 19. Jogie‐Brahim S, Min HK, Fukuoka Y, Xia HZ, Schwartz LB. Expression of alpha‐tryptase and beta‐tryptase by human basophils. J Allergy Clin Immunol. 2004;113:1086‐1092. [DOI] [PubMed] [Google Scholar]
- 20. Sala‐Cunill A, Cardona V, Labrador‐Horrillo M, et al. Usefulness and limitations of sequential serum tryptase for the diagnosis of anaphylaxis in 102 patients. Int Arch Allergy Immunol. 2013;160:192‐199. [DOI] [PubMed] [Google Scholar]
- 21. Fellinger C, Hemmer W, Wöhrl S, Sesztak‐Greinecker G, Jarisch R, Wantke F. Clinical characteristics and risk profile of patients with elevated baseline serum tryptase. Allergol Immunopathol. 2014;42:544‐552. [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez‐Quintela A, Vizcaino L, Gude F, et al. Factors influencing serum total tryptase concentrations in a general adult population. Clin Chem Lab Med. 2010;48:701‐706. [DOI] [PubMed] [Google Scholar]
- 23. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sabato V, Van De Vijver E, Hagendorens M, et al. Familial hypertryptasemia with associated mast cell activation syndrome. J Allergy Clin Immunol. 2014;134:1448‐1450. [DOI] [PubMed] [Google Scholar]
- 25. Brown SG, Stone SF. Laboratory diagnosis of acute anaphylaxis. Clin Exp Allergy. 2011;41:1660‐1662. [DOI] [PubMed] [Google Scholar]
- 26. Desborough JP, Taylor I, Hattersley A, et al. Massive histamine release in a patient with systemic mastocytosis. Br J Anaesth. 1990;65:833‐836. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalo‐Garijo MA, Pérez‐Rangel I, Alvarado‐Izquierdo MI, Pérez‐Calderón R, Sánchez‐Vega S, Zambonino MA. Metrorrhagia as an uncommon symptom of anaphylaxis. J Investig Allergol Clin Immunol. 2010;20:540‐541. [PubMed] [Google Scholar]
- 28. Sucker C, Mansmann G, Steiner S, et al. Fatal bleeding due to a heparin‐like anticoagulant in a 37‐year‐old woman suffering from systemic mastocytosis. Clin Appl Thromb Hemost. 2008;14:360‐364. [DOI] [PubMed] [Google Scholar]
- 29. Samoszuk M, Corwin M, Hazen SL. Effects of human mast cell tryptase and eosinophil granule proteins on the kinetics of blood clotting. Am J Hematol. 2003;73:18‐25. [DOI] [PubMed] [Google Scholar]
- 30. Hansson R, Holmberg CG, Tibbling G, Tryding N, Westling H, Wetterqvist H. Heparin‐induced diamine oxidase increase in human blood plasma. Acta Med Scand. 1966;180:533‐536. [DOI] [PubMed] [Google Scholar]
- 31. Hansson R, Thysell H. Diamine oxidase in blood plasma in some vertebrates and Anodonta cygnea before and after injection of heparin. Acta Physiol Scand. 1968;74:533‐542. [DOI] [PubMed] [Google Scholar]
- 32. Baylin SB, Beaven MA, Krauss RM, Keiser HR. Response of plasma histaminase activity to small doses of heparin in normal subjects and patients with hyperlipoproteinemia. J Clin Invest. 1973;52:1985‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D'Agostino L, Daniele B, Pallone F, Pignata S, Leoni M, Mazzacca G. Postheparin plasma diamine oxidase in patients with small bowel Crohn's disease. Gastroenterology. 1988;95:1503‐1509. [DOI] [PubMed] [Google Scholar]
- 34. Rokkas T, Vaja S, Murphy GM, Dowling RH. Postheparin plasma diamine oxidase in health and intestinal disease. Gastroenterology. 1990;98:1493‐1501. [DOI] [PubMed] [Google Scholar]
- 35. Klocker J, Drasche A, Sattler J, Bodner E, Schwelberger HG. Postheparin plasma diamine oxidase activity and the anticoagulant effect of heparin. Inflamm Res. 2000;49(suppl 1):S53‐S54. [DOI] [PubMed] [Google Scholar]
- 36. Rose B, Leger J. Serum histaminase during rabbit anaphylaxis. Proc Soc Exp Biol Med. 1952;79:379‐381. [DOI] [PubMed] [Google Scholar]
- 37. Logan GB. Release of a histamine‐destroying factor during anaphylactic shock in guinea pigs. Proc Soc Exp Biol Med. 1961;107:466‐469. [DOI] [PubMed] [Google Scholar]
- 38. Code CF, Cody DT, Hurn M, Kennedy JC, Strickland MJ. The simultaneous release of histamine and a histamine‐destroying factor during anaphylaxis in rats. J Physiol. 1961;156:207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmutzler W, Hahn F, Seseke G, Bernauer W. On the origin of plasma histaminase in the anaphylactic shock in guinea pigs. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1966;252:332‐338. [PubMed] [Google Scholar]
- 40. Logan GB. Histamine and histaminase release by guinea pigs at various intervals during anaphylactic shock. J Allergy. 1967;40:207‐214. [DOI] [PubMed] [Google Scholar]
- 41. Hahn F, Pröhle F, Mitze R, Degand L. Changes in the histaminolytic activity of the guinea pig liver and plasma during anaphylaxis and after heparin injection. Int Arch Allergy Appl Immunol. 1971;40:340‐350. [DOI] [PubMed] [Google Scholar]
- 42. Hahn F, Kretzschmar R, Teschendorf HJ, Mitze R. Role of histaminase (diamine oxidase) in disappearance of plasma histamine in anaphylaxis and after histamine injection. Int Arch Allergy Appl Immunol. 1970;39(5–6):449‐458. [DOI] [PubMed] [Google Scholar]
- 43. Boehm T, Pils S, Gludovacz E, et al. Quantification of human diamine oxidase. Clin Biochem. 2017;50:444‐451. [DOI] [PubMed] [Google Scholar]
- 44. McGrath AP, Hilmer KM, Collyer CA, et al. Structure and inhibition of human diamine oxidase. Biochemistry. 2009;48:9810‐9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Niggemann B, Beyer K. Time for a new grading system for allergic reactions? Allergy. 2016;71:135‐136. [DOI] [PubMed] [Google Scholar]
- 47. Cox LS, Sanchez‐Borges M, Lockey RF. World Allergy Organization systemic allergic reaction grading system: is a modification needed? J Allergy Clin Immunol Pract. 2017;5:58‐62. [DOI] [PubMed] [Google Scholar]
- 48. Alter SC, Metcalfe DD, Bradford TR, Schwartz LB. Regulation of human mast cell tryptase. Effects of enzyme concentration, ionic strength and the structure and negative charge density of polysaccharides. Biochem J. 1987;248:821‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwelberger HG, Hittmair A, Kohlwein SD. Analysis of tissue and subcellular localization of mammalian diamine oxidase by confocal laser scanning fluorescence microscopy. Inflamm Res. 1998;47(suppl 1):S60‐S61. [DOI] [PubMed] [Google Scholar]
- 50. Roberts IS, Brenchley PE. Mast cells: the forgotten cells of renal fibrosis. J Clin Pathol. 2000;53:858‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Francis A, Fatovich DM, Arendts G, et al. Serum mast cell tryptase measurements: sensitivity and specificity for a diagnosis of anaphylaxis in emergency department patients with shock or hypoxaemia. Emerg Med Australas. 2017;30:366‐374. [DOI] [PubMed] [Google Scholar]
- 52. Borer‐Reinhold M, Haeberli G, Bitzenhofer M, et al. An increase in serum tryptase even below 11.4 ng/mL may indicate a mast cell‐mediated hypersensitivity reaction: a prospective study in Hymenoptera venom allergic patients. Clin Exp Allergy. 2011;41:1777‐1783. [DOI] [PubMed] [Google Scholar]
- 53. Laroche D, Gomis P, Gallimidi E, Malinovsky JM, Mertes PM. Diagnostic value of histamine and tryptase concentrations in severe anaphylaxis with shock or cardiac arrest during anesthesia. Anesthesiology. 2014;121:272‐279. [DOI] [PubMed] [Google Scholar]
- 54. Hansson R. Diamine oxidase isoenzymes in human blood plasma. Scand J Clin Lab Invest. 1970;25:33‐39. [DOI] [PubMed] [Google Scholar]
- 55. Carrington ER, Frishmuth GJ, Oesterling MJ, Adams FM, Cox SE. Gestational and postpartum plasma diamine oxidase values. Obstet Gynecol. 1972;39:426‐430. [PubMed] [Google Scholar]
- 56. Morel AM, Delaage MA. Immunoanalysis of histamine through a novel chemical derivatization. J Allergy Clin Immunol. 1988;82:646‐654. [DOI] [PubMed] [Google Scholar]
- 57. Almeida AP, Flye W, Deveraux D, Horakova Z, Beaven MA. Distribution of histamine and histaminase (diamine oxidase) in blood of various species. Comp Biochem Physiol C. 1980;67C:187‐190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


