Abstract
Background
Lobar pneumonia is an occupational disease of welders. This is the first report of global deployment of a pneumococcal vaccination program for welders within a multi‐national corporation.
Methods
Global webinars were conducted to introduce the program. Communication packages translated into all location languages were deployed. All employee welders who had not previously been vaccinated were offered a free single dose of pneumococcal polysaccharide vaccine (PPV23) by on‐site location medical centers during normal working hours. Numbers of vaccinated welders were reported by each location each month.
Results
Twelve months after starting the program, 241 of 767 welders have been vaccinated (31%) across six countries.
Conclusions
Global deployment of pneumococcal vaccination for welders can be successfully undertaken by a multi‐national corporation. Adoption of this practice by corporations could reduce the incidence and mortality of pneumonia among welders globally.
Keywords: pneumococcal, pneumonia, vaccination, welder, welding
1. INTRODUCTION
Lobar pneumonia is an occupational disease of welders.1 There is both an increased incidence and mortality in this population.2 Evidence for this is remarkably consistent and comes from studies conducted in the United Kingdom, the United States, Sweden, and Canada.1, 2, 3, 4, 5, 6, 7, 8 Reported odds ratios (ORs) and standardized mortality ratios (SMRs) are typically about 2‐3.1, 2, 3, 4, 5, 6, 7, 8 This recognized risk of lobar pneumonia is predominantly due to Streptococcus pneumoniae (pneumococcus), where the risk is clearly elevated in welders and is thought to be related to the inhalation of ferrous metal fumes.2 The mechanism is not yet completely understood. It has been suggested that free iron may promote infections either by acting as a growth nutrient for bacteria or by causing free radical injury.2, 5 There is evidence that welding fume induces hyper‐susceptibility to pneumococcal infection by increasing platelet‐activating factor receptor (PAFR) expression in lower airway cells.9, 10 PAFR is a host receptor to which pneumococci adhere when infecting lower airway cells.9
Contemporary exposure controls including local exhaust ventilation and respiratory protection are important in welding to reduce the risk of various respiratory diseases. However, evidence that exposure controls reduce the risk of pneumonia is lacking. Despite implementation of environmental control measures and the availability of respiratory protective equipment (utilisation unknown), multiple cases of pneumococcal pneumonia (caused by multiple pneumococcal strains) occurred at a Belfast shipyard.11 It is possible that many of the shipyard welders did not use these controls and it is also possible that more cases may have occurred in the absence of controls.
The increased risk of pneumonia among welders is limited to the 12‐month period immediately following occupational exposures and does not persist during periods of post‐welding work activity or into retirement, an observation that makes confounding by smoking unlikely.2
In 2011 the Department of Health in England recommended a single dose of pneumococcal polysaccharide vaccine (PPV23) for welders who have not received PPV previously.2 Eligibility based on the type of welding or welder was not specified, with the recommendation suggested broadly for anyone undertaking welding on a regular basis. This recommendation remains current.12 The vaccine contains polysaccharides derived from 23 capsular types of pneumococcus.
In 2012 Palmer and Cosgrove published an in‐depth review on vaccinating welders against pneumonia.2 They presented a risk assessment, which estimated vaccination of 588 welders would prevent one case of pneumonia in the ensuing 10 years. They also estimated vaccination of 4900 welders would be expected to prevent one fatal case of pneumonia in the ensuing 10 years, based on a case fatality rate of 12%.
We aim to describe the global deployment of a pneumococcal vaccination program for welders within a multi‐national corporation. Alcoa Corporation (Alcoa) operates bauxite mines, alumina refineries, aluminum smelters, and an aluminum rolling mill, and employs welders to fabricate and maintain plant and equipment at operating locations. The welded base metal is mostly steel. The deployment of the pneumococcal vaccination program was voluntary and proactive, not reactive, with no known outbreak of pneumococcal disease. Plant‐site medical centers were the venue for vaccine delivery. We believe this is the first published report of such a global deployment. The vaccination program adhered to the recommendation of the Department of Health in England: a single dose of pneumococcal polysaccharide vaccine (PPV23) for welders who have not received PPV previously.12
2. MATERIALS AND METHODS
Initially we estimated the number of employees undertaking welding by asking our occupational hygienists to review the number of employees in “significant” and “unacceptable” similar exposure groups (SEGs) for welding related exposures. SEGs are classified by the company as “significant” if 5% or more of the samples exceed 50% of the company Occupational Exposure Limit (OEL) and are classified as “unacceptable” if 5% or more of the samples exceed the company OEL. The welding related SEGs were: welding fume, hexavalent chromium, chromium metals, iron oxide, manganese, and vanadium pentoxide. This resulted in an estimate of 693 employees undertaking welding. A simple business case was prepared, with an estimate of the cost of program deployment, assuming complete uptake by all eligible employees, and delivery by existing on‐site location medical centers. The unit cost of the vaccine varies by country but was estimated to be US$50. No additional resources were required by the onsite medical centers, so the estimated cost was approximately 700 employees x US$50 each = $US35 000. The cost was presented as insignificant in comparison with the value the company places on health protection, with the likely benefit being prevention of at least one case of occupational pneumonia.
We sought and received senior management approval and sponsorship for the program in January 2017. The scope of the program was global, defined as all currently operating locations, operated by Alcoa. This included bauxite mines in Australia (2) and Brazil (2), alumina refineries in Australia (3), Brazil (2) and Spain (1), aluminum smelters in Australia (1), Canada (3), Iceland (1), Norway (2), Spain (3) and the United States (3) and one rolling mill in the United States. Three communications packages were created: one for Business Unit (BU) managers and Environment, Health and Safety (EHS) staff, one for onsite medical center healthcare professionals and one for employees who undertake welding. The materials were written in English and then translated by the company's EHS Global Services Unit into five other languages spoken at operating locations: French, Icelandic, Norwegian, Portuguese, and Spanish. All three versions outlined in varying degrees of detail the research evidence for an increased risk of pneumonia in welders, the nature of the vaccine, the plan for global deployment, the need to continue to use local exhaust ventilation, and respiratory protection, and the importance of participating in available smoking cessation programs, given that smoking is an additional risk factor for pneumonia.
Two global webinars were conducted in April 2017 to familiarize EHS management, EHS professionals, occupational hygienists, and onsite medical center healthcare professionals with the program. Also, AMD presented directly to BU and location management, EHS management, EHS professionals, occupational hygienists, and onsite medical center healthcare professionals at locations in Australia, Brazil and Spain.
Any employee undertaking any amount of welding was eligible provided he/she had not previously received any pneumococcal vaccination and had no personal contraindications. We did not define a threshold for the amount of welding required to be eligible because the exposure‐response relationship is unknown at this time.2 We also considered but rejected inclusion of pneumococcal conjugate vaccine (PCV 13) in our vaccination campaign so as to remain aligned with the Department of Health in England recommendation and the published literature relating to welders wherein the focus was exclusively on PPV23.
Onsite medical center healthcare professionals were asked to liaise with location occupational hygienists to compile a list of employees who undertook welding, so they could be called in to the medical centers. This list comprised 767 employees, a modest increase (11%) in the total number of eligible employees originally estimated from the “significant” and “unacceptable” SEG data. Some of these employees only welded occasionally and would have been classified as being within “insignificant” SEGs.
Our methodology fixed the number of eligible welders and did not subtract those who left employment or add those who commenced employment during the observation period. Any new employees who were vaccinated were counted. With a global employee turnover rate of less than 6% during the observation period, assuming the cohort to be static is unlikely to have distorted the reported vaccination participation rate.
Onsite medical center healthcare professionals were asked to seek informed consent from eligible employees prior to vaccination, in line with normal medical practice. An information, questionnaire, and consent form was provided for this purpose, translated into all relevant languages. The questionnaire was designed to elicit any contraindications to vaccination or reasons for the vaccination to be delayed. The information, questionnaire, and consent forms were retained in the onsite medical center medical records and were not collected for this paper. The communication material for welders, outlined above, was made available in the onsite medical centers and eligible employees were also provided a copy of the vaccine manufacturer's patient information sheet to review. Employees were invited to ask any questions.
The onsite medical center healthcare professionals were asked to commence the program in June 2017. Eligible employees were called in to the medical centers specifically to be offered vaccination. If they did not respond, they were offered vaccination when next presenting for a routine occupational health evaluation (such as annual audiometry), or when next presenting with a medical problem.
A tracking and performance indicator was developed by Alcoa's Global Health Center of Excellence (Health CoE), and implemented by Alcoa's EHS Systems Manager in Microsoft Power BI. This cloud‐based business analytics tool is accessible to specified Alcoa EHS staff and tracks a broader range of EHS key performance indicators. Onsite medical center healthcare professionals were asked to enter the number of eligible employees at their respective locations and to update the cumulative number of vaccinated employees each month. No personal identifying data were entered.
Communication of the program included articles in electronic newsletters emailed to employees, including recommendations from AMD, Alcoa's Health CoE and a welder vaccination champion.
There was no ethics review and approval for the following reasons. Vaccination of welders was undertaken in accordance with the recommendation of the Department of Health in England. Informed consent was sought by clinical staff at the location medical centers in accordance with normal medical practice, with an information, contraindication questionnaire and consent form and the vaccine manufacturer's patient information sheet. The information, contraindication questionnaire and consent forms were retained by the onsite medical centers and were not collected for this paper. The data collected for the paper were simply the numbers of eligible employees at each location and the numbers of employees vaccinated at each location per month. There were no employee identifying data collected.
3. RESULTS
Twelve months after commencement of the vaccination program in June 2017, 241 employees have been vaccinated. This represents 31% of the 767 eligible employees. Table 1 shows the distribution by country. At many locations most of the welding is undertaken by contracting company employees. In Iceland, all welding is undertaken by contracting company employees, and so there were no eligible employees.
Table 1.
Pneumococcal vaccination by country
| Eligible employees (n) | Vaccinated employees (n) | Vaccinated employees (%) | |
|---|---|---|---|
| Australia | 334 | 97 | 29 |
| Brazil | 76 | 76 | 100 |
| Canada | 15 | 13 | 87 |
| Iceland | 0 | 0 | N/A |
| Norway | 40 | 14 | 35 |
| Spain | 3 | 2 | 67 |
| USA | 299 | 39 | 13 |
| Total | 767 | 241 | 31 |
Table 2 shows the distribution of eligible and vaccinated employees by business unit: bauxite mining, alumina refining, and aluminum smelting and rolling. Figure 1 shows the cumulative number of employees vaccinated globally by month of deployment.
Table 2.
Pneumococcal vaccination by business unit
| Eligible employees (n) | Vaccinated employees (n) | Vaccinated employees (%) | |
|---|---|---|---|
| Bauxite | 153 | 89 | 58 |
| Alumina | 193 | 77 | 40 |
| Aluminium | 421 | 75 | 18 |
| Total | 767 | 241 | 31 |
Figure 1.
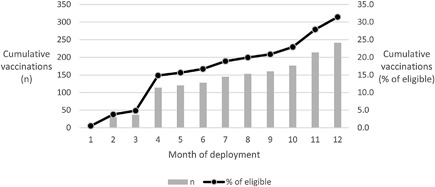
Cumulative vaccinations by month of deployment
4. DISCUSSION
The overall vaccination participation was 31%. This compares with a mean of 37% (range 19‐57%) for injectable seasonal influenza vaccine across 18 United State non‐healthcare companies in 2008‐2009.13
Our program design incorporated several features known to improve influenza vaccination rates, including: no cost to employees for vaccination; vaccination available on‐site; vaccination available on an ongoing basis during working hours; and vaccination promoted to eligible employees through workplace communication channels.14 We speculate that greater use of welders as vaccination champions may be useful.
Previous vaccination is unlikely to have been a reason for many welders declining or avoiding vaccination. Following an outbreak of pneumococcal disease at a Belfast shipyard only 0.7% (5/680) of attendees at a prophylaxis and vaccination clinic recalled vaccination against pneumococcal disease in the past five years.11 Only 6.3% (43/680) had medical conditions associated with an increased risk of invasive pneumococcal disease and might therefore have been vaccinated at some time. Consequently, we conclude that most of the welders who did not get vaccinated were not adequately persuaded of the merits of vaccination by our campaign.
Vaccination progressed steadily through the first 12 months of the program (Figure 1). It is difficult to rapidly recall an entire work group at any given location and more difficult to do so across an entire enterprise. With a voluntary initiative, employees may not respond to a recall and may only address the offer of vaccination when next at the medical center for a mandatory routine occupational health evaluation or a medical problem. Given the trajectory in Figure 1, it is likely that vaccination participation will continue to increase for some months.
Variation in vaccination participation by nation (Table 1) may be due to cultural differences, with employees less likely to accept employer recommendations in some countries. For example, participation in the US (13%) was less than half that in Australia (29%). This may also explain lower participation in the aluminum business unit compared to the bauxite and alumina business units (Table 2), as all eligible employees in the US were in the aluminum business unit and they represented the majority of those in this business unit (71%). Staff supporting onsite medical centers at United State locations have undertaken efforts to encourage vaccination among eligible employees.
While the initial phase of our vaccination campaign was limited to company employees, our company plans to expand the availability of the pneumococcal vaccine by requiring all contracting companies to offer an analogous voluntary vaccination program to their welder employees.
We will also share our experience with the operators of the company's joint ventures in Saudi Arabia and Guinea.
Our report indicates global deployment of pneumococcal vaccination for welders can be undertaken by a multi‐national corporation. Adoption of this practice by corporations would reduce the incidence and mortality of pneumonia in welders globally. We plan to promote pneumococcal vaccination of welders by seeking to influence practices at other companies in the primary aluminum industry, in the wider mining and minerals processing industries, and across industry more broadly. To this end we recently briefed the Health Committee of the International Aluminium Institute. With reference to the estimate from Palmer and Cosgrove that pneumococcal vaccination of 4900 welders could prevent one fatal case, a campaign to promote pneumococcal vaccination could be described as a fatality prevention campaign. Framing this type of initiative in such a way might capture the attention of senior management and cultivate their support. Irrespective of the exact number of employees needed to vaccinate in order to prevent a death, it is certain that the number of pneumonia cases prevented will increase with increased vaccination of this vulnerable population.
AUTHORS’ CONTRIBUTIONS
Dr Donoghue initially conceived of the project. Both authors contributed to the design and deployment of the project. Dr Wesdock obtained corporate sponsorship and conceived of using the Microsoft Power BI data collection tool. Both authors contributed to the data analysis, data interpretation and writing of the manuscript. Both authors gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
We acknowledge the dedication of Alcoa location medical center staff who recalled employees and undertook the vaccinations. We also thank Alcoa's occupational hygienists who assessed the numbers of employees in welding related SEGs. We acknowledge staff of Alcoa's EHS Global Services Unit who undertook translations of the communication material and we also thank John Rind, Alcoa's EHS Systems Manager, for his assistance implementing the Microsoft Power BI vaccination tracking tool.
FUNDING
Alcoa Corporation.
ETHICS APPROVAL AND INFORMED CONSENT
There was no ethics review and approval for the following reasons. Vaccination of welders was undertaken in accordance with the recommendation of the Department of Health in England. Informed consent was sought by clinical staff at the location medical centers in accordance with normal medical practice, with an information, contraindication questionnaire and consent form and the vaccine manufacturer's patient information sheet. The information, contraindication questionnaire and consent forms were retained by the onsite medical centers and were not collected for this paper. The data collected for the paper were simply the numbers of eligible employees at each location and the numbers of employees vaccinated at each location per month. There were no employee identifying data collected.
DISCLOSURE (AUTHORS)
Dr Donoghue is the Global Medical Director of Alcoa Alumina and Dr Wesdock is the Global Health Director of Alcoa Corporation. Both are full‐time employees of Alcoa Corporation and hold shares in the company.
DISCLOSURE BY AJIM EDITOR OF RECORD
Steven B. Markowitz declares that he has no conflict of interest in the review and publication decision regarding this article.
DISCLAIMER
None.
Donoghue AM, Wesdock JC. Pneumococcal vaccination for welders: Global deployment within a multi‐national corporation. Am J Ind Med. 2019;62:69–73. 10.1002/ajim.22934
Institution at which the work was performed: Alcoa Corporation.
REFERENCES
- 1. Coggon D, Inskip H, Winter P, Pannett B. Lobar pneumonia: an occupational disease in welders. Lancet. 1994; 344:41–43. [DOI] [PubMed] [Google Scholar]
- 2. Palmer KT, Cosgrove. Vaccinating welders against pneumonia. Occup Med. 2012; 62:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaumont JJ, Weiss NS. Mortality of welders, shipfitters, and other metal trades workers in boilermakers Local No. 104, AFL‐CIO. Am J Epidemiol. 1980; 112:775–786. [PubMed] [Google Scholar]
- 4. Newhouse ML, Oakes D, Woolley AJ. Mortality of welders and other craftsmen at a shipyard in NE England. Br J Ind Med 1985; 42:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palmer K, Coggon D. Does occupational exposure to iron promote infection?. Occup Environ Med. 1997; 54:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palmer KT, Poole J, Ayers JG, Mann J, Burge PS, Coggon D. Exposure to metal fume and infectious pneumonia. Am J Epidemiol. 2003; 157:227–233. [DOI] [PubMed] [Google Scholar]
- 7. Torén K, Qvarfordt I, Bergdahl IA, Järvholm B. Increased mortality from infectious pneumonia after occupational exposure to inorganic dust, metal fumes and chemicals. Thorax. 2011; 66:992–996. [DOI] [PubMed] [Google Scholar]
- 8. Wong A, Marrie TJ, Garg S, Kellner JD, Tyrrell GJ. Welders are at increased risk for invasive pneumococcal disease. Int J Infect Dis 2010; 14:e796–e799. [DOI] [PubMed] [Google Scholar]
- 9. Suri R, Periselneris J, Lanone S, et al. Exposure to welding fumes and lower airway infection with Streptococcus Pneumoniae. J Allergy Clin Immunol. 2016; 137:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grigg J, Miyashita L, Suri R. Pneumococcal infection of respiratory cells exposed to welding fumes; Role of oxidative stress and HIF‐1 alpha. PLoS ONE. 2017; 12:e0173569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ewing J, Patterson L, Irvine N, et al. Serious pneumococcal disease outbreak in men exposed to metal fume‐ detection, response and future prevention through pneumococcal vaccination. Vaccine. 2017; 35:3945–3950. [DOI] [PubMed] [Google Scholar]
- 12.Public Health England. Immunisation Against Infectious Disease: Pneumococcal 2017. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/596441/green_book_chapter__25.pdf Accessed June 7, 2018.
- 13. Nowalk MP, Lin CJ, Toback SL, et al. Improving influenza vaccination rates in the workplace: a randomized trial. Am J Prev Med. 2010; 38:237–246. [DOI] [PubMed] [Google Scholar]
- 14. Graves MA, Harris JR, Hannon PA, Hammerback K, Ahmed F, Zhou C. Workplace‐based influenza vaccination promotion practices among large employers in the United States. J Occup Environ Med. 2014; 56:397–402. [DOI] [PubMed] [Google Scholar]


