Abstract
Ex situ dual hypothermic oxygenated machine perfusion (DHOPE) and normothermic machine perfusion (NMP) of donor livers may have a complementary effect when applied sequentially. While DHOPE resuscitates the mitochondria and increases hepatic adenosine triphosphate (ATP) content, NMP enables hepatobiliary viability assessment prior to transplantation. In contrast to DHOPE, NMP requires a perfusion solution with an oxygen carrier, for which red blood cells (RBC) have been used in most series. RBC, however, have limitations and cannot be used cold. We, therefore, established a protocol of sequential DHOPE, controlled oxygenated rewarming (COR), and NMP using a new hemoglobin‐based oxygen carrier (HBOC)‐based perfusion fluid (DHOPE‐COR‐NMP trial, NTR5972). Seven livers from donation after circulatory death (DCD) donors, which were initially declined for transplantation nationwide, underwent DHOPE‐COR‐NMP. Livers were considered transplantable if perfusate pH and lactate normalized, bile production was ≥10 mL and biliary pH > 7.45 within 150 minutes of NMP. Based on these criteria five livers were transplanted. The primary endpoint, 3‐month graft survival, was a 100%. In conclusion, sequential DHOPE‐COR‐NMP using an HBOC‐based perfusion fluid offers a novel method of liver machine perfusion for combined resuscitation and viability testing of suboptimal livers prior to transplantation.
Keywords: clinical research/practice, donors and donation: extended criteria, ischemia reperfusion injury (IRI), liver allograft function/dysfunction, liver transplantation/hepatology, organ perfusion and preservation, organ procurement, organ procurement and allocation
Short abstract
This clinical cohort study indicates that a combination of hypo‐ and normothermic machine perfusion, using a preservation fluid containing an hemoglobin‐based oxygen carrier, is feasible and provides a tool to resuscitate and select initially declined high‐risk donor livers that can be transplanted successfully.
Abbreviations
- ALT
alanine aminotransferase
- ATP
adenosine triphosphate
- COR
controlled oxygenated rewarming
- DHOPE
dual hypothermic oxygenated machine perfusion
- DRI
donor risk index
- ET‐DRI
Eurotransplant donor risk index
- HBOC
hemoglobin‐based oxygen carrier
- HOPE
hypothermic oxygenated machine perfusion
- NMP
normothermic machine perfusion
- RBC
red blood cells
- SCS
static cold storage
- UW
University of Wisconsin
1. INTRODUCTION
Ex situ machine perfusion is increasingly investigated as a tool to increase the number of donor livers for transplantation and to reduce posttransplant complications. Machine perfusion, was recently introduced in clinical practice using two different temperature protocols: hypothermic (4‐12°C) or normothermic (37°C) machine perfusion.1, 2, 3, 4, 5 (Dual) hypothermic oxygenated perfusion ([D]HOPE) can be applied to resuscitate the mitochondria and increase hepatic adenosine triphosphate (ATP) content, resulting in less cell injury, including less choliangiocyte injury. Normothermic machine perfusion (NMP) allows for ex situ functional testing of (extended criteria) donor livers prior to transplantation and suboptimal donor livers have been successfully transplanted after NMP.2, 6, 7, 8 (D)HOPE and NMP may therefore have a complementary effect when applied sequentially.9, 10 In preclinical studies using human donor livers, it was previously shown that a short period of DHOPE prior to NMP results in increased ATP concentrations, less hepatobiliary injury and improved function during the NMP phase, compared to direct end‐ischemic NMP.9, 10
Different perfusion solutions have been used for (D)HOPE and NMP. A perfusion fluid based on human red blood cells (RBC) is frequently used for NMP.11, 12, 13 The use of RBC, however, has several drawbacks. Firstly, RBC are a relatively scarce human blood product.14 Secondly, RBC may induce an immune reaction or cause an infection.14, 15 Lastly, RBC cannot be used during (D)HOPE due to increased stiffness of the erythrocyte lipid membranes and hemolysis at low temperatures. These drawbacks press the need for an alternative oxygen carrier, especially when hypothermic and normothermic machine perfusion are combined. Hemoglobin‐based oxygen carriers (HBOC) are a suitable alternative for the use of RBC in ex situ liver machine perfusion. The bovine derived HBOC‐201 (Hemopure) has previously been used successfully in experimental and preclinical studies of liver machine perfusion.14, 16, 17
Based on the presumed complementary effect of (D)HOPE and NMP we have combined these two techniques in a clinical machine perfusion protocol using an HBOC‐201‐based solution. The use of this perfusion solution eliminates the need to change the perfusion fluid during different temperature phases. Donor livers that were initially declined for transplantation nationwide were subjected to a combined protocol of DHOPE, controlled oxygenated rewarming (COR), and subsequent viability testing during NMP (DHOPE‐COR‐NMP Trial). This report describes the first transplantations of initially nation‐wide declined livers that underwent ex situ machine perfusion with the HBOC‐201‐based perfusion fluid.
2. MATERIALS AND METHODS
2.1. Study protocol
Between August 2017 and April 2018, 20 livers were offered for inclusion in the DHOPE‐COR‐NMP study. All livers were declined for regular transplantation by the three liver transplant centers in the Netherlands. Thirteen livers were secondarily declined because of logistical reasons, long agonal phase (in case of donation after circulatory death), or macroscopic fibrosis/cirrhosis (Figure 1 and Figure S1). Seven livers were accepted to undergo DHOPE‐COR‐NMP. All seven livers were initially declined for transplantation because of a combination of risk factors, as described in Table 1. The median donor risk index was 2.82 (IQR 2.52‐2.97), reflecting the suboptimal quality of these livers.
Figure 1.
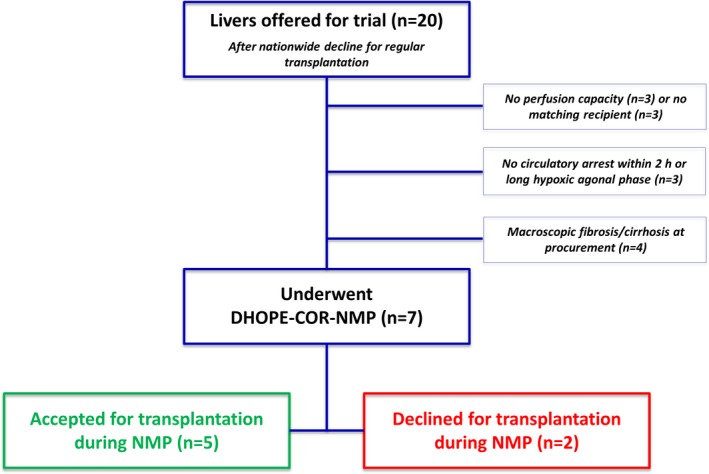
Flow chart of livers offered in the context of the DHOPE‐COR‐NMP Trial. After initial decline by all Dutch liver transplant centers a total number of 20 livers were offered for inclusion in this trial. Thirteen livers did not undergo machine perfusion due to logistical reasons, long agonal phase, or macroscopic findings. Seven livers underwent machine perfusion for resuscitation and viability assessment. DHOPE, dual hypothermic oxygenated machine perfusion; COR, controlled oxygenated rewarming; NMP, normothermic machine perfusion [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Donor characteristics of livers that were accepted to undergo machine perfusion
| Liver 1 | Liver 2 | Liver 3 | Liver 4 | Liver 5 | Liver 6 | Liver 7 | |
|---|---|---|---|---|---|---|---|
| Age (y) | 42 | 63 | 47 | 52 | 46 | 62 | 63 |
| DBD/DCD donor | DCD | DCD | DCD | DCD | DCD | DCD | DCD |
| BMI / degree of steatosis | BMI 21 | BMI 28 | BMI 33, Histologically >60% steatosis | BMI 28 | BMI 27 | BMI 23 | BMI 25 |
| Notably increased laboratory values in the donor | Peak AST 1676 U/L, peak ALT 1375 U/L, peak γGT 166 U/L | – | – | Peak γGT 340 U/L | Peak AST 161 U/L, peak ALT 270 U/L, peak γGT 254 U/L | Peak AST 201 U/L, peak ALT 175 U/L | – |
| Intoxications | Binge drinking | – | – | Frequent alcohol consumption | Alcohol, heroin, speed cocaine, xtc | – | – |
| dWIT (min)a | 29 | 23 | 30 | 33 | 27 | 35 | 25 |
| CIT (min)b | 289 | 306 | 525 | 294 | 256 | 278 | 221 |
| Donor hepatectomy time (min)c | 59 | 82 | 96 | 28 | 11 | 44 | 36 |
| DRI30 | 2.53 | 2.82 | 2.46 | 2.92 | 2.50 | 3.75 | 3.03 |
| ET‐DRI31 | 2.65 | 2.92 | 2.47 | 3.31 | 2.85 | 2.88 | 2.87 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body‐mass index; CIT, cold ischemia time; DBD, donation after brain death; DCD, donation after circulatory death; DRI, donor risk index; dWIT, donor warm ischemia time; ET‐DRI, Eurotransplant donor risk index; γGT, γ‐glutamyltransferase.
dWIT is defined as the time from withdrawal of mechanical support until the start of in situ cold perfusion.
CIT is defined as the time from in situ cold perfusion until the start of machine perfusion.
Donor hepatectomy time is defined as the time from in situ cold perfusion until the time of hepatectomy.
The study protocol was approved by the medical ethical review committee of our center (METc2016.281) and published in the national registry of clinical trials (www.trialregister.nl; NTR5972). The primary outcome parameter was 3‐month graft survival. All recipients gave written informed consent.
2.2. Procurement of donor livers
All donor livers were procured in a standard manner by a dedicated procurement team. After withdrawal of mechanical support, circulatory death was awaited, followed by a mandatory 5 minutes “no touch” period before procurement surgery was started. Cold in situ flush was performed with UW cold storage solution with the addition of 50 000 IU of heparin. After procurement, the livers were transported to our center using static cold storage. Upon arrival, the livers were prepared for machine perfusion, as described previously.11
2.3. Machine perfusion settings
A combined machine perfusion protocol of 1 hour of DHOPE (resuscitation phase), 1 hour of COR, and subsequent NMP (viability testing phase) was established (Figure 2). For machine perfusion at different temperatures the Liver Assist (Organ Assist, Groningen, the Netherlands) perfusion device was used. DHOPE was performed at 10°C. During COR the temperature was gradually increased about 1°C per 2 minutes, to 37°C at the start of NMP. Portal vein and hepatic artery pressures were set at 5 and 11 mm Hg during DHOPE, and gradually increased during COR to 11 and 70 mm Hg at the start of NMP, respectively.
Figure 2.
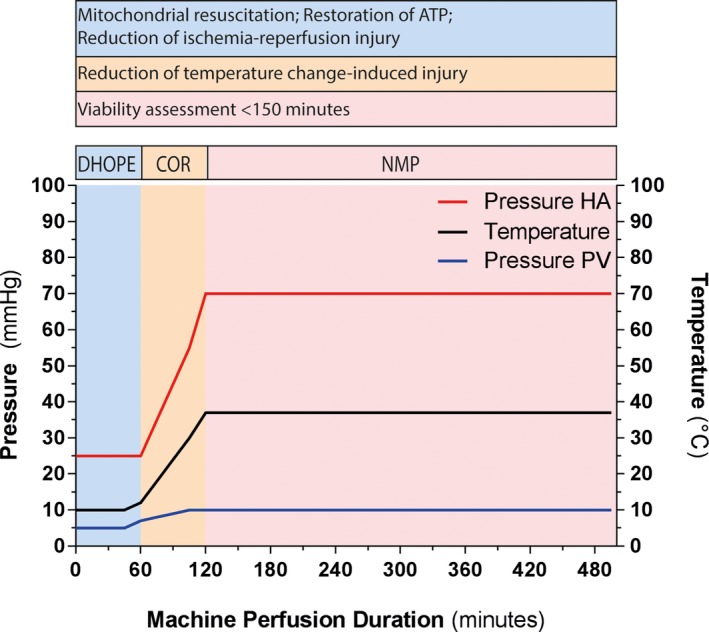
Overview of the machine perfusion protocol. A, The machine perfusion protocol included 1 h of DHOPE, 1 h of COR, and subsequent NMP for at least 150 minutes. Each phase of machine perfusion served a different purpose as described in the upper part of the figure. Machine perfusion settings were adjusted according to the perfusion temperature. The temperature was kept at 10°C during DHOPE and was gradually increased to 37°C during the COR phase, after which the liver was functionally tested during NMP. PV and mean HA pressure were set at 5 and 25 mm Hg, respectively, during DHOPE and were gradually increased during COR to 10 and 70 mm Hg, respectively, at the start of NMP. DHOPE, dual hypothermic oxygenated machine perfusion; COR, controlled oxygenated rewarming; HA, hepatic artery; NMP, normothermic machine perfusion; PV, portal vein [Color figure can be viewed at wileyonlinelibrary.com]
During DHOPE, the perfusion fluid was oxygenated with 1 L/min 100% O2, resulting in a perfusate pO2 > 80 kPa, as described previously.2 During NMP an air/oxygen mixture was used aimed to reach an arterial perfusate pO2 of 10.0‐13.3 kPa and a venous oxygen saturation of 55% to 75%. To obtain these targets, FiO2 was varied between 21% and 40%.
Arterial perfusate samples were collected every half hour and analyzed using the ABL 90 Flex analyzer (Radiometer, Brønhøj, Denmark). In addition, venous perfusate samples were collected and analyzed every hour, to determine oxygen consumption. Oxygen consumption was calculated based on the difference between arterial and venous oxygen content. The following equation was used, ([{ApO2−VpO2} × K /760] × total flow) + ([{AsO2−VsO2} × Hb × c × 0.0001 ] × total flow) / Liver weight × 100.18 Where pO2 was in mm Hg, sO2 in %, Hb in g/dL, total flow (sum of arterial and portal flow) in mL/min and liver weight in g. K was a constant (0.0225) and c the oxygen binding capacity of HBOC (1.26).
Bile was collected from COR‐NMP onwards and its quantity measured. Additionally, every half hour bile was collected under mineral oil to determine biliary pH and HCO3 ‐, as described previously.8, 19 Mineral oil prevented exposure of bile to ambient air, thus preventing the exchange of CO2 molecules, which influences biliary pH via HCO3 ‐.
2.4. Perfusion fluid
To facilitate perfusion at different temperatures, an acellular perfusion solution based on a bovine‐derived HBOC (HBOC‐201, HBO2 Therapeutics, Souderton, PA) was used. In addition to HBOC‐201, the perfusion fluid contained gelofusine, albumin, metronidazole, cefazolin, nutrients, glutathione, insulin, heparin, and NaHCO3. Details of the perfusion solution composition are provided in Table S1. As of liver #6, taurocholate was added to the perfusion fluid (50 mg at baseline), followed by a continuous infusion of 7.7 mg/h during the NMP phase.20 Taurocholate was produced according to GMP by our hospital pharmacy.
2.5. Viability testing—assessment of hepatobiliary function
Liver viability and function were assessed during the NMP phase using predefined viability criteria, including sufficient bile production with a biliary pH of >7.45, and normalization of perfusate pH and lactate (Table 2).5, 7, 8, 21, 22 The liver was considered acceptable for transplantation, if all criteria were met within the first 150 min of NMP. If the liver did not meet the predefined viability criteria, machine perfusion was terminated. If the liver did meet the predefined viability criteria, machine perfusion was continued and the recipient was brought to the operating room. When the hepatectomy of the native liver was almost complete, machine perfusion was terminated and the donor liver flushed out with 2 L of cold UW cold storage solution to remove the HBOC‐based machine perfusion fluid. As routinely performed in our center, the first 400 mL of venous blood from the liver was drained and discarded to avoid spill of UW Cold Storage Solution into the recipient circulation.
Table 2.
Viability criteria for donor liver assessment during NMP phase
| Viability criteria |
|---|
|
All predefined viability criteria had to be met in order to consider a liver transplantable.
NMP, normothermic machine perfusion.
3. RESULTS
3.1. Machine perfusion characteristics
Five of the seven livers (liver #1, and #4‐#7) that underwent DHOPE‐COR‐NMP were identified as transplantable based on functional assessment during NMP (Table 3). Median cold ischemia time of all livers was 289 minutes (IQR 256‐306 minutes). Machine perfusion times per liver are provided in Table 4. Median total duration of machine perfusion was shorter for the non‐transplanted livers due to termination of machine perfusion after these livers did not meet all predefined viability criteria within 150 min of NMP.
Table 3.
Viability criteria overview and transplantation decision per liver
| Liver #1 | Liver #2 | Liver #3 | Liver #4 | Liver #5 | Liver #6 | Liver #7 | |
|---|---|---|---|---|---|---|---|
| Perfusate pH | + | + | − | + | + | + | + |
| Perfusate latate | + | + | − | + | + | + | + |
| Bile production | + | + | + | + | + | + | + |
| Biliary pH | + | − | − | + | + | + | + |
| Transplantation (yes/no) | Yes | No | No | Yes | Yes | Yes | Yes |
+, the liver met this viability criterion; −, the liver did not meet this viability criterion.
Table 4.
Machine perfusion times per liver
| Liver #1 | Liver #2 | Liver #3 | Liver #4 | Liver #5 | Liver #6 | Liver #7 | |
|---|---|---|---|---|---|---|---|
| Total duration of NMP (min) | 347 | 163 | 180 | 397 | 301 | 373 | 391 |
| Duration of NMP from viability assessment onwards (min) | 197 | – | – | 247 | 157 | 223 | 241 |
| Total machine perfusion time (DHOPE‐COR‐NMP) (min) | 467 | 283 | 300 | 517 | 421 | 493 | 511 |
DHOPE, dual hypothermic oxygenated machine perfusion; COR, controlled oxygenated rewarming; NMP, normothermic machine perfusion.
Both portal vein and hepatic artery flow remained low during DHOPE and increased during COR. At 150 minutes of NMP, median portal vein flow was 1680 mL/min (IQR 1460‐1740 mL/min) (Figure 3A). Overall, resistance in the portal vein decreased towards NMP, but remained relatively high in liver #3 (Figure 3B). At 150 minutes of NMP, median hepatic artery flow was 547 mL/min (IQR 240‐737 mL/min). Furthermore, hepatic artery flow was variable between the livers (Figure 3C). Resistance in the hepatic artery, generally remained <0.2 mm Hg*min/L/g, except for resistance in liver #7 (Figure 3D). During COR, total flow increased as well, to a median of 2512 min (IQR 2133‐2570 min) at 150 minutes of NMP (Figure 3E).
Figure 3.
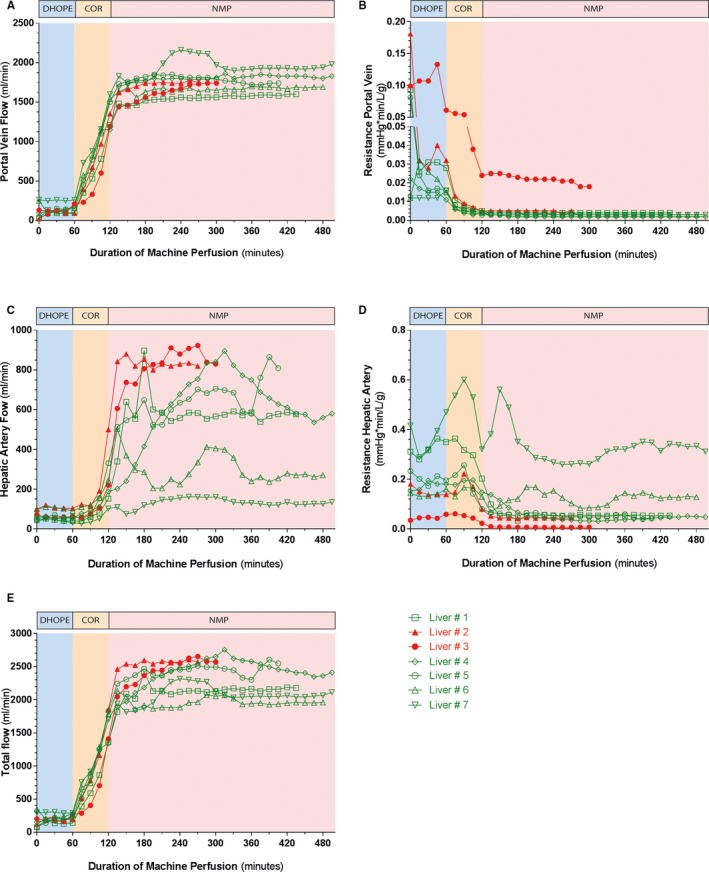
Flows and resistance during machine perfusion. A, PV flows were low during DHOPE. After 150 min of NMP, median portal vein flow was 1680 mL/min (IQR 1460‐1740 mL/min). B, Resistance in the portal vein was low, except for liver #3. C, Hepatic artery (HA) flows were low during DHOPE and COR. During NMP hepatic artery flows varied between 100 and 900 mL/min. At 150 min of NMP, median hepatic artery flow was 547 mL/min (IQR 240‐737 mL/min). D, Resistance in the hepatic artery was <0.2 mm Hg*min/L/g, except for liver #7. E, Total flow increased to a median of 2512 min (IQR 2133‐2570 min) at 150 minutes of NMP. The red lines represent the non‐transplanted livers and the green lines represent the transplanted livers. DHOPE, dual hypothermic oxygenated machine perfusion; COR, controlled oxygenated rewarming; HA, hepatic artery; IQR, interquartile range; NMP, normothermic machine perfusion; PV, portal vein; Tx, transplantation [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Hepatobiliary function and damage during machine perfusion
Perfusate pH normalized within 150 minutes of NMP of liver #1, #2, and #4 to #7, but not in liver #3, despite the addition of 25 mL 8.4% NaHCO3 (Figure 4A). Perfusate lactate normalized within 150 minutes of NMP in all livers, except for liver #3 (Figure 4B). Furthermore, all livers produced sufficient amounts of bile. Bile production of liver #6 appeared lower, however, this was caused by a cannulation problem of the bile duct (Figure 4C). Alanine aminotransferase (ALT) concentrations in perfusate of the transplanted livers were <2000 U/L. In the two non‐transplanted livers ALT concentrations were >2000 U/L, with a peak ALT concentration of 8460 U/L in liver #3 (Figure 4D). Livers #1 and #4 to #7 produced bile with a pH > 7.45, whereas livers #2 and #3 did not (Figure 4E). Bile duct biopsies of these two livers revealed signs of substantial histological injury (Figure S2).
Figure 4.
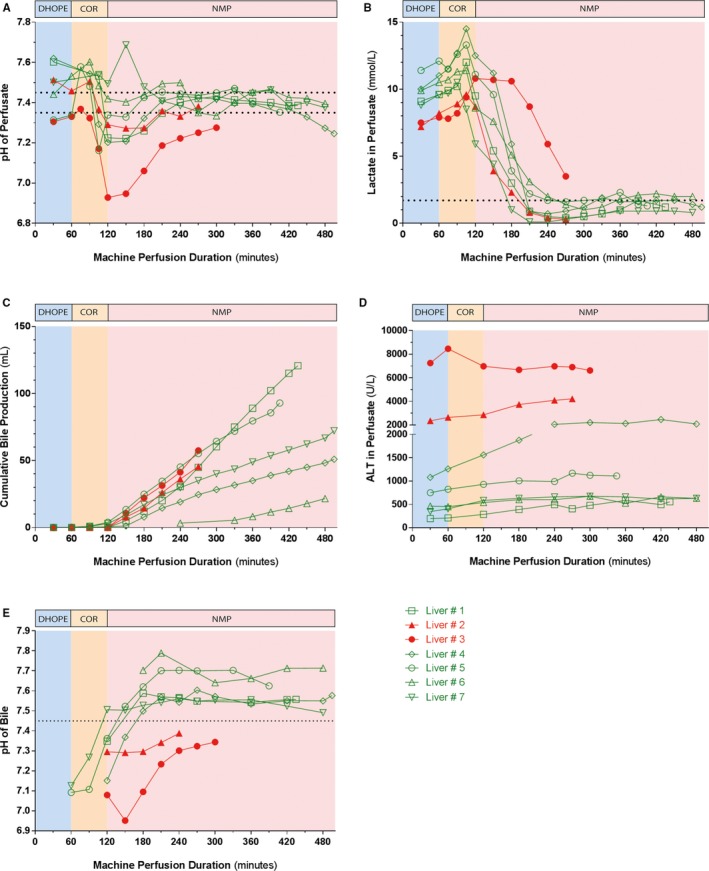
Machine perfusion fluid biochemistry. A‐B, Biochemical parameters used for viability assessment of the liver. In all but one liver, perfusate pH and lactate values normalized within 150 minutes after start of the NMP. C, All livers produced sufficient amounts of bile. Liver #6 seemingly produced less bile due to a cannulation problem of the bile duct. D, ALT perfusate levels were <2000 U/L in the transplanted livers and >2000 U/L in the nontransplanted livers. E, Biliary pH, a marker of biliary epithelial viability, increased to >7.45 in all livers that were transplanted, whereas biliary pH remained <7.45 in the livers that were not transplanted livers. The red lines represent the nontransplanted livers and the green lines represent the transplanted livers. ALT, alanine aminotransferase; NMP, normothermic machine perfusion [Color figure can be viewed at wileyonlinelibrary.com]
Median oxygen consumption was 0.14 mL O2/min/100 g liver weight during DHOPE and increased during COR to a median peak value of 2.77 mL O2/min/100 g liver weight during NMP.
3.3. Pre‐ and postoperative outcomes
Median follow up after transplantation was 197 days (IQR 152‐307 days). Laboratory values of the recipients are shown in Figure 6. Serum ALT levels rapidly decreased during the first week after transplantation and values were (nearly) normal at 30 and 90 days after transplantation. The recipients of liver #1 and #7 had remarkably low peak serum ALT concentrations of 201 and 337 U/L, respectively (Figure 5A). Serum total bilirubin levels rapidly decreased during the first postoperative days in all recipients. However, a temporary peak in serum bilirubin was noted at the end of the first week in recipients of liver #4 and #6 (Figure 5B). None of the transplanted livers classified for early allograft dysfunction.23
Figure 5.
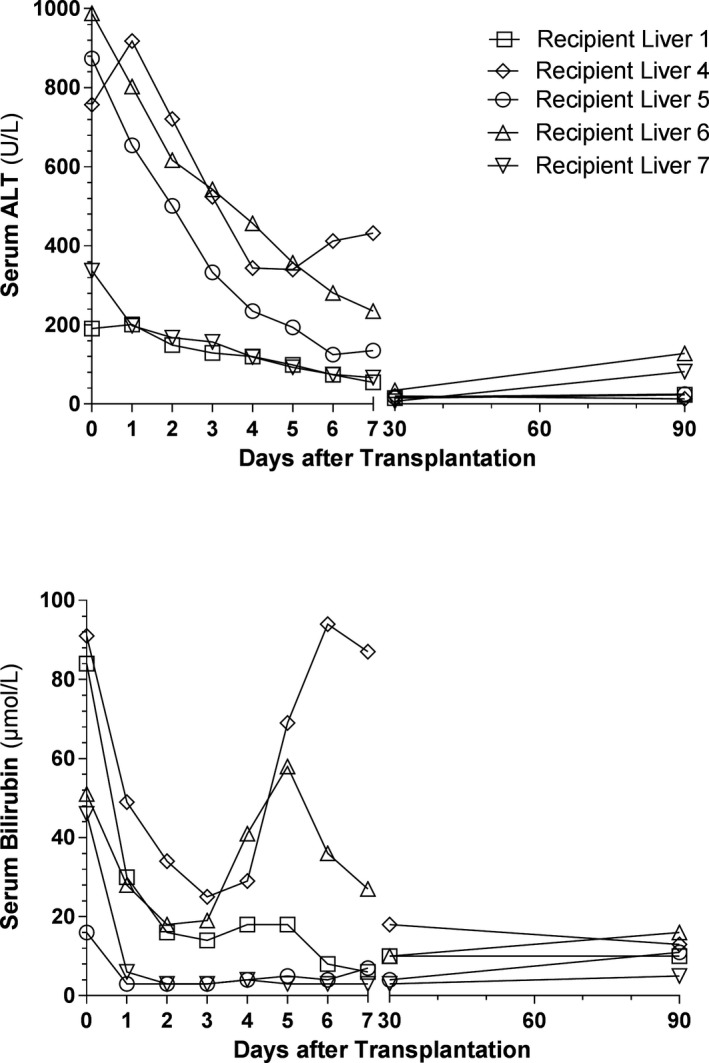
Posttransplantation serum ALT and total bilirubin. Laboratory values were recorded at postoperative day 0 until 7, and at 1 and 3 months. Postoperative day 0 was defined as the time from reperfusion in the recipient until midnight of the same day. A, Postoperative serum ALT concentrations rapidly decreased during the first week. The recipients of liver #1 and #7 had low peak serum ALT concentrations of 201 and 331 U/L, respectively. B, Postoperative total bilirubin concentration likewise decreased during the first week, except for a transient increase in the recipients of livers #4 and #6 at the end of the first week. Bilirubin levels of these livers, however, normalized during the weeks thereafter. ALT, alanine aminotransferase
Thus far we have observed a 100% patient and graft survival. None of the recipients has developed clinically evident non‐anastomotic strictures of the biliary tree during the median follow‐up of 197 days (6.5 months).
4. DISCUSSION
The clinical series described in this report provides two novel findings that are relevant for the further development of machine perfusion technology in organ transplantation. First, we have successfully used a combination of hypothermic and normothermic machine perfusion to resuscitate and select initially declined suboptimal livers for transplantation. The 100% graft survival at 3 months, which was the primary end point, indicated the safety and feasibility of the procedure. Secondly, we have shown that an HBOC‐based machine perfusion solution can be safely used in clinical liver transplantation.
Several groups have described the successful use of NMP to select suboptimal and initially declined donor livers.7, 12 Although the optimal parameters for viability assessment are still under debate, most groups have been using a combination of bile production, perfusate lactate levels, and pH as markers of hepatocellular function. We have previously suggested to use biliary pH and bicarbonate as markers of biliary epithelial (cholangiocellular) viability.22 Biliary epithelium actively modifies bile composition by the secretion of bicarbonate, resulting in an alkalotic biliary environment. This alkalotic environment protects biliary epithelial cells against the detergent effects of hydrophobic bile salts, a phenomenon known as the “bicarbonate umbrella.”24 In a clinical series of liver NMP, Watson et al have recently confirmed the potential usefulness of biliary pH as marker of biliary viability.12 We have, therefore, added biliary pH as a bile duct viability criterion to our protocol. Of the four predefined selection criteria, the criterion for biliary pH was the most frequent reason for secondary discard in these clinical series.
Although, favorable outcomes after NMP regarding graft and patient survival have been reported, it has not yet been demonstrated that NMP protects the cholangiocyte compartment.7, 12, 25 (D)HOPE in DCD liver grafts has, on the other hand, been described to reduce histological signs of biliary ischemia‐reperfusion injury and the incidence of posttransplant cholangiopathy.2, 3, 26 Furthermore, DHOPE has been shown to reduce ischemia reperfusion injury via resuscitation of the mitochondria and the increase in hepatic ATP content, thereby also protecting the hepatocytes.2, 3, 6 The latter might explain why postoperative peak ALT was lower in our recipients than in the studies that compared SCS to end‐ischemic NMP alone.7, 12 However, definitive conclusions on this topic cannot be drawn from the current study due to the lack of a control group.
In this study we combined the presumed benefits of DHOPE and NMP to resuscitate and select high‐risk donor livers that can be safely transplanted, despite initial nationwide decline. Besides a 100% graft survival at a median follow up of 197 days, none of the recipients has developed clinical signs of posttransplant cholangiopathy so far. The development of posttransplant cholangiopathy was, however, not the primary outcome of this study and was based on clinical symptoms and laboratory findings, rather than on imaging studies. Therefore, subclinical cases of cholangiopathy may have been missed and final conclusions on the efficacy of combined DHOPE, COR, and NMP in the prevention of posttransplant cholangiopathy require longer follow‐up in a larger series.
We applied sequential DHOPE and NMP linked by a controlled rewarming phase, as sudden temperature shifts may contribute to cellular injury.27, 28 A previous clinical study has indicated that a short period of COR prior to implantation of donor livers results in less hepatocellular injury, compared to direct implantation of a cold stored donor liver, as evidenced by lower postoperative peak transaminases and a higher graft survival rate at 6 months after transplantation.28 Based on the relative small number of livers included in the current study and the absence of a control group, we cannot draw conclusions on the value of the COR phase. Previous preclinical studies have shown that (D)HOPE and NMP can also be combined without a COR phase.9, 10
For the application of this combined machine perfusion protocol we have developed a perfusion fluid that can be used at various temperatures and eliminates the use of a third party human blood product. While during hypothermic machine perfusion, oxygen can be dissolved in the perfusion solution, during NMP an oxygen carrier is necessary. Perfusion solutions based on RBC, which are mostly used for NMP, cannot be used at low temperatures due to increasing lipid membrane stiffness and the risk of hemolysis. In contrast to RBC, HBOC can be used at low temperatures. In addition, HBOC‐201, used in this study, has a lower oxygen affinity than human Hb in erythrocytes and thus gives off the oxygen more easily. In the cold, the affinity of HBOC‐201 for oxygen increases, similar to that of human Hb in erythrocytes, but is still less.
HBOC‐201 has previously been used in experimental and pre‐clinical studies on ex‐situ liver machine perfusion, but not in clinical practice.14, 16, 17 In a study with discarded human livers, NMP with an HBOC‐201‐based perfusion fluid resulted in similar outcome compared to NMP with RBC, indicating HBOC‐201 as a suitable alternative for RBC.14 Our group reported higher ATP concentration, and cumulative bile production in discarded human livers undergoing NMP with an HBOC‐201‐based solution, compared to perfusion with an RBC‐based perfusion fluid.16 Altogether these preclinical studies and the currently presented first, clinical application indicate that HBOC‐201 can be used as a substitute for RBC in fluids for machine perfusion of donor organs.
A limitation of this series is a lack of a control group. However, livers of suboptimal quality with a perceived high risk of primary nonfunction or early allograft dysfunction were included in the current study, making it unethical, in our opinion, to transplant these livers without resuscitation and functional assessment. Furthermore, this study cannot discriminate between the beneficial effects of DHOPE, COR, and NMP separately. Finally, extrahepatic bile duct biopsies of the two livers that were secondary declined for transplantation, based on their failure to produce bile with a pH > 7.45 during NMP, were taken. Yet, biopsies of higher level bile ducts were not taken. Although we have previously shown that the degree of histological injury of the extrahepatic bile duct of a donor liver after cold storage is representative for the degree of injury of the proximal biliary tree, including larger intrahepatic ducts,29 we do not formally know whether this is also true after NMP.
A potential limitation of HBOC is its susceptibility to a conversion into methemoglobin, especially in the venous phase with low oxygen saturation. In contrast to erythrocytes, HBOC do not contain NADH‐dependent enzyme methemoglobin reductase, which is responsible for converting methemoglobin back to hemoglobin. We have noted a gradual increase in methemoglobin during NMP, but not during DHOPE when the perfusion fluid was oxygenated with an FiO2 of 100% (Figure S3). In a separate experiment, we have noted that the percentage of methemoglobin can be corrected or slowed down by the addition of extra HBOC‐201, glutathione, or vitamin C to the perfusion fluid (Figure S3). However, we do not prefer the use of vitamin C due to its effects on the pH and osmolality of the perfusion fluid.
In conclusion, this first clinical experience demonstrates the feasibility of combined hypo‐ and normothermic machine perfusion after traditional static cold storage of suboptimal liver grafts. The combination of oxygenated hypothermic and normothermic perfusion protects livers against ischemia‐reperfusion injury and enables hepatobiliary viability assessment prior to transplantation. The use of a novel HBOC‐201‐based perfusion fluid eliminated the need to change perfusion fluid during various temperature phases and appeared to be a safe alternative for RBC as oxygen carrier in ex situ donor organ machine perfusion. This new protocol of ex situ machine perfusion provides a tool to safely expand the pool of organs for liver transplantation.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
ACKNOWLEDGMENTS
We are grateful to Zafiris Zafirelis (HBO2 Therapeutics) for providing HBOC‐201 free of charge and for his advice on the use of this product. Moreover, we want to thank our organ perfusionists (Rinse Ubbink, Maureen Werner, Gert‐Jan Pelgrim and Leonie Venema) for their help during the machine perfusion procedures. Funding was obtained by the Dutch Ministry of Health, Welfare and Sport and the Jan Kornelis de Cock Foundation, Groningen, the Netherlands. HBOC‐201 was provided free of charge by HBO2 Therapeutics.
de Vries Y, Matton APM, Nijsten MWN, et al. Pretransplant sequential hypo‐ and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin‐based oxygen carrier perfusion solution. Am J Transplant. 2019;19:1202–1211. 10.1111/ajt.15228
Funding Information
Funding was obtained by the Dutch Ministry of Health, Welfare and Sport and the Jan Kornelis de Cock Foundation, Groningen, the Netherlands
REFERENCES
- 1. Karangwa SA, Dutkowski P, Fontes P, et al. Machine perfusion of donor livers for transplantation: a proposal for standardized nomenclature and reporting guidelines. Am J Transplant. 2016;16(10):2932‐2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Rijn R, Karimian N, Matton APM, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104(7):907‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dutkowski P, Polak WG, Muiesan P, et al. First comparison of hypothermic oxygenated PErfusion versus static cold storage of human donation after cardiac death liver transplants: an international‐matched case analysis. Ann Surg. 2015;262(5):1. [DOI] [PubMed] [Google Scholar]
- 4. Perera T, Mergental H, Stephenson B, et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transpl. 2016;22(1):120‐124. [DOI] [PubMed] [Google Scholar]
- 5. Watson CJ, Kosmoliaptsis V, Randle LV, et al. Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant. 2016;16(1):353‐357. [DOI] [PubMed] [Google Scholar]
- 6. Schlegel A, Rougemont O, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end‐ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58(2):278‐286. [DOI] [PubMed] [Google Scholar]
- 7. Mergental H, Perera M, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex‐situ evaluation. Am J Transplant. 2016;16(11):3235‐3245. [DOI] [PubMed] [Google Scholar]
- 8. Sutton ME, op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS ONE. 2014;9(11):e110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boteon YL, Laing RW, Schlegel A, et al. Combined hypothermic and normothermic machine perfusion improves functional recovery of extended criteria donor livers. Liver Transpl. 2018;24(12):1699‐1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Westerkamp AC, Karimian N, Matton AP, et al. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation. 2016;100(4):825‐835. [DOI] [PubMed] [Google Scholar]
- 11. op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13(5):1327‐1335. [DOI] [PubMed] [Google Scholar]
- 12. Watson CJE, Kosmoliaptsis V, Pley C, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18(8):2005‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Selzner M, Goldaracena N, Echeverri J, et al. Normothermic ex vivo liver perfusion using steen solution as perfusate for human liver transplantation: first north american results. Liver Transpl. 2016;22(11):1501‐1508. [DOI] [PubMed] [Google Scholar]
- 14. Laing RW, Bhogal RH, Wallace L, et al. The use of an acellular oxygen carrier in a human liver model of normothermic machine perfusion. Transplantation. 2017;101(11):2746‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buttari B, Profumo E, Rigano R. Crosstalk between red blood cells and the immune system and its impact on atherosclerosis. Biomed Res Int. 2015;2015:616834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matton APM, Burlage LC, van Rijn R, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl. 2018;24(4):528‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fontes P, Lopez R, van der Plaats A, et al. Liver preservation with machine perfusion and a newly developed cell‐free oxygen carrier solution under subnormothermic conditions. Am J Transplant. 2015;15(2):381‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koetting M, Luer B, Efferz P, et al. Optimal time for hypothermic reconditioning of liver grafts by venous systemic oxygen persufflation in a large animal model. Transplantation. 2011;91(1):42‐47. [DOI] [PubMed] [Google Scholar]
- 19. Hardison WG, Wood CA. Importance of bicarbonate in bile salt independent fraction of bile flow. Am J Physiol. 1978;235(2):E158‐E164. [DOI] [PubMed] [Google Scholar]
- 20. Imber CJ, St Peter SD, de Lopez Cenarruzabeitia I, et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 2002;73(5):701‐709. [DOI] [PubMed] [Google Scholar]
- 21. Watson CJ, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers prior to transplantation: hyperoxia and vasoplegia ‐ important lessons from the first 12 cases. Transplantation. 2017;101(5):1084‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weeder PD, van Rijn R, Porte RJ. Machine perfusion in liver transplantation as a tool to prevent non‐anastomotic biliary strictures: rationale, current evidence and future directions. J Hepatol. 2015;63(1):265‐275. [DOI] [PubMed] [Google Scholar]
- 23. Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16(8):943‐949. [DOI] [PubMed] [Google Scholar]
- 24. Hohenester S, Wenniger LM, Paulusma CC, et al. A biliary HCO3‐ umbrella constitutes a protective mechanism against bile acid‐induced injury in human cholangiocytes. Hepatology. 2012;55(1):173‐183. [DOI] [PubMed] [Google Scholar]
- 25. Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557(7703):50‐56. [DOI] [PubMed] [Google Scholar]
- 26. van Rijn R, van Leeuwen OB, Matton APM, et al. Hypothermic oxygenated machine perfusion reduces bile duct reperfusion injury after transplantation of donation after circulatory death livers. Liver Transpl. 2018;24(5):655‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minor T, Efferz P, Fox M, Wohlschlaeger J, Luer B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13(6):1450‐1460. [DOI] [PubMed] [Google Scholar]
- 28. Hoyer DP, Mathe Z, Gallinat A, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation: first clinical application of a new concept. Transplantation. 2016;100(1):147‐152. [DOI] [PubMed] [Google Scholar]
- 29. Karimian N, Weeder PD, Bomfati F, et al. Preservation injury of the distal extrahepatic bile duct of donor livers is representative for injury of the intrahepatic bile ducts. J Hepatol. 2015;63(1):284‐287. [DOI] [PubMed] [Google Scholar]
- 30. Schaubel DE, Sima CS, Goodrich NP, et al. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419‐425. [DOI] [PubMed] [Google Scholar]
- 31. Braat AE, Blok JJ, Putter H, et al. The Eurotransplant donor risk index in liver transplantation: ET‐DRI. Am J Transplant. 2012;12(10):2789‐2796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


