Abstract
Aims
To describe global patterns of insulin treatment and to assess the impact of patient, provider, health system and economic influences on treatment decisions for patients with insulin‐treated type 2 diabetes (T2D).
Methods
This prospective cohort study of insulin‐treated patients with T2D was conducted across 18 countries categorized as high, upper‐middle or lower‐middle income regions. Information collected from patients included knowledge of diabetes, experiences and interactions with their healthcare provider. Physician information included specialty, practice size, availability of diabetes support services, volume of diabetes patients treated and time spent per patient. Physicians determined an individualized haemoglobin A1c (HbA1c) target for each patient by the start of the study. Changes in T2D therapies and HbA1c were recorded for 2 years.
Results
Complete treatment data were available for 2528 patients. Median age was 61 years and median duration of diabetes was 11.4 years. Changes to treatment regimen occurred in 90.0% of patients, but changes were less common in countries with a higher economic status (P < 0.001). Most treatment changes involved insulin, with changes in dose the most common. Overall predictors of change in insulin therapy included younger age, use of any insulin regimen other than basal only, higher mean baseline HbA1c and longer duration of T2D. HbA1c levels remained constant regardless of regional economic status. At baseline, 20.6% of patients were at their HbA1c target; at 2 years this was 26.8%.
Conclusions
Among insulin‐treated patients with T2D, treatment changes were common; however, only approximately one‐fourth of individuals achieved their HbA1c target.
Keywords: glycaemic targets, haemoglobin A1c, insulin, patient outcomes, type 2 diabetes mellitus
1. INTRODUCTION
The prevalence of diabetes mellitus is increasing and the global disease burden is estimated to exceed 693 million cases by the year 2045.1, 2 Notably, the prevalence is increasing more rapidly in lower‐ and upper‐middle income countries.2 Diabetes and its complications account for substantial morbidity and mortality and involve a considerable cost burden to healthcare systems3; direct costs alone are estimated to exceed US$ 827 billion.4
Critical to long‐term management of diabetes is maintenance of adequate glycaemic control, which limits the risk of complications over time.5 However, the natural history of type 2 diabetes (T2D) is generally one of deteriorating glycaemic control, with many individuals requiring a stepwise intensification of pharmacotherapy to achieve and maintain blood glucose control.6 Escalation of therapy is necessary for many patients, in particular those treated with insulin, to achieve desired glycaemic targets.7, 8
To date, few studies have systematically quantified the interplay among patient, physician and healthcare system and the use of insulin therapy over time. Some studies have reported on barriers to insulin initiation and progression of therapies to maintain glycaemic control, indicating that implementation of guidelines for T2D treatment remains inconsistent in a real‐world setting.9, 10 The Translating Research Into Action for Diabetes (TRIAD) study identified several factors from patient and physician perspectives that were associated with lack of insulin initiation among T2D patients with poorly controlled disease; however, this study included a single country and focused on insulin initiation rather than intensification.9, 11 No study has yet compared these factors across diverse regional, geographical, economic and healthcare settings.
The objective of the Multinational Observational Study Assessing Insulin Use: Understanding the Challenges Associated with Progression of Therapy (MOSA1c; NCT01400971) study was to describe the determinants of diabetes‐related treatment changes in a prospective real‐world cohort across a global population of insulin‐treated patients with T2D, and to describe the extent to which patient‐specific glycaemic goals were achieved.12, 13 The study was designed to elucidate the specific challenges and the patient, physician and healthcare system factors associated with progression from initial to more advanced insulin treatment regimens. As such, this article describes observed treatment changes and associated predictors, as well as haemoglobin A1c (HbA1c) levels and hypoglycaemia events reported in this global, insulin‐treated population of patients with T2D.
2. METHODS
2.1. Study design
The MOSA1c study was a non‐interventional, prospective cohort study in 18 countries; complete methods were reported previously.12, 13 Patient enrolment started in 2011, with a 2‐year follow‐up for each patient; the study concluded in 2015. Data captured at the baseline visit included retrospective data up to 6 months prior to baseline. Prospective data were collected at 6, 12, 18 and 24 months, with a window of ± 3 months around each time point.
The study protocol, informed consent forms and other applicable documents were approved by local ethical review boards as required by local regulations. The study was conducted according to the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with good clinical practices and applicable laws and regulations of the country or countries where the study was conducted, as appropriate. All patients gave written informed consent before any study‐specific procedure was conducted.
2.2. Study setting
Participating countries were classified on a post‐hoc basis into three economic groups, according to 2015 World Bank categories, to account for the impact of a country's economic status, which has been shown to be associated with substantial differences in patient characteristics, access to treatment, use of medications, healthcare systems and health outcomes.14, 15, 16 The study included 11 high‐income countries (Canada, Germany, Israel, Italy, Japan, Saudi Arabia, South Korea, Spain, United Arab Emirates, United Kingdom, United States), six upper‐middle‐income countries (Argentina, Brazil, China, Mexico, Russian Federation, Turkey) and one lower‐middle‐income country (India). General medicine, primary care and specialty sites were recruited based on geographic region and prevalence of T2D; additional information regarding site selection has been published.12
2.3. Study population
Eligible patients were at least 18 years of age, had been diagnosed with T2D, were being seen in a primary care or diabetes specialty clinic as part of normal care, and had maintained an initial insulin regimen for at least 3 months, with or without other non‐insulin glucose‐lowering therapies. Patients undergoing intensive basal‐bolus therapy, defined as basal plus three mealtime insulin injections, were excluded from the study. Initial insulin formulation could be human, analogue or animal, depending on regional variations, and could consist of basal, intermediate‐acting, mixed, short‐acting or rapid‐acting insulin.
2.4. Data sources and collection
Details on the approach for enrolment of a representative sample that reflects the underlying population with T2D, using insulin type and physician practice type in each participating country, have been described.12 Baseline measures included demographic characteristics of age, gender and ethnicity, in addition to type of health insurance. Clinical characteristics included duration of diabetes and of insulin use, height, weight, body mass index (BMI), history of microvascular and macrovascular complications, self‐reported history of hypoglycaemic episodes in the past month and baseline HbA1c levels, if measured as part of usual clinical care. Use of baseline glucose‐lowering therapy was classified as basal insulin only, prandial insulin only, mixed only and any combination, as well as use of any non‐insulin glucose‐lowering therapy.
Physicians were asked to set a target HbA1c level for each patient over the next 2 years at study enrolment, which is consistent with current recommendations. Physicians completed surveys concerning their specialty, size of practice, availability of diabetes support services, volume of diabetes patients treated and time spent per patient.
Patients responded to surveys regarding their knowledge of diabetes, their experiences and their interactions with their provider. Patients' knowledge of diabetes was assessed using the Diabetes Knowledge Test, a nine‐item test that is scored as the number of correct responses (0‐9).17 The Diabetes Distress Scale (DDS) contains 17 items asking about patients' level of concern with various aspects of diabetes treatment and care; mean item scores range from 1 to 6, with a higher score indicating greater distress.18 The 25‐item Interpersonal Process of Care survey assessed patients' perception of their providers' behaviour in six domains: compassionate and respectful behaviour, discrimination, elicited concerns, explained results, patient‐centred decision‐making and hurried communication. Each domain was scored from 1 to 5, with higher mean item scores indicating more of the behaviour.19
Insulin type, dose and frequency of injection, as well as prescriptions for any non‐insulin glucose‐lowering therapies, were recorded at each visit. HbA1c levels were recorded when collected as part of standard patient care. Patients' hypoglycaemic episodes were self‐reported at each visit.
2.5. Statistical analysis
The primary analysis was a complete case analysis, restricted to patients with complete treatment data for all five visits. Patients for whom data concerning non‐treatment‐related descriptive variables were missing were included in the complete case analysis. As a secondary analysis, multiple imputation was conducted for selected variables with missing values; ten imputed datasets were created, analyses were performed separately in each dataset, and were then combined.
Baseline patient characteristics were compared across country economic groups, with P values for trend estimated from Spearman's correlation for continuous variables and the Cochran‐Armitage test for categorical variables. Changes in treatment during the follow‐up period were classified into insulin‐related change (increase or decrease of any magnitude in insulin total daily dose, frequency, or addition or discontinuation of insulin type) and non‐insulin‐related change (addition or discontinuation of glucagon‐like peptide‐1 receptor agonist [GLP‐1 RA] or oral glucose‐lowering therapy). A patient may have had both increases and decreases, either at the same visit (eg, increasing insulin dose and discontinuing an oral medication) or at different visits (eg, injection frequency increasing at one visit and decreasing at another).
Multivariable logistic regression models examined baseline patient‐, physician‐ and healthcare system‐related factors associated with any change in insulin therapy and any change in glucose‐lowering therapies. Variables were selected from univariate logistic models for each candidate factor, retaining those significant at P < 0.05. As a sensitivity analysis, personal income relative to country‐specific, per capita gross domestic product (GDP) was included in the regression model.
Among patients with complete HbA1c measurements at baseline and at least one follow‐up visit, change in HbA1c levels from baseline to each follow‐up visit was summarized by change in insulin therapy and country economic group. Additionally, the percentage of patients reporting any hypoglycaemic episode since the last visit was reported by changes in insulin therapy and country economic group.
3. RESULTS
3.1. Patient demographic and clinical characteristics
Baseline data were collected from a total of 4299 eligible patients from 192 sites; complete treatment data were available for 2528 patients (58.8%). Counts of patients enrolled per region are provided in Table S1. The main reason for exclusion from analysis was missing treatment data from one or more visits.
Patients for whom complete treatment data were available represented a typical population for insulin‐treated T2D patients, with a median age of approximately 61 years and BMI of 28.3 kg/m2 (Table 1). Approximately half of the patients were female and 20% were Hispanic/Latino. All economic regions were well represented, with approximately 25% of patients from the one lower‐middle‐income country, almost 30% from upper‐middle‐income countries and the remainder from high‐income countries. Median duration of T2D across the entire cohort was 11.4 years, and median baseline HbA1c was 7.8%. Almost 50% of patients had evidence of microvascular complications and approximately 25% reported a history of macrovascular disease. Close to two‐thirds (64%) of the cohort were undergoing treatment with concomitant oral glucose‐lowering therapies. At study entry, 55% of patients were utilizing basal insulin only and 37% were utilizing mixed insulins only.
Table 1.
Baseline patient, physician and health care system characteristics
| Baseline characteristic | All patients (N = 2528) | Lower‐middle‐income economies (N = 631) | Upper‐middle‐income economies (N = 788) | High‐income economies (N = 1109) |
|---|---|---|---|---|
| Demographics | ||||
| Age, median years (IQR) | 61.0 (54.0‐68.0) | 58.0 (52.0‐64.0) | 61.0 (54.0‐68.0) | 64.0 (56.0‐71.0)** |
| Female gender, n (%) | 1270 (50.2) | 283 (44.8) | 469 (59.5) | 518 (46.7) |
| Hispanic/Latino ethnicity, n (%) | 497 (19.7) | 5 (0.8) | 282 (35.8) | 210 (18.9)** |
| Clinical characteristics | ||||
| Duration of diabetes, median years (IQR) | 11.4 (6.2‐17.0) | 10.6 (5.2‐16.5) | 11.0 (6.1‐16.2) | 12.1 (7.2‐17.8)** |
| Time since insulin initiation, median years (IQR) | 1.0 (0.0‐4.0) | 1.0 (0.0‐2.0) | 1.0 (0.0‐3.0) | 2.0 (0.0‐5.0)** |
| BMI, median kg/m2 (IQR) | 28.3 (25.1‐32.4) | 26.7 (23.9‐29.7) | 28.0 (24.9‐32.2) | 30.1 (26.3‐34.3)** |
| Microvascular complication, n (%) | 1105 (46.1) | 224 (36.7) | 301 (41.4) | 580 (54.7)** |
| Macrovascular complication, n (%) | 609 (25.2) | 83 (13.4) | 212 (28.9) | 314 (29.7)** |
| ≥1 hypoglycaemic episode in last month, n (%) | 507 (23.0) | 85 (14.2) | 135 (20.3) | 287 (30.4)** |
| Baseline HbA1c level, median % (IQR) | 7.8 (6.9‐8.9) | 8.2 (7.5‐9.6) | 7.5 (6.5‐8.5) | 7.8 (7.0‐8.8)* |
| Insulin type taken at baseline | ||||
| Basal only, n (%) | 1385 (54.8) | 193 (30.6) | 428 (54.3) | 764 (68.9)** |
| Prandial only, n (%) | 76 (3.0) | 15 (2.4) | 29 (3.7) | 32 (2.9) |
| Mixed only, n (%) | 924 (36.6) | 405 (64.2) | 288 (36.5) | 231 (20.8)** |
| Combination, n (%) | 143 (5.7) | 18 (2.9) | 43 (5.5) | 82 (7.4)** |
| Oral antidiabetic medication taken at | ||||
| baseline, n (%) | 1623 (64.2) | 540 (85.6) | 316 (40.1) | 767 (69.2)** |
| HbA1c target | ||||
| Individualized HbA1c target, median % (IQR) | 7.0 (6.5‐7.0) | 7.0 (7.0‐7.0) | 6.5 (6.5‐7.0) | 7.0 (6.5‐7.0) |
| Physician characteristics | ||||
| Endocrinology physician specialty, n (%) | 1129 (68.9) | 450 (79.5) | 297 (75.2) | 382 (56.4)** |
| Years physician has treated patients with diabetes, median (IQR) | 14 (10‐21) | 13 (11‐20) | 12 (9‐15) | 20 (12‐25)** |
| Number of diabetes patients treated in last month, median (IQR) | 200 (84‐400) | 350 (200‐500) | 100 (30‐200) | 200 (80‐500)** |
| Minutes physician spends with patients, median (IQR) | 20 (15‐35) | 20 (15‐45) | 40 (20‐60) | 15 (10‐25)** |
| Healthcare system characteristic | ||||
| Public insurance type, n (%) | 1309 (55.3) | 111 (18.1) | 546 (73.7) | 652 (64.2)** |
| Presence of diabetes support service, n (%) | 1473 (89.9) | 555 (97.7) | 326 (82.5) | 592 (87.7)** |
| Patient‐reported patient and physician characteristics | ||||
| Diabetes Knowledge Test score (range 0‐9), median (IQR) | 5.0 (3.0‐6.0) | 4.0 (3.0‐5.0) | 6.0 (4.0‐7.0) | 5.0 (3.0‐6.0)** |
| Diabetes Distress Scale (range 1‐6), median (IQR) | 1.9 (1.3‐3.1) | 1.9 (1.3‐3.4) | 1.9 (1.4‐3.1) | 1.8 (1.4‐2.8)* |
| Interpersonal Process of Care (range 1‐5) | ||||
| Compassionate and respectful (higher = better), median (IQR) | 4.2 (3.6‐5.0) | 4.0 (3.4‐4.8) | 4.0 (3.6‐4.8) | 4.4 (3.8‐5.0)** |
| Discrimination (higher = worse), median (IQR) | 1.0 (1.0‐2.0) | 1.5 (1.0‐2.3) | 1.0 (1.0‐1.8) | 1.0 (1.0‐1.5)** |
| Elicited concerns (higher = better), median (IQR) | 4.0 (3.3‐5.0) | 4.3 (3.7‐5.0) | 4.0 (3.0‐4.7) | 4.3 (3.3‐5.0) |
| Explained results (higher = better), median (IQR) | 4.0 (3.3‐4.8) | 4.0 (3.3‐4.8) | 4.0 (3.5‐4.8) | 4.3 (3.3‐5.0)* |
| Patient‐centred decision making (higher = better), median (IQR) | 3.5 (2.5‐4.3) | 3.5 (2.3‐4.3) | 3.8 (2.5‐4.3) | 3.5 (2.3‐4.5)* |
| Hurried communication (higher = worse), median (IQR) | 1.2 (1.0‐2.0) | 1.2 (1.0‐1.8) | 1.4 (1.0‐2.0) | 1.2 (1.0‐1.8) |
Abbreviations: BMI, body mass index; HbA1c, glycated haemoglobin; IQR, interquartile range.
Median + interquartile range unless otherwise noted.
P < 0.05.
P < 0.001; P values for trend calculated by Spearman's correlation for continuous variables and the Cochran‐Armitage test for categorical variables.
Variability was observed in some baseline characteristics across different economic regions. Higher country economic status was associated with a modestly older median age (64 years), longer median duration of diabetes (12 years), higher median BMI (30.1 kg/m2), longer time since insulin initiation (2 years), higher rates of reported baseline microvascular (55%) and macrovascular (30%) complications, higher rate of hypoglycaemia (30%), and greater use of basal insulin only (69%). Upper‐middle economic regions had the lowest median baseline HbA1c (7.5%), use of concomitant oral glucose‐lowering medications (40%), and median HbA1c target (6.5%). Patients from the lower‐middle‐income economy had higher median baseline HbA1c (8.2%), greater use of mixed insulin only (64%), and concomitant oral glucose‐lowering therapies (86%). Multiple imputation of variables with missing data produced results similar to those of the complete case analysis (Table S2).
3.2. Physician‐ and patient‐reported characteristics
A majority of individuals in the entire cohort (69%) received diabetes care from endocrine specialists and had public health insurance (55%) (Table 1). Fewer patients from high‐income countries were treated by endocrinologists (56%) compared to their lower‐middle and upper‐middle‐income counterparts (79% and 75%, respectively; P < 0.001); however, physicians in the high‐income regions tended to be more experienced, having treated patients with T2D for a longer time. Most patients with public insurance resided in upper‐middle (74%) and high‐income (64%) countries, with only 18% of patients utilizing public insurance in the lower‐middle‐income country.
Physicians from the lower‐middle‐income region treated the highest monthly volume of patients with T2D (median, 350) vs those in upper‐middle (median, 100) and high‐income (median, 200) regions. Physicians from upper‐middle‐income regions spent more time with patients (median, 40 minutes) compared to those in lower‐middle‐income (median, 20 minutes) and high‐income (median, 15 minutes) regions. Diabetes support services (eg, presence of a dietician, diabetes health educator, etc.) were prevalent across all regions, regardless of regional income status, but were nearly universal in the lower‐middle region (98%).
Variability among economic regions was also observed for patient‐reported characteristics. The highest Diabetes Knowledge Test scores were observed in upper‐middle‐income countries (median score, 6.0), and the lowest scores in the lower‐middle‐income country (median score, 4.0). Patients overall in all regions reported low levels of diabetes distress (median DDS scores, 1.8‐1.9). Patients' perception of interactions with their physicians varied by domain. In high‐income countries, patients perceived physicians as more compassionate and respectful, and reported that their physicians explained test results. In contrast, patients from the lower‐middle‐income country reported a higher level of perceived discrimination from their physicians (median score, 1.5) compared to upper‐middle and high‐income regions (median score, 1.0 for each). Patients from upper‐middle‐income countries reported fewer elicited concerns and more hurried communication, but more patient‐centred decision‐making. When multiple imputation was conducted, results were similar to non‐imputed results (Table S3).
3.3. Predictors of treatment change over time
During the 2‐year follow‐up period, changes in treatment with either insulin‐ or non‐insulin‐based glucose‐lowering therapies were made for 90.0% of patients in the overall cohort, with an inverse association between treatment change and country economic status (P < 0.001) (Figure 1).
Figure 1.
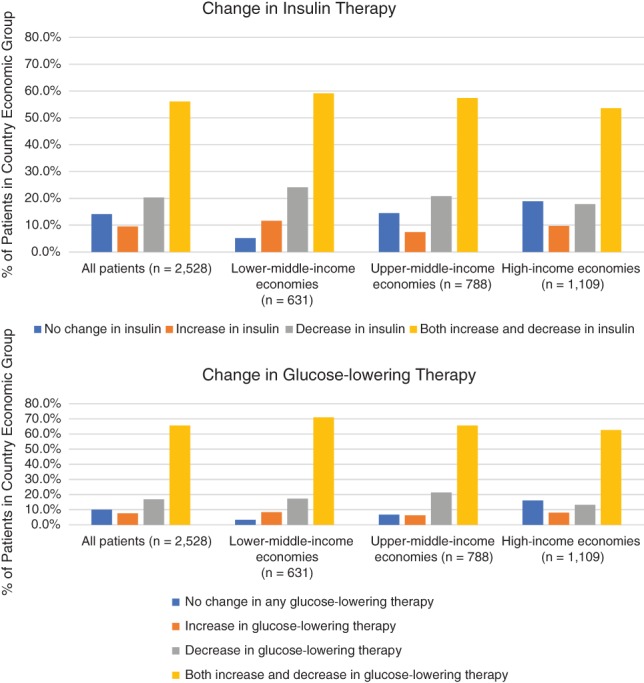
Distribution of patients with change in glucose‐lowering therapy by country economic group
Most treatment changes involved insulin, although more than 40% of patients experienced a change in non‐insulin glucose‐lowering therapies. Any change (increase, decrease or bidirectional) in insulin therapy was more common in lower‐middle‐income regions (94.8%). Patients from lower‐middle regions were also more likely to experience any change in glucose‐lowering therapies (96.7%) compared to other economic regions. Changes in insulin dose, either increase (67%) or decrease (50%), were the most common change across the entire cohort and were more frequent in lower‐middle economic regions (Table S4). Changes in dose were typically at least a 5% increase or decrease relative to the dose at the prior visit; only 1% of patients experienced dose changes less than 5% with no other change in treatment. The median total daily insulin dose at baseline was 30 units in lower‐middle and high‐income countries and 34 units in upper‐middle‐income countries; by the end of the follow‐up period, median doses were approximately four units higher than baseline in all regions (Table S5). Among patients experiencing a change in dose, the median change from baseline to the end of the follow‐up period was eight units for lower‐middle and high‐income countries and 10 units for upper‐middle‐income regions.
Predictors of change in insulin therapy among all patients included younger age, higher mean baseline HbA1c (>7.80%; 1.85 [1.38‐2.49]), longer duration of diabetes (1.46 [1.13‐1.88]), use of any insulin regimen other than basal only (prandial only, OR [95% CI], 5.71 [1.77‐18.37]; mixed only, OR [95% CI], 4.16 [3.02‐5.73]; combination, OR [95% CI], 10.50 [3.30‐33.41]), lower diabetes distress score (OR [95% CI], 0.74 [0.57‐0.95]), lower perceived discrimination from the physician (OR [95% CI], 0.50 [0.39‐0.65]), presence of diabetes support services (OR [95% CI], 1.92 [1.50‐2.46]) and non‐public insurance (OR [95% CI], 0.70 [0.54‐0.92]) (Figure 2). Some differences in predictors of change in treatment were observed according to country economic status. For example, duration of diabetes was a predictor of change in insulin treatment in high‐income countries, but it showed no impact in other economic regions. Baseline microvascular comorbidities predicted changes in insulin treatment in the lower‐middle economic region only. Baseline hypoglycaemia predicted changes in insulin treatment in upper‐middle economies but, alternatively, predicted absence of change in high‐income regions. Furthermore, a perceived compassionate and/or respectful physician predicted changes in insulin treatment for the lower‐middle economic region, but was associated with an absence of change in insulin treatment in high‐income economic regions. Predictors of changes in insulin therapy using multiple imputation (Figure S1) and predictors of change in any glucose‐lowering therapy (Figure S2) were generally similar to those in the primary analysis. The sensitivity analysis, adding personal income relative to country economic status, resulted in no change to the findings of the primary model.
Figure 2.
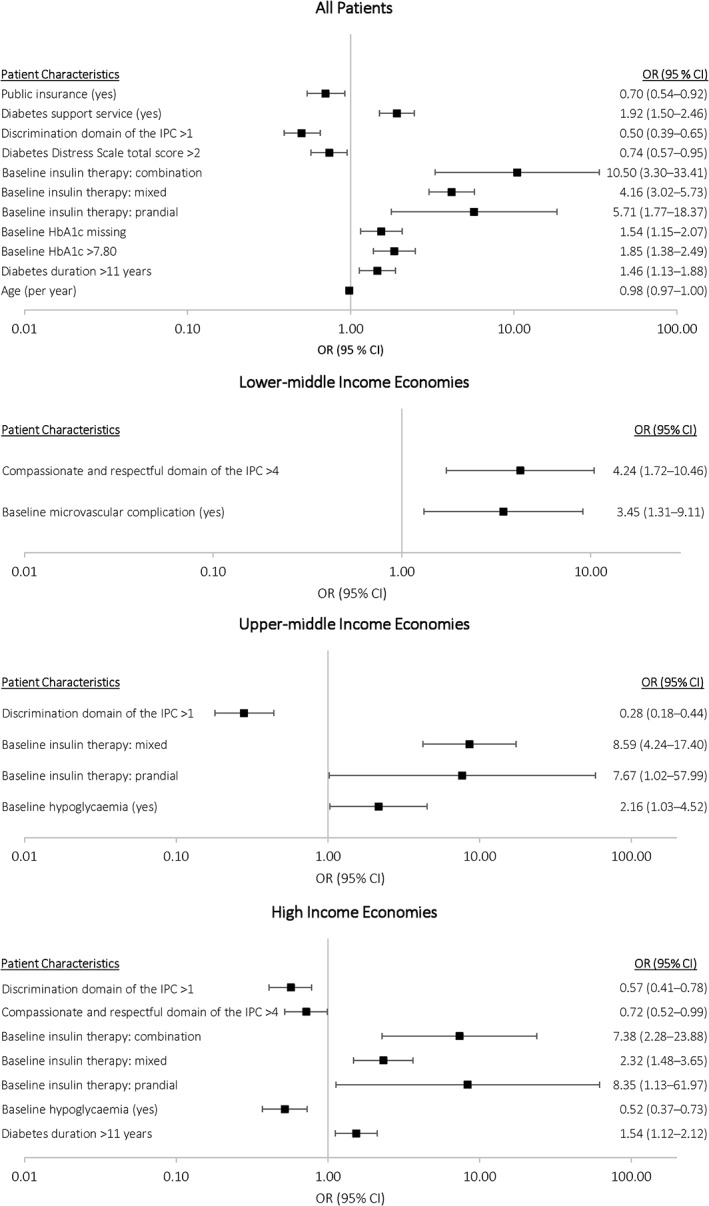
Logistic regression models examining predictors of insulin changes at any time during follow‐up, overall and by country economic group. CI, confidence interval; HbA1c, glycated haemoglobin; IPC, interpersonal process of care; OR, odds ratio
3.4. Change in HbA1c levels and hypoglycaemic events
Among patients with individualized HbA1c targets and HbA1c measurements at baseline (N = 1609) or at Visit 5 (N = 1465), 20.6% of patients were at target at baseline and 26.8% achieved target at the end of the 2‐year follow‐up period. Average HbA1c levels generally remained constant throughout the study, with a median change from baseline of −0.1% at each follow‐up visit and with minimal variation observed with changes in insulin treatment (Figure 3). Taking missing data into account, results were similar to those of the complete case analysis (Figure S3).
Figure 3.
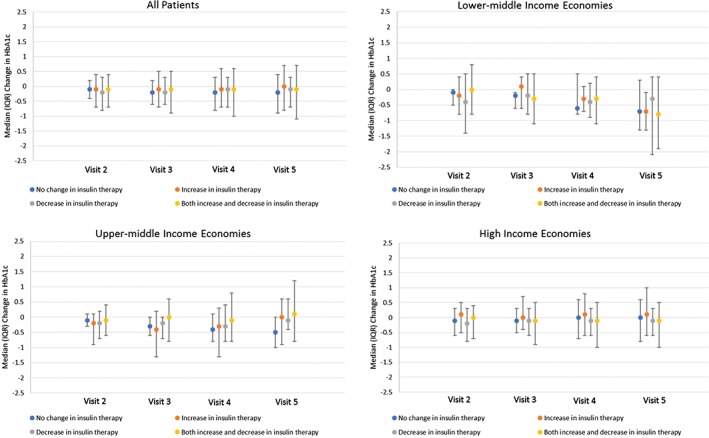
Change in HbA1c levels from baseline to each visit, by insulin‐related treatment change. HbA1c, glycated haemoglobin; IQR, interquartile range
Figure 4 illustrates the proportion of patients with a reported hypoglycaemic episode since the last visit, according to change in treatment during the follow‐up period. Across the entire cohort, 1199 out of 2528 patients (47.4%) experienced a hypoglycaemic event at any time during the study; approximately 20% to 25% of patients reported a hypoglycaemic episode between each visit. Patterns differed, depending on economic grouping. In the lower‐middle economic region, hypoglycaemia was more commonly reported among patients who had a decrease in insulin dosing at any time, whereas, in high‐income countries, hypoglycaemia was more common among patients with no change in insulin therapy.
Figure 4.
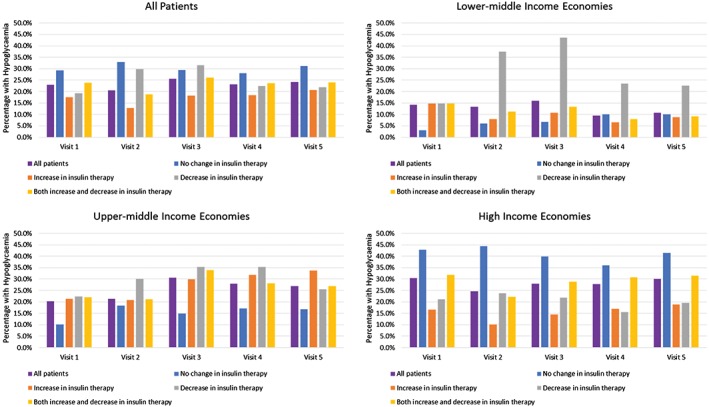
Percentage of patients with reported hypoglycaemic episodes since last visit, by insulin‐related treatment change
4. DISCUSSION
The MOSA1c study is the first long‐term, observational, prospective study to systematically assess diabetes‐related changes in treatment in a multinational adult population undergoing insulin therapy. The results demonstrate that changes in diabetes treatment are common among patients with insulin‐treated T2D across lower‐middle, upper‐middle and high‐income countries. The most commonly observed changes in insulin therapy over 2 years of follow‐up were bidirectional, that is, both decreases and increases in insulin treatment for the same patient, probably reflecting active management of patients. Addition and discontinuation of non‐insulin glucose‐lowering therapies were less commonly observed. Although these data suggest that insulin‐treated T2D patients who receive care in general medicine or specialty clinics (endocrinology/diabetology) are generally actively managed, approximately 10% of patients experienced no changes and 1% experienced only small changes in dose (<5%) of diabetes medication; insulin type and dose, frequency of injections and use of oral agents or GLP‐1 RA remained largely the same throughout the 2‐year period.
Treatment decisions regarding therapeutic changes in glucose‐lowering therapies should be based, ideally, on a composite of patient needs and patient‐related clinical, social, cultural and lifestyle factors.7 Other variables, such as physician training and behaviour, nature of the healthcare system and socioeconomic environment of the patient and physician may also influence these treatment decisions.20, 21
A unique aspect of this study was the investigation of the complex relationship among patient, physician, healthcare system, and individual changes in treatment. Indeed, the present study found a wide range of characteristics that predicted changes in insulin therapy, including age, baseline HbA1c level, duration of diabetes, baseline insulin type, diabetes distress level, patient‐reported perception of physician discrimination, diabetes support services and type of health insurance. Across country economic groups, differences in predictors of change in insulin treatment were observed, not only in levels of significance (eg, duration of diabetes significantly predicting change in insulin treatment in high‐income countries but not in other regions), but also in the direction of association for some variables (eg, baseline hypoglycaemia was a positive predictor in upper‐middle economies, but a negative predictor in high‐income regions). These differences further highlight how geographies associated with varying levels of income can differ significantly in the patient, provider and healthcare system characteristics that are associated with diabetes treatment decisions, and they suggest that no single approach to patient care is necessarily universally applicable.
Individualized targets for HbA1c levels during the study were similar across all regions. Observed changes in HbA1c levels were modest over time and similar across regions. Only approximately one‐fourth of patients for whom HbA1c data were available reached desired treatment targets at the end of the follow‐up period, despite changes in treatment regimens for most patients. Changes in therapy may fail to have a positive impact because of factors such as treatment adherence, clinical characteristics, treatment‐related costs and access to care.12, 22 Hypoglycaemia was common and occurred at least once in half the cohort. Unexplainably, the reported rates of hypoglycaemia were highest among patients who experienced no change in their glucose‐lowering therapies.
The major global diabetes treatment guidelines recommend setting personalized HbA1c target levels and modifying therapy if targets are not achieved within 3‐6 months.7, 23 Achieving and maintaining glycaemic control and assuring timely and appropriate adjustments in therapy for insulin‐treated T2D patients remain significant clinical challenges.7, 9, 11, 24, 25 Although the MOSA1c study was not designed to examine the impact of treatment changes on glycaemic control, the observed trend of relatively stable but not improved glycemic control, despite frequent treatment changes, may indicate that more targeted and more effective changes in therapy for T2D patients are necessary on a global scale. Thus, proper education of healthcare providers concerning effective approaches to patient management is important. Patients and physicians should be provided with specific point‐of‐care information concerning treatment options and titration. Further educational opportunities and simulation exercises for tailored treatment approaches should be offered, so that healthcare providers can be better informed when making treatment decisions for individual patients.
Limitations of this study include its observational nature. The study protocol did not specify treatment prescribing or monitoring measures for patients, and data on variables of interest were missing for many patients; however, analyses of imputed data provided results similar to those of the complete case analysis. Changes in treatment and achievement of HbA1c targets were examined in a snapshot view across a 2‐year period and do not reflect the continuity of treatment changes and HbA1c levels. Still, these data reflect real‐world patterns of prescribing behaviours and glycaemic control, and they include an array of patient‐reported factors relevant to physician management of insulin‐treated T2D patients. Caution is needed in interpreting data across economic regions because of the heterogeneous socio‐economic gradient for diabetes in developed vs developing countries.26, 27, 28, 29, 30, 31 However, recent studies have shown that differences in the prevalence of diabetes according to country income group persist after adjusting for socio‐economic status, and the socio‐economic gradient in developing countries is reversing to become similar to their developed counterparts.32, 33, 34, 35, 36, 37 Furthermore, the objective of this study was not to understand the impact of changes in treatment on HbA1c levels; future studies should address this question at a multinational level.
The data presented here are the first to longitudinally demonstrate the patterns of diabetes care and the factors associated with treatment decisions among insulin‐treated T2D patients across diverse geographic regions. Most patients in this study underwent a change in treatment during a 2‐year period. A wide range of patient, physician and healthcare system characteristics predicted changes in insulin therapy, with variation across country economic groups. Only one‐fourth of individuals achieved HbA1c targets over the 2‐year study period, even in an active management setting, suggesting that individualized, tailored changes in treatment to optimize glycaemic control may not occur as recommended. These data support the need for a deeper understanding of patient behaviour and patient‐physician interaction within a local diabetes care experience, and they strongly suggest the need for novel approaches to educating providers and patients and to informing healthcare systems to improve outcomes for individuals within the context of insulin‐treated T2D.
CONFLICTS OF INTEREST
K. K. R. reports receipt of personal fees from Lilly, Cerenis, AbbVie, Astra Zeneca, Boehringer Ingelheim, Novo Nordisk, Medco, Resverlogix, Esperion, Takeda, AKCEA, Cipla, Algorithm, and Kowa and reports receipt of grants and personal fees from Amgen, Sanofi, MSD, Pfizer and Regeneron. D. M. K. was an employee of Eli Lilly and Company at the time of study implementation and drafting of this paper; he is currently an employee of MannKind Corporation. A. E. C. has been a member of scientific advisory boards for Eli Lilly, Boehringer Ingelheim, Astra Zeneca, Novo Nordisk, Sanofi and Janssen. W. H. P. has served as a consultant for Eli Lilly, Novo Nordisk, Sanofi, Astra Zeneca, Abbott, Dexcom, Intarcia, Merck and Mannkind. Z. Z., L. F., B. H. C. and X. P. are employees of Lilly and own company stock options. B. L. N. is employed by Evidera, which received funding from Eli Lilly for the data analysis and statistical support in this study. M. J. D. reports receipt of personal fees from Novo Nordisk, Sanofi‐Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca, Janssen, Servier, Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc. and receipt of grants from Novo Nordisk, Sanofi‐Aventis, Lilly, Boehringer Ingelheim and Janssen.
Author contributions
All authors participated in the design and conduct of this study, data analysis and data interpretation. D. M. K. and B. H. C. were involved in data collection. All authors were responsible for the integrity of the data and accuracy of the data analysis, and have drafted, revised, critically reviewed and approved the final manuscript.
Supporting information
Table S1. Counts of patients from each Country.
Table S2. Baseline demographic and clinical characteristics (with missing data imputed).
Table S3. Baseline physician‐ and patient‐reported characteristics (with missing data imputed).
Table S4. Number of patients experiencing each type of treatment change during 2 years of follow‐up.
Table S5. Median insulin daily dose and dose change.
Figure S1. Logistic regression models examining predictors of insulin changes at any time during follow‐up, overall, and by country economic group (with missing data imputed).
Figure S2. Logistic regression models examining predictors of any glucose‐lowering therapy changes at any time during follow‐up, overall, and by Country Economic Group.
Figure S3. Change in HbA1c levels from baseline to each visit, by insulin‐related treatment change (with missing data imputed).
ACKNOWLEDGMENTS
Medical writing and editing support were provided by Melanie J Jardim, PhD (Evidera, Raleigh, North Carolina) and were funded by Eli Lilly and Company. Data analysis and statistical support were provided by Kathy Fraeman, SM (Evidera, Bethesda, Maryland). Manuscript formatting and editorial support were provided by Michael Grossi and Janet Dooley (Evidera, Waltham, Massachusetts).
Ray KK, Kendall DM, Zhao Z, et al. A multinational observational study assessing insulin use: Understanding the determinants associated with progression of therapy. Diabetes Obes Metab. 2019;21:1101–1110. 10.1111/dom.13622
Funding information Eli Lilly and Company provided funding for this project and article and had a role in study design, data analysis, data interpretation and critical review of the manuscript.
REFERENCES
- 1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271‐281. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Diabetes fact sheet. http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed December 6, 2017.
- 3. Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293‐301. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Global report on diabetes. 2016. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1. Accessed December 6, 2017.
- 5. Blonde L, Aschner P, Bailey C, et al. Gaps and barriers in the control of blood glucose in people with type 2 diabetes. Diab Vasc Dis Res. 2017;14:172‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Diabetes Association . 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S73‐S85. [DOI] [PubMed] [Google Scholar]
- 8. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427‐2443. [DOI] [PubMed] [Google Scholar]
- 9. Karter AJ, Subramanian U, Saha C, et al. Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care. 2010;33:733‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polinski JM, Smith BF, Curtis BH, et al. Barriers to insulin progression among patients with type 2 diabetes: a systematic review. Diabetes Educ. 2013;39:53‐65. [DOI] [PubMed] [Google Scholar]
- 11. Ratanawongsa N, Crosson JC, Schillinger D, Karter AJ, Saha CK, Marrero DG. Getting under the skin of clinical inertia in insulin initiation: the translating research into action for diabetes (TRIAD) insulin starts project. Diabetes Educ. 2012;38:94‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polinski JM, Curtis BH, Seeger JD, Choudhry NK, Zagar A, Shrank WH. Rationale and design of the multinational observational study assessing insulin use: the MOSA1c study. BMC Endocr Disord. 2012;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polinski JM, Kim SC, Jiang D, et al. Geographic patterns in patient demographics and insulin use in 18 countries, a global perspective from the multinational observational study assessing insulin use: understanding the challenges associated with progression of therapy (MOSA1c). BMC Endocr Disord. 2015;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low‐, middle‐, and high‐income countries. N Engl J Med. Aug 28 2014;371(9):818‐827. [DOI] [PubMed] [Google Scholar]
- 15. World Bank . World Bank country and lending groups. 2015. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed January 16, 2018.
- 16. Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high‐income, middle‐income, and low‐income countries (the PURE study): a prospective epidemiological survey. Lancet. 2011;378:1231‐1243. [DOI] [PubMed] [Google Scholar]
- 17. Fitzgerald JT, Funnell MM, Hess GE, et al. The reliability and validity of a brief diabetes knowledge test. Diabetes Care. 1998;21:706‐710. [DOI] [PubMed] [Google Scholar]
- 18. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626‐631. [DOI] [PubMed] [Google Scholar]
- 19. Stewart AL, Napoles‐Springer AM, Gregorich SE, Santoyo‐Olsson J. Interpersonal processes of care survey: patient‐reported measures for diverse groups. Health Serv Res. 2007;42:1235‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stuckey HL, Dellasega C, Graber NJ, Mauger DT, Lendel I, Gabbay RA. Diabetes nurse case management and motivational interviewing for change (DYNAMIC): study design and baseline characteristics in the chronic care model for type 2 diabetes. Contemp Clin Trials. 2009;30:366‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2‐4. [PubMed] [Google Scholar]
- 22. Saundankar V, Peng X, Fu H, et al. Predictors of change in adherence status from 1 year to the next among patients with type 2 diabetes mellitus on oral antidiabetes drugs. J Manag Care Spec Pharm. 2016;22:467‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. International Diabetes Foundation (IDF) . Recommendations for managing type 2 diabetes in primary care, 2017. https://www.idf.org/e‐library/guidelines/128‐idf‐clinical‐practice‐recommendations‐for‐managing‐type‐2‐diabetes‐in‐primary‐care.html. Accessed March 30, 2018.
- 24. Davies M, Storms F, Shutler S, Bianchi‐Biscay M, Gomis R, ATLANTUS Study Group . Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282‐1288. [DOI] [PubMed] [Google Scholar]
- 25. Holman RR, Farmer AJ, Davies MJ, et al. Three‐year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736‐1747. [DOI] [PubMed] [Google Scholar]
- 26. Whiting D, Unwin N, Roglic G. Diabetes: equity and social determinants. Chapter 5 In: Blas E, Kurup AS, eds. Equity, Social Determinants and Public Health Programmes. Geneva, Switzerland: World Health Organization; 2010:77‐94. [Google Scholar]
- 27. Pan XR, Yang WY, Li GW, Liu J. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and control cooperative group. Diabetes Care. 1997;20:1664‐1669. [DOI] [PubMed] [Google Scholar]
- 28. abu Sayeed M, Ali L, Hussain MZ, Rumi MA, Banu A, Azad Khan AK. Effect of socioeconomic risk factors on the difference in prevalence of diabetes between rural and urban populations in Bangladesh. Diabetes Care. 1997;20:551‐555. [DOI] [PubMed] [Google Scholar]
- 29. Diez‐Roux AV, Northridge ME, Morabia A, Bassett MT, Shea S. Prevalence and social correlates of cardiovascular disease risk factors in Harlem. Am J Public Health. 1999;89:302‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohan V, Shanthirani S, Deepa R, et al. Intra‐urban differences in the prevalence of the metabolic syndrome in southern India ‐‐ the Chennai urban population study (CUPS no. 4). Diabet Med. 2001;18:280‐287. [DOI] [PubMed] [Google Scholar]
- 31. Robbins JM, Vaccarino V, Zhang H, Kasl SV. Excess type 2 diabetes in African‐American women and men aged 40‐74 and socioeconomic status: evidence from the third National Health and Nutrition and Examination Survey. J Epidemiol Community Health. 2000;54:839‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ajay VS, Prabhakaran D, Jeemon P, et al. Prevalence and determinants of diabetes mellitus in the Indian industrial population. Diabet Med. 2008;25:1187‐1194. [DOI] [PubMed] [Google Scholar]
- 33. Anjana RM, Deepa M, Pradeepa R, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR‐INDIAB population‐based cross‐sectional study. Lancet Diabetes Endocrinol. 2017;5:585‐596. [DOI] [PubMed] [Google Scholar]
- 34. Misra A, Pandey RM, Devi JR, Sharma R, Vikram NK, Khanna N. High prevalence of diabetes, obesity and dyslipidaemia in urban slum population in northern India. Int J Obes Relat Metab Disord. 2001;25:1722‐1729. [DOI] [PubMed] [Google Scholar]
- 35. Dagenais GR, Gerstein HC, Zhang X, et al. Variations in diabetes prevalence in low‐, middle‐, and high‐income countries: results from the Prospective Urban and Rural Epidemiological Study. Diabetes Care. 2016;39:780‐787. [DOI] [PubMed] [Google Scholar]
- 36. Deepa M, Anjana RM, Manjula D, Narayan KM, Mohan V. Convergence of prevalence rates of diabetes and cardiometabolic risk factors in middle and low income groups in urban India: 10‐year follow‐up of the Chennai Urban Population Study. J Diabetes Sci Technol. 2011;5:918‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsu CC, Lee CH, Wahlqvist ML, et al. Poverty increases type 2 diabetes incidence and inequality of care despite universal health coverage. Diabetes Care. 2012;35:2286‐2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Counts of patients from each Country.
Table S2. Baseline demographic and clinical characteristics (with missing data imputed).
Table S3. Baseline physician‐ and patient‐reported characteristics (with missing data imputed).
Table S4. Number of patients experiencing each type of treatment change during 2 years of follow‐up.
Table S5. Median insulin daily dose and dose change.
Figure S1. Logistic regression models examining predictors of insulin changes at any time during follow‐up, overall, and by country economic group (with missing data imputed).
Figure S2. Logistic regression models examining predictors of any glucose‐lowering therapy changes at any time during follow‐up, overall, and by Country Economic Group.
Figure S3. Change in HbA1c levels from baseline to each visit, by insulin‐related treatment change (with missing data imputed).


