Abstract
Objective
Novel treatments are needed to control treatment‐resistant status epilepticus (SE). We report a summary of clinical cases where perampanel was used in established SE, refractory SE (RSE), or super‐refractory SE (SRSE).
Methods
Medical records were retrospectively reviewed for perampanel administration in SE at five European hospitals between 2011 and 2015.
Results
Of 1319 patients identified as experiencing SE, 52 (3.9%) received perampanel. Median latency from SE onset to perampanel initiation was 10 days. Patients with SE had previously failed benzodiazepines (when received) and a median of five other antiepileptic drugs (AEDs). Median initial perampanel dose was 6 mg/d, up‐titrated to a median maximum dose of 10 mg/d. Perampanel was the last drug added in 32/52 (61.5%) patients, with response attributed to perampanel in 19/52 (36.5%) patients. A greater proportion of perampanel non‐responders had SRSE (51.5%; 17/33) vs perampanel responders (31.6%; 6/19), and had failed a higher mean number of AEDs before initiating perampanel (5.9 vs 5.1, respectively). Most commonly reported adverse effects during perampanel treatment were dizziness (n = 1 [1.9%]) and somnolence (n = 1 [1.9%]). No serious adverse effects were documented, and none led to discontinuation of perampanel.
Conclusions
Perampanel was administered to patients with established SE, RSE, or SRSE at greater initial doses than those administered in clinical practice to patients with epilepsy. The SE cases reported here represent a refractory and heterogeneous population, and rate of seizure cessation attributed to perampanel treatment (36.5%) represents a notable response. These data should be confirmed in a larger patient population.
Keywords: AMPA, perampanel, refractory status epilepticus, summary of cases, super‐refractory status epilepticus
1. INTRODUCTION
Status epilepticus (SE) involves prolonged, self‐sustaining, or repeated seizures lasting ≥5 minutes.1 These arise either from the failure of seizure termination mechanisms or from the initiation of mechanisms that lead to abnormally prolonged seizures. SE is unpredictable, has serious long‐term consequences (including neuronal damage), and is potentially fatal.1, 2, 3 Although country‐specific guidelines for the treatment of SE are available, there are no universal treatment guidelines.4, 5 Differences also exist in terms of diagnostic criteria such as the application of electroencephalography [EEG] to monitor seizure activity, as well as doses and combinations of antiepileptic drugs (AEDs) delivered to patients with SE.4, 5, 6 This lack of consistent guidance, together with variation in the definitions of SE, complicates a physician's understanding and ability to effectively manage SE.6 In addition, there is a lack of clinical trial data available for the treatment of refractory SE (RSE) or super‐refractory SE (SRSE).6, 7
Intravenous benzodiazepines, currently the most effective treatment for early SE, act by increasing the frequency of γ‐aminobutyric acid A receptor (GABAAR) channel opening and can control SE prior to arrival at an emergency department in up to two‐thirds of patients.7 However, decreased inhibitory signaling through GABAAR, due to internalization of the receptor over the course of SE, results in failure of the mechanisms required for seizure termination and decreased efficacy of benzodiazepines in the later stages of SE.7, 8 Established SE that does not respond to benzodiazepines may be treated with AEDs such as phenytoin/fosphenytoin, valproate, levetiracetam, phenobarbital, and lacosamide. Unfortunately, 31%‐43% of patients with established SE will become refractory to first‐line AEDs.3, 9, 10, 11 RSE is defined as SE that has not responded to first‐ or second‐line therapy, and commonly requires general anesthesia, although many cases are treated without coma induction.7, 12, 13 SRSE is SE that has continued or recurred despite 24 hours of general anesthesia and has an estimated mortality rate of 30%‐50%.2, 11 Both RSE and SRSE can cause long‐term neurological disability and reduced quality of life compared with those in seizure remission,2, 11, 14 highlighting the unmet need for novel and effective SE treatments.
In terms of the underlying pathophysiology, SE might arise from the decreased inhibitory activity of GABAAR.15 Additionally, increased trafficking of N‐methyl‐d‐aspartate (NMDA) and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptor subunits to the synaptic membrane contributes to increased glutamate‐mediated excitatory activity and uncontrolled seizures.8, 15, 16 Therefore, targeting NMDA and AMPA receptors may offer alternative mechanisms to existing treatments for patients with SE.8, 15
In Europe, perampanel, a selective, non‐competitive AMPA receptor antagonist, is indicated as an adjunctive treatment for focal seizures, with or without secondarily generalized seizures, in patients aged ≥12 years, and for the adjunctive treatment of primary generalized tonic‐clonic seizures in patients with idiopathic generalized epilepsy aged ≥12 years. Perampanel is also approved for monotherapy use for focal seizures in the United States. Studies in the rat lithium‐pilocarpine model of SE have suggested that perampanel may have efficacy in terminating prolonged seizures17, 18 while providing neuroprotection, though the level of neuroprotective effects varied by region.18 To our knowledge, this has not been demonstrated in humans with SE. Furthermore, modified expression of the AMPA subunits GluA1 and GluA2 and altered Ca2+ permeability have been observed in animal models of SE.15 However, while clinical cases of perampanel use for the treatment of patients with SE have been reported,19, 20, 21, 22 there are currently few published case reports assessing the effectiveness of perampanel in SE, as reviewed previously.7 Given the limited clinical trial data available to guide clinicians in the treatment of SE, here we report a summary of cases where perampanel was used to treat patients with SE in clinics across Europe.
2. METHODS
2.1. Retrospective chart review of medical records
We reviewed the medical records from a cohort of adult patients (aged ≥18 years) with SE. The evaluation period was between January 1, 2011 and December 31, 2015, and records were assessed for any mention of the administration of perampanel, irrespective of whether or not this was the last drug administered for the treatment of an episode of SE. In total, 52 patients were treated with PER at five university hospitals in: Salzburg (n = 3), Austria; Frankfurt (n = 18) and Marburg (n = 18), Germany; Kuopio (n = 4), Finland; and Barcelona (n = 9), Spain. Patients from Salzburg already reported by Rohracher et al in 201523 were not included in the current analysis to avoid double reporting, but the three patients reported were later included into the single‐center analysis by Rohracher et al in 2018.20
Evaluation of all patients with SE is part of a study on SE outcomes in Frankfurt, Salzburg, and Marburg, and was approved by the local ethics committees in Barcelona, Frankfurt, Salzburg, and Marburg, and as a registry study by the responsible authorities in the hospital administration in Kuopio. Due to the retrospective study design, an informed consent into the data analysis was not necessary.
The 2015 International League Against Epilepsy (ILAE) definition and classification of SE were applied retrospectively to classify cases by etiology and semiology.1 In brief, the ILAE definition outlines two separate timepoints to determine when a seizure is abnormally prolonged (t 1), and when ongoing seizure activity may cause long‐term consequences (t 2).1 RSE was defined as recurrent seizure activity notwithstanding administration of two appropriately selected and dosed AEDs, including a benzodiazepine, and SRSE was defined as SE that continues or recurs ≥24 h after initiation of treatment with an anesthetic.10, 11 SE in comatose patients was diagnosed according to the validated Salzburg EEG criteria for non‐convulsive SE.24
2.2. Data collection and assessments performed
Patient data were collected for etiology, semiology, clinical diagnosis, demographics, history of seizures or SE, total length of stay in hospital, ventilation time, modified Rankin Scale (mRS) score, and the Status Epilepticus Severity Score (STESS) at time of admission.25 SE duration before initiation of perampanel and number of AEDs previously used were analyzed. Timing of perampanel administration in relation to SE onset and cessation, and incidence of adverse effects were also ascertained. Response to perampanel was defined as SE cessation for ≥24 hours based on clinical recovery, or cessation of EEG pattern fulfilling the Salzburg consensus criteria of SE within 24 hours of perampanel administration,24 when perampanel was the last drug added with no further administration of AEDs or anesthetic drugs before SE cessation, and last changes to AED regimen were >24 hours before initiation of perampanel.
Statistical analysis was performed using SPSS Statistics 25 (IBM Corp., Armonk, NY, USA) and BiAS for Windows Version 10.01 (epsilon‐Verlag, Frankfurt/Main, Germany). Data were grouped for patients who were perampanel responders and patients who were perampanel non‐responders. Among these groups, univariate comparisons of proportions were performed using Pearson's chi‐squared test. The Mann‐Whitney U test was used for comparisons of variables with non‐normal distribution. Two‐sided P‐values <0.05 were considered significant. Adjustments for multiple comparisons were not made.
3. RESULTS
3.1. Baseline demographics and clinical characteristics
During the evaluation period, 1319 patients with SE were treated across the five hospitals. Of these, perampanel was used to treat 52 (3.9%) patients with SE (Table 1). Across all patients, the mean age was 60.5 years (SD, 19.7; range, 19‐91), and 28 (53.8%) were female. For perampanel‐treated patients, RSE was present in 28 (53.8%) patients, SRSE in 23 (44.2%) patients, and an established SE was found in 1 (1.9%) patient. Prior to treatment with perampanel, patients had already failed a median of five AEDs (range, 0‐13); benzodiazepines had failed in all 51 patients who received them (the patient with established SE received perampanel as his/her first drug instead of a benzodiazepine). Other AEDs administered prior to perampanel and without cessation of SE included levetiracetam (n = 50; 96.2%), lacosamide (n = 38; 73.1%), valproate (n = 37; 71.2%), and phenytoin/fosphenytoin (n = 24, 46.2%).
Table 1.
Baseline demographics and clinical characteristics of patients with SE treated with perampanel
| All patients (n = 52) | Perampanel responders (n = 19) | Perampanel non‐responders (n = 33) | |
|---|---|---|---|
| Baseline demographics | |||
| Mean age (SD), y | 60.5 (19.7) | 55.6 (21.0) | 62.2 (18.7) |
| Female, n (%) | 28 (53.8) | 10 (52.6) | 18 (54.5) |
| Clinical characteristics | |||
| mRS score before admission | |||
| Median (range) | 3 (0‐5) | 3 (0‐5) | 3 (0‐4) |
| STESS score at admission | |||
| Median (range) | 3 (0‐6) | 3 (0‐6) | 3 (0‐6) |
| Pre‐existing epilepsy, n (%) | 26 (50.0) | 10 (52.6) | 16 (48.5) |
| SE etiology, n (%) | |||
| Structural | 45 (86.5) | 15 (78.9) | 30 (90.9) |
| Hypoxic | 5 (9.6) | 2 (10.5) | 3 (9.1) |
| Other | 2 (3.8) | 2 (10.5) | 0 (0.0) |
| SE semiology, n (%) | |||
| GTCSE | 20 (38.5) | 5 (26.3) | 15 (45.5) |
| NCSE | 13 (25.0) | 5 (26.3) | 8 (24.2) |
| Other | 19 (36.5) | 9 (47.4) | 10 (30.3) |
| SE refractoriness, n (%) | |||
| SE | 1 (1.9) | 1 (5.3) | 0 (0.0) |
| RSE | 28 (53.8) | 12 (63.2) | 16 (48.5) |
| SRSE | 23 (44.2) | 6 (31.6) | 17 (51.5) |
| Number of failed AEDs before perampanel | |||
| Median (range) | 5 (0‐13) | 5 (0‐13) | 6 (3‐10) |
AED, antiepileptic drug; GTCSE, generalized tonic‐clonic status epilepticus; mRS, modified Rankin Scale; NCSE, non‐convulsive status epilepticus; RSE, refractory status epilepticus; SD, standard deviation; SE, status epilepticus; SRSE, super‐refractory status epilepticus; STESS, status epilepticus severity score.
3.2. Perampanel treatment
For all patients, the median latency from onset of SE to initiation of perampanel treatment was 10 days (range, 0.5‐51; Table 2). The median initial perampanel dose was 6 mg/d (range, 2‐24), and perampanel was up‐titrated to a median maximum dose of 10 mg/d (range, 4‐24), corresponding to a median dose of 0.15 mg/kg body weight (range, 0.04‐0.32). In patients unable to swallow, ground perampanel tablets were administered through a nasogastric tube; all patients with SRSE (n = 23) received perampanel via this route, as they were typically intubated and/or in an induced coma.
Table 2.
Perampanel treatment parameters and outcomes
| All patients (n = 52) | Perampanel responders (n = 19) | Perampanel non‐responders (n = 33) | P value (responders vs non‐responders) | |
|---|---|---|---|---|
| Treatment parameters | ||||
| Latency from SE onset to first perampanel administration, d | ||||
| Mean (SD) | 12.6 (11.3) | 12.4 (9.7) | 12.7 (12.3) | 0.610 |
| Median (range) | 10 (0.5‐51) | 9 (2‐39) | 11 (0.5‐51) | |
| Initial perampanel dose, mg/d | ||||
| Mean (SD) | 7.3 (3.9) | 5.8 (2.5) | 8.1 (4.3) | 0.032 |
| Median (range) | 6 (2‐24) | 6 (2‐12) | 8 (2‐24) | |
| Maximal perampanel dose, mg/d | ||||
| Mean (SD) | 10.4 (4.2) | 8.8 (4.4) | 11.3 (3.9) | 0.005 |
| Median (range) | 10 (4‐24) | 8 (4‐24) | 12 (4‐24) | |
| Treatment outcomes | ||||
| Ventilation duration, ha | ||||
| Mean (SD) | 299.4 (378.9) | 192.5 (284.0) | 361.0 (415.6) | 0.223 |
| Total length of stay in ICU/IMC, d | ||||
| Mean (SD) | 23.7 (23.6) | 23.4 (17.9) | 23.9 (26.6) | 0.562 |
| Median (range) | 18.5 (0‐118) | 20 (0‐69) | 14 (0‐118) | |
| Total length of stay, d | ||||
| Mean (SD) | 33.1 (22.8) | 32.3 (20.8) | 33.6 (24.1) | 0.917 |
| Median (range) | 29.5 (4‐118) | 31 (4‐71) | 26 (6‐118) | |
| mRS score at discharge | ||||
| Median (range) | 5 (0‐6) | 4 (0‐6) | 5 (2‐6) | 0.034 |
| Mortality, n (%) | 14 (26.9) | 3 (15.8) | 11 (33.3) | 0.294 |
ICU, intensive care unit; IMC, intermediate care; mRS, modified Rankin Scale; SD, standard deviation; SE, status epilepticus.
Median values and ranges have not been reported for ventilation duration, since these data are skewed due to the low number of patients who were ventilated.
3.3. Efficacy outcomes
Perampanel was added as the last drug in 32/52 (61.5%) patients, and response was attributed to perampanel in 19/52 (36.5%) patients (Table 1). The remaining 33/52 (63.5%) patients were classified as perampanel non‐responders.
As patients with a hypoxic etiology (n = 5) and PER given as first drug (n = 1) might have diverging outcomes and therefore distort the efficacy numbers, excluding this patients would result in response attributed to PER in 16 out of 46 patients (34.8%). In these patients with refractory and super‐refractory course, PER was the last drug in 27 out of 46 (58.7%) patients.
Among the responders, resolution of SE on EEG was seen after a median time of 72 hours (range, 24‐336). A greater proportion of perampanel non‐responders had SRSE (51.5%; 17/33 patients) compared with perampanel responders (31.6%; 6/19 patients; P = 0.27), and had failed a higher mean number of AEDs before perampanel treatment compared with perampanel responders (5.9 vs 5.1, respectively; P = 0.11; Table 1). The mean initial perampanel dose was higher in perampanel non‐responders (8.1 mg/d) than perampanel responders (5.8 mg/d; P = 0.03), as was the mean maximal perampanel dose (11.3 mg/d vs 8.8 mg/d, respectively; P = 0.005).
Although distributions of mRS scores were similar between perampanel responders and non‐responders at admission (P = 0.75), perampanel non‐responders demonstrated higher mRS scores at discharge compared with perampanel responders (P = 0.03; Table 1, Figure 1). The mean latency from onset of SE to initiation of perampanel treatment was similar between the two groups (12.7 days vs 12.4 days for perampanel non‐responders and responders, respectively; P = 0.61; Table 2). Final outcomes were discharged to a rehabilitation facility for 19/52 (36.5%) patients, to home for 8/52 (15.4%) patients, into palliative/nursing care for 8/52 (15.4%) patients, and to another hospital for 3/52 (5.8%) patients. Of the 14/52 (26.9%) patients who died in hospital, a greater proportion of perampanel non‐responders were represented (11/33 [33.3%]) than perampanel responders (n = 3/19 [15.8%]; P = 0.29; Table 2).
Figure 1.
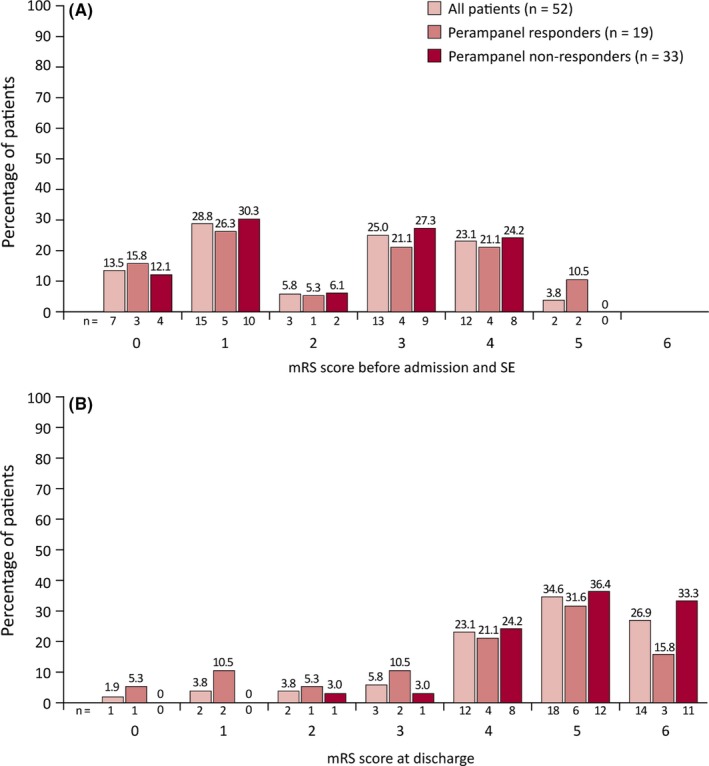
mRS scores (A) before admission and SE, and (B) at discharge for patients with SE treated with perampanel. mRS, modified Rankin Scale; SE, status epilepticus
3.4. Safety outcomes
In two patients, treatment‐related adverse effects were attributed to perampanel treatment, including dizziness (n = 1 [1.9%], while receiving perampanel 12 mg) and somnolence (n = 1 [1.9%], while receiving perampanel 8 mg). No serious adverse effects were reported, and no adverse effects led to discontinuation of perampanel. Respiratory insufficiency was reported in one (1.9%) patient (who had received phenytoin, valproic acid, and levetiracetam in addition to perampanel); however, this was due to pneumonia and considered unrelated to perampanel treatment. In addition, respiratory tract and urinary tract infections requiring antibiotic treatment were observed, and these caused complications in intensive care treatment. Transient liver enzyme and creatinine elevation were also reported when patients were taking AED polytherapy; these may not have been directly related to perampanel.
4. DISCUSSION
In this retrospective chart review of our clinical practice, perampanel was used to treat adults with established SE (n = 1; treated with perampanel as first drug), RSE (n = 28), and SRSE (n = 23). Although the perampanel Summary of Product Characteristics recommends up‐titration from an initial dose of 2 mg/d every 2 weeks in patients with focal seizures or generalized tonic‐clonic seizures, therapeutic levels of perampanel are required more immediately to control SE, thus a higher median initial dose of 6 mg/d was not unexpected (consistent with other cases of perampanel treatment of SE). Redecker et al19 reported a median initial perampanel dose of 6 mg across 10 episodes of RSE (in nine patients). The pharmacokinetic properties of perampanel with a half‐life of approximately 105 hours might delay the onset of action, and higher initial doses may be necessary. Rohracher et al20 reported a higher median initial perampanel dose of 32 mg (range, 16‐32) used in 14 patients with RSE or SRSE; the remaining 16 patients received a median initial dose of 4 mg (range, 2‐12). In two further cases of perampanel treatment for SE, perampanel was initiated at doses of 2 mg21 and 6 mg.22
Route of drug administration is another important consideration, in terms of rapid attainment of therapeutic drug levels, and the practicalities of administration while SE is ongoing and patients are potentially under general anesthesia.3 Nasogastric delivery of perampanel has previously been reported in SE,20 and intravenous delivery has been achieved in rats.17, 18 In our cases, nasogastric delivery of ground perampanel tablets was utilized in patients unable to swallow. In phase I studies, perampanel 12 mg (single doses) administered orally to healthy volunteers (n = 45) demonstrated a mean maximum concentration (C max) of 335.7 µg/L and median time to C max (T max) of 1.0 hours (range, 0.5‐4.0). Multiple daily doses of perampanel 12 mg produced a mean C max of 1138.5 µg/L and median T max of 1.0 hours (range, 0.5‐6.0; n = 93).26 Study 050 (NCT03376997) was a phase I study evaluating bioavailability of single doses of perampanel 12 mg delivered via intravenous infusions of different durations, compared with a single dose of perampanel 12 mg delivered as an oral tablet in healthy participants.27 A further study (Study 051) is planned to investigate bioequivalence of this intravenous formulation. Perampanel can also be delivered as an oral suspension with comparable bioavailability to tablets. As of September 2018, perampanel oral suspension is only approved for use in the United States.
Patients in the current study had failed a median of five other AEDs prior to perampanel initiation, representing a particularly refractory population. The SE cessation attributed to perampanel in 19/52 (36.5%) patients therefore represents a notable response. In the cases summarized here, latency from SE onset to perampanel initiation is unlikely to account for differences in response to perampanel, given the similarity in mean latencies between the two groups (12.7 vs 12.4 days for non‐responders and responders, respectively). Mean initial and mean maximal doses of perampanel administered to responders were lower than those administered to non‐responders. This could be indicative of the response in these patients circumventing further dose increases, and may also reflect the increased refractoriness of SE in non‐responders.
There are few published cases of perampanel treatment of SE.19, 20, 21, 22 Rohracher et al reported treatment response in 5/30 (16.7%) perampanel‐treated patients with RSE and SRSE, of whom two received higher doses (20 and 24 mg); however, decreased bioavailability and late administration may have diminished the potential effects of perampanel.20 In a retrospective study of 10 episodes of epilepsia partialis continua or non‐convulsive SE, perampanel was considered effective in three or four episodes, dependent on the criteria applied to determine the AED leading to SE cessation.19 For one of these cases (also published as a case report), perampanel was the final AED administered prior to cessation of focal SE, 24 hours after its initial administration.21 Additionally, a case study documented perampanel administration for treatment of Lance‐Adams syndrome, resulting in myoclonic seizure suppression.28 The case studies presented here provide further support for perampanel as a treatment for RSE.
Previous studies have described varied outcomes with other AEDs used off‐label for treatment of SE. A systematic review of lacosamide for treatment of SE demonstrated overall efficacy of 57% across 522 episodes of SE.29 In cases of RSE treated with lacosamide or phenytoin after failure of two previous AEDs, SE ceased in seven (33.3%) patients receiving lacosamide and six (40.0%) patients receiving phenytoin.30 Treatment of RSE and SRSE with brivaracetam, after failure of 1‐8 AEDs, resulted in SE cessation in three (27.3%) patients within 24 hours of administration.31 In a recent case series of patients with various stages of SE, brivaracetam led to SE cessation in four (57.1%) patients after a median of four previously failed AEDs.32 Across three retrospective reviews, off‐label ketamine use resulted in termination of 22.0% of RSE cases (N = 82).33 In a retrospective study of (S)‐ketamine treatment for RSE and SRSE, after a median of three failed AEDs, (S)‐ketamine was the final drug administered before SE cessation in 27 (64.3%) patients; however, four (14.8%) of these were also receiving propofol concurrently.34 A review of all published studies of ketamine treatment for SE found that 153 (73.9%) adult patients were responders.33 In a report of five patients with SRSE, stiripentol treatment after failure of 5‐8 AEDs was followed by SE cessation in three (60.0%) patients within 4 days of administration.35
It is important to consider inherent limitations associated with case studies and retrospective reviews. The SE cases reported here comprise a heterogeneous population of varying age, diagnosis, and cause and severity of SE; such cases may therefore respond differently to treatments and have contrasting prognoses.13, 36 For example, the current study included five patients with a hypoxic etiology, which has a greater case fatality rate than many other etiologies.13 Differences in treatment guidelines between institutions and AEDs already administered prior to perampanel initiation may produce further variation. Further, these data should be considered in the context of small sample size (N = 52). Finally, this was a retrospective review, rather than a prospective study, with no control arm.
Despite these limitations, we report to our knowledge the largest number of cases of patients with SE treated with perampanel. Such case reports may allow for development of improved treatment strategies by identifying factors associated with better outcomes in patients with SE after treatment with specific AEDs. These case studies may be of clinical value, given that practical and ethical issues preclude clinical trials being conducted for evaluation of perampanel as a treatment for SE. As SE is a potentially fatal medical emergency, high‐class, randomized, controlled trials have only been carried out in patients with the early stages of SE,7 although a recent phase III trial assessed an experimental treatment (brexanolone, SAGE‐547) as a third‐line therapy for SRSE. While perampanel is not currently licensed for treatment of SE, the cases described here add to evidence from previous case reports and animal studies that perampanel might be a therapeutic option for treatment of established SE, RSE, and SRSE; this should be confirmed by further research.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
CONFLICT OF INTEREST
Adam Strzelczyk reports personal fees and grants from Desitin Arzneimittel, Eisai, GW pharma, LivaNova, Medtronic, Sage Therapeutics, UCB Pharma, and Zogenix. Susanne Knake reports speaker's honoraria from Desitin Arzneimittel and UCB Pharma, and educational fees from AD tech, BESA, Bial, Brainlab, Desitin Arzneimittel, Eisai, Epilog, GW Pharma, Novartis, Siemens, and UCB Pharma. Reetta Kälviäinen reports speaker's honoraria from Eisai, Orion, and UCB Pharma, and honoraria for membership of advisory boards/consultation from Eisai, GW Pharma, Takeda, and UCB Pharma. Estevo Santamarina reports personal fees and grants from Bial, Eisai, Esteve, and UCB Pharma. Manuel Toledo reports personal fees from Bial, Eisai, Esteve, Novartis, Shire, and UCB Pharma, and grants from Bial, Eisai, and UCB Pharma. Alexandra Rohracher has received travel support and speaker's honoraria from Eisai. Eugen Trinka has acted as a paid consultant to Bial, Biogen Idec, Eisai, Ever Pharma, LivaNova, Medtronics, Novartis, Sanofi Genzyme, and UCB Pharma; has received speaker's honoraria from Bial, Boehringer Ingelheim, Eisai, GL Pharma, LivaNova, Newbridge, and UCB Pharma in the past 3 years; and has received research funding from Bayer, Biogen Idec, Bundesministerium für Wissenschaft und Forschung, the European Union, FWF Österreichischer Fond zur Wissenschaftsförderung, Merck, Novartis, Red Bull, and UCB Pharma. Eugen Trinka is also one of the investigators planning the ESET‐Trial and is a member of the Medical Therapies Commission of the ILAE, the Task Force on Classification of Status Epilepticus of the ILAE, and the Task Force on Definitions and Nosology. Felix Rosenow reports personal fees from Cerbomed, Desitin Arzneimittel, Eisai, GW Pharma, Medtronic, Novartis, Shire, UCB Pharma, and ViroPharma; and grants from Desitin Arzneimittel, the Detlev‐Wrobel‐Fonds for Epilepsy Research, Deutsche Forschungsgemeinschaft, the European Union, and within the LOEWE‐Programme from the State of Hesse. Sophia Willig has no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors were involved in the data collection and interpretation of the results. All authors contributed to the writing of the manuscript, critically reviewed each draft, approved the final manuscript for submission, and were involved in the decision to submit the article for publication. All authors also confirm accountability for the accuracy and integrity of the work.
ACKNOWLEDGMENTS
There is no funding to report for this study. Medical writing support, under the direction of the authors, was provided by David Pertab, PhD, of CMC AFFINITY, a division of Complete Medical Communications Ltd., Glasgow, UK, funded by Eisai Inc., in accordance with Good Publication Practice (GPP3) guidelines.
Strzelczyk A, Knake S, Kälviäinen R, et al. Perampanel for treatment of status epilepticus in Austria, Finland, Germany, and Spain. Acta Neurol Scand. 2019;139:369–376. 10.1111/ane.13061
REFERENCES
- 1. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus–Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515‐1523. [DOI] [PubMed] [Google Scholar]
- 2. Strzelczyk A, Ansorge S, Hapfelmeier J, et al. Costs, length of stay, and mortality of super‐refractory status epilepticus: a population‐based study from Germany. Epilepsia. 2017;58:1533‐1541. [DOI] [PubMed] [Google Scholar]
- 3. Trinka E, Höfler J, Leitinger M, et al. Pharmacologic treatment of status epilepticus. Expert Opin Pharmacother. 2016;17:513‐534. [DOI] [PubMed] [Google Scholar]
- 4. Glauser T, Shinnar S, Gloss D, et al. Evidence‐based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American epilepsy society. Epilepsy Curr. 2016;16:48‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence . Epilepsies: diagnosis and management Epub 11/1/2012. [PubMed]
- 6. Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3‐23. [DOI] [PubMed] [Google Scholar]
- 7. Trinka E, Kalviainen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. 2017;44:65‐73. [DOI] [PubMed] [Google Scholar]
- 8. Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724‐7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holtkamp M, Othman J, Buchheim K, et al. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry. 2005;76:534‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayer SA, Claassen J, Lokin J, et al. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205‐210. [DOI] [PubMed] [Google Scholar]
- 11. Shorvon S, Ferlisi M. The treatment of super‐refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134:2802‐2818. [DOI] [PubMed] [Google Scholar]
- 12. Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia. 2010;51:251‐256. [DOI] [PubMed] [Google Scholar]
- 13. Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol. 2015;14:615‐624. [DOI] [PubMed] [Google Scholar]
- 14. Kortland LM, Knake S, von Podewils F, et al. Socioeconomic outcome and quality of life in adults after status epilepticus: a multicenter, longitudinal, matched case‐control analysis from Germany. Front Neurol. 2017;8:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leo A, Giovannini G, Russo E, et al. The role of AMPA receptors and their antagonists in status epilepticus. Epilepsia. 2018;59:1098‐1108. [DOI] [PubMed] [Google Scholar]
- 16. Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246‐256. [DOI] [PubMed] [Google Scholar]
- 17. Hanada T, Ido K, Kosasa T. Effect of perampanel, a novel AMPA antagonist, on benzodiazepine‐resistant status epilepticus in a lithium‐pilocarpine rat model. Pharmacol Res Perspect. 2014;2:e00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu T, Ido K, Osada Y, et al. The neuroprotective effect of perampanel in lithium‐pilocarpine rat seizure model. Epilepsy Res. 2017;137:152‐158. [DOI] [PubMed] [Google Scholar]
- 19. Redecker J, Wittstock M, Benecke R, et al. Efficacy of perampanel in refractory nonconvulsive status epilepticus and simple partial status epilepticus. Epilepsy Behav. 2015;45:176‐179. [DOI] [PubMed] [Google Scholar]
- 20. Rohracher A, Kalss G, Neuray C, et al. Perampanel in patients with refractory and super‐refractory status epilepticus in a neurological intensive care unit: A single‐center audit of 30 patients. Epilepsia. 2018;59(Suppl 2):234‐242. [DOI] [PubMed] [Google Scholar]
- 21. Rösche J, Kampf C, Benecke R. Possible effect of perampanel on focal status epilepticus after generalized tonic‐clonic status epilepticus. Acta Neurol Belg. 2014;114:243‐244. [DOI] [PubMed] [Google Scholar]
- 22. Santamarina E, Sueiras M, Lidon RM, et al. Use of perampanel in one case of super‐refractory hypoxic myoclonic status: Case report. Epilepsy Behav Case Rep. 2015;4:56‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rohracher A, Hofler J, Kalss G, et al. Perampanel in patients with refractory and super‐refractory status epilepticus in a neurological intensive care unit. Epilepsy Behav. 2015;49:354‐358. [DOI] [PubMed] [Google Scholar]
- 24. Leitinger M, Beniczky S, Rohracher A, et al. Salzburg Consensus Criteria for Non‐Convulsive Status Epilepticus–approach to clinical application. Epilepsy Behav. 2015;49:158‐163. [DOI] [PubMed] [Google Scholar]
- 25. Rossetti AO, Logroscino G, Milligan TA, et al. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol. 2008;255:1561‐1566. [DOI] [PubMed] [Google Scholar]
- 26. Patsalos PN. The clinical pharmacology profile of the new antiepileptic drug perampanel: A novel noncompetitive AMPA receptor antagonist. Epilepsia. 2015;56:12‐27. [DOI] [PubMed] [Google Scholar]
- 27. ClinicalTrials.gov. Study to Evaluate Bioavailability of a Single 12 mg Dose of Perampanel for Three Intravenous Infusion Durations Relative to a Single 12 mg Perampanel Oral Tablet in Healthy Subjects Epub 2017. https://clinicaltrials.gov/ct2/show/NCT03376997. Accessed February 1, 2018.
- 28. Steinhoff BJ, Bacher M, Kurth C, et al. Add‐on perampanel in Lance‐Adams syndrome. Epilepsy Behav Case Rep. 2016;6:28‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strzelczyk A, Zöllner JP, Willems LM, et al. Lacosamide in status epilepticus: Systematic review of current evidence. Epilepsia. 2017;58:933‐950. [DOI] [PubMed] [Google Scholar]
- 30. Kellinghaus C, Berning S, Stogbauer F. Intravenous lacosamide or phenytoin for treatment of refractory status epilepticus. Acta Neurol Scand. 2014;129:294‐299. [DOI] [PubMed] [Google Scholar]
- 31. Strzelczyk A, Steinig I, Willems LM, et al. Treatment of refractory and super‐refractory status epilepticus with brivaracetam: A cohort study from two German university hospitals. Epilepsy Behav. 2017;70:177‐181. [DOI] [PubMed] [Google Scholar]
- 32. Kalss G, Rohracher A, Leitinger M, et al. Intravenous brivaracetam in status epilepticus: A retrospective single‐center study. Epilepsia. 2018;59(Suppl 2):228‐233. [DOI] [PubMed] [Google Scholar]
- 33. Höfler J, Trinka E. Intravenous ketamine in status epilepticus. Epilepsia. 2018;59(Suppl 2):198‐206. [DOI] [PubMed] [Google Scholar]
- 34. Höfler J, Rohracher A, Kalss G, et al. (S)‐Ketamine in refractory and super‐refractory status epilepticus: a retrospective study. CNS Drugs. 2016;30:869‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strzelczyk A, Kortland LM, Knake S, et al. Stiripentol for the treatment of super‐refractory status epilepticus. Acta Neurol Scand. 2015;132:435‐439. [DOI] [PubMed] [Google Scholar]
- 36. Rossetti AO, Hurwitz S, Logroscino G, et al. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry. 2006;77:611‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]


