Abstract
Background
The current study investigated the role of radiotherapy (RT) in patients with primary nonmetastatic retroperitoneal liposarcomas.
Methods
A total of 607 patients with localized retroperitoneal well‐differentiated liposarcomas (WDLPS) and dedifferentiated liposarcomas (DDLPS) underwent surgical resection with or without RT at 8 high‐volume sarcoma centers (234 patients with WDLPS, 242 patients with grade 1 to 2 DDLPS, and 131 patients with grade 3 DDLPS; grading was performed according to the National Federation of Centers for the Fight Against Cancer [Federation Nationale des Centres de Lutte Contre le Cancer; FNCLCC]). RT was administered in 19.7%, 34.7%, and 35.1%, respectively, of these 3 cohorts. Overall survival (OS) was estimated using the Kaplan‐Meier method, and the incidences of local recurrence and distant metastasis (DM) were estimated in a competing risk framework. To account for bias consistent with nonrandom RT assignment, propensity scores were estimated. Cox univariable analysis of the association between RT and oncological endpoints was performed by applying inverse probability of treatment weighting (IPTW) using propensity scores.
Results
Age, tumor size, and the administration of chemotherapy were found to be significantly imbalanced between patients who did and did not undergo RT in all cohorts. IPTW largely removed imbalances in key prognostic variables. Although the 8‐year local recurrence incidences in patients treated with surgery plus RT versus surgery only were 11.8% and 39.2%, respectively, for patients with WDLPS (P = .011;); 29.0% and 56.7%, respectively, for patients with grade 1 to 2 DDLPS (P = .008); and 29.8% and 43.7%, respectively, for patients with grade 3 DDLPS (P = .025), this significant benefit was lost after IPTW analyses. There were no significant differences noted with regard to DM and OS between irradiated and unirradiated patients across all 3 cohorts.
Conclusions
Perioperative RT was found to be associated with better local control in univariable unadjusted analysis in all 3 cohorts, but not after accounting for imbalances in prognostic variables. RT did not impact on DM or OS. The appropriate selection of RT in this disease remains challenging. The results of the European Organization for Research and Treatment of Cancer (EORTC)–Soft Tissue and Bone Sarcoma Group (STBSG) 62092‐22092 prospective randomized trial are awaited.
Keywords: local recurrence, radiotherapy (RT), retroperitoneal sarcoma, sarcoma, survival
Short abstract
In the current study, the addition of radiotherapy to curative surgery is analyzed in a large, retrospective, multi‐institutional series of patients with primary retroperitoneal liposarcoma. Although there appears to be an association with better local control and outcome, this benefit is lost after propensity score adjustment. The authors currently are awaiting the results of the European Organization for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group 62092‐22092 prospective randomized trial examining the value of preoperative radiotherapy.
Introduction
To the best of our knowledge, the role of radiotherapy (RT) in the treatment of retroperitoneal sarcoma (RPS) remains unclear and there is controversy regarding its optimal timing. Although some high‐volume sarcoma centers routinely use RT as an adjunct in the management of patients with RPS, the majority do not. To our knowledge, there is no agreed upon consensus with regard to indications or benefit.1 When delivered, preoperative RT may be preferable because postoperative RT may be associated with increased toxicity and therefore the total dose delivered may be compromised.2 The results of the recently completed European Organization for Research and Treatment of Cancer (EORTC)–Soft Tissue and Bone Sarcoma Group (STBSG) 62092‐22092 prospective randomized trial (ClinicalTrials.gov identifier NCT01344018) addressing the role of preoperative RT are eagerly awaited.
The Transatlantic Retroperitoneal Sarcoma Working Group (TARPSWG) previously investigated outcomes in a large retrospective cohort of patients with RPS. Gronchi et al1 described the variability in patterns of disease recurrence after surgical resection in 1007 patients. The proposed risk factors for local recurrence (LR) differed from those associated with distant metastases (DM). Factors found to significantly predict LR were patient age, large tumor size, incomplete surgical resection, high grade, tumor rupture, multifocality, absence of RT, and histological subtype. For these 1007 patients, the addition of RT appeared to be associated with a reduced risk of LR, with a hazard ratio (HR) of 0.58 (95% confidence interval [95% CI], 0.42‐0.80).
The objective of RT in patients with RPS, as with other soft‐tissue sarcomas, is to increase local control (LC). For patients with histologic subtypes with a low LR rate but that are more prone to DM, such as leiomyosarcomas, RT may play a less prominent role. Conversely, grade 1 to 2 dedifferentiated liposarcomas (DDLPD) and well‐differentiated liposarcomas (WDLPS) tend to have a higher rate of LR but with a much lower rate of DM. Typically, grade 1 to 2 DDLPS demonstrate rapid local failures (approximately 20% at 2 years and approximately 40% at 5 years), whereas the DM rate at 5 years most likely is <10%. WDLPS behave differently. They rarely metastasize, but exhibit a steady LR rate of 4% to 5% per year of follow‐up. Furthermore, for WDLPS, the local failure rate was found to be lowest in participating centers that administered RT more frequently. However, this finding did not translate into any significant difference in overall survival (OS), at least by 5 years of follow‐up.
The current study investigated the effect of RT on LR in the TARPSWG series of patients with primary retroperitoneal DDLPS and WDLPS and its association with eventual death from sarcoma.
Materials and Methods
The current series included 3 groups of patients (those with WDLPS, those with grade 1 to 2 DDLPS [pooled], and those with grade 3 DDLPS; grading was performed according to the National Federation of Centers for the Fight Against Cancer [Federation Nationale des Centres de Lutte Contre le Cancer; FNCLCC]) with localized disease at the time of diagnosis who underwent surgical resection with or without RT between January 2002 and December 2011 at 8 high‐volume sarcoma centers as previously described.1 All patients in the current study were included in our prior report, but the current analysis was new and focused on RT for patients with liposarcomas only. The 3 liposarcoma groups were analyzed separately. Patients were categorized into those with macroscopically complete (R0 or R1) or incomplete (R2) surgical resections. In patients who underwent R2 surgical resections, local disease progression rather than LR was considered an event. RT was planned and delivered or not, as previously described. In brief, RT was delivered by photon beams, with doses ranging from 36 Gy to 65 Gy (median, 50 Gy); RT predominantly was delivered preoperatively and by 3‐dimensional conformal techniques. Patients were followed prospectively by history and physical examination and surveillance imaging every 3 to 4 months for the first 2 years, biannually for the next 3 years, and yearly thereafter.
Statistical Analysis
The binary association between continuous and categorical variables was assessed using the Mann‐Whitney‐Wilcoxon or the Kruskal‐Wallis tests, as appropriate. The Fisher‐Freeman‐Halton test was used when testing the association between 2 categorical variables.3
The study outcomes were OS and the incidence of LR and DM. OS curves were estimated using the Kaplan‐Meier method and crude cumulative incidence (CCI) curves of LR or DM were estimated in a competing risk framework; synchronous DM and LR were accounted for as DM and were included in the corresponding CCI estimation. To account for biases consistent with nonrandom RT assignment, we estimated a propensity score as a balancing score4 and performed univariable Cox model analyses of the association between RT and the endpoints by applying an inverse probability of treatment weighting (IPTW). In particular, the RT propensity score was estimated in a multivariable logistic model with a binary response of RT performed/not performed and including treatment center, patient age, patient sex, tumor size and grading (in the analysis of patients with grade 1 to 2 DDLPS), completeness of surgical resection, multifocality, intraoperative tumor rupture, and administration of chemotherapy. With regard to IPTW, we used the method of matching weights5 proposed as a more advantageous analog to 1‐to‐1 pair matching without replacement on the propensity score with a caliper. To account for the lack of independence in replications of subjects induced by weighting,6 the 95% CI and Wald test P value related to the RT Cox coefficient were computed using the bootstrap estimate of the standard error. The standardized mean difference (SMD)7 was used to quantify differences in means (numerical variables) and proportions (categorical variables) between the 2 RT treatment groups (performed/not performed), with SMDs ≥0.3 considered to be indicative of a relevant between‐group imbalance. The SMD was calculated before and after IPTW adjustment.
Multivariable analyses of OS, LR, and DM were performed using Cox regression models. Patient age and tumor size were modeled as continuous variables using 3‐knot restricted cubic splines to obtain a flexible fit.8 All other variables were modeled as categorical using dummy variables. Overfitting due to the high dimensionality of the model in relation to the low number of events was controlled through the penalized maximum likelihood estimation method.9 Statistical analyses were performed using SAS statistical software (SAS Institute Inc, Cary, North Carolina) and R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
We identified 234 patients with WDLPS, 242 patients with grade 1 to 2 DDLPS, and 131 patients with grade 3 DDLPS. The demographic, clinical, and pathological characteristics of the 3 groups are shown in Table 1.
Table 1.
Demographic, Clinical, and Pathological Characteristics
| WDLPS N = 234 | Grade 1 to 2 DDLPS N = 242 | Grade 3 DDLPS N = 131 | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sex | ||||||
| Female | 101 | 43.2 | 104 | 43.0 | 57 | 43.5 |
| Male | 133 | 56.8 | 138 | 57.0 | 74 | 56.5 |
| Patient age, y | ||||||
| Median (first and third quartile) | 59 (50‐70) | 60 (50‐68) | 61 (54‐68) | |||
| Tumor size, cm | ||||||
| Median (first and third quartile) | 27 (18.6‐35.8) | 27 (19.0‐35.0) | 22 (16.5‐30.0) | |||
| Follow‐up from surgery, mo | ||||||
| Median (first and third quartile) | 59 (34‐94) | 58 (38‐87) | 56 (34‐86) | |||
| FNCLCC grade | ||||||
| 1 | 234 | 100.0 | 12 | 5.0 | – | – |
| 2 | – | – | 230 | 95.0 | – | – |
| 3 | – | – | – | – | 131 | 100.0 |
| Completeness of surgical resection | ||||||
| R0/R1 | 224 | 95.7 | 231 | 95.5 | 116 | 88.5 |
| R2 | 10 | 4.3 | 11 | 4.5 | 15 | 11.5 |
| Multifocality | ||||||
| No | 215 | 91.9 | 216 | 89.3 | 111 | 84.7 |
| Yes | 19 | 8.1 | 26 | 10.7 | 20 | 15.3 |
| Tumor rupture | ||||||
| No | 224 | 95.7 | 232 | 95.9 | 113 | 86.3 |
| Yes | 10 | 4.3 | 10 | 4.1 | 18 | 13.7 |
| Preoperative/postoperative chemotherapy | ||||||
| Done (pre‐/post‐/pre‐ and postoperative) | 12 (10/1/1) | 5.1 | 37 (34/3/0) | 15.3 | 29 (21/8/0) | 22.1 |
| Not done | 222 | 94.9 | 205 | 84.7 | 102 | 77.9 |
| Preoperative/postoperative RT | ||||||
| Done (pre‐/post‐/pre‐ and postoperative) | 46 (32/11/3) | 19.7 | 84 (68/14/2) | 34.7 | 46 (24/17/5) | 35.1 |
| Not done | 188 | 80.3 | 158 | 65.3 | 85 | 64.9 |
Abbreviations: DDLPS, dedifferentiated liposarcomas; FNCLCC, National Federation of Centers for the Fight Against Cancer (Federation Nationale des Centres de Lutte Contre le Cancer); R0, macroscopically complete surgical resection with negative microscopic margins; R1, macroscopically complete surgical resection with positive microscopic margins; R2; macroscopically incomplete surgical resection; RT, radiotherapy; WDLPS, well‐differentiated liposarcomas.
The numbers of patients who received RT were 46 (19.7%), 84 (34.7%), and 46 (35.1%), respectively, in the 3 subgroups. Table 2 shows the distribution of RT administration according to different characteristics, and the P values of the tests of association between treatment group (surgery plus RT [S+RT] vs surgery only) and the different characteristics, together with SMD as a measure of imbalance in the characteristics between the 2 treatment groups. Significant test results generally are associated with SMDs >30%, indicating a high imbalance. In patients with WDLPS, the most imbalanced factors were sex, age, tumor size, and administration of chemotherapy. For these variables, the SMD before IPTW yielded values >30%, and correspondingly low P values were observed. Among the patients with grade 1 to 2 DDLPS, the imbalance was observed for tumor size and administration of chemotherapy, and in the patients with grade 3 DDLPS the imbalance was found for age and tumor size. For all 3 histologic subtypes, IPTW largely removed imbalances in key prognostic variables between the groups treated with RT and those who were untreated (low SMD values) (Table 2).
Table 2.
RT Administration According to Demographic, Clinical, and Pathological Characteristics
| WDLPS | Grade 1 to 2 DDLPS | Grade 3 DDLPS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % RT | P | SMD Before/After IPTWa | % RT | P a | SMD Before/After IPTWa | % RT | P | SMD Before/After IPTWa | |
| Sex | .020 | 0.394/0.061 | .416 | 0.115/0.053 | .854 | 0.069/<0.00 | |||
| Female | 26.7 | 31.7 | 33.3 | 1 | |||||
| Male | 14.3 | 37.0 | 36.5 | ||||||
| Patient age, ya | .020 | 0.367/<0.001 | .083 | 0.223/0.026 | .008 | 0.443/0.030 | |||
| ≤40 | 16.0 | 21.7 | 37.5 | ||||||
| 40‐50 | 27.3 | 34.1 | 52.6 | ||||||
| 50‐60 | 31.9 | 33.8 | 44.7 | ||||||
| 60‐70 | 11.3 | 33.3 | 28.9 | ||||||
| >70 | 9.3 | 45.5 | 14.3 | ||||||
| Tumor size, cma | .001 | 0.541/0.033 | .009 | 0.402/0.007 | <.001 | 0.961/0.047 | |||
| ≤10 | 33.3 | 56.2 | 63.6 | ||||||
| 10‐20 | 29.2 | 37.0 | 52.0 | ||||||
| 20‐30 | 18.8 | 37.6 | 26.8 | ||||||
| 30‐40 | 11.5 | 32.1 | 5.6 | ||||||
| >40 | 12.9 | 16.1 | 9.1 | ||||||
| FNCLCC grade | – | – | – | .551 | 0.101/0.053 | – | – | – | |
| 1 | 25.0 | ||||||||
| 2 | 35.2 | ||||||||
| 3 | – | ||||||||
| Completeness of surgical resection | 1 | 0.005/0.002 | .103 | 0.273/0.037 | .573 | 0.137/0.024 | |||
| R0/R1 | 19.6 | 35.9 | 36.2 | ||||||
| R2 | 20.0 | 9.1 | 26.7 | ||||||
| Multifocality | .381 | 0.189/0.001 | .275 | 0.185/0.022 | .620 | 0.090/<0.001 | |||
| No | 20.5 | 36.1 | 34.2 | ||||||
| Yes | 10.5 | 23.1 | 40.0 | ||||||
| Tumor rupture | 1 | 0.005/0.046 | .501 | 0.142/0.055 | .600 | 0.131/0.061 | |||
| No | 19.6 | 35.3 | 36.3 | ||||||
| Yes | 20.0 | 20.0 | 27.8 | ||||||
| Preoperative/postoperative chemotherapy | <.0001 | 0.611/0.023 | .025 | 0.302/0.015 | .271 | 0.224/0.055 | |||
| Not done | 16.7 | 31.7 | 32.4 | ||||||
| Done | 75.0 | 51.4 | 44.8 | ||||||
Abbreviations: DDLPS, dedifferentiated liposarcomas; FNCLCC, National Federation of Centers for the Fight Against Cancer (Federation Nationale des Centres de Lutte Contre le Cancer); IPTW, inverse probability of treatment weighting; R0, macroscopically complete surgical resection with negative microscopic margins; R1, macroscopically complete surgical resection with positive microscopic margins; R2; macroscopically incomplete surgical resection; RT, radiotherapy; SMD, standardized mean difference before/after inverse probability of treatment weighting; WDLPS, well‐differentiated liposarcomas.
P values were determined using the Mann‐Whitney‐Wilcoxon, the Kruskal‐Wallis, or the Fisher‐Freeman‐Halton Fisher tests, as appropriate.
Test and SMD were performed by comparing the distribution of the continuous variable in the 2 RT groups.
Local Recurrence
WDLPS group
LR developed in 52 of 234 patients (22.2%), all as a first event: 4 of 46 patients (8.7%) in the S+RT subgroup and 48 of 188 patients (25.5%) in the surgery‐only subgroup. The median time to first LR among those who developed disease recurrence was 34 months (interquartile range [IQR], 30‐41.8 months) in the S+RT subgroup and 40.5 months (IQR, 24.8‐62.3 months) in the surgery‐only subgroup. The CCIs of LR at 5 years and 8 years were 21.8% and 33.6%, respectively; the CCI of LR was 11.8% at both 5 years and 8 years in the S+RT subgroup and 24.2% at 5 years and 39.2% at 8 years in the surgery‐only subgroup (P = .011) (Fig. 1 Top) (95% CIs are shown in Supporting Table 1).
Figure 1.
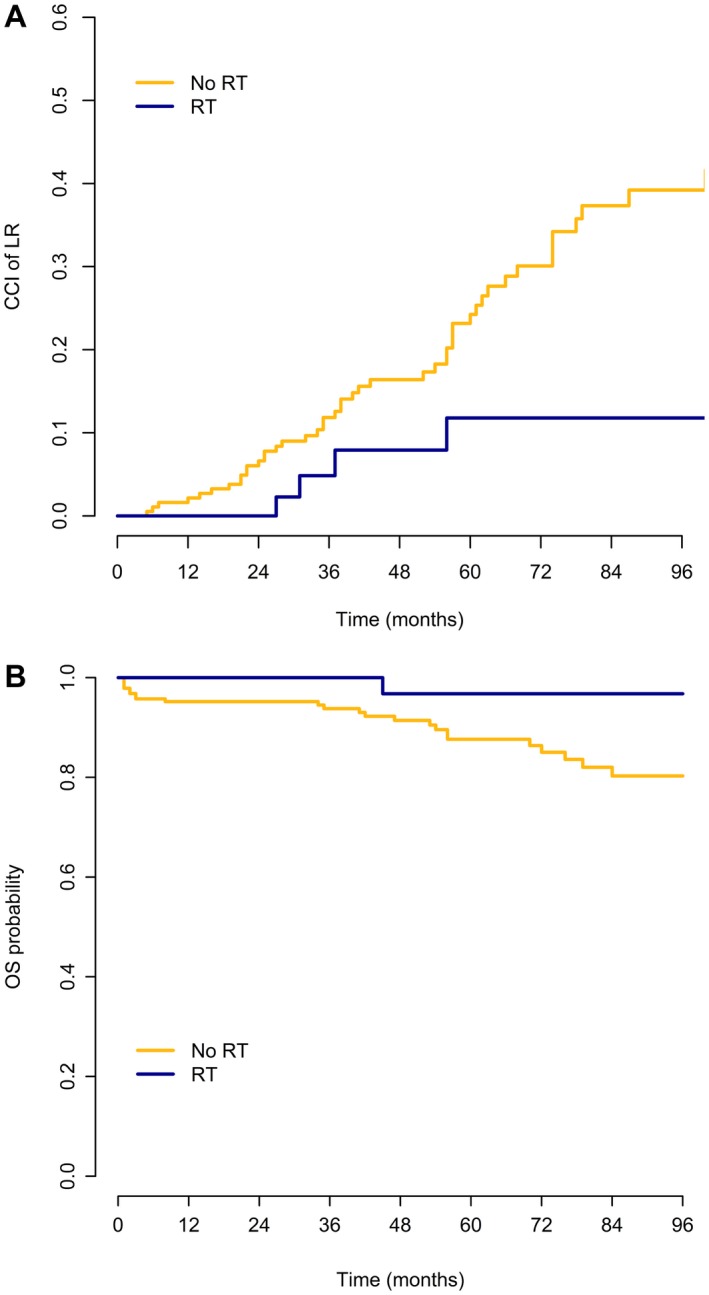
(Top) Crude cumulative incidence (CCI) of local recurrence (LR) and (Bottom) probability of overall survival (OS) among patients with well‐differentiated liposarcoma according to the administration of radiotherapy (RT).
The apparent association between RT and a reduced risk of LR was not found to be statistically significant after IPTW adjustment; the univariable Cox model HR estimate of S+RT versus surgery only was 0.28 (95% CI, 0.10‐0.77; P = .013) without adjustment and 0.54 (95% CI, 0.06‐4.8; Wald test P = .579) with IPTW adjustment.9
Multivariable Cox model analyses (Table 3) demonstrated that only completeness of surgical resection was significantly associated with LR.
Table 3.
WDLPS Subgroup: Results From the Multivariable Cox Models
| Local Recurrence | Overall Survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, y | .654 | <.0001 | ||||
| 70 vs 50a | 1.20 | 0.73‐1.99 | 5.27 | 2.73‐10.16 | ||
| Sex | .850 | .990 | ||||
| Male vs female | 1.05 | 0.62‐1.80 | 1.01 | 0.47‐2.15 | ||
| Tumor size, cm | .057 | .865 | ||||
| 35.8 vs 18.6a | 1.65 | 1.04‐2.62 | 0.92 | 0.51‐1.65 | ||
| Completeness of surgical resection | .038 | .081 | ||||
| R2 vs R0/R1 | 2.47 | 1.05‐5.82 | 3.64 | 0.85‐15.57 | ||
| Tumor rupture | .339 | .424 | ||||
| Yes vs no | 1.50 | 0.65‐3.47 | 0.55 | 0.13‐2.38 | ||
| Multifocality | .158 | .086 | ||||
| Yes vs no | 1.67 | 0.82‐3.42 | 2.61 | 0.87‐7.78 | ||
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio; R0, macroscopically complete surgical resection with negative microscopic margins; R1, macroscopically complete surgical resection with positive microscopic margins; R2; macroscopically incomplete surgical resection; WDLPS, well‐differentiated liposarcomas.
P value was determined using the 2‐sided Wald test.
The 2 values were the third and first quartiles, respectively, of the variable distribution.
Grade 1 to 2 DDLPS group
LR developed in 105 of 242 patients (43.4%): 25 of 84 patients (29.8%) in the S+RT subgroup and 80 of 158 patients (50.6%) in the surgery‐only subgroup. In 96 of 242 patients, LR was the first event (22 of 84 patients in the S+RT subgroup and 74 of 158 patients in the surgery‐only subgroup), whereas 8 of 242 patients developed concurrent LR and DM (3 of 84 patients in the S+RT subgroup and 5 of 158 patients in the surgery‐only subgroup), and 1 of the 242 patients in the surgery‐only group developed LR after DM. The median time to first LR among those who developed disease recurrence was 16.5 months (IQR, 11.5‐28.3 months) in the S+RT subgroup and 21.5 months (IQR, 14‐40.5 months) in the surgery‐only subgroup. The CCIs of LR at 5 years and 8 years were 42.2% and 48.4%, respectively; the CCI was 29.0% at both 5 years and 8 years in the S+RT subgroup and was 48.5% and 56.7%, respectively, at 5 years and 8 years in the surgery‐only subgroup (P = .008) (Fig. 2 Top).
Figure 2.
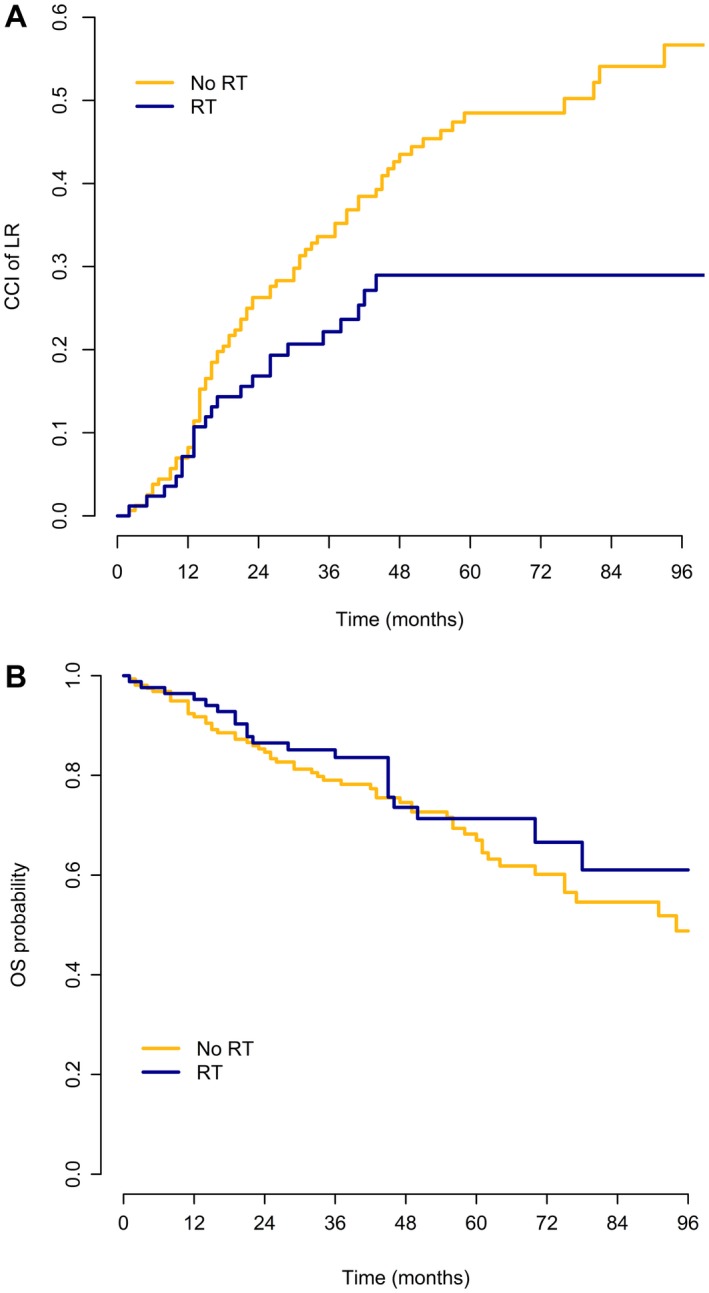
(Top) Crude cumulative incidence (CCI) of local recurrence (LR) and (Bottom) probability of overall survival (OS) among patients with grade 1 to 2 dedifferentiated liposarcoma according to the administration of radiotherapy (RT).
Prior to IPTW, RT appeared to be associated with better LC. The significance of this association was lost after IPTW. The univariable Cox model HR estimate for LR after S+RT versus surgery only was 0.53 (95% CI, 0.33‐0.86; P = .009) without adjustment and 0.70 (95% CI, 0.36‐1.39; Wald test P = .312) with IPTW adjustment.
Multivariable Cox model analyses demonstrated that the factors significantly associated with LR were tumor size, completeness of surgical resection, and multifocality (Table 4).
Table 4.
Grade 1 to 2 DDLPS Subgroup: Results From the Multivariable Cox Models
| Local Recurrence | Distant Metastasis | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age, y | .740 | .965 | .048 | ||||||
| 68 vs 50a | 1.00 | 0.72‐1.39 | 0.91 | 0.43‐1.94 | 1.57 | 1.10‐2.25 | |||
| Sex | .639 | .388 | .066 | ||||||
| Male vs female | 0.91 | 0.60‐1.36 | 1.26 | 0.75‐2.12 | 1.48 | 0.98‐2.24 | |||
| Tumor size, cm | .005 | .684 | .619 | ||||||
| 35 vs 19a | 1.73 | 1.24‐2.43 | 0.87 | 0.50‐1.51 | 1.17 | 0.85‐1.63 | |||
| Completeness of surgical resection | .002 | .851 | .129 | ||||||
| R2 vs R0/R1 | 4.07 | 1.71‐9.70 | 1.06 | 0.58‐1.95 | 1.66 | 0.86‐3.22 | |||
| FNCLCC grade | .323 | .322 | .750 | ||||||
| 2 vs 1 | 0.65 | 0.28‐1.53 | 0.74 | 0.41‐1.34 | 0.90 | 0.47‐1.73 | |||
| Tumor rupture | .419 | .902 | .572 | ||||||
| Yes vs no | 0.68 | 0.27‐1.73 | 1.04 | 0.57‐1.90 | 1.22 | 0.62‐2.39 | |||
| Multifocality | .004 | .928 | .735 | ||||||
| Yes vs no | 2.36 | 1.31‐4.25 | 1.03 | 0.58‐1.83 | 1.10 | 0.64‐1.90 | |||
Abbreviations: 95% CI, 95% confidence interval; DDLPS, dedifferentiated liposarcomas; FNCLCC, National Federation of Centers for the Fight Against Cancer (Federation Nationale des Centres de Lutte Contre le Cancer); HR, hazard ratio; R0, macroscopically complete surgical resection with negative microscopic margins; R1, macroscopically complete surgical resection with positive microscopic margins; R2; macroscopically incomplete surgical resection.
P value was determined using a 2‐sided Wald test.
The 2 values were the third and first quartiles, respectively, of the variable distribution.
Grade 3 DDLPS group
LR developed in 64 of 131 patients (48.9%): 15 of 46 patients (32.6%) in the S+RT subgroup and 49 of 85 patients (57.6%) in the surgery‐only subgroup. In 48 of 131 patients, LR was the first event: 11 of 46 patients in the S+RT subgroup and 37 of 85 patients in the surgery‐only subgroup. Concurrent LR and DM developed in 14 of 131 patients (2 of 46 patients in the S+RT subgroup and 12 of 85 patients in the surgery‐only subgroup). The median time to first LR among those patients who developed disease recurrence was 20 months (IQR, 9.5‐27.5 months) in the S+RT subgroup and 11 months (IQR, 4‐22 months) in the surgery‐only subgroup. The CCIs of LR at 5 years and 8 years were 36.1% and 38.2%, respectively; the CCIs of LR were 22.8% and 29.8%, respectively, at 5 years and 8 years in the S+RT subgroup and 43.7% for both 5 years and 8 years in the surgery‐only subgroup (P = .025) (Fig. 3 Top).
Figure 3.
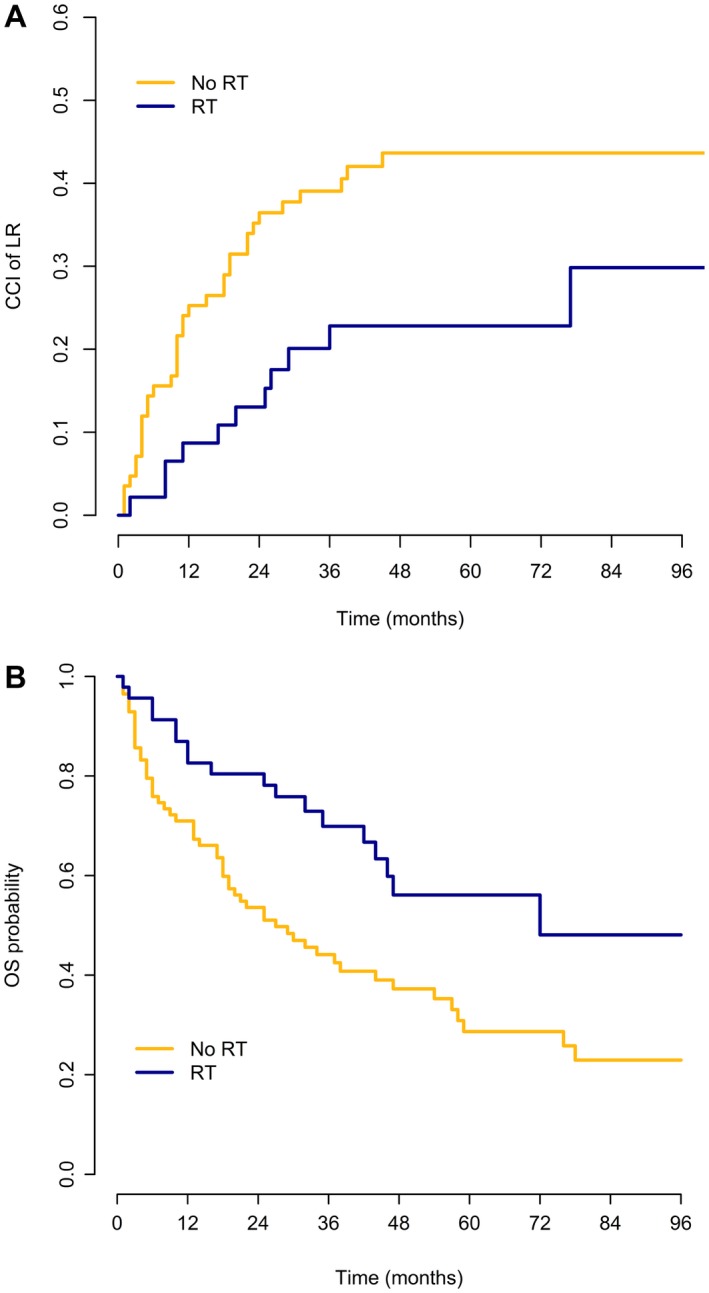
(Top) Crude cumulative incidence (CCI) of local recurrence (LR) and (Bottom) probability of overall survival (OS) among patients with grade 3 dedifferentiated liposarcoma according to the administration of radiotherapy (RT).
Again, the RT prognostic effect on LR was lost after IPTW adjustment; the univariable Cox model HR estimate of S+RT versus surgery only was 0.36 (95% CI, 0.18‐0.72; P = .004) without adjustment and 0.34 (95% CI, 0.07‐1.58; Wald test P = .170) with IPTW adjustment.
Multivariable Cox model analyses (Table 5) demonstrated that the factors significantly associated with LR were completeness of surgical resection and intraoperative tumor rupture.
Table 5.
Grade 3 DDLPS Subgroup: Results From the Multivariable Cox Models
| Local Recurrence | Distant Metastasis | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age, y | .809 | .935 | .597 | ||||||
| 68 vs 54a | 0.97 | 0.59‐1.60 | 0.95 | 0.59‐1.55 | 1.20 | 0.85‐1.70 | |||
| Sex | .256 | .636 | .352 | ||||||
| Male vs female | 1.38 | 0.79‐2.39 | 1.12 | 0.71‐1.75 | 1.22 | 0.80‐1.85 | |||
| Tumor size, cm | .405 | .246 | .736 | ||||||
| 30 vs 16a | 1.39 | 0.85‐2.28 | 1.49 | 0.84‐2.62 | 1.09 | 0.76‐1.58 | |||
| Completeness of surgical resection | .027 | .975 | .160 | ||||||
| R2 vs R0/R1 | 2.32 | 1.10‐4.89 | 1.01 | 0.57‐1.79 | 1.51 | 0.85‐2.68 | |||
| Tumor rupture | .004 | .774 | .082 | ||||||
| Yes vs no | 2.77 | 1.40‐5.49 | 1.08 | 0.62‐1.88 | 1.61 | 0.94‐2.75 | |||
| Multifocality | .795 | .324 | .157 | ||||||
| Yes vs no | 1.09 | 0.56‐2.11 | 1.30 | 0.77‐2.18 | 1.43 | 0.87‐2.36 | |||
Abbreviations: 95% CI, 95% confidence interval; DDLPS, dedifferentiated liposarcomas; HR, hazard ratio; R0, macroscopically complete surgical resection with negative microscopic margins; R1, macroscopically complete surgical resection with positive microscopic margins; R2; macroscopically incomplete surgical resection.
P value was determined using a 2‐sided Wald test.
The 2 values were the third and first quartiles, respectively, of the variable distribution.
Distant Metastases
WDLPS group
DM developed in 2 of 234 patients (0.85%), both of whom were in the surgery‐only subgroup; in 1 patient, this was the first event at 20 months and 1 patient developed DM after LR. The CCI of DM at both 5 years and 8 years was 0% in the S+RT subgroup and 0.4% in the surgery‐only subgroup. Due to the low number of metastatic events, univariable IPTW‐adjusted and multivariable Cox analyses were not performed.
Grade 1 to 2 DDLPS group
DM developed in 23 of 242 patients (9.5%): 8 of 84 patients (9.5%) in the S+RT subgroup and 15 of 158 patients (9.5%) in the surgery‐only subgroup. In 18 patients, this was the first event (7 of 84 patients in the S+RT subgroup and 11 of 158 patients in the surgery‐only subgroup); 8 of 242 patients developed concurrent LR and DM (3 of 84 patients in the S+RT subgroup and 5 of 158 patients in the surgery‐only subgroup), and 5 of 242 patients developed DM after LR (1 of 84 patients in the S+RT subgroup and 4 of 158 patients in the surgery‐only subgroup). The median time to first DM among those who developed DM was 14 months (IQR, 8.5‐20.0 months) in the S+RT subgroup and 22 months (IQR, 7.5‐38 months) in the surgery‐only subgroup. The CCI of DM both at 5 years and 8 years was 8.6% (95% CI, 5.5%‐13.6%): 9.3% in the S+RT subgroup and 8.1% in the surgery‐only subgroup.
There was no association noted between treatment with RT and DM, either before or after IPTW adjustment. The estimated HR (S+RT vs surgery only) was 1.17 (95% CI, 0.45‐3.01; P = .749) in the univariable Cox model without adjustment and 1.04 (95% CI, 0.15‐7.34; Wald test P = .966) with IPTW adjustment.
Multivariable Cox model analyses (Table 4) identified no factors as being significantly associated with DM.
Grade 3 DDLPS group
DM developed in 44 of 131 patients (33.6%): 15 of 46 patients (32.6%) in the S+RT subgroup and 29 of 85 patients (34.1%) in the surgery‐only subgroup. In 39 patients, DM was the first event (14 of 46 patients in the S+RT subgroup and 25 of 85 patients in the surgery‐only subgroup), whereas 14 of 131 patients developed concurrent LR and DM (2 of 46 patients in the S+RT subgroup and 12 of 85 patients in the surgery‐only subgroup), and 5 of 131 patients developed DM after LR (1 of 46 patients in the S+RT subgroup and 4 of 85 patients in the surgery‐only subgroup). The median time to first DM among those who developed disease recurrence was 11.5 months (IQR, 3.8‐30 months) in the S+RT subgroup and 8 months (IQR, 3‐15 months) in the surgery‐only subgroup. The CCIs of DM at 5 years and 8 years were 30.5% and 32.2%, respectively; the CCIs of DM were 30.1% and 35.1%, respectively, at 5 years and 8 years in the S+RT subgroup and 30.6% and 30.6%, respectively, at 5 years and 8 years in the surgery‐only subgroup.
The RT prognostic effect on DM was not found to be statistically significant either before or after IPTW adjustment; the univariable Cox model HR estimate of S+RT versus surgery only was 0.70 (95% CI, 0.36‐1.36; P = .296) without adjustment and 1.30 (95% CI, 0.25‐6.67; Wald test P = .750) with IPTW adjustment.
Multivariable Cox model analyses (Table 5) identified no factors as being significantly associated with DM.
Overall Survival
WDLPS group
A total of 29 of 234 patients (12.4%) died: 1 of 46 patients (2.2%) in the S+RT subgroup and 28 of 188 patients (14.9%) in the surgery‐only subgroup. The 5‐year and 8‐year OS estimates were 90.1% and 83.6%, respectively (96.8% at both 5 years and 8 years in the S+RT subgroup and 87.6% and 80.3%, respectively, at 5 years and 8 years in the surgery‐only subgroup) (Fig. 1 Bottom).
Treatment with RT was associated with a lower risk of death, but this was statistically significant only before and not after IPTW adjustment; the univariable Cox model HR estimate of S+RT versus surgery only was 0.25 (95% CI, 0.07‐0.90; P = .034) without adjustment and 0.50 (95% CI, 0.06‐4.01; Wald test P = .517) with IPTW adjustment.
On multivariable Cox model analyses (Table 3), only patient age was found to be significantly associated with OS.
Grade 1 to 2 DDLPS group
A total of 78 of 242 patients (32.2%) died: 21 of 84 patients (25%) in the S+RT subgroup and 57 of 158 patients (36.1%) in the surgery‐only subgroup.
The 5‐year and 8‐year OS estimates were 66.5% and 51.1%, respectively: 71.4% and 61.0%, respectively, at 5 years and 8 years in the S+RT subgroup and 67.0% and 48.8%, respectively, at 5 years and 8 years in the surgery‐only subgroup (Fig. 2 Bottom).
RT demonstrated no statistically significant effect on OS, neither before nor after IPTW adjustment; the univariable Cox model HR estimate for S+RT versus surgery only was 0.74 (95% CI, 0.45‐1.22; P = .236) without adjustment and 0.72 (95% CI, 0.32‐1.59; Wald test P = .412) with IPTW adjustment.
On multivariable Cox model analyses (Table 4), only patient age was found to be significantly associated with OS.
Grade 3 DDLPS group
A total of 74 of 131 patients (56.5%) died: 19 of 46 patients (41.3%) in the S+RT subgroup and 55 of 85 patients (64.7%) in the surgery‐only subgroup.
The 5‐year and 8‐year OS estimates were 36.7% and 30.2%, respectively (56.1% and 48.1%, respectively, at 5 years and 8 years in the S+RT subgroup and 28.7% and 22.9%, respectively, at 5 years and 8 years in the surgery‐only subgroup) (Fig. 3 Bottom). The median survival was 72 months and 27 months, respectively.
The RT effect on OS was not found to be statistically significant after IPTW adjustment; the univariable Cox model HR estimate for S+RT versus surgery only was 0.50 (95% CI, 0.29‐0.84; P = .009) without adjustment and 0.72 (95% CI, 0.22‐2.30; Wald test P = .576) with IPTW adjustment.
On multivariable Cox model analyses (Table 5), neither of the factors was found to be significantly associated with OS.
Discussion
In the current series of patients with retroperitoneal WDLPS and DDLPS who were treated at 8 major referral institutions over a 10‐year time span, the administration of perioperative RT was found to be associated with better LC on univariable analyses but not after propensity score matched analyses. Obviously, the current study is retrospective, with all the caveats therein. Given the complexity of decision making in RPS management, it may well have been that RT administration was chosen for the patients with a better prognosis, and the usual prognostic variables were not enough to control for this. Indeed, in the WDLPS subgroup, RT predominantly was administered to younger patients and/or those with smaller tumors; this also was true for the cohort of patients with grade 3 DDLPS, among whom patients with smaller tumors were more likely to receive RT. In addition, the extent of surgery was not homogeneous among the participating institutions over the study period, as previously reported.1
Despite these caveats, the current series comprised only primary, localized retroperitoneal liposarcomas, and to the best of our knowledge is the largest study to date to examine the role of RT in these patients. For patients with WDLPS and grade 1 to 2 DDLPS, tumors that have a limited tendency to metastasize, LR is the leading cause of death. Therefore, we examined our multi‐institutional series to gain a better understanding of the possible role of RT in the subgroups of patients who might be expected to benefit the most from multimodality treatment. The results of the multivariable Cox regression analyses in the current study have suggested that the receipt of RT is not a major determinant of LC, but rather LC rates appear to be determined by the completeness of surgical resection (in the case of patients with WDLPS), tumor size, completeness of surgical resection and multifocality (in patients with grade 1 to 2 DDLPS), and completeness of surgical resection and tumor rupture (in patients with grade 3 DDLPS). The question of whether preoperative RT would play a role in facilitating complete surgical resection and/or avoiding tumor rupture (preferably performed in experienced high‐volume centers10) cannot be determined in this type of retrospective study.
The administration of preoperative and/or postoperative RT in retrospective series such as the current study appears to be a proxy for smaller tumors, which are easier to irradiate as well as to resect.11, 12 Its presumed association with better LC and survival is lost at propensity matched analysis, which, although not perfect, controls better for prognostic variables. Furthermore, data regarding the morbidity of adding preoperative RT to abdominal surgery and the potential risk of disease progression while receiving RT, among others, are lacking. All these issues will be addressed in the forthcoming STRASS (EORTC 62092‐22092) analysis, which is seeking level I evidence regarding the role of preoperative RT in this disease. For now, we cannot exclude some effect in subgroups, but this remains to be proven.
To our knowledge to date, the use of RT for the treatment of patients with RPS is highly variable. In a population‐based study of 2348 patients with RPS in the Surveillance, Epidemiology, and End Results (SEER) program published in 2006, Porter et al13 reported that in general practice, approximately 25.9% of patients received RT, and of these, 85.5% were treated postoperatively. In a more recent SEER database analysis, published in 2015, approximately 30% of patients received RT, all postoperatively.14 Although the percentage of patients receiving RT was similar in the present TARPSWG series (29%), it was by contrast predominantly given preoperatively (76%).
The retrospective National Cancer Data Base study by Nussbaum et al15 involved 9068 patients with RPS and was performed using case‐control, propensity score–matched principles to minimize selection biases. In this cohort, 563 patients received preoperative RT, 2215 patients received postoperative RT, and 6290 patients received no RT. It is interesting to note that the delivery of preoperative RT was associated with management in an academic medical center in recent years. Both preoperative RT (HR, 0.70) and postoperative RT (HR, 0.78) were found to be significantly associated with improved OS compared with surgery alone. Unfortunately, the National Cancer Data Base analysis does not capture data regarding histologic subtypes, disease recurrences, toxicity, RT details, extent of surgery, or disease‐specific survival. Given its retrospective nature, selection biases are likely present. In addition, even in propensity matched analyses, the administration of RT was found to be associated with a higher possibility of undergoing surgery in academic centers. Furthermore, an association between RT delivery and tumors with an inherently better prognosis could not be ruled out. Thus, conclusions regarding the possible benefit of the combined modality (S+RT) are considered tentative.
We acknowledge that despite the use of propensity score analyses to compensate for known biases inherent in retrospective data, this methodology is limited because it cannot account for unknown factors. The lack of an RT effect in the current study cohort does not provide definitive proof that no patients would ever benefit from RT for retroperitoneal liposarcomas. Conversely, if the current study had found a positive effect of RT on LC, it still would be an overinterpretation to suggest that it was enough evidence of a true RT benefit. Ultimately, these data remain hypothesis‐generating only in the absence of results from phase 3 randomized controlled trials. Furthermore, several advances in the delivery of RT for patients with RPS have been described and/or are subject to prospective trials. Kelly et al investigated intensity‐modulated RT alone or in combination with proton beam therapy in 172 patients with RPS, and noted improved LC compared with patients who were treated with surgery alone.16 DeLaney et al have suggested, based on their phase 1 study, that increasing the RT dose to the posterior abdominal wall by means of proton beam therapy is feasible up to an equivalent dose of 63 Gy17; this finding currently is being explored further in a phase 2 study (ClinicalTrials.gov identifier NCT01659203). Instructions regarding how to delineate this high‐risk volume have been published previously by Baldini et al.18
Although the use of RT in patients with RPS still is under investigation, there is growing evidence that a potential benefit is not going to be homogeneous across all histologic variants of the disease. Patients with WDLPS and grade 1 to 2 DDLPS are the RPS patient subgroups with the highest LR risk, whereas patients with grade 3 DDLPS also have a substantial risk of metastases. It is in these patients that individualized consideration of RT still has a potential rationale. In other words, an association between RT and a better outcome may just be the result of selection and not causation. The results of the current study demonstrate that other determinants of local failure may be inextricably linked with the use of RT, thereby emphasizing the need for level I evidence.
Funding Support
No specific funding was disclosed.
Conflict of Interest Disclosures
Piotr Rutkowski has received honoraria for lectures from and acted as a paid member of the Advisory Boards of Novartis, MSD, Bristol‐Myers Squibb, Roche, and Amgen and has received honoraria for lectures from Pfizer and Blueprint Medicines for work performed outside of the current study. Dario Callegaro has received honoraria from Eli Lilly and Company for work performed outside of the current study.
Author Contributions
Conceptualization: Rick L.M. Haas, Sylvie Bonvalot, Rosalba Miceli, and Alessandro Gronchi. Methodology: Rick L.M. Haas, Sylvie Bonvalot, Rosalba Miceli, and Alessandro Gronchi. Formal analysis: Rick L.M. Haas, Sylvie Bonvalot, Rosalba Miceli, and Alessandro Gronchi. Patient data contribution: All authors. Writing–original draft: Rick L.M. Haas, Sylvie Bonvalot, Rosalba Miceli, and Alessandro Gronchi. Writing–review and editing: All authors.
Supporting information
References
- 1. Gronchi A, Strauss DC, Miceli R, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the Multi‐institutional Collaborative RPS Working Group. Ann Surg. 2016;263:1002‐1009. [DOI] [PubMed] [Google Scholar]
- 2. Haas RL, Baldini EH, Chung PW, van Coevorden F, DeLaney TF. Radiation therapy in retroperitoneal sarcoma management. J Surg Oncol. 2018;117:93‐98. [DOI] [PubMed] [Google Scholar]
- 3. Freeman GH, Halton JH. Note on exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38:141‐149. [PubMed] [Google Scholar]
- 4. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516‐524. [Google Scholar]
- 5. Li L, Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat. 2013;9:215‐234. [DOI] [PubMed] [Google Scholar]
- 6. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642‐5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flury BK, Reidwyl H. Standard distance in univariate and multivariate analysis. Am Stat. 1986;40:249‐251. [Google Scholar]
- 8. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551‐561. [DOI] [PubMed] [Google Scholar]
- 9. Moons KG, Donders AR, Steyerberg EW, Harrell FE. Penalized maximum likelihood estimation to directly adjust diagnostic and prognostic prediction models for overoptimism: a clinical example. J Clin Epidemiol. 2004;57:1262‐1270. [DOI] [PubMed] [Google Scholar]
- 10. Bonvalot S, Gaignard E, Stoeckle E, et al. Survival impact of surgical management in reference centers for retroperitoneal sarcoma: a nationwide study of FSG‐GETO and NETSARC. J Clin Oncol. 2018;36:S11568. [Google Scholar]
- 11. O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120:2866‐2875. [DOI] [PubMed] [Google Scholar]
- 12. Dagan R, Indelicato DJ, McGee L, et al. The significance of a marginal excision after preoperative radiation therapy for soft tissue sarcoma of the extremity. Cancer. 2012;118:3199–3207. [DOI] [PubMed] [Google Scholar]
- 13. Porter GA, Baxter NN, Pisters PW. Retroperitoneal sarcoma: a population‐based analysis of epidemiology, surgery, and radiotherapy. Cancer. 2006;106:1610‐1616. [DOI] [PubMed] [Google Scholar]
- 14. Bates JE, Dhakal S, Mazloom A, Constine LS. The benefit of adjuvant radiotherapy in high‐grade nonmetastatic retroperitoneal soft tissue sarcoma: a SEER analysis. Am J Clin Oncol. 2018;41:274‐279. [DOI] [PubMed] [Google Scholar]
- 15. Nussbaum DP, Rushing CN, Lane WO, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case‐control, propensity score–matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17:966‐975. [DOI] [PubMed] [Google Scholar]
- 16. Kelly KJ, Yoon SS, Kuk D, et al. Comparison of perioperative radiation therapy and surgery versus surgery alone in 204 patients with primary retroperitoneal sarcoma: a retrospective 2‐institution study. Ann Surg. 2015;262:156‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeLaney TF, Chen YL, Baldini EH, et al. Phase 1 trial of preoperative image guided intensity modulated proton radiation therapy with simultaneously integrated boost to the high risk margin for retroperitoneal sarcomas. Adv Radiat Oncol. 2017;2:85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baldini EH, Abrams RA, Bosch W, et al. Retroperitoneal sarcoma target volume and organ at risk contour delineation agreement among NRG sarcoma radiation oncologists. Int J Radiat Oncol Biol Phys. 2015;92:1053‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


