Summary
Background
Prospectively designed studies assessing the exposure‐response profile of vedolizumab are lacking. Observational exposure‐response data for vedolizumab are limited and have not been adjusted for potential confounding factors, particularly those that may affect vedolizumab clearance.
Aims
To (a) investigate the vedolizumab exposure‐response relationship after adjusting for potential confounding variables; (b) propose potential target serum vedolizumab concentrations for future study; (c) ascertain whether early vedolizumab serum concentrations were associated with short‐ and long‐term clinical outcomes in adults with ulcerative colitis in GEMINI 1.
Methods
Propensity‐score‐based case‐matching analysis was performed using data from GEMINI 1 and an earlier large population pharmacokinetic study, with vedolizumab clearance or concentration as predictors of clinical remission and response, adjusted for age, weight, anti‐tumour necrosis factor alpha therapy history, serum albumin and faecal calprotectin concentrations. Potential vedolizumab concentration targets at weeks 6, 14 and steady state were proposed. Association between early vedolizumab concentrations at weeks 2, 4 and 6 and clinical remission at weeks 14 and 52 was evaluated.
Results
Among 693 patients with pharmacokinetic data at week 6, potential target vedolizumab concentrations at weeks 6, 14 and steady state were 37.1, 18.4 and 12.7 µg/mL respectively. Week 6 was identified as the earliest time at which vedolizumab concentrations were consistently associated with clinical remission at weeks 14 and 52.
Conclusions
In this comprehensively adjusted analysis, vedolizumab concentrations at week 6 were associated with short‐ and long‐term remission. Potential induction and maintenance target concentrations were proposed for further study.
1. INTRODUCTION
Ulcerative colitis (UC) and Crohn's disease (CD) are chronic idiopathic inflammatory bowel diseases (IBDs) for which no medical cure presently exists. Patients with moderate‐to‐severe IBD typically require treatment with immunosuppressive medications, and biologic agents are gaining favour due to their efficacy and safety profiles.1, 2 Hypothesised mechanisms of lack or loss of response to monoclonal antibodies include increased serum clearance and development of immunogenicity, both of which may lead to a decrease in serum concentrations and decrease in clinical response rates.3 Numerous studies of anti‐tumour necrosis factor alpha (anti‐TNFα) therapy have documented an association between low serum drug concentrations and low rates of favourable outcomes, such as clinical response, clinical remission and endoscopic mucosal healing.4, 5, 6, 7 As such, exposure‐response evaluations with measurement of drug concentrations and anti‐drug antibody concentrations have been increasingly utilised in an effort to optimise the use of these agents.3, 8, 9, 10
Vedolizumab is a humanised monoclonal antibody that specifically binds to the α4β7 integrin and blocks lymphocyte interaction with mucosal addressin cell adhesion molecule‐1 expressed on the endothelium of mesenteric lymph nodes and gastrointestinal mucosa.11 As a result, vedolizumab impairs the migration of gut‐homing lymphocytes into gastrointestinal mucosa and acts via a gut‐selective mechanism of action.11 The efficacy of vedolizumab for the treatment of active UC was demonstrated in the large, randomised, controlled trial GEMINI 1.12 Among patients in GEMINI 1 treated with vedolizumab, 300 mg every 8 weeks (the US Food and Drug Administration [FDA]‐approved labelled dosing), a trend towards increasing clinical response at week 52 with increasing vedolizumab concentration quartiles at week 46 was observed.12 In addition, a post‐hoc analysis of GEMINI 1 reported that rates of endoscopic mucosal healing at weeks 6 and 52 improved with increasing vedolizumab concentration quartiles at weeks 6 and 46 respectively.13 A post‐hoc analysis of data from the wider GEMINI clinical trial programme including patients with either UC or CD found that higher vedolizumab concentrations were associated with higher clinical remission rates.14 Although several small real‐world observational studies have evaluated vedolizumab concentrations and clinical outcomes,15, 16, 17, 18 the studies did not control for the influence of confounding factors on potential associations. Therefore, there is a need for further exploration in an established vedolizumab treatment population such as the participants in the vedolizumab GEMINI trials.
To date, exposure‐response studies of biologics in IBD have had two important limitations: (a) they were not designed prospectively with the primary aim of making inferences about the drug exposure‐response relationship; and (b) data were analysed on a population level rather than an individual level, without adjustment for variables that could potentially affect drug clearance and resulting drug concentrations, most notably disease activity/inflammatory burden, serum albumin concentration and body weight.3, 19 Therefore, studies demonstrating differences in drug concentrations between responders and nonresponders reveal considerable heterogeneity, not only in drug concentration cut‐off values predictive of response, but also in drug concentration ranges between responders and nonresponders.4, 7, 20, 21, 22, 23, 24
Thus, given the limited information on the exposure‐response relationship with vedolizumab and the paucity of adjusted exposure‐response data for biologic agents used to treat IBD, the analyses herein aimed to characterise the relationship between vedolizumab exposure and response in UC using patient‐level data from GEMINI 1 adjusted for variables known to affect drug clearance and serum concentration. Although methodologies such as quartile analyses and receiver operating characteristic analyses have historically been employed to evaluate the relationship between biologic drug exposure and response, these approaches have the major limitation of not accounting for confounding factors and correlations between endpoints. Consequently, propensity‐score‐based case‐matching was used in the current analysis because one of its unique strengths is the ability to account for confounding factors. Potential vedolizumab concentration targets at important time points during treatment (weeks 6, 14 and steady state) were also proposed. Of note, the data from the week 6 proposed concentration target were generated in part to help design the currently ongoing vedolizumab dose‐optimisation randomised controlled trial, ENTERPRET (NCT03029143). An additional aim was to identify whether early vedolizumab concentrations (at weeks 2, 4 and 6) were associated with improved short‐term (at week 14) and long‐term (at week 52) clinical outcomes in GEMINI 1.
2. METHODS
2.1. Patient population
In the induction phase of GEMINI 1, patients received either double‐blind or open‐label vedolizumab 300 mg at weeks 0 and 2. Patients with a clinical response at week 6 were re‐randomised 1:1:1 to receive vedolizumab 300 mg every 8 weeks, vedolizumab 300 mg every 4 weeks or placebo, and followed to week 52 (Figure S1).13 Patients without a response at week 6 received vedolizumab 300 mg every 4 weeks throughout the maintenance phase. Patients treated with placebo during induction continued to receive placebo during maintenance. Ethical guidelines have been previously published for GEMINI 1 (ClinicalTrials.gov number, NCT00783718).12
2.2. Outcome measures
In GEMINI 1, clinical assessments were performed using the complete Mayo Score at weeks 0, 6 and 52 and the 9‐point partial Mayo Score (eg, complete Mayo Score without the endoscopy sub‐score) at all other time points. To examine the vedolizumab exposure‐response relationship in the present analysis, two outcomes were used: (a) clinical response, defined as a reduction in complete or partial Mayo Score of ≥3 points and ≥30% from baseline, as well as a decrease of ≥1 point on the rectal bleeding sub‐score or an absolute rectal bleeding sub‐score ≤1 and (b) clinical remission, defined as a complete or partial Mayo Score of ≤2 points with no individual sub‐score >1. To examine the association between early vedolizumab concentrations at weeks 2, 4 and 6 and clinical outcomes at weeks 14 and 52, clinical remission was selected as the sole outcome measure, given that it is a more rigorous endpoint, and was defined for both time points as a partial Mayo Score of ≤2 points with no individual sub‐score >1.
2.3. Vedolizumab concentration measurement
In GEMINI 1, vedolizumab concentrations in serum samples were measured using a direct capture, pharmacokinetic, enzyme‐linked immunosorbent assay (ELISA; sandwich), with a lower limit of detection of 0.125 µg/mL. The time points at which these concentrations were drawn were just prior to vedolizumab infusions at weeks 2 and 6 and at the week 4 study visit.
2.4. Covariates and estimation of individual vedolizumab exposures
A number of variables that can potentially affect the clearance of vedolizumab have been identified in a prior population pharmacokinetic analysis and included patient age, weight, history of anti‐TNFα treatment, serum albumin concentration and faecal calprotectin concentration.19 In that analysis, which included approximately 20 000 serum samples from 2000 patients, vedolizumab clearance and serum concentrations were estimated based on multiple vedolizumab studies, including a phase 1 healthy volunteer study, a phase 2 study in UC and the large phase 3 GEMINI 1 and 2 randomised controlled trials (GEMINI 2 showed that vedolizumab was effective as induction and maintenance therapy for active CD).12, 19, 25, 26 These data led to the development of a full covariate model capable of characterising vedolizumab pharmacokinetics and pharmacodynamics more completely.19 In the current analysis, the relationship between patient‐specific covariates and estimated vedolizumab clearance and vedolizumab concentrations were expressed as P values for the monotonic trend analysis. P values for trends were also explored using the exact Cochran‐Armitage trend test.
2.5. Propensity‐score case‐matching adjustment of the exposure‐response relationship
The GEMINI 1 trial lacked a prospectively randomised dose‐ranging design, thus creating the potential for an imbalance in observed and unobserved patient‐specific covariates that could confound causal inference in the vedolizumab exposure‐response relationship. Candidate confounding covariates in the observed data were identified based on the prior multi‐study population pharmacokinetic analysis mentioned above.19 Imbalance in the observed covariates across exposure quartiles was evaluated graphically and with quartile‐specific data summaries.
To characterise the vedolizumab exposure‐response relationship, a propensity‐score‐based case‐matching analysis was performed, adjusting for potential imbalance in observed covariates (patient age, weight, history of anti‐TNFα treatment, serum albumin concentration and faecal calprotectin concentration) across the exposure range. For each vedolizumab exposure quartile at both week 6 and steady state, a logistic propensity‐score model was fitted to data from the vedolizumab‐treated patients and all control subjects (ie, those receiving placebo), using all measured covariates as predictors. The propensity score was used to match treated patients with controls to establish a reference point for response at zero drug exposure (eg, absence of vedolizumab). A robust estimate of the standard deviation (SD) of the propensity‐score distribution was then obtained based on the median absolute deviation of the fitted propensity scores. For each patient in the exposure quartile, a match was randomly selected with replacement from the subjects in the control arm with propensity scores within a calliper of 0.2 times the robust estimate of the SD obtained in the previous step. Treated patients without a matched control were excluded from the outcome analysis. The matching step was repeated 1000 times for each candidate match, and the absolute standardised difference in means (ASDM) was calculated for all covariate main effects and two‐way interactions. The optimal subset of matched controls was identified as the candidate match with the lowest maximum ASDM among interaction effects that satisfied an ASDM <0.2 for all main effects. This method resulted in an exposure‐response data subset that was balanced across observed covariates. The extent of remaining imbalance across unobserved factors was not possible to assess.
The exposure‐response analysis was conducted using the full data set and the case‐matched subset. Specifically, the rates of clinical response and remission and the distribution of the odds ratios of clinical response and remission were calculated for each quartile of estimated vedolizumab clearance at week 6 and estimated vedolizumab concentrations at week 6 and steady state (trough, during maintenance). Trends in the distribution of the odds ratios of clinical response and remission with increasing estimated vedolizumab clearance or estimated concentration quartiles were examined to determine the robustness of the exposure‐response relationship.
For this analysis, a clinically meaningful target response was defined as a reduction in partial Mayo Score of at least 3 units. The quartiles of clearance and exposure associated with this response magnitude were identified. Given the quartile‐based boundary on clearance, the FDA label‐specified dosing regimen with vedolizumab maintenance at 300 mg every 8 weeks, and the prior population pharmacokinetic model, potential target vedolizumab concentrations associated with a clinically meaningful response were proposed at therapeutically important time points: week 6 (during induction), week 14 (end of induction period) and steady state (representing trough, during maintenance after day 128 [ie, after five 25.5‐day linear elimination half‐lives of vedolizumab]).
To determine whether early vedolizumab concentrations at weeks 2, 4 and 6 were associated with clinical remission at week 14, the analysis cohort was restricted to GEMINI 1 patients in the intention‐to‐treat population who were randomised to receive maintenance vedolizumab 300 mg every 8 weeks, as this is the FDA‐approved dose and reflects current clinical practice. Two separate analyses were conducted. First, vedolizumab concentrations at weeks 2, 4 and 6 were stratified by clinical remission status at week 14. Second, clinical remission status at week 14 was stratified by vedolizumab concentration quartiles at weeks 2, 4 and 6. Patients were not case‐matched to maximise the number of samples for analysis. The nonparametric Wilcoxon rank‐sum test was used to compare vedolizumab concentrations at weeks 2, 4 and 6 stratified by remitters vs nonremitters at week 14. Trends in clinical remission status at week 14 or week 52 stratified by vedolizumab serum concentration quartiles at weeks 2, 4 and 6 were explored using the exact Cochran‐Armitage trend test.
Compared with week 6, vedolizumab concentrations at weeks 2 and 4 did not have as consistent an association with rates of clinical remission at week 14 (see Section 2.1). The association between clinical remission at week 52 and early vedolizumab concentrations was therefore restricted to week 6 concentrations and was calculated by vedolizumab concentration quartile. To increase the sample size for this analysis, the entire GEMINI 1 cohort12 was used, including subjects who received placebo and open‐label induction vedolizumab, and patients were not case‐matched.
Of note, clinical outcomes with respect to the presence of anti‐vedolizumab antibodies were not assessed in this analysis, as only 3.7% of GEMINI 1 patients were antibody positive at any time and only 1.0% were persistently positive (ie, at ≥2 consecutive visits).12 Thus, the sample size of the antibody‐positive population was too small to perform any meaningful analysis.
3. RESULTS
3.1. Vedolizumab exposure‐response relationship and proposed potential target concentrations
Of the 746 patients who received at least one dose of vedolizumab in GEMINI 1, 693 (93%) had vedolizumab concentrations assessed at week 6 and were included in the exposure‐response analysis. Characteristics of these patients are shown in Table 1. Of note, patients had a mean disease duration of 6.9 years, mean complete Mayo Score of 8.5 and a mean partial Mayo Score of 6.0; 347 (50%) had disease proximal to the splenic flexure, and 281 (41%) had received prior anti‐TNFα therapy.
Table 1.
Baseline demographics and disease characteristics: Patients in GEMINI 112 with available data on vedolizumab pharmacokinetics at week 6
| Characteristic | Vedolizumab pharmacokinetic population (n = 693) |
|---|---|
| Age (y), mean (SD) | 40.2 (13.3) |
| Female sex, n (%) | 290 (42) |
| Body weight (kg), mean (SD) | 73.6 (18.8) |
| Disease duration (y), mean (SD) | 6.9 (6.2)[Link] |
| Disease activity, mean (SD) | |
| Mayo Clinic Score | 8.5 (1.7) |
| Partial Mayo Clinic Score | 6.0 (1.6) |
| Disease localisation, n (%) | |
| Proctosigmoiditis | 83 (12) |
| Left‐sided colitis | 264 (38) |
| Extensive colitis | 86 (12) |
| Pancolitis | 260 (38) |
| Prior anti‐TNFα failure, n (%) | 281 (41) |
| Serum albumin (g/L), mean (SD) | 37.0 (4.8) |
| Faecal calprotectin (μg/g), median (range) | 880.5 (23.8‐20 000.0) |
SD, standard deviation; TNFα, tumour necrosis factor alpha.
n = 691.
Trends were observed between patient‐specific covariates and estimated clearance and concentrations of vedolizumab (Figure 1). Specifically, history of prior anti‐TNFα treatment (P < 0.0001), lower serum albumin concentration (P < 0.0001) and higher faecal calprotectin concentration (P < 0.0001) were each associated with higher estimated vedolizumab clearance quartiles at week 6 and lower estimated vedolizumab concentration quartiles at both week 6 and steady state. Similar but less consistent associations were also observed for patient age (P ≤ 0.0210) and weight (P ≤ 0.0013). These findings indicated a strong imbalance in the distribution of measured covariates across exposure or clearance quartiles, but it is unknown if similar imbalance existed across other unmeasured factors. For each vedolizumab concentration quartile at week 6 and steady state prior to covariate‐based case‐matching, the majority of the ASDMs for the patient‐specific covariate main effects were >0.1. However, these ASDMs were <0.1 after case‐matching, indicating good matching between patients receiving vedolizumab and those receiving placebo.
Figure 1.
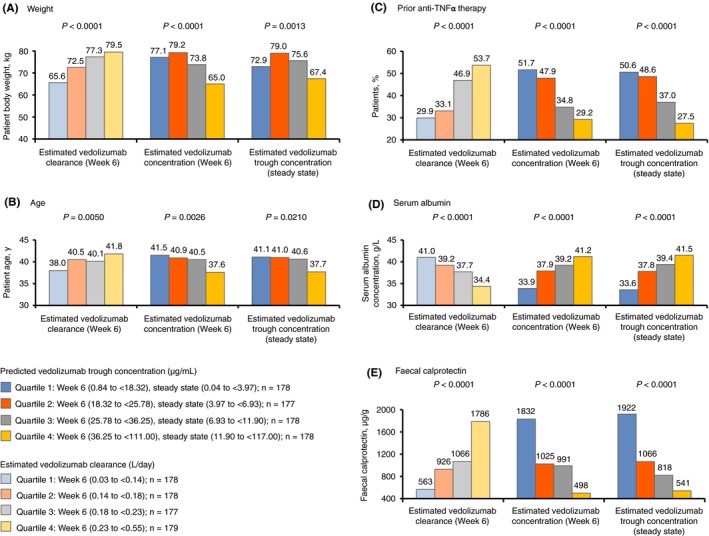
Patient‐specific covariates (A) weight, (B) age, (C) prior anti‐TNFα therapy, (D) serum albumin and (E) faecal calprotectin, stratified by quartiles from low (quartile 1) to high (quartile 4) for estimated concentration and estimated clearance of vedolizumab at week 6 and at steady state. The relationship between patient‐specific covariates and estimated vedolizumab clearance and vedolizumab concentrations was expressed as P values for the monotonic trend analysis (A, B, D, E). P values for prior anti‐TNFα therapy were determined using the exact Cochran‐Armitage trend test (C). TNFα, tumour necrosis factor alpha
Given the case‐matching‐adjusted data, rates of clinical response and remission decreased with increasing estimated vedolizumab clearance quartiles at week 6, whereas these rates rose with increasing estimated vedolizumab concentration quartiles at both week 6 and steady state (P < 0.0001; Figure 2). Specifically, clinical response rates ranged from 27%‐32% in the highest clearance/lowest vedolizumab concentration quartiles to 62%‐66% in the lowest clearance/highest vedolizumab concentration quartiles. Clinical remission rates ranged from 6%‐8% in the highest clearance/lowest vedolizumab concentration quartiles to 33%‐36% in the lowest clearance/highest vedolizumab concentration quartiles. When examining the distribution of the odds ratios of clinical response and remission by estimated vedolizumab clearance quartiles at week 6 and by estimated vedolizumab concentration quartiles at week 6 and steady state, consistent trends towards increasing odds ratios with decreasing clearance/increasing vedolizumab concentration quartiles were observed. These trends were most apparent for clinical response with week 6 vedolizumab clearance and steady‐state vedolizumab concentrations, and for clinical remission with week 6 vedolizumab concentrations (Figure 3).
Figure 2.
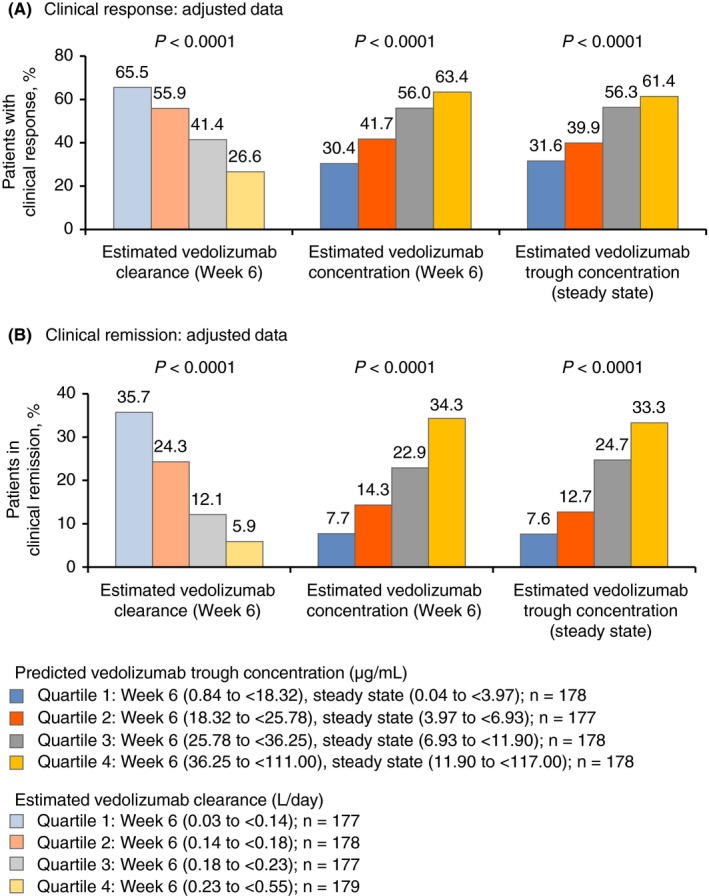
Clinical response (A) and remission (B) (adjusted for age, weight, history of prior anti‐TNFα therapy, serum albumin concentration and faecal calprotectin concentration), stratified by quartiles from low (quartile 1) to high (quartile 4) for estimated concentration and estimated clearance of vedolizumab at week 6 and steady state (n = 170‐177). P values were determined using the exact Cochran‐Armitage trend test. Clinical response was defined as a reduction in partial Mayo Score of ≥3 points and a ≥30% decrease from baseline, with a decrease of ≥1 point on the rectal bleeding sub‐score or an absolute rectal bleeding score ≤1. Clinical remission was defined as a partial Mayo Score of ≤2 points with no individual sub‐score >1 point and mucosal healing (endoscopic sub‐score ≤1). TNFα, tumour necrosis factor alpha
Figure 3.
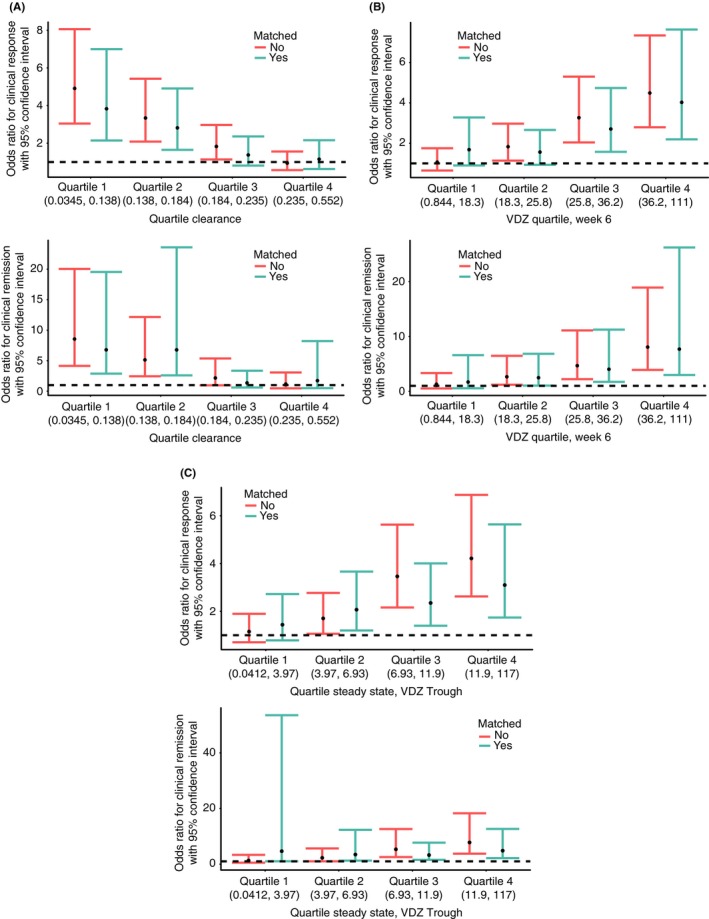
Odds ratios for: (A) clinical response and remission by estimated vedolizumab clearance quartiles at week 6; (B) vedolizumab concentration quartiles at week 6; and (C) estimated vedolizumab trough concentration quartiles at steady state from low (quartile 1) to high (quartile 4). Dashed lines indicate zero
Because an estimated vedolizumab clearance of <0.14 L/d was associated with high rates of clinical response, potential target vedolizumab concentrations at the clinically important time points of week 6 (during induction), week 14 (end of induction period) and steady state (representing trough, during maintenance) were proposed based on this value. Using the vedolizumab pharmacokinetic model and the standard FDA‐approved every‐8‐weeks maintenance dosing schedule of vedolizumab, the following concentrations were calculated for a clearance of ≤0.14 L/d: >37.1 μg/mL at week 6, >18.4 μg/mL at week 14 and >12.7 μg/mL at steady‐state trough.
3.2. Association of early vedolizumab concentrations and clinical remission at weeks 14 and 52
To determine the earliest time point at which measurement of vedolizumab concentrations was associated with clinical remission at week 14, vedolizumab concentration data at weeks 2, 4 and 6 were assessed in patients randomised to vedolizumab 300 mg every 8 weeks in the maintenance arm of GEMINI 1. Patients in this arm were similar to those receiving vedolizumab 300 mg every 4 weeks or to those receiving placebo in terms of baseline characteristics (including disease activity and prior immunomodulator use), except for a numerically longer disease duration for patients receiving vedolizumab every 4 weeks (disease duration of 7.6 years) compared with vedolizumab every 8 weeks (disease duration of 6.2 years) (Table S1). Patients who achieved clinical remission at week 14 showed higher week 4 (P = 0.0328) and week 6 (P = 0.0418) vedolizumab concentrations compared with those who did not achieve remission at week 14; at week 2, vedolizumab concentrations were generally similar between patients who later achieved remission at week 14 and those who did not (P = 0.4592; Figure 4). When examining clinical remission status at week 14 by vedolizumab concentration quartiles at weeks 2, 4 and 6, a consistent trend for increasing remission with increasing vedolizumab concentration was observed at week 4 (P = 0.0469) and week 6 (P = 0.0098), but not at week 2 (P = 0.6766; Figure 5). At week 6, a 40% remission rate was reported in the lowest concentration quartile vs 74% in the highest quartile.
Figure 4.
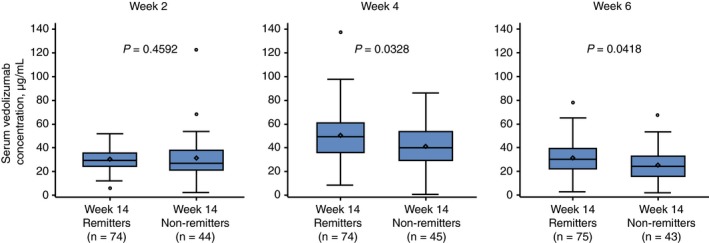
Vedolizumab concentrations at weeks 2, 4 and 6, stratified by remission status at week 14.a P values were determined using the Wilcoxon rank‐sum test. aPatients positive for human anti‐human antibodies were excluded from this analysis; patients received vedolizumab at weeks 0, 2, 6 and 14; clinical remission was defined as a partial Mayo Score of ≤2 points with no individual sub‐score >1 point. Patients were not case‐matched for this analysis. Midlines represent medians, and individual points inside the boxes represent means. Box limits represent 25th and 75th percentiles. Whiskers (error bars) represent highest and lowest points within 1.5× interquartile range. Individual points above and below whiskers represent outliers
Figure 5.
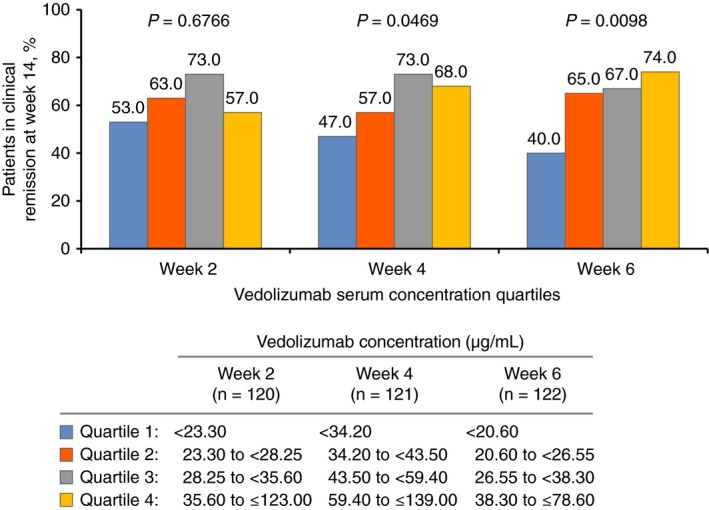
Clinical remission statusa at week 14 in patients with ulcerative colitis, stratified by estimated vedolizumab concentration quartiles from low (quartile 1) to high (quartile 4) at weeks 2, 4 and 6. P values were determined using the exact Cochran‐Armitage trend test. aClinical remission was defined as a partial Mayo Score of ≤2 points with no individual sub‐score >1 point. Patients were not case‐matched for this analysis
Given that it was only at week 6 that vedolizumab concentrations exhibited a consistent association with rates of clinical remission at week 14, the association between clinical remission at week 52 and early vedolizumab concentrations was restricted to week 6, but was expanded to include the entire GEMINI 1 cohort. Clinical remission rates at week 52 increased consistently with increasing quartiles of vedolizumab concentration at week 6, with a 15% remission rate in the lowest concentration quartile and 37% in the highest quartile (Figure 6).
Figure 6.
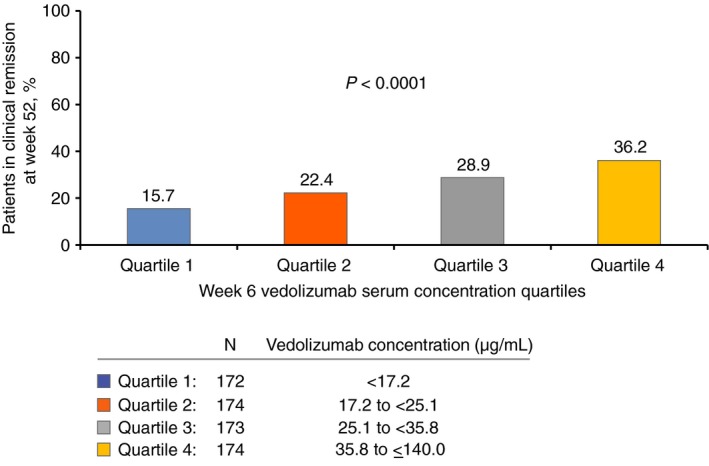
Clinical remissiona rates at week 52 in patients with ulcerative colitis, stratified by estimated vedolizumab concentration quartiles from low (quartile 1) to high (quartile 4) at week 6. P values were determined using the exact Cochran‐Armitage trend test. aClinical remission was defined as a partial Mayo Score of ≤2 points with no individual sub‐score >1 point. Patients were not case‐matched for this analysis
4. DISCUSSION
Exposure‐response metrics have increasingly been used to optimise IBD treatment with anti‐TNFα agents, but the vast majority of the reported analyses were based on studies not prospectively designed from exposure‐response inferences or adjusted for potential confounding factors.3, 8, 9, 10 In fact, only recently was the first study with patient‐level covariate adjustment of the exposure‐response relationship for anti‐TNFα therapy published, by Vande Casteele et al,27 in patients with CD treated with certolizumab pegol. Very limited data on the vedolizumab exposure‐response relationship are currently available to guide clinicians. Our analysis of the large GEMINI 1 randomised controlled trial data set identified a clear exposure‐response relationship for vedolizumab in UC, even after adjusting for potential confounding factors. Potential target vedolizumab concentrations during therapy were proposed using pharmacokinetic and pharmacodynamic data from multiple trials as follows: >37.1, >18.4 and >12.7 μg/mL at week 6, week 14 and steady state (ie, after day 128) respectively. Additionally, when examining early measurements of vedolizumab, unadjusted concentrations at week 6 were consistently associated with short‐ and long‐term clinical remission (at weeks 14 and 52 respectively), implying that the early use of exposure‐response data during treatment may provide an opportunity for dose optimisation sooner than week 10, as was previously described.28
The association between observed vedolizumab concentrations and clinical efficacy is not surprising because similar trends have been observed with anti‐TNFα treatment of patients with IBD, and a wealth of data demonstrates that higher infliximab and adalimumab concentrations during maintenance are associated with improved clinical outcomes.3, 4, 5, 6, 7, 8, 9, 21, 22, 23, 24 Furthermore, recent data have shown that higher induction and/or immediate post‐induction serum concentrations of infliximab and adalimumab, starting as early as week 2, are associated with improved outcomes in IBD.7, 29, 30 The interpretation of these results, however, is limited by the lack of prospective exposure‐response designs, and it is not known if a true causal relationship between exposure and response exists at the doses studied.
The results of the current analysis are consistent with those of several recent observational studies that examined a potential relationship between vedolizumab concentration and treatment response. Additional published data regarding vedolizumab concentrations and clinical outcomes include a recent retrospective analysis of a real‐world population of 179 patients with IBD (UC, 66; CD, 113), which demonstrated a significant correlation between vedolizumab exposure and treatment response. Vedolizumab trough concentrations >30 μg/mL at week 2, >24 μg/mL at week 6 and >14 μg/mL during maintenance therapy were associated with a higher probability of achieving improved efficacy (endoscopic healing, clinical response, biologic response or remission; P < 0.05).15 A prospective, real‐world study demonstrated that patients with IBD who achieved clinical remission had higher vedolizumab concentrations compared to those with active disease.17 Similarly, a prospective, single‐centre, observational study reported a significant correlation between higher vedolizumab trough levels and improved clinical response in patients with IBD.16 Finally, a prospective, multicentre, observational study demonstrated that early vedolizumab trough levels predict mucosal healing in patients with IBD.18 Collectively, these results support the monitoring of vedolizumab trough concentrations in patients with IBD as a means of predicting clinical outcomes and suggest that early monitoring may be helpful in identifying patients who may derive benefit from an intensified dosing regimen. However, these were small observational studies that showed consistent results despite the lack of adjustment for confounding factors. To our knowledge, the current study represents the largest vedolizumab patient data set available for this type of analysis, and the analyses were adjusted for variables that could potentially affect drug clearance and resulting drug concentrations.
The current analysis was undertaken to extend these observations and evaluate the vedolizumab exposure‐response profile, with adjustment for variables known to affect vedolizumab clearance. Previously reported exposure‐response data on anti‐TNFα therapy have been almost exclusively population‐based rather than at an individual patient level, without adjustment for factors, such as disease activity and serum albumin, which can potentially confound the drug exposure‐response relationship. In contrast, the present analysis was adjusted for the five variables that have been previously identified as being independently influential on vedolizumab drug clearance (patient age, weight, history of anti‐TNFα therapy, serum albumin concentration and faecal calprotectin concentration).19 The propensity‐score‐based case‐matching algorithm used in this analysis created well‐matched data that allowed analysis of the drug exposure‐response relationship without confounding due to these variables.
Interestingly, the potential vedolizumab concentration target of >37.1 μg/mL at week 6 that we proposed in this analysis to optimise clinical response is very similar to that observed in a recent post‐hoc analysis of endoscopic mucosal healing in GEMINI 1.13 In that study, in which rates of mucosal healing improved with increasing vedolizumab concentration quartiles, the highest vedolizumab concentration quartile at week 6 corresponded to a concentration of >35.7 μg/mL. In contrast, a study by Williet et al reported that vedolizumab trough concentrations of <18.5 µg/mL at week 6 were associated with a need for drug optimisation within 6 months, with an AUROC of 72%.31 However, that study differed from the present analysis in a number of ways: the Williet et al study was rather small, with only 47 patients in total; two‐thirds of the patients in that study had CD, which may be important because the observed vedolizumab dose‐response relationship was less consistent in GEMINI 2 (CD)25 compared with GEMINI 1 (UC)12; and that study did not adjust for factors known to affect vedolizumab clearance. Nevertheless, the median serum vedolizumab concentration in primary responders was 42.5 µg/mL at week 6 in the Williet et al study, which is similar to the week 6 value of >37.1 μg/mL derived in our analysis. Furthermore, based on the results of our analysis, week 6 appears to be the earliest time point to consider therapeutic drug monitoring with vedolizumab, given the consistent association and statistical trends between vedolizumab concentration quartiles and clinical outcomes.
The strength of the present analysis is that the largest data sets of vedolizumab pharmacokinetic data (derived from the multi‐study population pharmacokinetic analysis19) and clinical response data (from GEMINI 112) were combined to propose potential target vedolizumab concentrations during treatment and to identify the earliest time point at which exposure‐response analysis might be beneficial in clinical practice. Additionally, data were taken from randomised controlled trials, which represent the least biased method of data collection currently available.
Although the present analysis is limited by its post‐hoc design, it likely provides the most robust insight into vedolizumab exposure‐response to date. An ongoing, phase 4, open‐label, randomised controlled trial (ENTERPRET; NCT03029143) is the first clinical study to prospectively evaluate vedolizumab exposure‐response; this study is comparing the efficacy and safety of intravenous vedolizumab dose optimisation vs standard vedolizumab intravenous dosing in patients with moderately to severely active UC who are nonresponders and have high vedolizumab clearance. ENTERPRET, which was designed using the results of the present analysis as a guide for dose adjustment in the dose‐optimisation arm, began enrolling patients in April 2017.
In conclusion, this analysis of the vedolizumab exposure‐response relationship used patient‐level data from a large data set and adjusted for factors known to affect vedolizumab clearance. Potential target vedolizumab concentrations at weeks 6, 14 and steady state during treatment were proposed to be >37.1, >18.4 and >12.7 µg/mL respectively. Additionally, week 6 was identified as the earliest time point at which vedolizumab concentrations were consistently associated with short‐ and long‐term remission. Until the ongoing ENTERPRET randomised controlled trial is completed, the results of the present analysis provide the most comprehensively adjusted description of the exposure‐response relationship for vedolizumab in UC to date.
AUTHORSHIP
Guarantor of the article: Dr Osterman serves as the guarantor of the article.
Author contributions: In the preparation of this article, all authors made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data. Dr Osterman prepared the initial draft and all authors participated in preparing the full draft of this manuscript or revised it critically for important intellectual content. All authors also approved the final version of the manuscript, including the authorship list, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
ACKNOWLEDGEMENTS
Declaration of personal interests: Mark T. Osterman has served as an advisory board consultant for AbbVie, Janssen, Lycera, Merck, Pfizer, Takeda, UCB; and has received research grant support from UCB. Maria Rosario is an employee of Takeda International Co. Karen Lasch is an employee of Takeda Pharmaceuticals USA, Inc. Morris Barocas was an employee of Takeda Pharmaceuticals USA, Inc. at the time the study was conducted. Jayson D. Wilbur is an employee of Metrum Research Group, LLC, which was a contract research provider to Takeda for the vedolizumab development programme. Nathanael L. Dirks is an employee of Metrum Research Group, LLC, which was a contract research provider to Takeda for the vedolizumab development programme. Marc R. Gastonguay is an employee of Metrum Research Group, LLC, which was a contract research provider to Takeda for the vedolizumab development programme.
Osterman MT, Rosario M, Lasch K, et al. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther. 2019;49:408–418. 10.1111/apt.15113
Morris Barocas performed this work while employed with Takeda Pharmaceuticals USA.
The Handling Editor for this article was Professor Ailsa Hart, and it was accepted for publication after full peer‐review.
Funding information
Financial support was provided by Takeda.
This study was sponsored by Takeda. Medical writing support was provided by Claudia Wiedemann, PhD, of Chameleon Communications International Ltd, UK (a Healthcare Consultancy Group Company) and funded by Takeda.
The copyright line for this article was changed on 30 May 2019 after original online publication.
REFERENCES
- 1. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence‐based consensus on the diagnosis and management of Crohn's disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3‐25. [DOI] [PubMed] [Google Scholar]
- 2. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence‐based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11:769‐784. [DOI] [PubMed] [Google Scholar]
- 3. Colombel JF, Feagan BG, Sandborn WJ, Van Assche G, Robinson AM. Therapeutic drug monitoring of biologics for inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:349‐358. [DOI] [PubMed] [Google Scholar]
- 4. Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C‐reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721‐1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reinisch W, Colombel JF, Sandborn WJ, et al. Factors associated with short‐ and long‐term outcomes of therapy for Crohn's disease. Clin Gastroenterol Hepatol. 2015;13:539‐547 e532. [DOI] [PubMed] [Google Scholar]
- 6. Ungar B, Levy I, Yavne Y, et al. Optimizing anti‐TNF‐alpha therapy: Serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:550‐557. [DOI] [PubMed] [Google Scholar]
- 7. Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long‐term outcome of adalimumab therapy in Crohn's disease. Gastroenterology. 2009;137:1628‐1640. [DOI] [PubMed] [Google Scholar]
- 8. Vaughn BP, Martinez‐Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20:1996‐2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320‐1329. [DOI] [PubMed] [Google Scholar]
- 10. Mosli MH, Sandborn WJ, Kim RB, Khanna R, Al‐Judaibi B, Feagan BG. Toward a personalized medicine approach to the management of inflammatory bowel disease. Am J Gastroenterol. 2014;109:994‐1004. [DOI] [PubMed] [Google Scholar]
- 11. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437‐1444. [DOI] [PubMed] [Google Scholar]
- 12. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699‐710. [DOI] [PubMed] [Google Scholar]
- 13. Rosario M, Abhyankar B, Sankoh S, Dirks N, Lasch K, Sandborn W. Relationship between vedolizumab pharmacokinetics and endoscopic outcomes in patients with ulcerative colitis. J Crohns Colitis. 2015;9(Suppl. 1):S46. [Google Scholar]
- 14. Rosario M, French JL, Dirks NL, et al. Exposure‐efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn's disease. J Crohns Colitis. 2017;11:921‐929. [DOI] [PubMed] [Google Scholar]
- 15. Dreesen E, Verstockt B, Bian S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:1937‐1946.e8. [DOI] [PubMed] [Google Scholar]
- 16. Schulze H, Esters P, Hartmann F, et al. A prospective cohort study to assess the relevance of vedolizumab drug level monitoring in IBD patients. Scand J Gastroenterol. 2018;53:670‐676. [DOI] [PubMed] [Google Scholar]
- 17. Ungar B, Kopylov U, Yavzori M, et al. Association of vedolizumab level, anti‐drug antibodies, and alpha4beta7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:697‐705e697. [DOI] [PubMed] [Google Scholar]
- 18. Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment. Pharmacol. Ther. 2018;47:906‐912. [DOI] [PubMed] [Google Scholar]
- 19. Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics‐pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn's disease. Aliment Pharmacol Ther. 2015;42:188‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Assche G, Magdelaine‐Beuzelin C, D'Haens G, et al. Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861‐1868. [DOI] [PubMed] [Google Scholar]
- 21. Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohns Colitis. 2013;7:736‐743. [DOI] [PubMed] [Google Scholar]
- 22. Imaeda H, Bamba S, Takahashi K, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with Crohn's disease under scheduled maintenance treatment. J. Gastroenterol. 2014;49:674‐682. [DOI] [PubMed] [Google Scholar]
- 23. Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:80‐84e82. [DOI] [PubMed] [Google Scholar]
- 24. Sandborn WJ, Van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology. 2012;142:257‐265. [DOI] [PubMed] [Google Scholar]
- 25. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711‐721. [DOI] [PubMed] [Google Scholar]
- 26. Rosario M, Wyant T, Leach T, et al. Vedolizumab pharmacokinetics, pharmacodynamics, safety, and tolerability following administration of a single, ascending, intravenous dose to healthy volunteers. Clin Drug Investig 2016;36:913‐923. [DOI] [PubMed] [Google Scholar]
- 27. Vande Casteele N, Feagan BG, Vermeire S, et al. Exposure‐response relationship of certolizumab pegol induction and maintenance therapy in patients with Crohn's disease. Aliment Pharmacol Ther 2018;47:229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618‐627. [DOI] [PubMed] [Google Scholar]
- 29. Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short‐term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:543‐549. [DOI] [PubMed] [Google Scholar]
- 30. Papamichael K, Baert F, Tops S, et al. Post‐induction adalimumab concentration is associated with short‐term mucosal healing in patients with ulcerative colitis. J Crohns Colitis. 2017;11:53‐59. [DOI] [PubMed] [Google Scholar]
- 31. Williet N, Boschetti G, Fovet M, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol. 2017;15:1750‐1757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


