Abstract
Background and Objectives
Some patients with early‐stage oral cancer have a poor prognosis owing to the delayed neck metastasis (DNM). Tumor budding is reportedly a promising prognostic marker in many cancers. Moreover, the tissue surrounding a tumor is also considered to play a prognostic role. In this study, we evaluated whether tumor budding and adjacent tissue at the invasive front can be potential novel predictors of DNM in early tongue cancer.
Methods
In total, 337 patients with early‐stage tongue squamous cell carcinoma were retrospectively reviewed. The patient characteristics and histopathological factors were evaluated for association with DNM. DNM rates were calculated; items which were significant in the univariate analysis were used as explanatory variables, and independent factors for DNM were identified by the multivariate analysis.
Results
The univariate analysis identified T classification, depth of invasion, tumor budding, vascular invasion, and adjacent tissue at the invasive front as significant predictors of DNM; the multivariate analysis using these factors revealed all the above variables except vascular invasion, which are independent predictors of DNM.
Conclusion
In addition to conventional predictors, high grade tumor budding and adjacent tissue at the invasive front can serve as useful predictors of DNM in early tongue cancer.
Keywords: metachronous neck metastasis, prognostic indicator, small cancer‐cell clusters, tongue cancer, tumor‐adjacent tissue
1. INTRODUCTION
Oral cavity squamous cell carcinoma (OSCC) represents a heterogeneous group of head and neck cancers. OSCC is one of the leading causes of cancer‐related deaths worldwide.1, 2, 3 Tongue squamous cell carcinoma (TSCC) is the most common cancer diagnosed in the oral cavity, comprising 25%‐40% of oral carcinomas, and along with the closely related cancer of the floor of the mouth (15%‐20%) accounts for more than half of all oral carcinomas when excluding those affecting the lips.4, 5 TSCC has a high recurrence rate, and is significantly more aggressive than other forms of oral cancer, with a propensity for rapid local invasion and spread.6, 7 Tumor stage represented by the tumor, node, and metastasis staging system (TNM) is routinely regarded as the strongest prognostic parameter for patients with TSCC. However, similarly staged patients with TSCC may present with markedly different prognoses, indicating that the TNM stage cannot completely explain the tumor behavior. The detection of TSCC at an early‐stage (T1/T2N0M0) does not always lead to a good prognosis, as 20%‐40% of patients show occult metastasis at the presentation.7, 8, 9 In general, oral cancers that have clinically evident cervical lymph node metastasis are treated with tumor resection and modified radical neck dissection, whereas early‐stage oral cancers (cT1/T2N0 cases) are treated with primary tumor resection alone. In our experience, however, some cases classified as cT1‐2N0M0 have delayed neck metastasis (DNM) during the early period after tumor resection. The resection margin and pathological differentiation of tumors are unsatisfactory in many cases, with respect to predicting DNM and poor prognosis of patients.10
Therefore, it is important to identify novel predictors of metastasis for the TSCC treatment. Many investigators have proposed that detailed histopathological grading of the tumors with specific histopathological scoring systems may help clinicians in the individualization of the treatment and in prognostication of patients with OSCC.10, 11, 12, 13, 14 Although some studies have found such systems useful for prognostication of TSCC,15, 16, 17 most of the models are either too cumbersome for clinical diagnosis or have no prognostic significance, particularly for TSCC.18, 19, 20, 21
Tumor budding, defined as the presence of small single‐cancer‐cell clusters of fewer than five cells at the tumor invasive front,22, 23 has been reported to be an independent prognostic factor for several cancers such as lung, gastrointestinal, colorectal, pancreatic, and esophageal cancers.24, 25, 26, 27, 28 There are few reports on the role of tumor budding in oral cancer.29, 30, 31, 32, 33 Tumor budding represents two main features of a malignancy: loss of cell adhesion and active tumor invasion.34, 35, 36 These features represent a more aggressive and malignant tumor potential.37 As tumor budding is easily identified in hematoxylin and eosin (H&E) or in immunohistochemically stained sections, it may be useful to screen patients who are candidates for elective neck lymph node dissection or multimodal therapy for this phenomenon after the surgery. The depth of tumor invasion has also been described as an important prognostic parameter for OSCCs, and a depth of ≥ 4 mm was found to be associated with lymph node metastasis; lymph node metastasis is considered to be the most important marker for guiding therapy and for predicting prognosis in OSCC.31, 38, 39 Previous studies have reported that the response of the tissue surrounding the tumor, including the lymphocytic host response (LHR)40, 41 and cancer‐associated fibroblasts (CAFs),42, 43, 44 was correlated with the prognosis of OSCC. Therefore, we hypothesized that the adjacent tissue‐type at the invasive front may be associated with lymph node metastasis and prognosis. We, therefore, chose previously suggested histomorphological parameters and two other novel, practical, and easy‐to‐evaluate parameters, namely, tumor budding and adjacent tissue at the invasive front, and evaluated them for any prognostic relevance for TSCC. We also analyzed the correlation of tumor budding and adjacent tissue at the invasive front with clinicopathologic features and DNM in clinical early‐stage TSCC.
2. MATERIALS AND METHODS
2.1. Patients
Patients with Stage I‐II TSCC who underwent surgical resection of the primary tumor without elective neck dissection (END) between January 2008 and December 2014 were retrospectively reviewed. All the patients were at least 15 years of age and had an Eastern Cooperative Oncology Group performance status score of 0 to 3. The staging was based on the TNM classification of the Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC; 7th edition). In all patients, neck metastasis was evaluated using palpation, computed tomography (CT), cervical ultrasonography, and 18F‐fluorodeoxyglucose‐positron emission tomography/CT. As an auxiliary procedure, magnetic resonance imaging was also performed. As a rule in all institutions, if neck metastasis was not detected after the examination and imaging procedures, the patient was treated according to the “wait and see” policy without END. A total of 432 patients were included in this study. We excluded 60 patients who underwent END for reasons such as reconstructive surgery, 26 patients who had the recurrent disease at the primary site, and 9 patients for whom the sufficient pathological data were not available, leaving a total of 337 patients eligible for the enrollment in this study. The following factors were evaluated as background factors: age, sex, and disease stage. The medical records of all patients treated at the Department of Oral and Maxillofacial Surgery, Nara Medical University Hospital, Osaka University Hospital, Nagasaki University Hospital, Kobe University Hospital, Tokai University, and Shinshu University Hospital were evaluated. The study protocol was approved by the Ethics Committee of Nara Medical University and was in accordance with the Helsinki Declaration of 1975, and its revision in 1983. Informed consent was obtained from all the patients enrolled in the study.
2.2. Histopathological analyses
The formalin‐fixed and paraffin‐embedded specimens or slices archived at each institution were used for pathological analyses. Paraffin specimens were sectioned to a thickness of 4 µm and stained with H&E. All specimens were assessed by more than two pathologists who were blinded to the clinical data.
The depth of invasion (DOI) was measured from the tumor surface to the deepest point of invasion.29, 31 The cut‐off point for DOI was set at 4 mm.29, 31, 38, 45
Tumor budding is defined as the presence of a single‐cancer‐cell or small cluster of < 5 cancer cells at the invasive front.22, 23, 31, 46, 47 Tumor specimens were initially scanned with a 4× objective lens (and a 10× ocular lens) to select the appropriate areas. The number of budding foci was counted in a selected field using the 20× objective lens at the tumor invasive front (Figure 1). The patients were classified into three grades according to the number of tumor buds: low‐grade group in which the patient had no buds/field, intermediate grade group in which the patient had 1 to 4 buds/field, or the high‐grade group in which the patient had ≥ 5 buds/field.
Figure 1.
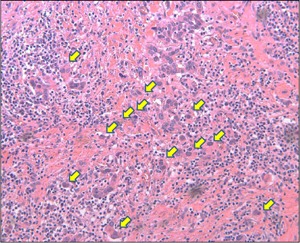
Histopathological analysis of tumor budding at the tumor invasive front (arrow; 20× magnification) [Color figure can be viewed at wileyonlinelibrary.com]
The presence of lymphoid, fibrous, muscle, and fatty tissues was assessed to evaluate the adjacent tissue at the invasive front. Representative examples of histopathological parameters are shown in Figure 2.
Figure 2.
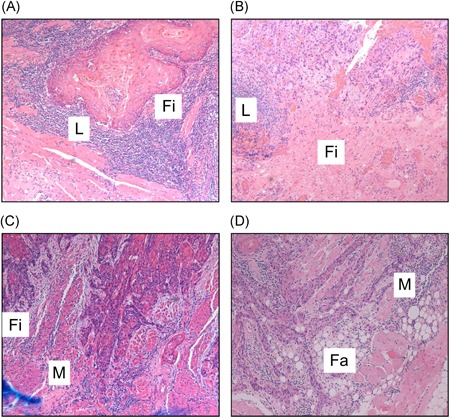
Representative examples of histopathological parameters for adjacent tissue at the invasive front. A,B, Presence of lymphocytes and fibrous tissue. C, Presence of fibrous tissue and muscle tissue. D, Presence of muscle tissue and fatty tissue. L, lymphocytes; Fi, fibrous tissue; M, muscle tissue; Fa, fatty tissue [Color figure can be viewed at wileyonlinelibrary.com]
The histological tumor differentiation was classified according to the World Health Organization (WHO) criteria, and the presence of vascular invasion and perineural invasion was evaluated.
Tumor budding and the adjacent tissue were determined at the tumor invasion front. Most other parameters were determined by conventional methods.
2.3. Statistical analyses
All analyses were performed using the StatFlex ver.6 software (Artech Co., Osaka, Japan). The DNM rates and overall survival (OS) rates were calculated by the Kaplan‐Meier method and compared using the log‐rank test. Variables with significant differences detected in the univariate analysis were used as explanatory variables to extract independent factors related to DNM by the multivariate analysis. The multivariate analysis was performed using the Cox proportional hazards model. For all statistical analyses, P values of < 0.05 were considered significant.
3. RESULTS
The patients included 192 (57.0%) men and 145 (43.0%) women, with a mean age of 61.9 years (range, 15‐92 years). Clinicopathological characteristics of the patients are summarized in Table 1. According to the UICC/AJCC TNM classification (7th edition), T1 was detected in 221 patients (65.6%) and T2 in 116 (34.4%) patients. The median follow‐up time for patients was 58.2 months (range, 4‐116 months). Based on the WHO grading system, 224 tumors (66.5%) were classified as well‐differentiated, 107 (31.7%) as moderately differentiated, and 6 (1.8%) as poorly differentiated. The DOI ranged from 0.2 to 13.0 mm (median: 2.5 mm). A DOI of < 4 mm was observed in 215 patients (63.8%), whereas a DOI of ≥ 4 mm was observed in 122 patients (36.2%). Tumor budding was not present in 243 patients (72.1%). Budding was of intermediate grade (1‐4 buds/field) in 46 patients (13.7%) and of high grade ( ≥ 5 buds/field) in 48 patients (14.2%). Venous invasion, lymphovascular invasion, and perineural invasion were observed in 59 (17.5%), 31 (9.2%), and 31 patients (9.2%), respectively. In the adjacent tissue at the invasive front, lymphocytes, fibrous tissue, muscle tissue, and fat tissue were present in 312 (92.6%), 198 (58.7%), 152 (45.1%), and 49 patients (14.5%), respectively.
Table 1.
Clinicopathological characteristics of the patients enrolled in the study
| No. | % | |
|---|---|---|
| Age | ||
| <65 | 163 | 48.4 |
| ≥65 | 174 | 51.6 |
| Sex | ||
| Male | 192 | 57.0 |
| Female | 145 | 43.0 |
| T classification | ||
| T1 | 221 | 65.6 |
| T2 | 116 | 34.4 |
| Tumor Budding grades (buds/field) | ||
| Low: 0 | 243 | 72.1 |
| Intermediate: 1‐4 | 46 | 13.7 |
| High: ≥ 5 | 48 | 14.2 |
| Differentiation | ||
| Well | 224 | 66.5 |
| Moderate | 107 | 31.7 |
| Poor | 6 | 1.8 |
| Depth of invasion (mm) | ||
| <4 | 215 | 63.8 |
| ≥4 | 122 | 36.2 |
| Venous invasion | ||
| v(‐) | 278 | 82.5 |
| v(+) | 59 | 17.5 |
| Lymphovascular invasion | ||
| ly(‐) | 306 | 90.8 |
| ly(+) | 31 | 9.2 |
| Perineural invasion | ||
| neu(‐) | 306 | 90.8 |
| neu(+) | 31 | 9.2 |
| Adjacent tissue at invasive front | ||
| Lymphocytes | ||
| Absent | 25 | 7.4 |
| Present | 312 | 92.6 |
| Fibrous tissue | ||
| Absent | 139 | 41.3 |
| Present | 198 | 58.8 |
| Muscle tissue | ||
| Absent | 185 | 54.9 |
| Present | 152 | 45.1 |
| Fatty tissue | ||
| Absent | 288 | 85.5 |
| Present | 49 | 14.5 |
DNM was detected in 58 (17.2%) of 337 patients during the overall study period. The 5‐year cumulative DNM rate for the entire cohort was 17.8% (Figure 3) and the 5‐year OS rate was 90.9%. The DNM rate was 12.6% in T1 and 28.7% in T2 cases, demonstrating a significant difference (P = 0.0001; Figure 4A, Table 2). The DNM rate according to DOI was significantly different between < 4 mm and ≥ 4 mm cases (P < 0.0001; Figure 4B, Table 2). The patients who had no buds/field had significantly different DNM rates at 10.4%, compared with DNM rates of 26.2% for patients who had intermediate grade (1‐4 buds/field) (P = 0.0012) and 47.2% for patients who had high grade (≥5 buds/field) (P < 0.0001) budding (Figure 4C, Table 2). The DNM rates were not significantly different for the lymphocytes and fibrous tissue at the adjacent tissue at invasive front (Figure 5A, B, Table 2). However, the DNM rates in the adjacent tissue at the invasive front were significantly different between cases with muscle tissue (31.6%) and those without muscle tissue (6.6%) (P < 0.0001; Figure 5C, Table 2). Furthermore, a significant difference was seen between cases with fat tissue (37.2%) and those without fat tissue (14.4%) (P < 0.0001; Figure 5D, Table 2). Patients with venous invasion and lymphovascular invasion had significantly higher DNM rates (P < 0.05, Table 2). No significant differences were observed based on sex, age, pathologic differentiation, or perineural invasion (Table 2).
Figure 3.
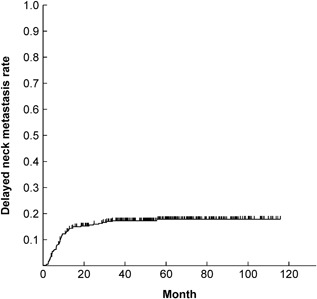
Kaplan‐Meier curves for delayed neck metastatic rate in all patients
Figure 4.
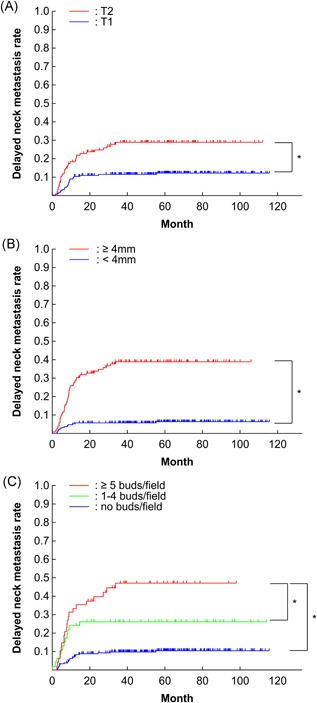
(A) Kaplan‐Meier curves for delayed neck metastatic rate according to T classification, B, depth of invasion, and C, tumor budding. *P < 0.05 [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Results of univariate and multivariate analyses
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variable | DNM rates (%) | P | bHR | 95% | P |
| Age | |||||
| <65 | 19.3 | ||||
| ≥65 | 16.3 | 0.4083 | |||
| Sex | |||||
| Male | 14.8 | ||||
| Female | 21.8 | 0.0977 | |||
| T classification | |||||
| T1 | 12.2 | Ref. | |||
| T2 | 28.7 | 0.0001* | 2.02 | 1.17‐3.47 | 0.0112* |
| Tumor Budding (buds/field) | |||||
| Low: 0 | 10.4 | Ref. | Ref. | ||
| Intermediate: 1‐4 | 26.2 | 0.0012* | 1.24 | 0.59‐2.61 | 0.5738 |
| High: ≥ 5 | 47.2 | <0.0001* | 2.22 | 1.15‐4.30 | 0.0179* |
| Differentiation | |||||
| Well | 17.9 | Ref. | |||
| Moderate | 15.9 | 0.5234 | |||
| Poor | 33.3 | 0.3118 | |||
| Depth of invasion (mm) | |||||
| <4 | 6.4 | Ref. | |||
| ≥4 | 38.7 | <0.0001* | 3.91 | 1.95‐7.85 | 0.0001* |
| Venous invasion | |||||
| v(‐) | 15.2 | Ref. | |||
| v(+) | 30.5 | 0.008* | 0.86 | 0.46‐1.60 | 0.6299 |
| Lymphovascular invasion | |||||
| ly(‐) | 16.2 | Ref. | |||
| ly(+) | 33.9 | 0.0249* | 1.07 | 0.52‐2.22 | 0.8484 |
| Perineural invasion | |||||
| Neu(‐) | 16.5 | ||||
| Neu(+) | 30.1 | 0.1009 | |||
| Adjacent tissue at invasive front | |||||
| Lymphocytes | |||||
| Absent | 16.6 | ||||
| Present | 17.9 | 0.7955 | |||
| Fibrous tissue | |||||
| Absent | 19.8 | ||||
| Present | 16.4 | 0.4002 | |||
| Muscle tissue | |||||
| Absent | 6.6 | Ref. | |||
| Present | 31.6 | <0.0001* | 2.59 | 1.27‐5.26 | 0.0087* |
| Fatty tissue | |||||
| Absent | 14.4 | Ref. | |||
| Present | 37.2 | <0.0001* | 1.41 | 0.75‐2.65 | 0.2912 |
Abbreviations: DNM, delayed neck metastasis; HR, hazard ratio; CI, confidence interval.
P < 0.05 < 0.05.
Figure 5.
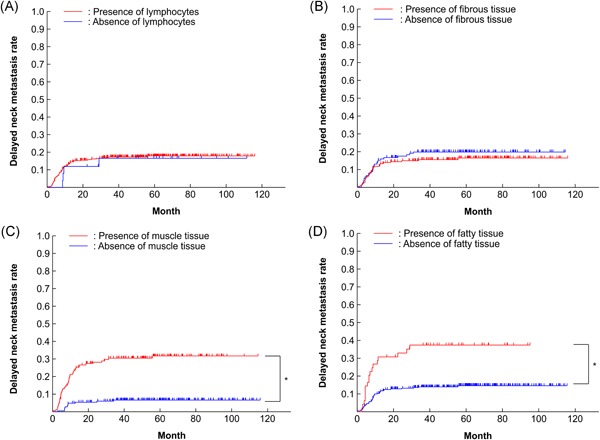
Kaplan‐Meier curves for delayed neck metastatic rate according to adjacent tissue at the invasive front. A, Lymphocytes, B, fibrous tissue, C, muscle tissue, D, and fatty tissue *P < 0.05 [Color figure can be viewed at wileyonlinelibrary.com]
Multivariate analysis using the seven parameters that correlated significantly with DNM in the univariate analyses revealed that T classification (T2, P = 0.0112, hazard ratio (HR): 2.02; 95% confidence interval (CI): 1.17‐3.47), tumor budding (high grade: ≥ 5 buds/field, P = 0.0179, HR: 2.22; 95% CI: 1.15‐4.30), DOI ( ≥ 4 mm, P = 0.0001, HR: 3.91; 95% CI: 1.95‐7.85), and presence of muscle tissue at the adjacent tissue at the invasive front (P = 0.0087, HR: 2.59; 95% CI: 1.27‐5.26) to be independent risk factors for DNM (Table 2). Tumor budding (intermediate grade: 1‐4 buds/field), venous invasion, lymphovascular invasion, and presence of fat tissue in the adjacent tissue at the invasive front were not independent factors for DNM.
4. DISCUSSION
Even patients with early‐stage oral cavity cancer (cStage I‐II) often have poor outcomes due to DNM. In particular, the prognosis of tongue cancer is relatively worse than that of oral cancers occurring at other subsites.48 In early‐stage tongue cancer, surgical resection of the primary lesion alone is often performed after patients are diagnosed as having low‐risk of cervical metastasis. However, DNM has been reported in 20%‐40% of such cases.7, 8, 9 Therefore, to lower the risk of DNM, END is indicated, with the aim of improving regional control rate. However, patients undergoing unnecessary END are at risk of developing complications. In patients with early‐stage tongue cancer, there are no clear criteria for performing END as of date, and the treatment method remains controversial.49 Thus, neck dissection is often performed without confirmed evidence of cervical lymph node metastasis.38 In recent years, sentinel node navigation surgery (SNNS) has been reported to be effective for early‐stage tongue cancer,50, 51 but SNNS is not widely used at present, and requires further studies to be accepted and performed as a routine procedure. In early‐stage tongue cancer, differentiation between low‐risk patients who can be adequately treated by resection of the primary lesion alone, and high‐risk patients in whom resection of the primary lesion alone is inadequate, is important for follow‐up and determination of the necessity for END and multimodal treatment.
To identify new predictors of DNM in patients with early‐stage tongue cancer, we evaluated several novel parameters, in addition to TNM classification and conventional histopathological parameters. As new candidate predictors, tumor budding and adjacent tissue at the invasive front were used because they can be evaluated using H&E staining alone; this analysis procedure is performed routinely, and thus saves time and is cost‐effective.
The DOI has been reported as a major predictor of occult neck metastasis or DNM in early‐stage tongue cancer. Many studies have demonstrated a marked increase in DNM when the DOI was more than 4 mm.29, 31, 38, 39, 45 In this study, we observed that a DOI ≥ 4 mm was an independent predictor of DNM.
Tumor budding has been reported to be associated with poor prognosis and lymph node metastasis in several types of carcinomas.24, 25, 26, 27, 28 In particular, in colorectal cancer, tumor budding is recognized as a prognostic factor by the WHO, and the International Tumor Budding Consensus Conference (ITBCC) achieved consensus on 10 statements regarding diagnosis and assessment.52 In addition, as≥ 5 tumor buds/field has been reported to be associated with a poor prognosis,29, 30, 31, 32 we classified patients according to the number of buds into groups with ≥ 5 buds/field (high grade), 1 to 4 buds/field (intermediate grade) or no bud/field (low grade). In this study, high‐grade tumor budding ( ≥ 5 buds/field) was an independent predictor of DNM. For more accurate evaluation of tumor budding, immunohistochemical staining of pan‐cytokeratin has been recommended by a previous study.30 However, according to the ITBCC's recommendations, “tumor budding is counted by H&E” and “tumor budding is assessed in one hotspot at the invasive front.” In this study, this assessment method was adopted for evaluating TSCC. Neck metastasis was also reported to be predicted by evaluating tumor budding in biopsy specimens.53 However, evaluation in biopsy specimens is not equivalent to the assessment at the tumor invasive front, and may not allow for accurate evaluation of other parameters. Cancer metastasis requires cancer cell invasion into the stroma, cancer cell invasion/migration into the vessels, and cancer cell colonization/proliferation in the lymph nodes.54 The initiation of invasion is known to be induced by the epithelial‐mesenchymal transition (EMT).55, 56 As a correlation between EMT and tumor budding has been reported,32, 57, 58 tumor budding can be regarded as an early step in cancer metastasis.59 Therefore, in patients exhibiting tumor budding, the risk of DNM may be high. In this study, many patients had no tumor budding. This may have been because the patients in this study had early‐stage tongue cancer and this metastasis step had not yet occurred.
Adjacent tissue at the invasive front was detected as a new candidate predictor in this study. Previous studies have reported parameters such as LHR40, 41 and CAFs,42, 43, 44 which comprise the responses of tissue surrounding the tumor, can be used as prognostic factors. LHR has been reported to be associated with a favorable prognosis10, 41, 60; CAFs, in contrast, have been reported to be correlated with a poor prognosis,42, 61 but not in early‐stage tongue cancer.31 In this study, focusing on tissue surrounding the tumor, we evaluated the types of adjacent tissue (lymphoid, fibrous, muscle, and fat tissues) at the tumor invasive front and the association between the presence of each type of tissue and the DNM rate. Univariate analysis demonstrated significant correlations between the presence of muscle/fat tissue and the DNM rate. Based on multivariate analysis, only muscle tissue was an independent predictor of DNM. The incidence of DNM was high in patients with muscle tissue at the tumor invasive front. This may have been because in addition to deep invasion, invasion along muscle fibers was also present in cases showing muscle tissue invasion. Therefore, there is a possibility that delayed metastasis was not prevented by the routine resection method. Tissues adjacent to the tumor may be evaluated accurately using immunostaining. For example, myosin staining allows for more accurate evaluation of muscle tissue. Immunostaining may thus affect the proportion of patients exhibiting each type of tissue, and influence the results of the evaluation. In other words, the presence of fat tissue may be identified as an independent prognostic factor when the presence of the muscle tissue decreases.
The main limitation of this study is that H&E staining was the only investigative method used for the analysis of resected specimens. At most institutions, H&E staining of resected specimens is used to evaluate conventional parameters. Therefore, no additional efforts in cost and time were necessary for evaluating the parameters in this study. However, there is a possibility that tumor budding and adjacent tissues at the invasive front can be more accurately evaluated by immunostaining, which requires further studies. A detailed investigation about OS was not important in this study because cases with local recurrence were excluded and the follow‐up period was short. In studies of patients with early‐stage tongue cancer, a long follow‐up period and the inclusion of local recurrence cases are necessary to investigate the correlation of OS with each parameter. Furthermore, in this study, the DOI was evaluated using resected specimens (surgical specimens), similar to previous studies.29, 31, 38, 39, 45 Preoperative imaging‐based DOI assessment was not included in the criteria for determining a therapeutic strategy and whether END should be performed. For patients who did not require reconstruction with a vascular pedicle free flap, the “wait and see” policy without END was the selected treatment method. Future studies should probably include DOI assessment with various preoperative imaging procedures to determine how significant a factor it is in predicting occult metastases.
Based on multivariate analysis, the clinical T stage (T2), DOI (≥ 4 mm), tumor budding (≥ 5 buds/field), and tumor‐adjacent tissue (muscle tissue) correlated strongly with and were independent predictors of DNM. Patients with early‐stage tongue cancer may thus be stratified based on these factors to identify those at a high‐risk for DNM. A careful follow‐up of the high‐risk patients thus identified is indicated. In addition, in patients with more than one of these risk factors, a frequent and stringent follow‐up is indicated, along with the inclusion of END or postoperative adjuvant therapy in the treatment regimen.
5. CONCLUSION
In this retrospective multi‐institutional analysis, we demonstrated that five or more tumor buds (high grade) and presence of muscle tissues adjacent to the tumor invasive front are independent predictors of DNM in Stage I and Stage II TSCC. These results suggest that, in addition to conventional predictors, tumor budding and type of adjacent tissue at the invasive front can serve as useful predictors of DNM in early‐stage TSCC.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
LIST OF AUTHORS
Nobuhiro Yamakawa, DDS, PhD, Tadaaki Kirita, DDS, PhD, Masahiro Umeda, DDS, PhD, Souichi Yanamoto, DDS, PhD, Yoshihide Ota, DDS, PhD,Mitsunobu Otsuru, DDS, PhD, Masaya Okura, DDS, PhD, Hiroshi Kurita, DDS, PhD, Shin‐ichi Yamada, DDS, PhD, Takumi Hasegawa, DDS, PhD, Tomonao Aikawa, DDS, PhD, Takahide Komori, DDS, PhD, Michihiro Ueda, DDS, PhD and the Japan Oral Oncology Group.
All authors are Japan Oral Oncology Group members.
SYNOPSIS
Although the TNM staging system has been used for predicting the prognosis in patients with early tongue cancer, the final outcome is different for each patient. Here we report that high‐grade tumor budding and the presence of muscle tissue at the invasive front are predictive of DNM, and can identify patients at high‐risk of DNM.
ACKNOWLEDGMENTS
We would like to thank the following pathologists for their help with the histopathological analyses: Dr. Chihoko Hirai from the Department of Diagnostic Pathology, Kobe University Graduate School of Medicine, Dr. Yuri Noda from the Department of Oral Pathology, Graduate School of Dentistry, Osaka University, and Dr. Tetsuya Kitamura from the Department of Oral Pathology and Biology, Division of Oral Pathobiological Science, Hokkaido University Graduate School of Dental Medicine. This study did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
We would like to thank Editage (http://www.editage.jp/) for English‐language editing.
Yamakawa N, Kirita T, Umeda M, et al. Tumor budding and adjacent tissue at the invasive front correlate with delayed neck metastasis in clinical early‐stage tongue squamous cell carcinoma. J Surg Oncol. 2019;119:370‐378. 10.1002/jso.25334
References
REFERENCES
- 1. Palme CE, Gullane PJ, Gilbert RW. Current treatment options in squamous cell carcinoma of the oral cavity. Surg Oncol Clin North Am. 2004;13:47‐70. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int J Cancer. 2010;127:2893‐2917. [DOI] [PubMed] [Google Scholar]
- 4. Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (I). Oral Oncol. 2010;46:630‐635. [DOI] [PubMed] [Google Scholar]
- 5. Regezi JA, Sciubba JJ, Jordan RCK. Oral pathology: clinical, pathologic correlations. 5th ed St Louis (MO): Saunders Elsevier; 2008. [Google Scholar]
- 6. Lydiatt DD, Robbins KT, Byers RM, Wolf PF. Treatment of stage I and II oral tongue cancer. Head Neck. 1993;15:308‐312. [DOI] [PubMed] [Google Scholar]
- 7. Yuen APW, Lam KY, Chan ACL, et al. Clinicopathological analysis of elective neck dissection for N0 neck of early oral tongue carcinoma. Am J Surg. 1999;177:90‐92. [DOI] [PubMed] [Google Scholar]
- 8. Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue—clinicopathologic features affecting outcome. Cancer. 2012;118:101‐111. [DOI] [PubMed] [Google Scholar]
- 9. Ho CM, Lam KH, Wei WI, Lau SK, Lam LK. Occult lymph node metastasis in small oral tongue cancers. Head Neck. 1992;14:359‐363. [DOI] [PubMed] [Google Scholar]
- 10. Brandwein‐Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease‐free and overall survival. Am J Surg Pathol. 2005;29:167‐178. [DOI] [PubMed] [Google Scholar]
- 11. Jakobsson PÅ, Eneroth CM, Killander D, Moberger G, Mårtensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol. 1973;12:1‐8. [DOI] [PubMed] [Google Scholar]
- 12. Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987;95:229‐249. [DOI] [PubMed] [Google Scholar]
- 13. Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18:432‐437. [DOI] [PubMed] [Google Scholar]
- 14. Vila CN, Martínez‐Gimeno C, Rodríguez EM, Varela CL. Squamous cell carcinoma of the oral cavity: a clinicopathologic scoring system for evaluating risk of cervical lymph node metastasis. Laryngoscope. 1995;105:728‐733. [DOI] [PubMed] [Google Scholar]
- 15. Odell EW, Jani P, Sherriff M, et al. The prognostic value of individual histologic grading parameters in small lingual squamous cell carcinomas. The importance of the pattern of invasion. Cancer. 1994;74:789‐794. [DOI] [PubMed] [Google Scholar]
- 16. Kantola S, Parikka M, Jokinen K, et al. Prognostic factors in tongue cancer‐relative importance of demographic, clinical and histopathological factors. Br J Cancer. 2000;83:614‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurokawa H, Zhang M, Matsumoto S, et al. The high prognostic value of the histologic grade at the deep invasive front of tongue squamous cell carcinoma. J Oral Pathol Med. 2005;34:329‐333. [DOI] [PubMed] [Google Scholar]
- 18. Po Wing Yuen A, Lam KY, Lam LK, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma‐a comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez‐Gimeno score, and pathologic features. Head Neck. 2002;24:513‐520. [DOI] [PubMed] [Google Scholar]
- 19. Brandwein‐Gensler M, Smith RV, Wang B, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34:676‐688. [DOI] [PubMed] [Google Scholar]
- 20. Weijers M, Snow GB, Dick bezemer P, van der Waal I. Malignancy grading is no better than conventional histopathological grading in small squamous cell carcinoma of tongue and floor of mouth: retrospective study in 128 patients. J Oral Pathol Med. 2009;38:343‐347. [DOI] [PubMed] [Google Scholar]
- 21. Dantas da silveira EJ, Pina godoy G, Alvesuchôa lins RD, et al. Correlation of clinical, histological, and cytokeratin profiles of squamous cell carcinoma of the oral tongue with prognosis. Int J Surg Pathol. 2007;15:376‐383. [DOI] [PubMed] [Google Scholar]
- 22. Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factor for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385‐394. [DOI] [PubMed] [Google Scholar]
- 23. Prall F. Tumour budding in colorectal cancer. Histopathology. 2007;50:151‐162. [DOI] [PubMed] [Google Scholar]
- 24. Masuda R, Kijima H, Imamura N, et al. Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. Mol Med Rep. 2012;6:937‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koelzer VH, Langer R, Zlobec I, Lugli A. Tumor budding in upper gastrointestinal carcinomas. Front Oncol. 2014;4:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: a promising parameter in colorectal cancer. Br J Cancer. 2012;106:1713‐1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karamitopoulou E, Zlobec I, Born D, et al. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur J Cancer. 2013;49:1032‐1039. [DOI] [PubMed] [Google Scholar]
- 28. Brown M, Sillah K, Griffiths EA, et al. Tumour budding and a low host inflammatory response are associated with a poor prognosis in oesophageal and gastro‐oesophageal junction cancers. Histopathology. 2010;56:893‐899. [DOI] [PubMed] [Google Scholar]
- 29. Almangush A, Coletta RD, Bello IO, et al. A simple novel prognostic model for early stage oral tongue cancer. Int J Oral Maxillofac Surg. 2015;44:143‐150. [DOI] [PubMed] [Google Scholar]
- 30. Xie N, Wang C, Liu X, et al. Tumor budding correlates with occult cervical lymph node metastasis and poor prognosis in clinical early‐stage tongue squamous cell carcinoma. J Oral Pathol Med. 2015;44:266‐272. doi: 10.1111/jop.12242 [DOI] [PubMed] [Google Scholar]
- 31. Almangush A, Bello IO, Keski‐Säntti H, et al. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early‐stage oral tongue cancer. Head Neck. 2014;36:811‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie N, Wang C, Liu X, et al. Tumor budding correlates with poor prognosis and epithelial‐mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med. 2015;44:266‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirita T, Yamakawa N, Ueda N, Yagyuu T. Tumor budding as a useful prognostic indicator in early oral squamous cell carcinoma. J Cancer Sci Ther. 2018;10:162‐167. [Google Scholar]
- 34. Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour 'budding' as an index to estimate the potential of aggressiveness in rectal cancer. Histophathology. 2002;40:127‐132. [DOI] [PubMed] [Google Scholar]
- 35. Wang LM, Kevans D, Mulcahy H, et al. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol. 2009;33:134‐141. [DOI] [PubMed] [Google Scholar]
- 36. Wang C, Huang H, Huang Z, et al. Tumor budding correlates with poor prognosis and epithelial‐mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med. 2011;40:545‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dawson H, Koelzer VH, Karamitopoulou E, et al. The apoptotic and proliferation rate of tumour budding cells in colorectal cancer outlines a heterogeneous population of cells with various impacts on clinical outcome. Histopathology. 2014;64:577‐584. [DOI] [PubMed] [Google Scholar]
- 38. Huang SH, Hwang D, Lockwood G, Goldstein DP, O'Sullivan B. Predictive value of tumor thickness for cervical lymph‐node involvement in squamous cell carcinoma of the oral cavity: a meta‐analysis of reported studies. Cancer. 2009;115:1489‐1497. [DOI] [PubMed] [Google Scholar]
- 39. Wang K, Veivers D. Tumour thickness as a determinant of nodal metastasis in oral tongue carcinoma. ANZ J Surg. 2017;87:720‐724. [DOI] [PubMed] [Google Scholar]
- 40. Brandwein‐Gensler M, Smith RV, Wang B, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34:676‐688. [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Bai S, Carroll W, et al. Validation of the risk model: high‐risk classification and tumor pattern of invasion predict outcome for patients with low‐stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013;7:211‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bello IO, Vered M, Dayan D, et al. Cancer‐associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma‐associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47:33‐38. [DOI] [PubMed] [Google Scholar]
- 43. Kellermann MG, Sobral LM, Silva SD, et al. Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosis. Histopathology. 2007;51:849‐853. [DOI] [PubMed] [Google Scholar]
- 44. Marsh D, Suchak K, Moutasim KA, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223:470‐481. 10.1002/path.2830 [DOI] [PubMed] [Google Scholar]
- 45. Kurokawa H, Yamashita Y, Takeda S, Zhang M, Fukuyama H, Takahashi T. Risk factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002;24:731‐736. [DOI] [PubMed] [Google Scholar]
- 46. Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004;240:832‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127‐132. [DOI] [PubMed] [Google Scholar]
- 48. Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer. 2008;112:345‐351. [DOI] [PubMed] [Google Scholar]
- 49. Grabenbauer GG, Rödel C, Ernst‐Stecken A, et al. Neck dissection following radiochemotherapy of advanced head and neck cancer—for selected cases only? Radiother Oncol. 2003;66:57‐63. [DOI] [PubMed] [Google Scholar]
- 50. Alkureishi LWT, Ross GL, Shoaib T, et al. Sentinel node biopsy in head and neck squamous cell cancer: 5‐year follow‐up a European multicenter trial. Ann Surg Oncol. 2010;17:2459‐2464. [DOI] [PubMed] [Google Scholar]
- 51. Yamauchi K, Fujioka Y, Kohno N. Sentinel node navigation surgery versus observation as a management strategy for early tongue carcinoma. Head Neck. 2012;34:568‐572. [DOI] [PubMed] [Google Scholar]
- 52. Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299‐1311. [DOI] [PubMed] [Google Scholar]
- 53. Seki M, Sano T, Yokoo S, Oyama T. Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck. 2016;38:E1582‐E1590. [DOI] [PubMed] [Google Scholar]
- 54. Hart IR, Goode NT, Wilson RE. Molecular aspects of the metastatic cascade. Biochim Biophys Acta. 1989;989:65‐84. [DOI] [PubMed] [Google Scholar]
- 55. Brabletz T. To differentiate or not ‐routes towards metastasis. Nat Rev Cancer. 2012;12:425‐436. [DOI] [PubMed] [Google Scholar]
- 56. Kalluri R, Weinberg RA. The basics of epithelial‐mesenchymal transition. J Clin Investig. 2009;119:1420‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Prall F. Tumour budding in colorectal carcinoma. Histopathology. 2007;50:151‐162. [DOI] [PubMed] [Google Scholar]
- 58. Liang F, Cao W, Wang Y, Li L, Zhang G, Wang Z. The prognostic value of tumor budding in invasive breast cancer. Pathol Res Pract. 2013;209:269‐275. [DOI] [PubMed] [Google Scholar]
- 59. Grigore A, Jolly M, Jia D, Farach‐Carson M, Levine H. Tumor budding: the name is EMT. Partial EMT. J Clin Med. 2016;5:E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 61. Thode C, Jørgensen TG, Dabelsteen E, Mackenzie I, Dabelsteen S. Significance of myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:201‐207. [DOI] [PubMed] [Google Scholar]


