Abstract
Remote ischemic postconditioning (RIPC) is a promising neuroprotective strategy for ischemic stroke. Here, we employed a focal ischemic stroke mouse model to test the hypothesis that poststroke collateral circulation as a potent mechanism of action underlying the therapeutic effects of immediate RIPC. During reperfusion of cerebral ischemia, the mice were randomly assigned to receive RIPC, granulocyte colony‐stimulating factor (G‐CSF) as a positive control, or no treatment. At 24 hr, we found RIPC and G‐CSF increased monocytes/macrophages in the dorsal brain surface and in the spleen, coupled with enhanced leptomeningeal collateral flow compared with nontreatment group. Blood monocytes depletion by 5‐fluorouracil (5‐FU) significantly limited the neuroprotection of RIPC or G‐CSF treatment. The protein expression of proangiogenic factors such as Ang‐2 was increased by ischemia, but treatment with either RIPC or G‐CSF showed no further upregulation. Thus, immediate RIPC confers neuroprotection, in part, by enhancing leptomeningeal collateral circulation in a mouse model of ischemic stroke.
Keywords: animal models, collateral circulation, ischemic stroke, monocytes/macrophages, remote ischemic postconditioning
1. INTRODUCTION
Acute cerebral ischemia is a leading cause of death and disability due to disturbance of blood supply to the brain. The only FDA‐approved treatment for this condition is intravenous thrombolysis therapy with tissue plasminogen activator, which is severely limited by its short therapeutic time window of <4.5 hr (Hacke et al., 2008).
Revascularization is a major endogenous defense mechanism in the brain after stroke. Accumulating evidence suggests that the early phase of cerebral blood volume (CBV) increase is likely due to the improvement in collateral flow, also known as arteriogenesis, whereas the late phase CBV increase is attributed to a surge of angiogenesis (J. Liu et al., 2014). Thus, therapeutic stimulation of vascular remodeling is a viable target for therapeutic interventions given this natural adaptive progress can be augmented by exogenous growth factors (van Oostrom, van Oostrom, Quax, Verhaar, & Hoefer, 2008). G‐CSF in particular has been fairly well investigated for treating cerebral ischemia (Dela Peña et al., 2015; England, Gibson, & Bath, 2009), likely in part due to its upregulation of collateral growth through monocyte recruitment (Sugiyama et al., 2011).
Remote ischemic postconditioning (RIPC) is an intriguing treatment modality whereby ischemia induced in a distant nonvital organ, when applied immediately after the ischemic insult, offers protection (Andreka et al., 2007). Numerous reports in animals and clinical trials have demonstrated the neuroprotective effect of RIPC against brain injury after stroke (Hess, Hoda, & Bhatia, 2013; Meng et al., 2012; Ren et al., 2009, 2011). Multiple mechanisms have been proposed for RIPC‐mediated protection, including decreased expression of inflammatory mediators (Kong, Rogers, & Qin, 2013), increase of AKT/GSK3β‐dependent autophagy (Qi et al., 2012), and attenuation of blood–brain barrier disruption by inhibition of MMP‐9 activity and occludin degradation (Ren et al., 2015). However, the underlying mechanisms contributing to RIPC are complex and remain poorly elucidated (Hess et al., 2015; Zhao, Ren, Chen, & Shen, 2012). Here, using G‐CSF as a positive control, we examined the effect of RIPC on monocyte recruitment and subsequent leptomeningeal collateral circulation in mice with ischemic stroke.
2. MATERIALS AND METHODS
2.1. Animal experimental protocol
Male C57BL/6J strain mice 9–10 weeks of age; Charles River Laboratories Inc., Beijing, China) were used in this study. The experimental procedures were approved by the Animal Care and Use Committee of Capital Medical University, China, and conducted according to National Institutes of Health guidelines.
Focal cerebral ischemia was generated on a modified 3‐vessel occlusion (3VO) method as reported by Z. Liu et al. (2017). Briefly, general anesthesia was induced and maintained with 1.0–2.5% enfluane in 70% N2O and 30% O2 (Bickford veterinary anesthesia equipment model no. 61010; AM Bickford Inc., Wales Center, NY). Under anesthesia, bilateral common carotid arteries (CCAs) were clipped for 15 min using surgical miniclips, whereas the stem of right distal middle cerebral artery (dMCA) was permanently cauterized using a bipolar coagulator (B. Braun Aesculap, Melsungen, Germany) through a 1‐mm‐diameter burr hole in the skull. Surgery was performed under a microscope (Carl Zeiss AG, Jena, Germany). A circulating heating pad and a heating lamp were used to keep rectal temperature at 37℃. Sham‐operated mice were exposed to the same conditions, but the dMCA and the CCAs were not manipulated. Stroke animals were randomly divided into three groups: (a) Stroke without treatment: 3VO followed by 24 hr of reperfusion; (b) Stroke&RIPC: RIPC was conducted immediately after CCAs release as described previously (Ren et al., 2011), generally by occluding and releasing the femoral arteries in bilateral lower limbs for three cycles; each occlusion/release lasted for 10 min; (c) Stroke&G‐CSF: recombinant human G‐CSF, 100 µg/kg body weight in 100 µl sterile saline, sc., immediately after the operation. All studies were performed in a blinded and randomized manner.
2.2. Neurological testing
The corner test was used to examine stroke severity and functional deficits (Zhang et al., 2002). Each mouse was tested for 20 trials and the laterality index was calculated according to the formula: laterality index = (turns to the right side − turns to the left side)/total number of turns. A laterality index of 0 is indicative of animals presenting total symmetry. This test detects integrated sensorimotor function as it involves both stimulation of the vibrissae (sensory/neglect) and rearing (motor response).
2.3. Infarct volume measurements
Twenty‐four hours after surgery, mice were anesthetized with 10% chloral hydrate. Coronal brain sections with a 1‐mm thickness were removed, and stained with 2% solution of 2,3,5‐triphenyltetrazolium chloride (Sigma‐Aldrich Corp., St. Louis, MO). To correct hemispheric edema, infarct volume of the ischemic region was normalized to the nonischemic region and expressed as a percentage of contralateral cortex volume.
2.4. Visualization of vessels by latex perfusion
The leptomeningeal anastomoses were visualized by latex perfusion technique as previously reported (Woitzik, Hecht, Schneider, Peña‐Tapia, & Vajkoczy, 2006). The mice were anesthetized with 1% enfluane in 70% N2O and 30% O2, and papaverine hydrochloride solution was slowly injected through the femoral vein at a rate of 100 µl/min (50 mg/kg body weight), then the right atrium of the heart was incised for the venous blood drain out. The left ventricle of the heart was cannulated and injected with 2 ml saline and subsequently 0.5 ml latex compound (Product No. 39210; UHU GmbH & Co. KG, Bühl/Baden, Germany), which was mixed with 50 µl/ml carbon black (Beijing Ink Product No. 1022–1144; Beijing, China) beforehand. Then, the brain was removed carefully from the skull, and photographs of the dorsal and ventral brain surface were taken by a scanner (Scanjet G3110; HP, Shanghai, China). The vessel diameters of the leptomeningeal anastomoses were measured at the point where the distal MCA joined the distal anterior cerebral artery (ACA). The vessel diameter of the Willis' circle was measured at the proximal point where the right ACA diverged the olfactory artery.
2.5. Immunostaining of monocytes/macrophages (MMs)
Immunostaining was carried out at 24 hr after ischemic stroke. Mice were transcardially perfused with 0.9% NaCl, followed by 4% paraformaldehyde. Five‐micron‐thick paraffin‐embedded slices were stained with anti‐macrophage antibody (MAC387; 1:100; Abcam, Cambridge, UK) and then with Alexa 594‐conjugated goat anti‐mouse secondary antibody (1:400; Thermo Fisher Scientific, Rockford). Images were captured using a microscope (Nikon, Tokyo, Japan).
2.6. Monocyte depletion
Monocyte depletion was achieved by a single intraperitoneal injection of 5‐fluorouracil (5‐FU) 4 days before cerebral ischemic injury (150 mg/kg). To verify the monocyte depletion, blood analysis was performed by flow cytometry at 4 and 7 days after the injection during preliminary test.
2.7. Western blot analysis
At 24 hr mice were transcardially perfused with 0.9% NaCl, and total protein was extracted from the bilateral cortices. Protein concentration determination and western blot analysis were performed as our previous report (Wei et al., 2016). The amount of proteins were quantified by densitometry and normalized to β‐actin, an internal standard. Primary antibodies purchased were as follows: rabbit anti‐angiopoietin 1 polyclonal antibody (1:500; Abcam), rabbit anti‐Angiopoietin‐2 polyclonal antibody (1:1000; Abcam); mouse anti‐β‐actin monoclonal antibody (1:10,000; Sigma‐Aldrich Corp., St. Louis, MO). The horseradish peroxidase conjugated goat anti‐rabbit or anti‐mouse IgGs were used as secondary antibodies (1:4,000; Stressgen Biotechnologies Corporation, Victoria, BC). The specific reaction signals were detected through the use of a chemiluminescence kit (GE Healthcare, Amersham Place, Buckinghamshire, UK), and imaged by using Fusion‐Capt Advance software on FUSION FX (Vilber Lourmat, Collégien, France).
2.8. Enzyme‐linked immunoassay (ELISA)
The levels of mouse G‐CSF and human G‐CSF in mouse plasma and brain cortex homogenate were determined by ultrasensitive ELISA kits (R&D Systems, Inc), according to the manufacturer's instructions. Before assay, mouse plasma samples were 10‐fold diluted whereas brain cortex homogenate two‐fold, and 50 µL or 100 µL of diluted sample was added per well for mouse G‐CSF or human G‐CSF detection, respectively. Absorbance was read using a microplate reader set to 450 nm, and wavelength correction at 540 nm.
2.9. Statistical analysis
All data were expressed as mean ± SEM. Statistical analysis was performed with SPSS for Windows (version 22, SPSS Inc., Armonk). The differences among groups were assessed using one‐way analysis of variance, followed by all pairwise multiple comparison procedures using the Bonferroni test. Statistical significance was set at p ≤ 0.05.
3. RESULTS
3.1. Reduction of cerebral infarct volume and neurological deficit by RIPC
After 24 hr of ischemic stroke, a significant increase in corner test score (asymmetry) was observed in the Stroke group (−0.7 ± 0.1), which was significantly reduced by both RIPC (−0.2 ± 0.1, p < 0.001 vs. Stroke) and G‐CSF treatments (−0.1 ± 0.1, p < 0.001 vs. Stroke; Figure 1a). Additionally, infarct volume in the Stroke group was 45.4% ± 0.7% whereas Stroke&RIPC mice displayed significantly reduced infarct volume (31.8 ± 1.8%) compared with nontreated animals (p < 0.001). The Stroke&G‐CSF group showed a greater decrease in cerebral infarct volume than RIPC treated stroke mice (26.4 ± 1.1%, p < 0.05 vs. Stroke&RIPC group; Figure 1b).
Figure 1.
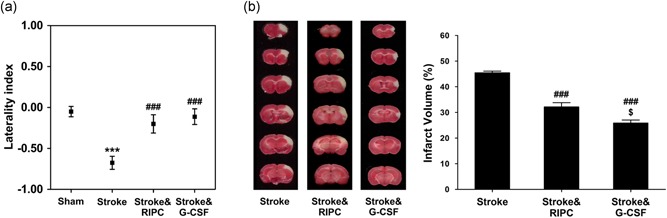
Infarct volume reduction and neurological function improvement by remote ischemic postconditioning. (a) At 24 hr, mice subjected to focal cerebral ischemic stroke showed asymmetry in turning preference, demonstrating impaired motor function, whereas the treatment groups reduced neurological deficit compared with nontreated animals; no further difference was observed between the two treatment groups (***p < 0.001 vs. Sham, ### p < 0.001 vs. Stroke, n = 8). (b) Representative brain slices stained by 2,3,5‐triphenyltetrazolium‐chloride (TTC) 24 hr after reperfusion and average infarct volumes in stroke with and without treatment. Both RIPC and G‐CSF treatments significantly reduced infarct volume when compared with nontreated animals (### p < 0.001), whereas G‐CSF exhibited a further reduction than RIPC ($ p < 0.05; n = 8). Data were expressed as mean ± SEM. G‐CSF: granulocyte colony‐stimulating factor [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Enhanced leptomeningeal anastomoses growth by RIPC
The effect of RIPC on leptomeningeal anastomoses development was assessed at 24 hr after 3VO by quantitative measurement of colored‐latex perfused vessel diameter. Both RIPC and G‐CSF treatments significantly enhanced the vessel diameter of ACA‐MCA anatomoses as compared with nontreated animals (Stroke&RIPC: 52.4 ± 2.7 µm, Stroke&G‐CSF: 54.8 ± 4.0 µm vs. Stroke: 45.0 ± 1.0 µm, p < 0.05; Figure 2a,c1). The vessel diameter of the circle of Willis showed no significant differences after 3VO (Stroke: 110.3 ± 5.4 µm, Sham: 118.8 ± 7.5 µm), nor by RIPC or G‐CSF treatment (114.0 ± 6.3 µm and 117.9 ± 7.2 µm, respectively; Figure 2b,c2).
Figure 2.
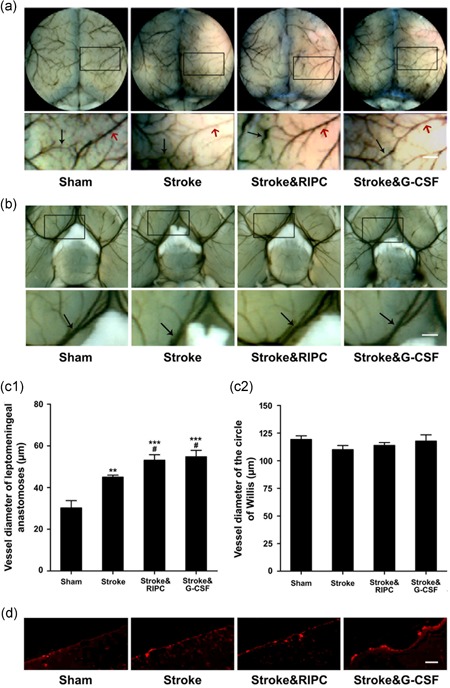
Enhanced leptomeningeal anastomoses flow by remote ischemic postconditioning as well as G‐CSF. Visualization of cerebral angioarchitecture by carbon black‐stained latex 24 hr after ischemic stroke with RIPC or G‐CSF treatment. Magnified images of the box in the upper panels were shown in the lower panels. (a) The black arrows indicate the vessels of anterior cerebral artery–middle cerebral artery anastomoses (ACA‐MCA), and the red arrows indicate the branch of distal MCA (scale bar = 200 µm). (b) The arrow heads indicate vessels of the circle of Willis (scale bar = 500 µm). (c1) RIPC as well as G‐CSF treatment promoted the leptomeningeal collateral flow after ischemic stroke (**p < 0.01, ***p < 0.001 vs. Sham, # p < 0.05 vs. Stroke, n = 6). (c2) Collateral flow at the circle of Willis was observed whereas there was no difference by any treatment (n = 6). (d) Macrophage staining for MAC387 in the dorsal brain surface 24 hr after 3VO stroke with RIPC or G‐CSF treatment was presented (scale bar = 100 µm). Data were expressed as mean ± SEM. G‐CSF: granulocyte colony‐stimulating factor [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Brain and spleen monocytes/macrophages
Macrophages in the dorsal brain surface were identified with MAC387 antibody. The number of MAC387 positive cells significantly increased by both RIPC and G‐CSF treatment (Stroke&RIPC: 13.0 ± 1.0, Stroke&G‐CSF: 14.4 ± 0.7 vs. Stroke: 8.6 ± 0.6, positive cells per field, n = 5, p < 0.05; Figure 2d). There was a >70% macrophage decrease in the spleens of mice at 24 hr after 3VO insult (Stroke vs. Sham, p < 0.01), and both RIPC and G‐CSF treatments completely abolished the macrophage depletion (Stroke&RIPC: 99.5 ± 9.9%, Stroke&G‐CSF: 143.1 ± 23.8% vs. Stroke: 27.4 ± 9.9% [% Sham], p < 0.01, p < 0.001, respectively), with G‐CSF treatment even eliciting a significant increase of macrophage in the spleen when compared with Sham group (p < 0.05; Figure 3).
Figure 3.
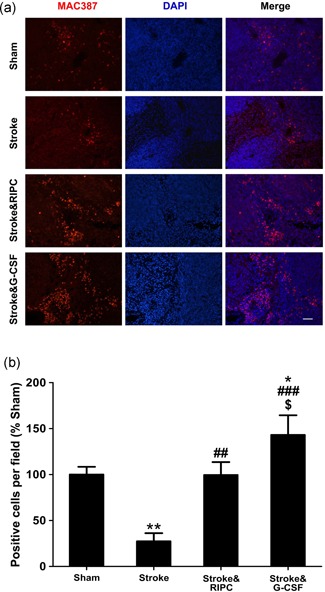
RIPC treatment induced monocytes/macrophages accumulation in the spleen similarly to G‐CSF. (a) Representative images of double immunostaining of anti‐macrophage antibody MAC387 (red) and DAPI (blue; scale bar = 100 µm). (b) Quantification of the number of MAC387 positive macrophages (data were expressed as mean ± SEM. *p < 0.05, **p < 0.01 vs. Sham, ## p < 0.01, ### p < 0.001 vs. Stroke, $ p < 0.05 vs. Stroke&RIPC, n = 5). G‐CSF: granulocyte colony‐stimulating factor; DAPI: 4′,6‐diamidino‐2‐phenylindole; RIPC: remote ischemic postconditioning [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Suppression of RIPC‐induced neuroprotection by monocyte depletion
To further validate the mechanistic correlation between monocyte/macrophage mobilization and collateral circulation increase, we tested the impact of monocyte depletion on leptomeningeal anastomoses growth by RIPC. A single dose of 5‐FU administration (150 mg/kg intraperitoneally) severely depleted blood monocytes at 4 days after injection (4 days: 4 ± 4/µl, 7 days: 6 ± 4/µl vs. normal: 276 ± 81/µl; n = 4 per day). This intervention significantly impeded leptomeningeal anastomoses growth by RIPC or G‐CSF treatment (5‐FU, Stroke&RIPC: 44.3 ± 3.8 µm, Stroke&G‐CSF: 42.0 ± 2.4 µm, vs. Stroke: 48.0 ± 3.6 µm; Figure 4a). Monocyte depletion by 5‐FU also interfered with the RIPC and G‐CSF‐mediated alleviation of spleen shrinkage (5‐FU, Stroke&RIPC: 0.18 ± 0.01%, Stroke&G‐CSF: 0.18% ± 0.01%, vs. Stroke: 0.18% ± 0.01%, spleen/BW (%); Figure 4c). Moreover, RIPC and G‐CSF mice pretreated with 5‐FU exhibited no reduction in cerebral infarct volume compared with nontreated stroke animals (Stroke&RIPC: 42.6% ± 1.0%, Stroke&G‐CSF: 41.6% ± 1.3%, vs. Stroke: 39.2% ± 1.3%; Figure 4d).
Figure 4.
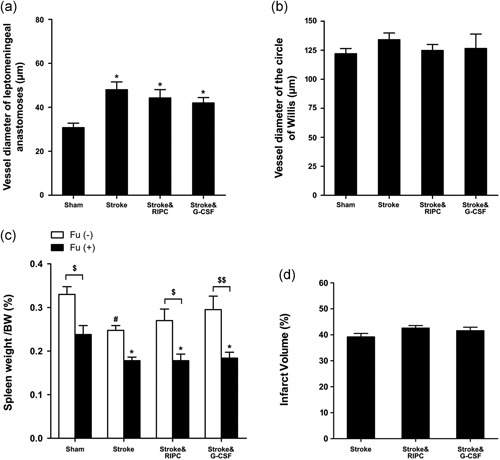
Monocyte depletion reversed the neuroprotective effects of RIPC. FU(+) indicates 5‐FU administration. FU(‐) indicates vehicle administration. (a) In 5‐Fu(+) monocyte depleted mice, ischemic cerebral injury promoted leptomeningeal collateral flow whereas RIPC or G‐CSF treatment exhibited no further increase (*p < 0.05 vs. Sham group, n = 4). (b) 5‐Fu treated mice of all groups showed no differences in collateral flow at the circle of Willis (n = 4). (c) Monocyte depletion by 5‐FU caused spleen shrinkage, and 3‐vessel occlusion (3VO) ischemia with or without RIPC or G‐CSF treatment aggravated the drop of spleen weight (*p < 0.05 vs. 5‐FU administrated Sham, # p < 0.05 vs. 5‐FU free Sham; $ p < 0.05, $$ p < 0.01 vs. 5‐FU free mice). (d) RIPC or G‐CSF treatment did not alter the cerebral infarct volume in monocyte depleted mice with ischemic stroke (n = 5). Data were expressed as mean ± SEM. FU: Fluorouracil; G‐CSF: granulocyte colony‐stimulating factor; RIPC: remote ischemic postconditioning
3.5. Angioprotein‐expression assay in ischemic cortex by western blot analysis
Ang‐1 and Ang‐2 were selected as markers of angiogenesis. There were no significant differences of Ang‐1 expression between groups (Figure 5a). Meanwhile ischemic insult upregulated Ang‐2 expression in the ischemic cortex, whereas both treatments of RIPC and G‐CSF showed no additional increase (Figure 5b).
Figure 5.
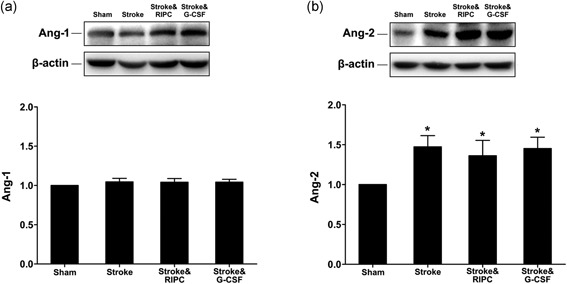
Western blot for angiopoietins Ang‐1 and Ang‐2. (a) There were no significant differences of Ang‐1 expression between all the groups (n = 5). (b) Ischemic insult upregulated Ang‐2 expression in the ischemic cortex, whereas both RIPC and G‐CSF treatment showed no further increase (1.8‐, 1.7‐, 1.8‐fold vs. Sham, *p < 0.05, n = 5). Data were expressed as mean ± SEM. Data were normalized to β‐actin. G‐CSF: granulocyte colony‐stimulating factor; RIPC: remote ischemic postconditioning
3.6. G‐CSF levels in mouse brain and plasma
Both mouse G‐CSF and human G‐CSF levels in brain and serum from 20 mice were determined by ELISA (n = 5 per group). Mouse G‐CSF in each contralateral cortex sample was 0 pg (Figure 6a). Mouse G‐CSF content in right (ischemic) cortex was 0 pg in Sham group, whereas Stroke&RIPC group (354.4 ± 61.0 pg) and Stroke&G‐CSF group (179.0 ± 36.5 pg) showed significantly lower levels compared with nontreatment group (720.4 ± 115.4 pg; p < 0.01, p < 0.001; Figure 6a). Within the plasma, mouse G‐CSF concentration in the Stroke group dropped significantly (1,266.0 ± 420.9 pg/ml) compared with Sham group (3,736.6 ± 1,319.1 pg/ml; p < 0.05), whereas Stroke&RIPC group (3,179.4 ± 1,369.0 pg/ml) and Stroke&G‐CSF group (3,223.4 ± 903.8 pg/ml) showed notable uptrends, though not reaching statistical significance compared with Stroke group (Figure 6b). As for human G‐CSF, it was only detected exclusively in the plasma of Stroke&G‐CSF group, at 1.4 ± 0.85 (pg/ml), and was not detected in the other groups.
Figure 6.
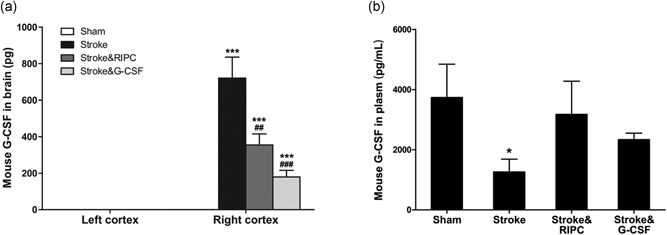
Enzyme‐linked immunoassay (ELISA) for mouse G‐CSF. (a) Mouse G‐CSF level in contralateral cortex of each mouse was 0, as well as the ipsilateral cortex of Sham group. There was an apparent increase of mouse G‐CSF in the ipsilateral cortex in Stroke mice, whereas remote ischemic postconditioning and G‐CSF significantly decreased the factor compared with Stroke without treatment (***p < 0.001 vs. Sham, ## p < 0.01, ### p < 0.001 vs. Stroke, n = 5). (b) Mouse G‐CSF level in plasma of Stroke group showed significant decrease compared with Sham group (*p < 0.05), whereas RIPC and G‐CSF treatment both attenuated the downtrend of mouse G‐CSF in plasma caused by cerebral ischemic insult (n = 5). Data were expressed as mean ± SEM. G‐CSF: granulocyte colony‐stimulating factor
4. DISCUSSION
The present study demonstrated that RIPC, which was conducted immediately after ischemic stroke in the bilateral hind limbs, conferred protection against focal cerebral ischemia in mice through enhancing leptomeningeal collateral circulation. Similar to G‐CSF positive‐control treatment, the collateral flow effect of immediate RIPC treatment was associated with increased localization of macrophages in the dorsal surface of the ischemic brain, and monocyte depletion by 5‐FU completely abolished RIPC‐induced collateral flow response and suppressed the neuroprotective effect of RIPC. Our data confirmed the vital role of MMs in the RIPC‐induced leptomeningeal anastomoses growth mechanism, complimenting recent reports that macrophages can directly form vessel walls under hypoxic microenvironments (Barnett et al., 2016). Postischemic immune cell migration is a major determinant of stroke outcome and peripheral‐to‐central organ interactions in stroke pathophysiology (S. Ma, Zhao, Ji, & Luo, 2015), especially the role of spleen‐derived MMs in brain injury, has attracted great attention (Kim, Yang, Beltran, & Cho, 2014; Miró‐Mur et al., 2016; Schmidt et al., 2017; Song et al., 2017). This study was designed to probe the effects of RIPC on leptomeningeal anastomoses growth, and our results support the concept of monocyte recruitment as playing a critical role in the enhancement of collateral flow (Heil et al., 2002; Sugiyama et al., 2011).
In addition to collateral flow, increased angiogenesis has been shown to promote neurological and histological improvement after stroke, in particular, demonstrating the outgrowth of new vessels from pre‐existing vasculature (Dela Peña et al., 2015; J. Liu et al., 2014). In this study the angiogenesis marker Ang‐1 and Ang‐2 were examined at 24 hr after ischemic stroke. The results indicated that neither RIPC nor human G‐CSF (100 µg/kg BW) treatment promoted angiogenesis via these factors. In a separate ongoing study, we are analyzing the angiogenic and neurogenic response by repeated daily RIPC in chronic phase after ischemic stroke. Recognizing that G‐CSF could exert direct neuroprotective action, we inquired whether remote limb ischemic postconditioning could produce endogenous G‐CSF into the peripheral blood and then into the brain. Using ultrasensitive ELISA, we examined mouse G‐CSF levels in animal plasma and cerebral tissue to answer this question. The results refuted the hypothesis that endogenous G‐CSF is mobilized by RIPC. Nevertheless, considering the duration of the second protection window of ischemic conditioning (Hausenloy et al., 2016), the ability of repeated daily RIPC to produce accumulative effects to trigger endogenous G‐CSF is worth future investigation.
Currently, the concept of remote ischemic conditioning‐induced neuroprotection includes remote ischemic preconditioning (RIPreC), remote ischemic perconditioning (RIPerC), and remote ischemic postconditioning (RIPostC) based on application timing either before ischemia, during ischemia, or during reperfusion of ischemia, respectively (Hess et al., 2013; Lim & Hausenloy, 2012; Wang et al., 2015). Our 3VO animal model represents a “mixed temporary/permanent” ischemia model that produces clear neocortical infarct and relevant to the most common type of human stroke. After 15 min of 3VO, when the clips for CCAs were removed, reperfusion could be confirmed visually. Using 3VO mouse model and visualization of vessels by colored latex perfusion, our data supported the hypothesis that immediate RIPC could enhance collateral flow after ischemic stroke. This was in agreement with a recent paper demonstrating pial collateral development by remote ischemic conditioning (RIC) in a similar 3VO rat model, involving distal MCA and ipsilateral CCA (tandem) permanent ligation with temporary (1 hr) occlusion of the contralateral CCA (J. Ma et al., 2017). RIC was immediately initiated after 60 min of 3VO by occluding and releasing the femoral arteries bilaterally for three cycles (each 15 min ON/OFF; J. Ma et al., 2017). However, another paper using suture MCAO mouse model reported that only RIPerC, but not RIPostC, afforded brain protection via enhancement of collateral circulation (Kitagawa, Saitoh, Ishizuka, & Shimizu, 2018). Hind limb RIC was accomplished by a tourniquet for four cycles (each 5 min occlusion/release; Kitagawa et al., 2018). Differences between femoral ligation RIC and noninvasive induction of RIC may account for discrepant stroke outcomes. Accordingly, vis‐à‐vis comparisons of RIC protocols in a variety of experimental stroke models may provide more reproducible translational data that can optimize RIC regimen for future clinical investigations.
RIC represents a highly translatable therapy for multiorgan protection against ischemia–reperfusion injury (IRI). A growing number of clinical trials have indicated that RIC exerts therapeutic effects, specifically in the heart (Cao, Zhang, Wang, Xia, & Yang, 2018; Cho et al., 2017) and brain (England et al., 2017; Pico et al., 2016). However, to date, a clear understanding of the mechanism through which RIC exerts its functional benefits remains a key hurdle in its clinical translation. Our present study showed that immediate RIPC reduced cerebral damage by leptomeningeal collateral flow augmentation via spleen‐derived MMs recruitment in ischemic stroke mice. Combined with its noninvasive and safe profile, the collateral flow enhancement property of RIPC may facilitate its clinical application for ischemic stroke patients.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGEMENTS
This work was supported by grants from National Key R&D Program of China (2017YFC1308401), and National Natural Science Foundation of China (grant no. 81401086, 31671205, 81871022).
Zhang Y, Ma L, Ren C, et al. Immediate remote ischemic postconditioning reduces cerebral damage in ischemic stroke mice by enhancing leptomeningeal collateral circulation. J Cell Physiol. 2018;234:12637–12645. 10.1002/jcp.27858
References
REFERENCES
- Andreka, G. , Vertesaljai, M. , Szantho, G. , Font, G. , Piroth, Z. , Fontos, G. , … Andreka, P. (2007). Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart (British Cardiac Society), 93(6), 749–752. 10.1136/hrt.2006.114504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett, F. H. , Rosenfeld, M. , Wood, M. , Kiosses, W. B. , Usui, Y. , Marchetti, V. , … Friedlander, M. (2016). Macrophages form functional vascular mimicry channels in vivo. Scientific Reports, 6, 36659 10.1038/srep36659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B. , Zhang, C. , Wang, H. , Xia, M. , & Yang, X. (2018). Renoprotective effect of remote ischemic postconditioning in patients with ST‐elevation myocardial infarction undergoing primary percutaneous coronary intervention. Therapeutics and Clinical Risk Management, 14, 369–375. 10.2147/TCRM.S158768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y. J. , Lee, E. H. , Lee, K. , Kim, T. K. , Hong, D. M. , Chin, J. H. , … Jeon, Y. (2017). Long‐term clinical outcomes of remote ischemic preconditioning and postconditioning outcome (RISPO) trial in patients undergoing cardiac surgery. International Journal of Cardiology, 231, 84–89. 10.1016/j.ijcard.2016.12.146 [DOI] [PubMed] [Google Scholar]
- Dela Peña, I. C. , Yoo, A. , Tajiri, N. , Acosta, S. A. , Ji, X. , Kaneko, Y. , & Borlongan, C. V. (2015). Granulocyte colony‐stimulating factor attenuates delayed tPA‐induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis. Journal of Cerebral Blood Flow and Metabolism, 35(2), 338–346. 10.1038/jcbfm.2014.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- England, T. J. , Gibson, C. L. , & Bath, P. M. W. (2009). Granulocyte‐colony stimulating factor in experimental stroke and its effects on infarct size and functional outcome: A systematic review. Brain Research Reviews, 62(1), 71–82. 10.1016/j.brainresrev.2009.09.002 [DOI] [PubMed] [Google Scholar]
- England, T. J. , Hedstrom, A. , O'sullivan, S. , Donnelly, R. , Barrett, D. A. , Sarmad, S. , … Bath, P. M. (2017). RECAST (remote ischemic conditioning after stroke trial): A pilot randomized placebo controlled Phase II trial in acute ischemic stroke. Stroke, 48(5), 1412–1415. 10.1161/STROKEAHA.116.016429 [DOI] [PubMed] [Google Scholar]
- Hacke, W. , Kaste, M. , Bluhmki, E. , Brozman, M. , Dávalos, A. , Guidetti, D. , … Toni, D. (2008). Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England Journal of Medicine, 359(13), 1317–1329. 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- Hausenloy, D. J. , Barrabes, J. A. , Bøtker, H. E. , Davidson, S. M. , Di Lisa, F. , Downey, J. , … Garcia‐Dorado, D. (2016). Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Research in Cardiology, 111(6), 70 10.1007/s00395-016-0588-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil, M. , Ziegelhoeffer, T. , Pipp, F. , Kostin, S. , Martin, S. , Clauss, M. , & Schaper, W. (2002). Blood monocyte concentration is critical for enhancement of collateral artery growth. American Journal of Physiology Heart and Circulatory Physiology, 283(6), H2411–H2419. 10.1152/ajpheart.01098.2001 [DOI] [PubMed] [Google Scholar]
- Hess, D. C. , Blauenfeldt, R. A. , Andersen, G. , Hougaard, K. D. , Hoda, M. N. , Ding, Y. , & Ji, X. (2015). Remote ischaemic conditioning‐a new paradigm of self‐protection in the brain. Nature Reviews Neurology, 11(12), 698–710. 10.1038/nrneurol.2015.223 [DOI] [PubMed] [Google Scholar]
- Hess, D. C. , Hoda, M. N. , & Bhatia, K. (2013). Remote limb perconditioning [corrected] and postconditioning: Will it translate into a promising treatment for acute stroke? Stroke, 44(4), 1191–1197. 10.1161/strokeaha.112.678482 [DOI] [PubMed] [Google Scholar]
- Kim, E. , Yang, J. , Beltran, C. D. , & Cho, S. (2014). Role of spleen‐derived monocytes/macrophages in acute ischemic brain injury. Journal of Cerebral Blood Flow and Metabolism, 34(8), 1411–1419. 10.1038/jcbfm.2014.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa, K. , Saitoh, M. , Ishizuka, K. , & Shimizu, S. (2018). Remote limb ischemic conditioning during cerebral ischemia reduces infarct size through enhanced collateral circulation in murine focal cerebral ischemia. Journal of Stroke and Cerebrovascular Diseases, 27(4), 831–838. 10.1016/j.jstrokecerebrovasdis.2017.09.068 [DOI] [PubMed] [Google Scholar]
- Kong, Y. , Rogers, M. R. , & Qin, X. (2013). Effective neuroprotection by ischemic postconditioning is associated with a decreased expression of RGMa and inflammation mediators in ischemic rats. Neurochemical Research, 38(4), 815–825. 10.1007/s11064-013-0984-5 [DOI] [PubMed] [Google Scholar]
- Lim, S. Y. , & Hausenloy, D. J. (2012). Remote ischemic conditioning: From bench to bedside. Frontiers in Physiology, 3, 27 10.3389/fphys.2012.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Wang, Y. , Akamatsu, Y. , Lee, C. C. , Stetler, R. A. , Lawton, M. T. , & Yang, G. Y. (2014). Vascular remodeling after ischemic stroke: Mechanisms and therapeutic potentials. Progress in Neurobiology, 115, 138–156. 10.1016/j.pneurobio.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Ran, Y. , Huang, S. , Wen, S. , Zhang, W. , Liu, X. , … Hu, X. (2017). Curcumin protects against ischemic stroke by titrating microglia/macrophage polarization. Frontiers in Aging Neuroscience, 9, 233 10.3389/fnagi.2017.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J. , Ma, Y. , Dong, B. , Bandet, M. V. , Shuaib, A. , & Winship, I. R. (2017). Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. Journal of Cerebral Blood Flow and Metabolism, 37(8), 3001–3014. 10.1177/0271678x16680636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, S. , Zhao, H. , Ji, X. , & Luo, Y. (2015). Peripheral to central: Organ interactions in stroke pathophysiology. Experimental Neurology, 272, 41–49. 10.1016/j.expneurol.2015.05.014 [DOI] [PubMed] [Google Scholar]
- Meng, R. , Asmaro, K. , Meng, L. , Liu, Y. , Ma, C. , Xi, C. , … Ji, X. (2012). Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology, 79(18), 1853–1861. 10.1212/WNL.0b013e318271f76a [DOI] [PubMed] [Google Scholar]
- Miró‐Mur, F. , Pérez‐De‐Puig, I. , Ferrer‐Ferrer, M. , Urra, X. , Justicia, C. , Chamorro, A. , & Planas, A. M. (2016). Immature monocytes recruited to the ischemic mouse brain differentiate into macrophages with features of alternative activation. Brain, Behavior, and Immunity, 53, 18–33. 10.1016/j.bbi.2015.08.010 [DOI] [PubMed] [Google Scholar]
- Pico, F. , Rosso, C. , Meseguer, E. , Chadenat, M. L. , Cattenoy, A. , Aegerter, P. , … Amarenco, P. (2016). A multicenter, randomized trial on neuroprotection with remote ischemic per‐conditioning during acute ischemic stroke: The REmote iSchemic Conditioning in acUtE BRAin INfarction study protocol. International Journal of Stroke, 11(8), 938–943. 10.1177/1747493016660098 [DOI] [PubMed] [Google Scholar]
- Qi, Z. F. , Luo, Y. M. , Liu, X. R. , Wang, R. L. , Zhao, H. P. , Yan, F. , … Ji, X. M. (2012). AKT/GSK3beta‐dependent autophagy contributes to the neuroprotection of limb remote ischemic postconditioning in the transient cerebral ischemic rat model. CNS Neuroscience & Therapeutics, 18(12), 965–973. 10.1111/cns.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, C. , Gao, M. , Dornbos, D., 3rd , Ding, Y. , Zeng, X. , Luo, Y. , & Ji, X. (2011). Remote ischemic post‐conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurological Research, 33(5), 514–519. 10.1179/016164111x13007856084241 [DOI] [PubMed] [Google Scholar]
- Ren, C. , Li, N. , Wang, B. , Yang, Y. , Gao, J. , Li, S. , … Ji, X. (2015). Limb ischemic perconditioning attenuates blood‐brain barrier disruption by inhibiting activity of MMP‐9 and occludin degradation after focal cerebral ischemia. Aging and Disease, 6(6), 406–417. 10.14336/ad.2015.0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, C. , Yan, Z. , Wei, D. , Gao, X. , Chen, X. , & Zhao, H. (2009). Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Research, 1288, 88–94. 10.1016/j.brainres.2009.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A. , Strecker, J. K. , Hucke, S. , Bruckmann, N. M. , Herold, M. , Mack, M. , … Minnerup, J. (2017). Targeting different monocyte/macrophage subsets has no impact on outcome in experimental stroke. Stroke, 48(4), 1061–1069. 10.1161/strokeaha.116.015577 [DOI] [PubMed] [Google Scholar]
- Song, S. , Kong, X. , Acosta, S. , Sava, V. , Borlongan, C. , & Sanchez‐Ramos, J. (2017). Effects of an inhibitor of monocyte recruitment on recovery from traumatic brain injury in mice treated with granulocyte colony‐stimulating factor. International Journal of Molecular Sciences, 18(7), 1418 10.3390/ijms18071418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama, Y. , Yagita, Y. , Oyama, N. , Terasaki, Y. , Omura‐Matsuoka, E. , Sasaki, T. , & Kitagawa, K. (2011). Granulocyte colony‐stimulating factor enhances arteriogenesis and ameliorates cerebral damage in a mouse model of ischemic stroke. Stroke, 42(3), 770–775. 10.1161/strokeaha.110.597799 [DOI] [PubMed] [Google Scholar]
- van Oostrom, M. C. , van Oostrom, O. , Quax, P. H. A. , Verhaar, M. C. , & Hoefer, I. E. (2008). Insights into mechanisms behind arteriogenesis: What does the future hold? Journal of Leukocyte Biology, 84(6), 1379–1391. 10.1189/jlb.0508281 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Reis, C. , Applegate, R., 2nd , Stier, G. , Martin, R. , & Zhang, J. H. (2015). Ischemic conditioning‐induced endogenous brain protection: Applications pre‐, per‐ or post‐stroke. Experimental Neurology, 272, 26–40. 10.1016/j.expneurol.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H. , Li, Y. , Han, S. , Liu, S. , Zhang, N. , Zhao, L. , … Li, J. (2016). cPKCgamma‐modulated autophagy in neurons alleviates ischemic injury in brain of mice with ischemic stroke through Akt‐mTOR pathway. Translational Stroke Research, 7(6), 497–511. 10.1007/s12975-016-0484-4 [DOI] [PubMed] [Google Scholar]
- Woitzik, J. , Hecht, N. , Schneider, U. C. , Peña‐Tapia, P. G. , & Vajkoczy, P. (2006). Increased vessel diameter of leptomeningeal anastomoses after hypoxic preconditioning. Brain Research, 1115(1), 209–212. 10.1016/j.brainres.2006.07.081 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Schallert, T. , Zhang, Z. G. , Jiang, Q. , Arniego, P. , Li, Q. , … Chopp, M. (2002). A test for detecting long‐term sensorimotor dysfunction in the mouse after focal cerebral ischemia. Journal of Neuroscience Methods, 117(2), 207–214. [DOI] [PubMed] [Google Scholar]
- Zhao, H. , Ren, C. , Chen, X. , & Shen, J. (2012). From rapid to delayed and remote postconditioning: The evolving concept of ischemic postconditioning in brain ischemia. Current Drug Targets, 13(2), 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]


