Abstract
In this study, we retrospectively compared the effectiveness of exenatide once‐weekly (ExeOW) versus liraglutide in non‐insulin treated patients with type 2 diabetes followed under routine care. We also present a meta‐analysis of similar observational studies available in the literature. In our multicentre retrospective study, patients initiating ExeOW (n = 204) or liraglutide (n = 410) had similar baseline clinical characteristics. Change in HbA1c at 6 months was superimposable in the two groups (−0.7% ± 1.0%), and changes in body weight were also similar (ExeOW ‐2.2 ± 3.7 kg; liraglutide −2.5 ± 4.3 kg; p = 0.457). Discontinuation rates were numerically but not significantly lower for ExeOW versus liraglutide. Pooling these data with those of observational studies available in the literature yielded superimposable effects between the two groups for the change in HbA1c and body weight, with a higher risk of discontinuation (mainly based on pharmacy refill rates) for ExeOW. We conclude that, in patients under routine care, initiation of ExeOW provides similar benefits on HbA1c and body weight as initiation of liraglutide. These data help view the results of randomized controlled trials from the perspective of their application in routine clinical practice.
Keywords: antidiabetic drug, cohort study, exenatide, liraglutide, type 2 diabetes
1. INTRODUCTION
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) differ in biochemical structure and pharmacokinetics, with injection schedules ranging from twice daily to once weekly (OW). In randomized controlled trials (RCTs) in patients with type 2 diabetes (T2D), GLP‐1RA improved glucose control, body weight, blood pressure and serum lipids.1 Because of these effects on multiple cardiovascular risk factors, GLP‐1RAs have great potential for protection against cardiovascular disease. This has been confirmed by RCTs showing reduction of major adverse cardiovascular events (MACE) with once‐daily liraglutide and OW semaglutide and albiglutide.2, 3, 4 The RCTs evaluating exenatide OW (ExeOW) reported a nearly significant reduction in MACE (P = 0.06) and a reduction in all‐cause mortality (P = 0.016).5 Based on this evidence, treatment guidelines recommend GLP‐1RAs for cardiovascular protection in T2D.6
According to a network meta‐analysis, some differences in the glucose‐lowering potency of the various GLP‐1RAs may exist.7 In the DURATION‐6 trial, liraglutide 1.8 mg was superior to ExeOW in reducing HbA1c.8 However, the 1.8 mg liraglutide dose is often not reached in clinical practice and is not recommended by some guidelines,9 such that it remains unclear whether liraglutide and ExeOW differ in their glucose‐lowering capacity in the real world. Since data coming from routine clinical practice can complement RCT findings,10 we compared the effectiveness of liraglutide and ExeOW in a retrospective study conducted in diabetes outpatient clinics in Italy.11 To view the results from the perspective of the available literature, we also performed a meta‐analysis of observational studies comparing liraglutide and ExeOW.
2. METHODS
2.1. Study design and data extraction
The DARWIN‐T2D was a retrospective multicentre study on data of patients initiating dapagliflozin, a dipeptidyl peptidase‐4 (DPP‐4) inhibitor, gliclazide, or a GLP‐1RA routinely accumulated in electronic charts. Ethical committee approval was obtained at all centres. Software interrogated the same electronic chart and automatically extracted anonymized data. The study design and main results were previously published.11, 12 We herein report a comparison within the group of non‐insulin treated patients who were first prescribed with a GLP‐1RA. We collected information only on liraglutide and ExeOW because, at the time the study was designed, lixisenatide was only being used in a minority (2%) of GLP‐1RA treated patients, twice‐daily exenatide had almost entirely switched to liraglutide or ExeOW, and dulaglutide, semaglutide and albiglutide were not marketed in Italy. Patients were retrospectively included if they had a diagnosis of T2D, were prescribed a GLP‐1RA for the first time between 15 March 2015 and 31 December 2016, and were still on the drug at the first available follow‐up visit 3–12 months after baseline. Patients concomitantly treated with insulin were excluded because, during the study period, only liraglutide was reimbursed in association with insulin.
Effectiveness endpoints were change from baseline to follow‐up in fasting glucose, HbA1c, body weight, systolic blood pressure, lipids (total cholesterol, HDL cholesterol, triglycerides and LDL cholesterol), liver enzymes, estimated glomerular filtration rate (eGFR) and urinary albumin excretion rate (AER). We also recorded presence or absence of chronic complications, information on concomitant medications for diabetes and cardiovascular risk factors, and the lifetime history of glucose‐lowering medications (GLM).
Drug discontinuation was defined when the drug was no longer prescribed at follow‐up. Reasons for discontinuation, as well as information on side effects, including hypoglycaemia, were not available.
2.2. Statistical analysis
Continuous data are presented as mean ± standard deviation, while categorical data are presented as percentages. Normality of continuous variables was checked using the Kolmogorov‐Smirnov test and non‐normal variables were log‐transformed before analysis. Between‐group differences in baseline variables were evaluated using unpaired 2‐tail Student's test for continuous variables and chi‐square test for categorical variables. In addition to P‐values, we report the standardized difference as a mean to evaluate the balance between groups. In the presence of P < 0.05, a standardized difference > 0.10 was considered suggestive of clinically relevant imbalance. Within‐group differences in effectiveness endpoints were assessed using paired 2‐tail Student's t test. Changes from baseline to follow‐up in effectiveness variables were computed in each group and compared using unpaired 2‐tail Student's test. To account for selection bias and adjust for eventual imbalanced variables, a multiple linear regression was planned with the desired effectiveness endpoint as the dependent variable and covariate(s) as independent variable(s). Statistical significance was accepted at P < 0.05 and SPSS v.21 or higher was used. Methods of the meta‐analysis are given in the online Appendix S1 (see the supporting information for this article).
3. RESULTS
3.1. Patients' characteristics
During the study period, 2247 T2D patients initiated a GLP‐1RA; after excluding patients without a follow‐up visit and those who were on insulin, the study included 410 patients on liraglutide and 204 patients on ExeOW (Figure S1A). The two groups were well balanced for age, sex, diabetes duration, body mass index (BMI), blood pressure, fasting glucose, HbA1c, renal function, concomitant and lifetime use of other GLM (Table S1). This allowed a direct comparison of the effectiveness of ExeOW versus liraglutide. However, 10% less of patients in the ExeOW group versus the liraglutide group were on angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ACEi/ARBs; P = 0.005). Table S1B shows the differences between patients with and without a follow‐up visit.
3.2. Effectiveness
Table S2 summarizes effectiveness results. After an average follow‐up of 5.6 months in both groups (median 5.9; interquartile range 4.2‐6.4), HbA1c declined significantly from 7.7% ± 0.8% to 7.0% ± 0.9% in the liraglutide group and from 7.6% ± 0.9% to 6.9% ± 0.9% in the ExeOW group. The change from baseline in HbA1c between the two groups was not significantly different (−0.7% ± 1.0% vs. −0.7% ± 1.0%; P = 0.752; Figure 1A). At follow‐up, 56.4% of patients in the ExeOW group versus 50.4% in the liraglutide group reached HbA1c < 7.0% (P = 0.199). Decline in fasting plasma glucose was also similar between the two groups (liraglutide −17.2 ± 35.1 mg/dL; ExeOW −21.1 ± 35.8 mg/dL; P = 0.321). Body weight declined by 2.5 ± 4.3 kg in the liraglutide group and by 2.2 ± 3.7 kg in the ExeOW group (P = 0.457; Figure 1B). Systolic and diastolic blood pressure did not significantly change in patients treated with either liraglutide or ExeOW, even after adjusting for baseline use of ACEi/ARBs. After adjusting for baseline outcome values and follow‐up time, there were still no differences in the change from baseline to follow‐up in HbA1c, body weight and systolic blood pressure (Table S3).
Figure 1.
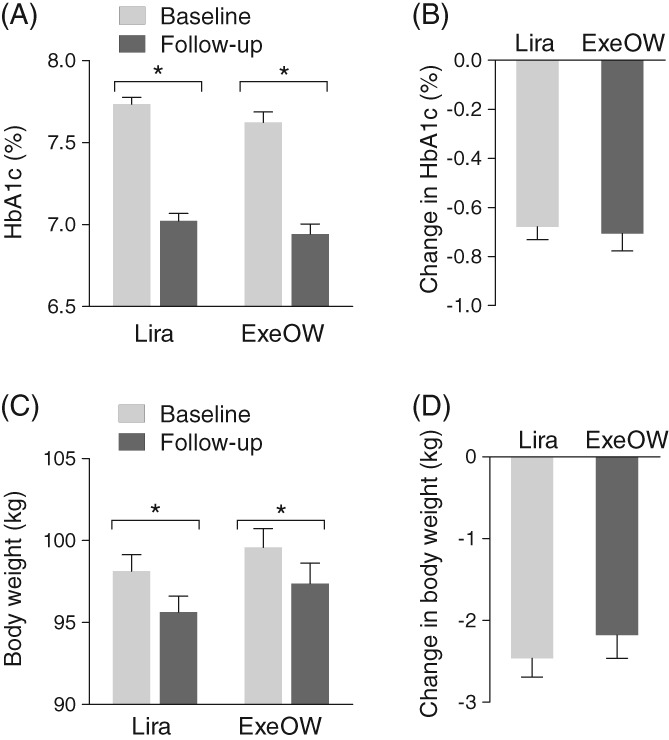
Comparative effectiveness. A, HbA1c values at baseline and at follow‐up are plotted for patients who received liraglutide (Lira) or exenatide OW (ExeOW); *p < 0.05 using paired Student's t test. B, The change versus baseline in HbA1c in the two groups. C, Body weight at baseline and at follow‐up are plotted for the two groups of patients (*p < 0.05 using paired Student's t test). D, The change versus baseline in body weight in the two groups. Error bars indicate standard error
Total cholesterol and LDL cholesterol levels improved significantly during treatment with both liraglutide and ExeOW and the changes from baseline were not significantly different between groups. The reduction of liver enzymes was also similar between the two groups. Also, eGFR and AER did not change during treatment of either GLP‐1RA.
3.3. Discontinuation
The rate of discontinuation was 8.7% in the liraglutide group versus 6.8% in the ExeOW group (P = 0.41). The corresponding projected yearly discontinuation rates were 18.4% and 14.5%, respectively.
3.4. Meta‐analysis
We identified 13 real world observational studies (Figure 2A). After excluding those not reporting poolable data for any of the three elected outcome measures and adding the results of the present study, we included in the meta‐analysis 3 studies13, 14 reporting the change in HbA1c (6035 patients) and the change in body weight (9384 patients), and 8 studies15, 16, 17, 18, 19, 20, 21 reporting discontinuation rates (110 232 patients). Follow‐up duration was 6 months in 5 studies14, 16, 17, 20, 21 and 12 months in the others.13, 15, 18, 19 Altogether, there was no difference between liraglutide and ExeOW for the change in HbA1c [0.01%; 95% confidence interval (CI) ‐0.08; 0.09; no heterogeneity, Figure 2B] or for the change in body weight (0.12 kg; 95% CI ‐0.26; 0.51; no heterogeneity, Figure 2C). The pooled risk ratio of discontinuation was 1.22 (95% CI 1.07; 1.39), indicative of a higher likelihood of discontinuation with ExeOW than with liraglutide, but with very high heterogeneity among studies (Figure 2D).
Figure 2.
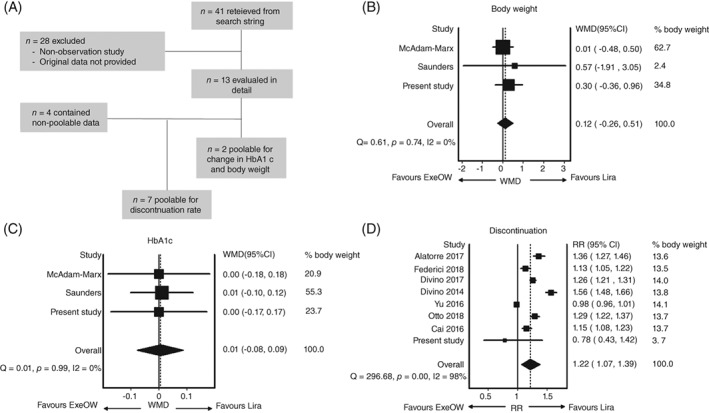
Meta‐analysis of observational studies comparing exenatide OW (ExeOW) and liraglutide (Lira). A, The meta‐analysis flowchart. Panels B‐D show the forest plots for the 3 outcomes considered: B, change in HbA1c, C, change in body weight and D, discontinuation rates. In the paper by McAdam‐Marx et al.,13 unadjusted data were recorded for the change in HbA1c and body weight, and, for the change in HbA1c, only the insulin‐free subgroup was considered. In the paper by Otto et al.,15 cohort 1 was considered, which was composed by GLP‐1RA initiators, while cohort 2 was composed by intra‐class switchers. In the paper by Yu et al20 the unmatched cohorts were considered. WMD, weighted mean difference; RR, risk ratio, Q and I2 are tests for homogeneity and heterogeneity of effect sizes
4. DISCUSSION
We show that, in the routine clinical treatment of T2D patients who were uncontrolled on oral agents, initiation of ExeOW provided similar reductions in HbA1c and body weight to initiation of liraglutide. These results are consistent with those of other studies included in the meta‐analysis, but contrast with results of the head‐to‐head comparative trial DURATION‐6,8 as summarized in Table S4. In DURATION‐6, the magnitude of HbA1c reduction at 6 months was larger (−1.48% with liraglutide and − 1.28% with ExeOW) and liraglutide, uptitrated to the maximum dose of 1.8 mg in all patients, reduced HbA1c more than ExeOW. The higher average HbA1c reduction in the DURATION‐6 trial than in our real‐world study could be explained by the higher baseline HbA1c (8.4‐8.5% vs. 7.6‐7.7%, respectively). Indeed, the percentages of patients reaching HbA1c < 7.0% was similar in the trial to the real world. The absence of any difference in the glycaemic effectiveness between liraglutide and ExeOW in observational studies is probably attributable to the doses of liraglutide used in clinical practice. The starting dose of liraglutide is 0.6 mg, and patients are instructed to increase the dose to 1.2 mg after at least 1 week. Most patients are not routinely uptitrated to 1.8 mg,22 a step not recommended by the NICE guidelines.9 Thus, we expected liraglutide dose to be closer to 1.2 mg than to 1.8 mg at the first follow‐up23; data from one pilot study centre yielded average liraglutide doses of 1.27 mg at ~6 months and of 1.48 mg at the last available visit. Therefore, dose titration emerges as a clinical practice issue to maximize liraglutide efficacy.
The use of ExeOW versus liraglutide may increase patient compliance and adherence, because of the need for less injections. In our study, the rate of permanent discontinuation at ~6 months was nominally, but not significantly, lower with ExeOW than with liraglutide. Rates of discontinuation at 6 or 12 months in other studies in the literature, defined based on drug pharmacy refill rates, were higher with ExeOW than with liraglutide, with very high heterogeneity. Discrepancy with our study is probably attributable to the different definition of discontinuation. A possible reason for higher discontinuation rates with ExeOW compared with liraglutide is the formation of subcutaneous nodules, which occurred more frequently with the first than with the newest ExeOW injection device.24
This study has limitations inherent in its observational nature. Assignment to liraglutide or ExeOW was based on clinical decision and not randomized. The two groups were very well balanced at baseline, thereby making a direct comparison in effectiveness easier without adjustments or matching. However, we cannot exclude confounding by unmeasured variables that drive the outcome, including self‐selection (the choice of a once‐daily or OW therapy by patient preference or compliance) and prescriber bias. In addition, differences between patients with and those without a follow‐up examination may limit the generalizability of the findings. Finally, several important items of information were not available, including lifestyle optimization prior to initiation of GLP‐1RA, liraglutide doses at all centres, discontinuation causes, tolerability and side effects.
In summary, we show that in T2D patients mildly uncontrolled on oral agents in the real world, initiation of ExeOW provided similar benefits on HbA1c and body weight at ~6 months as initiation of liraglutide. These data help to view the results of RCTs from the perspective of their application to routine clinical practice.
CONFLICTS OF INTEREST
G.P.F. received grant support, lecture or advisory board fees from AstraZeneca, Boehringer‐Ingelheim, Eli Lilly, NovoNordisk, Sanofi, Genzyme, Abbott, Novartis and Merck Sharp & Dohme. B.M.B. received lecture or advisory board fees from Novartis and Eli Lilly. A.L. received grant support, lectures or advisory board fees from Novo Nordisk, Sanofi, Abbott and Eli Lilly. P.S.M. received lecture or advisory board fees from AstraZeneca, Boehringer‐Ingelheim, NovoNordisk, Sanofi and Merck Sharp & Dohme. N.S. received lecture fees or other support from Eli Lilly, Abbott and NovoNordisk. A.A. received research grants, lecture or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boeringher‐Ingelheim, Sanofi, Mediolanum, Janssen and NovoNordisk. B.F. has nothing to disclose.
Author contributions
Study design: G.P.F. and A.A. Data collection and analysis: G.P.F., B.M.B., A.L., B.F., P.S.M. and N.S. Manuscript writing: G.P.F and A.A. Manuscript revision: G.P.F., B.M.B., A.L., B.F., P.S.M., N.S. and A.A. All authors approved the final version of the manuscript.
Supporting information
Appendix S1. Supplemental methods and results
ACKNOWLEDGMENTS
We wish to thank Alessia Russo, Italian Diabetes Society, for the invaluable technical support.
Composition of the DARWIN‐T2D network
Agostino Consoli and Gloria Formoso (Dipartimento di Medicina e Scienze dell'Invecchiamento ‐ Università Degli studi G. D'Annunzio di Chieti‐Pescara); Giovanni Grossi (Ospedale San Francesco di Paola ‐ Azienda Sanitaria Provinciale di Cosenza); Achiropita Pucci (Azienda Sanitaria Provinciale di Cosenza); Giorgio Sesti and Francesco Andreozzi (Azienda Ospedaliero Universitaria di Catanzaro); Giuseppe Capobianco (Azienda Sanitaria Locale Napoli 2 Nord); Adriano Gatti (Ospedale San Gennaro dei Poveri ‐ Azienda Sanitaria Locale Napoli 1 Centro); Riccardo Bonadonna, Ivana Zavaroni and Alessandra Dei Cas (Azienda Ospedaliero Universitaria di Parma); Giuseppe Felace (Ospedale di Spilimbergo ‐ Azienda per l'Assistenza Sanitaria n.5 Friuli Occidentale); Patrizia Li Volsi (Ospedale di Pordenone ‐ Azienda per l'Assistenza Sanitaria n.5 Friuli Occidentale); Raffaella Buzzetti and Gaetano Leto (Ospedale Santa Maria Goretti ‐ Azienda Sanitaria Locale di Latina); Gian Pio Sorice (Fondazione Policlinico Universitario A. Gemelli, Roma); Paola D'Angelo (Ospedale Sandro Pertini ‐ Azienda Sanitaria Locale Roma 2); Susanna Morano (Azienda Ospedaliera Universitaria Policlinico Umberto I, Roma); Antonio Carlo Bossi (Ospedale di Treviglio ‐ Azienda Socio Sanitaria Territoriale Bergamo Ovest); Edoardo Duratorre (Ospedale Luini Confalonieri di Luino ‐ Azienda Socio Sanitaria Territoriale Sette Laghi); Ivano Franzetti (Ospedale Sant'Antonio Abate di Gallarate ‐ Azienda Socio Sanitaria Territoriale Valle Olona); Paola Silvia Morpurgo (Ospedale Fatebenefratelli ‐ Azienda Socio Sanitaria Territoriale Fatebenefratelli Sacco); Emanuela Orsi (Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico di Milano); Fabrizio Querci (Ospedale Pesenti Fenaroli di Alzano Lombardo ‐ Azienda Socio Sanitaria Territoriale Bergamo Est); Massimo Boemi† and Federica D'Angelo (Presidio Ospedaliero di Ricerca INRCA‐IRCCS di Ancona); Massimiliano Petrelli (Azienda Ospedaliero Universitaria Ospedali Riuniti di Ancona); Gianluca Aimaretti and Ioannis Karamouzis (Azienda Ospedaliero Universitaria Maggiore della Carità di Novara); Franco Cavalot (Azienda Ospedaliero Universitaria San Luigi Gonzaga, Orbassano); Giuseppe Saglietti† (Ospedale Madonna del Popolo di Omegna ‐ Azienda Sanitaria Locale Verbano Cusio Ossola); Giuliana Cazzetta (Casa della Salute, Ugento ‐ Distretto Socio Sanitario Gagliano del Capo ‐ Azienda Sanitaria Locale di Lecce); Silvestre Cervone (Presidio ospedaliero San Marco in Lamis ‐ Distretto Socio Sanitario San Marco in Lamis ‐ Azienda Sanitaria Locale di Foggia); Eleonora Devangelio (Distretto Socio Sanitario di Massafra ‐ Azienda Sanitaria Locale di Taranto); Olga Lamacchia (Azienda Ospedaliero Universitaria Ospedali Riuniti di Foggia); Salvatore Arena (Ospedale Umberto I – Azienda Sanitaria Provinciale di Siracusa); Antonino Di Benedetto (Azienda Ospedaliera Universitaria Policlinico G. Martino di Messina); Lucia Frittitta (Azienda Ospedaliera di Rilievo Nazionale e di Alta Specializzazione Garibaldi di Catania); Carla Giordano (Azienda Universitaria Policlinico Paolo Giaccone di Palermo); Salvatore Piro (Azienda Ospedaliera di Rilievo Nazionale e di Alta Specializzazione Garibaldi di Catania); Manfredi Rizzo, Roberta Chianetta and Carlo Mannina (Azienda Universitaria Policlinico Paolo Giaccone di Palermo); Roberto Anichini (Ospedale San Jacopo di Pistoia – Azienda USL Toscana Centro); Giuseppe Penno (Azienda Ospedaliero Universitaria Pisana); Anna Solini (Azienda Ospedaliera Universitaria Pisana); Bruno Fattor (Comprensorio Sanitario di Bolzano ‐ Azienda Sanitaria della Provincia Autonoma di Bolzano); Enzo Bonora and Massimo Cigolini (Azienda Ospedaliero Universitaria Integrata di Verona); Annunziata Lapolla and Nino Cristiano Chilelli (Complesso Socio Sanitario Ai Colli ‐ Azienda ULSS n.6 Euganea); Maurizio Poli (Ospedale Girolamo Fracastoro di San Bonifacio ‐ Azienda ULSS n.9 Scaligera); Natalino Simioni and Vera Frison (Ospedale di Cittadella ‐ Azienda ULSS n.6 Euganea); Carmela Vinci (Azienda ULSS n.4 Veneto Orientale).
Fadini GP, Bonora BM, Lapolla A, et al. Comparative effectiveness of exenatide once‐weekly versus liraglutide in routine clinical practice: A retrospective multicentre study and meta‐analysis of observational studies. Diabetes Obes Metab. 2019;21:1255–1260. 10.1111/dom.13623
Funding information The study was partly supported by the Italian Diabetes Society, through a grant from AstraZeneca. The external sponsor had no role in study design, data analysis and interpretation, and decision to publish.
REFERENCES
- 1. Aroda VR. A review of GLP‐1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20(suppl 1):22‐33. [DOI] [PubMed] [Google Scholar]
- 2. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519‐1529. [DOI] [PubMed] [Google Scholar]
- 3. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 4. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in Type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon‐like peptide‐1 receptor agonists in type 2 diabetes: a systematic review and mixed‐treatment comparison analysis. Diabetes Obes Metab. 2017;19:524‐536. [DOI] [PubMed] [Google Scholar]
- 8. Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION‐6): a randomised, open‐label study. Lancet. 2013;381:117‐124. [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence (NICE) . Liraglutide for the treatment of type 2 diabetes mellitus. https://wwwniceorguk/guidance/ta203. Published Oct 27, 2010. Accessed May 4, 2015.
- 10. Chatterjee S, Davies MJ, Khunti K. What have we learnt from “real world” data, observational studies and meta‐analyses. Diabetes Obes Metab. 2018;20(suppl 1):47‐58. [DOI] [PubMed] [Google Scholar]
- 11. Fadini GP, Zatti G, Consoli A, Bonora E, Sesti G, Avogaro A. Rationale and design of the DARWIN‐T2D (DApagliflozin Real World evIdeNce in Type 2 Diabetes): a multicenter retrospective nationwide Italian study and crowdsourcing opportunity. Nutr Metab Cardiovasc Dis. 2017;27:1089‐1097. [DOI] [PubMed] [Google Scholar]
- 12. Fadini GP, Zatti G, Baldi I, et al. Use and effectiveness of dapagliflozin in routine clinical practice: an Italian multicentre retrospective study. Diabetes Obes Metab. 2018;20:1781‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAdam‐Marx C, Nguyen H, Schauerhamer MB, et al. Glycemic control and weight outcomes for exenatide once weekly versus liraglutide in patients with Type 2 diabetes: a 1‐year retrospective cohort analysis. Clin Ther. 2016;38:2642‐2651. [DOI] [PubMed] [Google Scholar]
- 14. Saunders WB, Nguyen H, Kalsekar I. Real‐world glycemic outcomes in patients with type 2 diabetes initiating exenatide once weekly and liraglutide once daily: a retrospective cohort study. Diabetes Metab Syndr Obes. 2016;9:217‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Otto T, Myland M, Jung H, Lebrec J, Richter H, Norrbacka K. Utilization patterns of glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes mellitus in Germany: a retrospective cohort study. Curr Med Res Opin. 2018;1‐19. [DOI] [PubMed] [Google Scholar]
- 16. Federici MO, McQuillan J, Biricolti G, et al. Utilization patterns of glucagon‐like peptide‐1 receptor agonists in patients with Type 2 diabetes mellitus in Italy: a retrospective cohort study. Diabetes Ther. 2018;9:789‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alatorre C, Fernandez Lando L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon‐like peptide‐1 receptor agonists: higher adherence and persistence with dulaglutide compared with once‐weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19:953‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Divino V, DeKoven M, Khan FA, Boye KS, Sapin H, Norrbacka K. GLP‐1 RA treatment patterns among Type 2 diabetes patients in five European Countries. Diabetes Ther. 2017;8:115‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai J, Wang Y, Baser O, Xie L, Chow W. Comparative persistence and adherence with newer anti‐hyperglycemic agents to treat patients with type 2 diabetes in the United States. J Med Econ. 2016;19:1175‐1186. [DOI] [PubMed] [Google Scholar]
- 20. Yu M, Xie J, Fernandez Lando L, Kabul S, Swindle RW. Liraglutide versus exenatide once weekly: persistence, adherence, and early discontinuation. Clin Ther. 2016;38:149‐160. [DOI] [PubMed] [Google Scholar]
- 21. Divino V, DeKoven M, Hallinan S, et al. Glucagon‐like peptide‐1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther. 2014;5:499‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rigato M, Avogaro A, Fadini GP. Effects of dose escalating liraglutide from 1.2 to 1.8 mg in clinical practice: a case‐control study. J Endocrinol Invest. 2015;38:1357‐1363. [DOI] [PubMed] [Google Scholar]
- 23. Lapolla A, Frison V, Bettio M, et al. Correlation between baseline characteristics and clinical outcomes in a large population of diabetes patients treated with liraglutide in a real‐world setting in Italy. Clin Ther. 2015;37:574‐584. [DOI] [PubMed] [Google Scholar]
- 24. Wysham CH, Rosenstock J, Vetter ML, Dong F, Ohman P, Iqbal N. Efficacy and tolerability of the new autoinjected suspension of exenatide once weekly versus exenatide twice daily in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:165‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental methods and results


