Summary
Background
Chronic constipation affects approximately 17% of the population worldwide and remains an important unmet need since patients are often dissatisfied with treatment. Kiwifruit may offer an alternative to traditional laxatives and have been shown to increase stool volume, frequency and improve consistency.
Aims
Using non‐invasive MRI techniques, we assessed the effect of ingestion of kiwifruit on fluid distribution in the intestines and bowel function.
Methods
Two period crossover trial of kiwifruit vs control in healthy adults. Intervention: two kiwifruits twice daily vs isocaloric control (maltodextrin) twice daily, consumed for a total of 3 days. Subjects underwent MRI scanning fasted and at hourly intervals for 7 hours on the third day. Primary outcome: T1 relaxation time of ascending colon (T1AC) using MRI. Secondary outcomes: Small bowel water content (SBWC), colonic volume, gut transit time, T1 of descending colon, stool frequency and form.
Results
Fourteen volunteers completed the study. T1AC was higher after kiwifruit ingestion (P = 0.029) during the second half of the day (when meal residue would be expected to reach the AC, AUC T1 T240‐420 minutes; mean (SD) 137 (39) s*minute with kiwifruit versus 108 (40) s*minute with control. SBWC (P < 0.001), colon volumes (P = 0.004), as well as stool frequency (1.46 ± 0.66 with kiwifruit vs 1.14 ± 0.46 stools per day with control; P = 0.034) and stool form score (Bristol Stool Chart score 4.1 (0.9) with kiwifruit versus 3.4 (0.7) with control; P = 0.011) were markedly increased in participants consuming kiwifruit compared to control.
Conclusion
Consumption of kiwifruit in healthy volunteers increases water retention in the small bowel and ascending colon and increases total colonic volume. The data may explain the observed increase in stool frequency and looser stool consistencies, suggesting that kiwifruit could be used as a dietary alternative to laxatives in mild constipation.
1. INTRODUCTION
Chronic constipation affects approximately 17% of the population worldwide1 and its management remains an important unmet need since patients are often dissatisfied with treatment.2 Current stimulant or osmotic laxatives are successful in increasing stool frequency3 but are often associated with diarrhoea, bloating, cramps and abdominal discomfort.4 Furthermore, such powerful treatments taken intermittently may result in alternation between diarrhoea and no stools, this is both inconvenient for the patient and potentially disturbs the microbiota with uncertain consequences.5 Many patients including those with mild symptoms who do not seek health care would benefit from a less powerful treatment which could be taken daily producing more regular stools of normal consistency.
Kiwifruit offer such an alternative and, in two open label and one randomised placebo controlled trials, have been shown to increase stool volume and frequency in patients with constipation.6, 7, 8 The mode of action remains unclear as kiwifruit have many potentially active ingredients.9
Kiwifruit are the berry of the Actinidia vine and there are two varieties that are widely commercially available. These are the “Hayward’ or green variety (Actinidia deliciosa) (marketed as Zespri® Green) and “Zesy002” or gold variety (Actinidia chinensis) (marketed as Zespri® SunGold).
The fruit are nutrient‐dense and are high in vitamins C, E, K, and in micronutrients potassium, folate, phytochemicals and carotenoids, as well as in fibre.10
A dose of two Kiwifruit weighing 300 g contains approximately 12 g fructose, 12 g glucose and 9 g of dietary fibre. The fibre comes primarily from the plant cell walls and is a mixture of soluble (one third) and insoluble fibre (two thirds). The soluble fibre is pectic polysaccharides and the insoluble fibre is cellulose and hemicellulose.11 Pig studies showed that while the soluble component of kiwifruit fibre is well digested in the small bowel, the insoluble component passes largely intact into the colon.12
Kiwifruit cell walls have a large swelling and water retention capacity both before and after digestion and in vitro tests (which involve suspension in 250 mL of water and allowing to settle overnight) found that it swells to over three times its original volume for both green and gold kiwifruit.11 This swelling is around 1.5 times that of psyllium, a commonly used laxative, and more than six times that of apple fibre. The water retention capacity of the fibre, defined as the amount of water which remains after centrifugation of kiwifruit pulp suspended in water, was around 12‐13 g of water per gram of insoluble fibre. Both this swelling and water retention are high compared to other readily available forms of dietary fibre such as apple, orange, wheat bran and sugar beet fibre.11
Soluble fibre is a proven laxative,13 softening stool and increasing stool frequency. Early studies in ileostomy patients showed that psyllium increases ileal flow14 but until recently its mode of action in the intact colon was unclear. However, recently we have shown using novel non‐invasive MRI techniques that psyllium 3.5 g taken three times daily substantially increases small bowel water content (SBWC) in healthy volunteers, retaining fluid in the small bowel. This increases the effect of the gastro‐ileal response to eating which propels the retained fluid from the ileum to the ascending colon immediately after eating a 1000 kcal meal.15 This is associated with an increase in colonic volume and water content. Kiwifruit appear clinically to be as effective as ispaghula as shown in a recent multi‐centre trial in Japan, Italy and New Zealand of 178 participants (n = 61 healthy, n = 57 FC and n = 60 IBS‐C). The effects of 4 weeks of kiwifruit (2 per day) and psyllium (7.5 g/day) were comparable, with an increase of 2.1 complete spontaneous bowel movements per week in the kiwifruit arm compared to 0.92 per week with psyllium compared to baseline.16
Kiwifruit also contains insoluble fibre, which in the form of 15 g bran has also been demonstrated to increase post‐prandial small bowel water content17 and accelerate small bowel transit.18
Until recently, defining the site of action of kiwifruit at the level of the small bowel and colon would require either invasive intubation studies or the study of ileostomists whose physiology may well differ significantly from the target population of healthy individuals. Using recently developed and validated novel non‐invasive MRI techniques, we have assessed transit and fluid distribution in the small intestine as well as colonic fluid and volume19, 20, 21, 22 in order to define the mechanisms behind the known laxative effects of kiwifruit consumption. We hypothesised that consuming kiwifruit would combine the effects of both soluble and insoluble fibre, increasing the water content of small and large intestine contents in healthy volunteers.
2. METHODS
2.1. Study design
This study was a two period, two treatment crossover study with randomisation of treatment order. The study protocol was approved by the University of Nottingham Medical School Ethics Committee (Ref. A200317) and all volunteers gave written informed consent. The study was carried out according to Good Clinical Practice as defined by the Declaration of Helsinki. The study was registered on clinicaltrials.gov, reference: NCT03303417.
2.2. Study participants
Adult participants (aged 18‐65), able to give consent and scoring <2 on the 15 individual items (ie <15/45 total) of the Gastrointestinal Symptom Rating Scale23 were recruited. Exclusion criteria were existing GI disorder or major GI surgery, inability to cease medication affecting GI transit or motility, known intolerance of kiwifruit, contraindication to MRI scanning or lifestyle factors likely to disrupt gut function or compliance with the protocol such as night shift working.
2.3. Test products and meals
Test product was either two large and ready to eat green variety kiwifruits without skins (approximate weight 150 g per kiwifruit) eaten twice per day or calorie matched control drink (28 g maltodextrin, average degree of polymerisation = 5, in 250 mL water providing 120 kcal and estimated osmolality of 124 mosmol/L). These were consumed for 48 hours prior to the study day and on the scanning day (12 kiwifruit/6 drinks total).
On the study day (see Figure 1 for an overview), participants were fed two standard meals that we have used in previous studies.17 Meal A consisted of 220 g creamed rice pudding (Sainsbury's, London, UK), 34 g seedless raspberry jam (Sainsbury's) and a glass of 100 mL pure orange juice from concentrate (Sainsbury's), providing 331 kcal. Meal B consisted of 400 g microwaveable macaroni cheese ready meal (Sainsbury's), 100 g cheesecake slice (Sainsbury's) and 250 mL bottled still water, providing a total of 1007 kcal.
Figure 1.
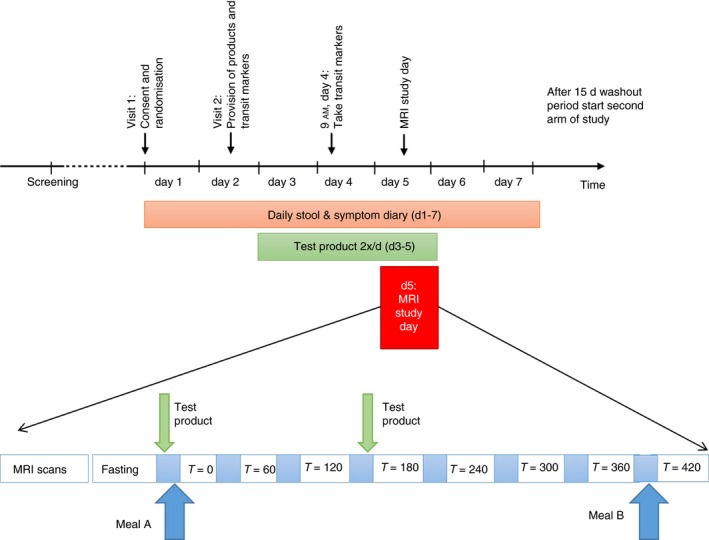
Schematic diagram of study
2.4. Study day protocol
Consent, screening and enrolment took place at the Nottingham Digestive Diseases Biomedical Research Unit, Nottingham. All subsequent study visits took place at the Sir Peter Mansfield Imaging Centre (SPMIC), University of Nottingham. Subjects visited the Imaging Centre on two separate occasions, with at least 2 weeks between each visit to minimise any carryover effect. Subjects were asked to abstain from alcohol, caffeine and strenuous exercise for 18 hours before the visit and did not eat after 8 PM on the evening beforehand. The day before the scan they ingested five MRI marker pills at 9 AM, which were imaged 24 hours later at the beginning of the study day. Ingestion was confirmed by a time‐stamped video. The marker pills, which were developed in‐house, are inert capsules containing the MRI contrast agent gadolinium, making the pills clearly visible on the MR scan. Each pill is allocated a score based on its position in the colon and a weighted average position score calculated as previously described.20 The same publication showed that this score correlates well with transit time in hours as measured using the well‐established radio‐opaque marker test.24
On the day of the visit, subjects fasted before attendance, with the exception of sips of water with essential medicines. On arrival they underwent a baseline MRI scan. They were then given 10 minutes to consume the test product. Thirty minutes later they consumed test Meal A followed by another MRI Scan (Time = 0). They then underwent hourly scanning for 7 hours (T = 0‐420). They had a second dose of test product immediately before the scan at T180 to simulate the effect of two doses daily, giving time to see any effect before the final Meal B between T = 360 and T = 420 (Figure 1).
Background diet was not controlled other than the instructions given for the day before and during the MRI day; however, participants were asked to remain on their normal diet and not eat any other kiwifruit during the study period (except those provided by the study team).
2.5. Questionnaires
Participants were asked to keep a diary of symptoms (abdominal pain, nausea, bloating, gas estimated on a scale of 0‐3: none, mild, moderate, severe) and bowel habit (frequency and form according to Bristol stool form chart) during the week of the study day—this included the 2 days prior to the consumption of the test product, the 3 days consuming (including study day) and 2 days after this. Data were analysed for kiwifruit or control on days 3‐6 of the respective study arm.
2.6. MRI protocol
Imaging was carried out on a fully research‐dedicated 3.0 T Philips Achieva scanner (Best, The Netherlands). Subjects were positioned supine in the scanner with a parallel imaging SENSE XL‐Torso receiver coil wrapped around the abdomen. Different imaging sequences were used to optimally image the different regions of the gut as follows.
A high resolution balanced gradient echo sequence to acquire sagittal images of the contents of the ascending colon, eight slices 5 mm thick, 0.58 mm gap was acquired to include the entire ascending colon. This was repeated for the descending colon.
The longitudinal relaxation time T1 was measured in the ascending colon and separately in the descending colon, using a single slice inversion recovery balanced turbo field echo sequence with a preparatory 180° inversion pulse applied before acquiring the imaging data. The slice for the T1 is chosen from the high resolution images and is chosen as the slice which provides the best cross‐section through the colonic segment maximising the colonic content to sample. The parameters for this sequence were a field of view of 400 (HF) × 280 (AP) mm; 7 mm slice thickness; half Fourier acquisition; 256 × 256 matrix; repetition time/echo time 2.4/1.2 milliseconds; flip angle 45°. Data were acquired from eight different inversion times (TI; time between inversion pulse and imaging pulses) ranging from 0.05 to 4.95 seconds.21 There was a 15 second gap between each acquisition to allow the system to return to equilibrium.
A single shot, fast spin echo sequence (rapid acquisition with relaxation enhancement; effective echo time TE = 400 milliseconds) to acquire in a single breath‐hold, 20 coronal images with in‐plane resolution interpolated to 0.78 mm × 0.78 mm and a slice thickness of 7 mm, with no gap between slices (acquired voxel size = 1.4 × 1.76 × 7 mm3), SENSE = 2.0. This sequence yields high‐intensity signals from areas with freely mobile fluid and little signal from body tissues and was used to measure small bowel water content.17, 19
Colonic volumes were assessed using a coronal 3D dual‐gradient echo sequence (TE1 = 1.07 milliseconds, TE2 = 1.9 milliseconds, repetition time = 3.0 milliseconds) and mDIXON readout.25 The field of view at each station was 250 mm (HF) × 375 mm (LR) by 200 mm (AP) with acquired slice thickness 3.6 mm, interpolated to 1.8 mm with a reconstruction matrix of 432. These two sets of images were combined into a single image of matrix size 543 (HF) × 432 (LR) and 37 slices of thickness 5.4 mm and were used to visualize the colonic anatomy. Four different image types are produced from this acquisition, water only, fat only, fat and water in‐phase and fat and water out‐of‐phase data. Slices were acquired in two overlapping coronal stacks (30 mm overlap), each acquired in a breath hold. Colonic volumes were measured manually as previously described.22 The water‐only images from this sequence were also used to locate the position of the transit pills within the GI tract.20
Data were all acquired on an expiration breath‐hold with duration between 13 and 24 seconds depending on the sequence, monitored using a respiratory belt. Including set‐up and imaging, the volunteers spent approximately 20 minutes inside the magnet for every time point and the rest of the time sitting upright in an adjacent room.
2.7. Outcomes
The primary outcome was longitudinal relaxation time (T1, an MRI constant reflecting water mobility) of the chyme in the ascending colon, this reflects the fluidity of the contents, with more fluid contents having a longer T1 time for example, liver has a T1 of 750 milliseconds while cerebrospinal fluid has a T1 of 4000 milliseconds at 3 T field strength. Secondary MRI outcomes were: T1 of descending colon, SBWC, colonic volume, gut transit time as assessed by WAPS at 24 hours and questionnaire‐based scores for symptoms and bowel habit. Stool consistency was measured using the Bristol stool form scale (ranges from 1 = hard lumpy stools to 7 = watery stools).
2.8. Randomisation and blinding
The order of the test products was randomised using www.randomisation.com. Neither subjects nor researchers were blind to the meal consumed on study days. All study data and images carried an anonymising study ID number and MRI analysis were conducted blind to the intervention.
2.9. Power calculation
Our data15 with a standard laxative dose of psyllium 7 g three times per day showed a change of the area under the curve of T1 vs time from time 0 to time 360 minutes (T1 AUC 0‐360) of mean (SD) 88 (55) seconds × minutes. Using these data, we calculated that n = 15 healthy volunteers will give us >90% power to detect a similar difference.
2.10. Data analysis
The image data set from each subject and each time point was stored on a password‐protected server and processed as previously described using either software written in house in IDL (version 6.4; Research Systems Inc, Boulder, CO, USA; for analysis of SBWC and T1 relaxation times) by Dr Caroline Hoad at the SPMIC, or commercial software (Analyze v.9, Biomedical Imaging Resources, Mayo Clinic, Rochester, MN, USA; for analysis of colon volumes).19, 21, 22
2.11. Statistical analysis
Graphpad Prism (v.7; San Diego, CA, USA) and spss (v.24; IBM; Armonk, NY, USA) were used for statistical analysis of the data. We assessed the changes over time and between treatments using a two‐way analysis of variance (ANOVA) test. Normal distribution of data was determined using the Shapiro‐Wilk test. Within each scanning time point, data were then compared with a paired t‐test, for parametric data, or with a paired Wilcoxon test, for nonparametric data. P = 0.05 was used as the threshold for statistically significant outcomes. When data were ordinal (stool frequency, stool form score), a Wilcoxon test (kiwifruit compared to control) or Friedman ANOVA (kiwifruit compared to control and baseline) was carried out. For the outcomes recorded in the questionnaires over several days (stool frequency, stool form, symptoms), the data within each period (kiwifruit and control) were summarised by their median for each participant, before applying the appropriate tests. Data are displayed as median and interquartile range.
3. RESULTS
Sixteen participants were recruited but two dropped out after a single visit due to unexpected work commitments so 14 volunteers completed the study. Of these 14, six were female, eight were male with a median age of 26 ± 4 (range 21‐33) years and a body mass index of 23 ± 4 (range 18‐30) kg/m2. Median score was 0.5 (range 0‐4) on Gastrointestinal Symptom Rating Scale. The study procedures were well tolerated by the subjects.
3.1. T1 relaxation time of chyme in the ascending colon
The primary outcome was defined as the area under the curve for T1 relaxation time measured in the ascending colon over the time from baseline until the end of the study day (T420), comprising of eight hourly scans, and was increased in the kiwifruit arm however did not reach significance (P = 0.056, Table 1). As individual data points measured at each scan, T1 relaxation time is not different as analysed by two‐way ANOVA, P = 0.75 (Appendix S1).
Table 1.
Data for MRI variables measured as AUC of hourly scans. AUC expressed as a function of time (SBWC and colon volume ml.min or T1 seconds.minutes)
| MRI endpoint | Kiwifruit | Control | P‐value |
|---|---|---|---|
| AUC T1 AC (seconds.minutes) Baseline‐420 | 356 ± 109 | 291 ± 110 | 0.056 |
| AUC T1 AC (seconds.minutes) T240‐420 | 137 ± 39 | 108 ± 40 | 0.029* |
| AUC T1 DC (seconds.minutes) Baseline‐420 | 216 ± 120 | 203 ± 114 | 0.3 |
| AUC T1 DC (seconds.minutes) T240‐420 | 96 ± 50 | 87 ± 52 | 0.4 |
| AUC SBWC (mL.minutes) Baseline‐420 | 85 530 ± 34 923 | 35 023 ± 15 557 | 6 × 10−6* |
| AUC colon volume (ml.min) Baseline‐420 | 283 583 ± 59 402 | 243 182 ± 46 682 | 0.004* |
AC, ascending colon; AUC, area under the curve; DC, descending colon; SBWC, small bowel water content; T1, T1 relaxation time.
Data are expressed as mean ± SD.
Analysing the T1 relaxation time data further, when the area under the curve was measured from the time point when the first meal and treatment products start reaching the colon, T240‐T420, this showed a statistically significant increase of T1 relaxation time from the control to the kiwifruit arm of the study (+27%, P = 0.029, Table 1 and Figure 2).
Figure 2.
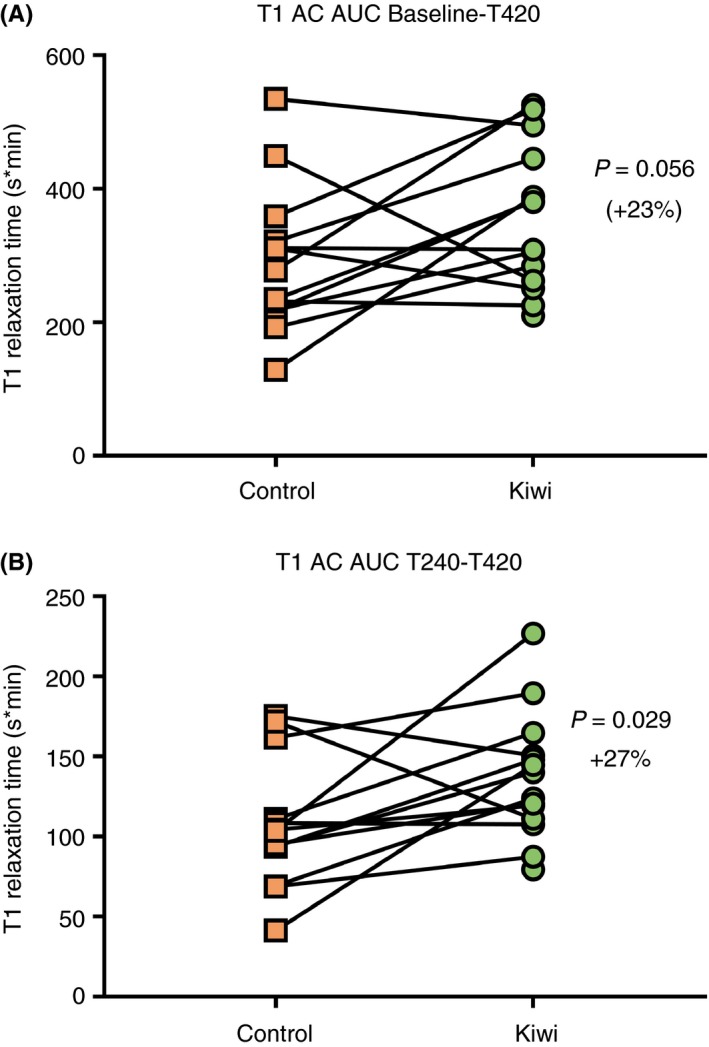
T1 relaxation time in the ascending colon expressed as area under the curve from baseline until the end of the scan day (Baseline‐T420, A); and from the time that the first meal is assumed to have reached the ascending colon until the end of the scan day (T240‐T420, B)
3.2. T1 relaxation time in the descending colon
In the descending colon measuring T1 relaxation time was challenging, as the descending colon was sometimes empty, therefore some data sets were excluded from the analysis (n = 9 for control and n = 12 for kiwifruit). There was no difference between the kiwifruit and the control arms of the study, regardless whether the area under the curves was considered over the whole day (P = 0.3, Table 1), over the later time points (T240‐T420, P = 0.4, Table 1) or when each time point was considered individually (two‐way ANOVA P = 1.0, Appendix S1).
3.3. Small bowel water content
Kiwifruit markedly increased SBWC, both when expressed as area under the curve (P = 6 × 10−6) (Table 1), and when measured throughout the day (two‐way ANOVA, overall P = 3 × 10−32, difference between treatment products P = 2 × 10−22, difference between time points P = 2 × 10−18, interaction P = 8 × 10−7). SBWC was increased at all time points except for baseline, when participants arrived fasted (paired tests, P < 0.05 indicated by asterisks, Figure 3, Appendix S1).
Figure 3.
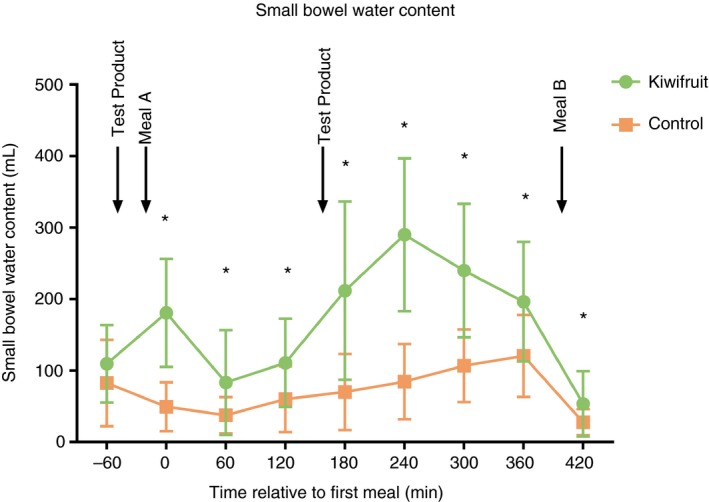
Small bowel water content shown over the course of the study day. Points represent mean ± SD and asterisks indicate P < 0.05
3.4. Regional and total colon volumes
As Table 1 shows, area under the curve from baseline‐T420 for the total colon volume is increased in the kiwifruit arm of the study (P = 0.004, see Figure 4 and Table 1), due to an increase in ascending colon volume (P = 0.003). Both transverse and descending colon volume AUCs were similar between the groups.
Figure 4.
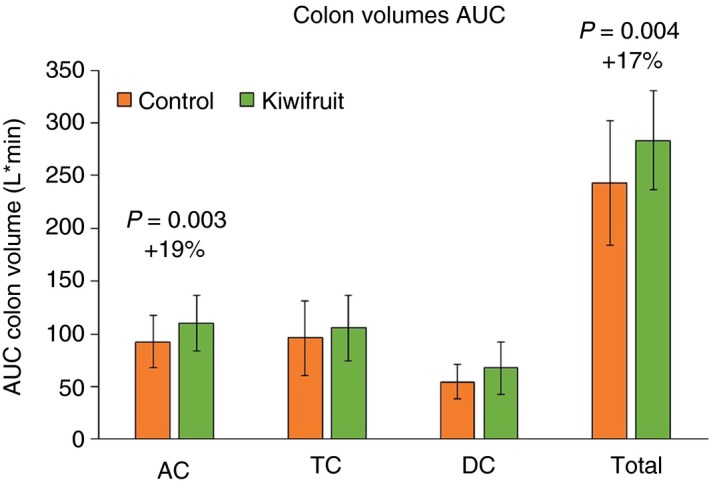
Total and sectional colon volumes expressed as the area under the curve throughout the study day (baseline to T420)
Two‐way ANOVA showed significant effects of treatment but not time for total colon volumes and ascending colon volumes, AUCbase‐420 minutes of total colon volumes (overall P = 0.011, difference between treatment products P = 2×10−7, difference between hourly time points P = 0.8, interaction P = 1.0) and AUCbase‐420 minutes of ascending colon volumes (overall P = 0.035, difference between treatment products P = 2 × 10−6, difference between time points P = 0.8, interaction P = 1.0). However, ANOVA for transverse and descending colon volumes showed no significant differences (P = 0.9 and P = 0.148 respectively).
Total colon volume was increased at all time points except baseline (paired tests, P < 0.05). Similarly, volumes of the ascending colon were increased at most time points (except baseline and T180), whereas transverse and descending colon volumes were only significantly increased at a few time points, which for the descending colon tended to be towards the end of the scanning session (Appendix S1 and S2).
3.5. Whole gut transit
Whole gut transit was similar on both arms of the study with weighted average position score (kiwifruit median score 0.8 [0‐1.4 IQR], control median score 1.0 [0.5‐3.1 IQR], P = 0.11).
3.6. Stool diary and symptom questionnaire
Daily stool frequency was significantly increased during the kiwifruit arm (defined as days when the treatment product is consumed plus the following day, ie days 3‐6, indicated by the shaded area in Figure 1) compared to the control arm (1.46 ± 0.66 vs 1.14 ± 0.46 stools per day; P = 0.034 Wilcoxon; Figure 5A).
Figure 5.
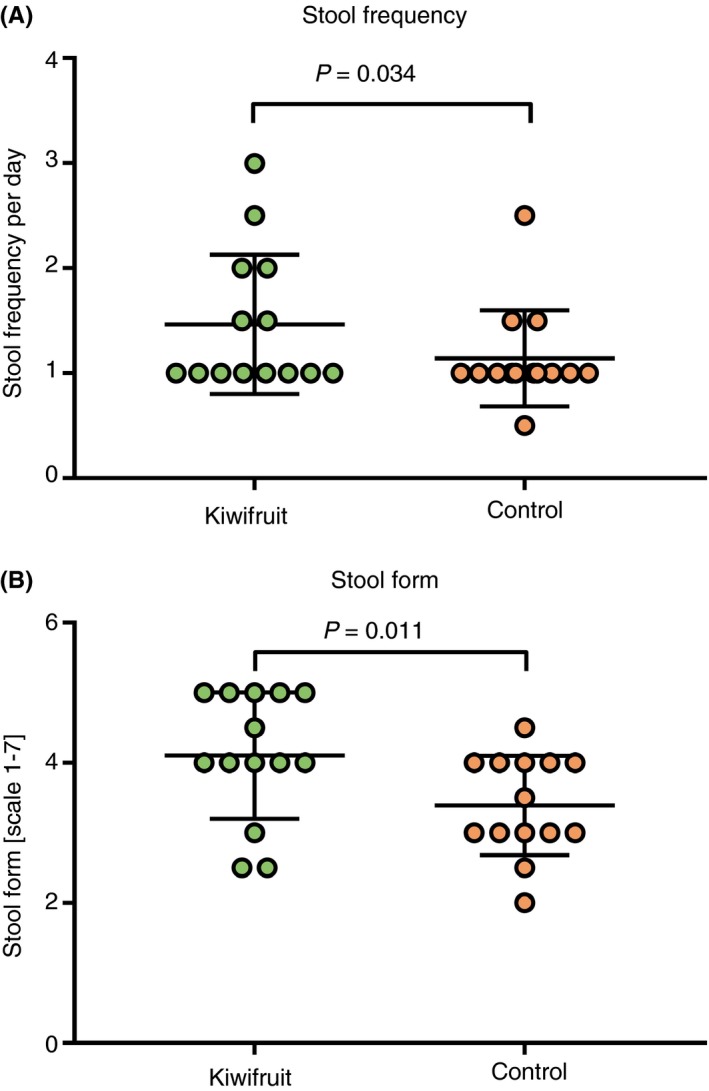
Stool frequency per day during each of the three study periods (A) and stool form, self‐assessed following the Bristol chart, ranging from 1 for severe constipation to 7 for extreme diarrhoea, during each of the three study periods (B)
The consistency scores of stools were different between groups (P = 0.049, Figure 5B), with higher scores (softer/more watery stools) in the kiwifruit arm compared to control (P = 0.011).
There was no significant difference in the scores for any adverse symptoms between the kiwifruit consumption, control consumption and baseline (Appendix S3). As both kiwifruit and the control maltodextrin drink were well tolerated, participants reported few instances of these symptoms, with no symptom reported to be higher in severity than level 2 (moderate).
4. DISCUSSION
This study aimed to use MRI techniques to identify the mechanism of action behind the therapeutic benefit seen from kiwifruit in previous studies. We hypothesised that some of the physicochemical properties of kiwifruit would increase intestinal water, and we have found this to be the case.
Colonic water content, reflected by T1 relaxation time, showed some increase in the ascending colon. This is likely due to water trapping by the fruit fibre in the small intestine. The timing of the increase in colonic water content in the second half of the study day is in accordance with when the meal contents would be predicted to reach the caecum.
In the descending colon measuring T1 relaxation time was challenging, as the descending colon was sometimes empty. There was no difference in T1 found here and the values were generally lower than those in the ascending colon. This is as previously reported15 and in keeping with the function of the colon in progressively dehydrating colonic contents as they move distally.
Regarding the SBWC, the pattern seen on the control arm was similar to that observed previously17, 26 with an initial drop in content likely to reflect the rapid absorption of water with the glucose, fructose and sucrose molecules in the orange juice in the test meal, followed by a later rise due to intestinal and pancreatic secretions accompanying the gastric emptying of the solid phase of the test meal into the small bowel. The maltodextrin did not appear to increase SBWC over baseline despite being given with 250 ml of water behaving as we have observed with pure glucose17 suggesting it was rapidly digested. In contrast, the water content on the kiwifruit arm rose initially with two peaks seen to coincide with the ingestion of the kiwifruit doses. This suggests kiwifruit impairs water absorption as it does for glucose absorption as previously demonstrated by reduced post‐prandial hyperglycaemia.27 The second peak was greater than the first—which may reflect retention of the initial fibre within the small bowel with additional water trapping by the second dose. The larger increase after the second dose may be also due to the fact that the sugars in Meal A may have enhanced water absorption immediately after ingestion of the first dose.
There was no significant difference in SBWC at the fasting time point despite pre‐dosing for 2 days, which suggests that the effect of the kiwifruit disappears overnight when the small bowel contents are emptied into the colon by the migrating motor complexes which occur during overnight fasting.28
Colonic volumes were increased by kiwifruit ingestion which may reflect the increased volume of water arriving in the colon. Although flow through the colon is not uniform, colonic volumes can be approximated from the formula: Volume = flow (24 hours stool excretion) × transit time. The rise in colonic volumes despite unchanged transit time implies therefore an increase in 24‐hour stool excretion in keeping with the more frequent passage of looser stools we observed. It is worth noting that while the colon volume average as assessed by the AUC 0‐420 minutes throughout the study day rose by 16% this is much less that the 50% increase in total colonic volume we previously reported after 10.5 of psyllium daily.15 This may suggest that kiwifruit has prokinetic effects in addition to simple water trapping and hence less colonic distension when compared to ispaghula. Whether this translates into less bloating would require a head to head direct comparison study.
Acceleration of transit, when compared to psyllium (7.5 g/day), by two kiwifruit daily was observed in the Japanese arm29 of the multicentre study already described above16. The fact that we did not observe a significantly accelerated transit time may in part be due to a "floor effect’ in this healthy volunteer population in whom transit, as we have previously observed, is relatively fast at 28 hours (IQR 4‐50) with some subjects with a low or zero weighted average position score and hence difficult to demonstrate further accleration.20 We did observe that there was a higher proportion of subjects who had expelled all the marker pills at the 24‐hour mark (giving a score of 0) in the kiwifruit arm (six participants) compared to control (two participants). Similar findings were seen in another of our recent studies assessing the effect of psyllium on transit in which transit times were not seen to be significantly increased in healthy volunteers as most markers had passed to the rectosigmoid colon or had been expelled at 24 hours, whereas a significant increase in speed was seen in participants with constipation whose baseline transit was slower.15
Despite using healthy volunteers rather than constipated patients, the change in bowel habit (increased frequency with a normal stool form) and low side‐effect profile are similar to what has been seen in previous patient studies.6, 7, 8
4.1. Limitations
This mechanistic study was limited by small sample size which was exacerbated by some participants’ withdrawal from the study. Our subjects were young adults so caution is needed in extrapolating our results to the elderly. However, this data will serve as a basis for power calculations for future MRI studies of digestive function.
We also did not control any other aspects of diet—for example, limiting any other fruit consumption (beyond asking not to eat kiwifruit in the control week) or other sources of fibres. We gave two meals on the study day to simulate how kiwifruit are normally consumed as part of a mixed diet. Both have been used in previous studies, so their effect is known, however the nutrients they contain may have interacted with the digestion of the kiwifruit and vice versa.
The kiwifruit we used were top quality and somewhat larger than the average on sale at present. Furthermore, the dose of two kiwifruit twice daily was large compared to normal consumption but ensured a clear mechanistic result in the limited intervention time with the small numbers it was feasible to scan. The clinical trials show that more reasonable doses that is, two daily are also clinically effective.6, 7, 8, 16 The maltodextrin acted as a control for calorie content but did not control for other aspects like chewing and salivation.
5. FUTURE WORK AND CONCLUSIONS
Future work may want to look at whether other fruits have similar mechanisms of action or whether these are unique to the kiwifruit. Kiwifruit contain substantial amounts of the protease actinidin which has the potential to activate protease‐activated receptors found on enterocytes, lymphocytes and enteric nerves which have been shown to play an important role in visceral sensitivity. Kiwifruit also contain raphides (needle‐shaped calcium oxalate crystals found in the tissues of kiwifruit). It is unclear whether these are all destroyed in the acid gastric environment or whether some enter the small bowel where they might stimulate water secretion. Whether raphides or actinidin alter colonic absorption, secretion or motility remains to be determined. The effect of the kiwifruit on the microbiota may also be another area worth studying in more detail since preliminary studies show its consumption increases lactobacilli and bifidobacteria.30
In conclusion, these MRI data suggest that consumption of kiwifruit in healthy volunteers increases water content in the small bowel and ascending colon, as well as an increase in colonic bulk. These data are consistent with the observations of an increase in stool frequency and looser stool consistencies, suggesting that kiwifruit could be used as a dietary alternative to laxatives in mild constipation.
AUTHORSHIP
Guarantor of the article: Professor Robin Spiller
Author contributions: R. Spiller, V. W‐Smith, P. Gowland, L. Marciani, C. Hoad and J. Ansell contributed to the conception and design of the research; V. W. Smith acquired the data; N. Dellschaft and V. W. Smith analysed the data; V. W. Smith, N. Dellschaft drafted the manuscript; R. S. acts as the guarantor for the article and all authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
ACKNOWLEDGEMENTS
Declaration of personal interests: This study and Dr Dellschaft’s post have been funded by Zespri International Limited, a consortium of kiwifruit growers. R. Spiller has received research funding from Norgine and Zespri. He has also acted on advisory boards for Allergan, Commonwealth Diagnostics International, Napo Pharmaceuticals, Ipsen, and Yuhan, and received speakers’ fees from Menarini and Alfawasserman. J Ansell is an employee of Zespri International. The remaining authors have no COI to declare.
Wilkinson‐Smith V, Dellschaft N, Ansell J, et al. Mechanisms underlying effects of kiwifruit on intestinal function shown by MRI in healthy volunteers. Aliment Pharmacol Ther. 2019;49:759–768. 10.1111/apt.15127
The Handling Editor for this article was Colin Howden, Spiller, and it was accepted for publication after full peer‐review.
V. Wilkinson‐Smith and N. Dellschaft assert joint first authorship.
Funding information
This study and Dr Dellschaft's post have been funded by Zespri International Limited, a consortium of kiwifruit growers.
REFERENCES
- 1. Peppas G, Alexiou VG, Mourtzoukou E, Falagas ME. Epidemiology of constipation in Europe and Oceania: a systematic review. BMC Gastroenterol. 2008;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Müller‐Lissner S, Tack J, Feng Y, et al. Levels of satisfaction with current chronic constipation treatment options in Europe – an internet survey. Aliment Pharmacol Ther. 2013;37:137‐145. [DOI] [PubMed] [Google Scholar]
- 3. Nelson AD, Camilleri M, Chirapongsathorn S, et al. Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta‐analysis. Gut. 2017;66:1611‐1622. [DOI] [PubMed] [Google Scholar]
- 4. Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta‐analysis. Gut. 2011;60:209‐218. [DOI] [PubMed] [Google Scholar]
- 5. Vandeputte D, Falony G, Vieira‐Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rush EC, Patel M, Plank LD, Ferguson LR. Kiwifruit promotes laxation in the elderly. Asia Pac J Clin Nutr. 2002;11:164‐168. [DOI] [PubMed] [Google Scholar]
- 7. Chan A‐O‐O, Leung G, Tong T, Wong N‐Y. Increasing dietary fiber intake in terms of kiwifruit improves constipation in Chinese patients. World J Gastroenterol. 2007;13:4771‐4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang C‐CC, Lin Y‐TT, Lu Y‐TT, et al. Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac J Clin Nutr. 2010;19:451‐457. [PubMed] [Google Scholar]
- 9. Bayer SB, Gearry RB, Drummond LN. Putative mechanisms of kiwifruit on maintenance of normal gastrointestinal function. Crit Rev Food Sci Nutr. 2017;1‐21. [DOI] [PubMed] [Google Scholar]
- 10. Drummond L.Chapter three – the composition and nutritional value of kiwifruit In: Boland M, Moughan PJ. eds. Advances in Food and Nutrition Research. Vol 68. Cambridge, MA: Academic Press; 2013:33‐57. [DOI] [PubMed] [Google Scholar]
- 11. Sims IM, Monro JA. Chapter five – fiber: composition, structures, and functional properties In: Boland M, Moughan PJ. eds. Advances in Food and Nutrition Research. Vol. 68. Cambridge, MA: Academic Press; 2013:81‐99. [DOI] [PubMed] [Google Scholar]
- 12. Montoya CA, Saigeman S, Rutherfurd SM, Moughan PJ. The digestion of kiwifruit (Actinidia deliciosa) fibre and the effect of kiwifruit on the digestibility of other dietary nutrients. Food Chem. 2016;197(Pt A):539‐545. [DOI] [PubMed] [Google Scholar]
- 13. Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther. 2011;33:895‐901. [DOI] [PubMed] [Google Scholar]
- 14. Newton CR. Effect of codeine phosphate, lomotil, and isogel on ileostomy function. Gut. 1978;19:377‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Major G, Murray K, Singh G, et al. Demonstration of differences in colonic volumes, transit, chyme consistency, and response to psyllium between healthy and constipated subjects using magnetic resonance imaging. Neurogastroenterol Motil. 2018;e13400. [DOI] [PubMed] [Google Scholar]
- 16. Barbara G, Fukudo S, Drummond L, Kuhn‐Sherlock B, Ansell J, Gearry R. Tu1644 – green kiwifruit compared to psyllium for the relief of constipation and improving digestive comfort in patients with functional constipation and constipation predominant irritable bowel syndrome — analysis of three international trial centres. Gastroenterology. 2018;154(6, Suppl. 1):S‐979‐S‐980. [Google Scholar]
- 17. Marciani L, Cox EF, Hoad CL, et al. Postprandial changes in small bowel water content in healthy subjects and patients with irritable bowel syndrome. Gastroenterology. 2010;138: 469‐477, 477.e1. [DOI] [PubMed] [Google Scholar]
- 18. McIntyre A, Vincent RM, Perkins AC, Spiller RC. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: the role of physical factors. Gut. 1997;40:223‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoad CL, Marciani L, Foley S, et al. Non‐invasive quantification of small bowel water content by MRI: a validation study. Phys Med Biol. 2007;52:6909‐6922. [DOI] [PubMed] [Google Scholar]
- 20. Chaddock G, Lam C, Hoad CL, et al. Novel MRI tests of orocecal transit time and whole gut transit time: studies in normal subjects. Neurogastroenterol Motil. 2014;26:205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoad C, Garsed K, Marciani L, et al. Measuring T1 of chyme in the ascending colon in health and diarrhoea predominant Irritable Bowel Syndrome (Abstract 1275). Proc Intl Soc Mag Reson Med. 20. 2012.
- 22. Pritchard SE, Marciani L, Garsed KC, et al. Fasting and postprandial volumes of the undisturbed colon: normal values and changes in diarrhea‐predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol Motil. 2014;26:124‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Svedlund J, Sjödin I, Dotevall G. GSRS – A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129‐134. [DOI] [PubMed] [Google Scholar]
- 24. Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40‐47. [DOI] [PubMed] [Google Scholar]
- 25. Eggers H, Brendel B, Duijndam A, Herigault G. Dual‐echo Dixon imaging with flexible choice of echo times. Magn Reson Med. 2011;65:96‐107. [DOI] [PubMed] [Google Scholar]
- 26. Murray K, Wilkinson‐Smith V, Hoad C, et al. Differential effects of FODMAPs (fermentable oligo‐, di‐, mono‐saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. 2014;109:110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mishra S, Willis J, Ansell J, Monro JA. Equicarbohydrate partial exchange of kiwifruit for wheaten cereal reduces postprandial glycaemia without decreasing satiety. J Nutr Sci. 2016;5:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spiller RC, Brown ML, Phillips SF, Azpiroz F. Scintigraphic measurements of canine ileocolonic transit. Gastroenterology. 1986;91:1213‐1220. [DOI] [PubMed] [Google Scholar]
- 29. Okawa Y, Nakaya K, Muratsubaki T, et al. Tu1639 – kiwifruit can reduce whole gut transit and symptoms in patients with functional constipation and patients with irritable bowel syndrome with constipation. Gastroenterology. 2018;154(6, Suppl. 1):S‐977. [Google Scholar]
- 30. Lee YK, Low KY, Siah K, Drummond LM, Gwee KA. Kiwifruit (Actinidia deliciosa) changes intestinal microbial profile. Microb Ecol Health Dis. 2012;23: 18572. https://doi.org/10.3402 /mehd.v23i0.18572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


