Abstract
Objective
To evaluate the effect of a moderate‐to‐high–intensity, aerobic and resistance exercise with person‐centered guidance in older adults with rheumatoid arthritis (RA), through a randomized controlled multicenter trial.
Methods
Older adults (ages 65–75 years) with RA (n = 74) were randomized to either a 20‐week exercise intervention at a gym (n = 36) or to home‐based exercise of light intensity (n = 38). Assessments were performed at baseline, at 20 weeks, and at 12 months. The primary outcome was the difference in the Health Assessment Questionnaire disability index (HAQ DI) score, and the secondary outcomes were the differences in physical fitness assessed by a cardiopulmonary exercise test, an endurance test, the timed up and go test, the sit to stand test, and an isometric elbow flexion force measurement.
Results
No significant differences between the groups were found for the primary outcome, HAQ DI score. Within the intervention group there was a significant improvement in the HAQ DI score when compared to baseline (P = 0.022). Aerobic capacity (P < 0.001) and 3 of 4 additional performance‐based tests of endurance and strength significantly improved (P < 0.05) in the intervention group when compared to the control group. In the intervention group, 71% of patients rated their health as much or very much improved compared to 24% of patients in the control group (P < 0.001). At the 12‐month follow‐up, there were no significant differences in change between the 2 groups on the HAQ DI score. A significant between‐group difference was found for change in an endurance test (P = 0.022).
Conclusion
Aerobic and resistance exercise with person‐centered guidance improved physical fitness in terms of aerobic capacity, endurance, and strength in older adults with RA.
Keywords: Patient‐centred, person‐centred, person‐centered, exercise, Rheumatoid arthritis, physiotherapy, physical therapy
Introduction
A major factor contributing to ill health in old age is the increase in systemic inflammation that occurs with physiologic aging, so‐called inflamm‐aging. Systemic inflammation also changes body composition, leading to increased fat mass and sarcopenia 1, with the latter contributing to impaired balance and falls, which are associated with deleterious outcomes 2. Physical activity has antiinflammatory effects by promoting the breakdown of fat, increasing the antiinflammatory and regulatory properties of the immune system, and increasing muscle‐produced interleukin 3, 4, 5, 6. Age‐related decline of physical function and ability to perform desired activities is a concern for patients with rheumatoid arthritis (RA) 7, especially since patients with RA of all ages, despite disease control, show a disease‐related loss of muscle mass and altered body composition 8 that is related to disability 9. Studies have shown improvements in aerobic capacity, muscle strength, and disability, as assessed with the Stanford Health Assessment Questionnaire disability index (HAQ DI), after an intervention involving aerobic and resistance training 3, 5. Therefore, it has been proposed that physical activity should be included in the routine management of middle‐aged patients with RA 5, 10, and the World Health Organization recommends both aerobic and resistance exercise each week, preferably of moderate‐to‐vigorous intensity, for adults ages >65 years 11. However, knowledge about benefits of exercise in older adults (ages >65 years) with RA is scarce.
Significance & Innovations.
Aerobic and resistance exercise with person‐centered guidance improved physical fitness in older adults with rheumatoid arthritis (RA).
Seventy‐one percent of patients in the intervention group rated their health as much or very much improved on the Patient's Global Impression of Change scale.
Older adults with RA were able to perform both aerobic and resistance exercise at a high intensity without any serious adverse events.
The intervention is recommended for inclusion as part of the management of RA for older adults with low‐to‐moderate disease activity.
The physical activity level among patients with RA, especially among those ages >55 years, is lower than the level recommended by international guidelines for health‐enhancing physical activity and is lower than that among healthy persons 12, 13. The reduced physical activity level among patients with RA is partly due to a worry that exercise could damage the joints 14, 15, but no harmful side effects from exercise have been documented 5, and no joint damage is seen at extended follow‐up after high‐intensity exercise 16. A person‐centered approach 17 is suggested to help identify and assuage worries of this type 18. The principles underlying this approach include the establishment of a partnership between the care giver and the patient, based on the patient narrative, and shared information and decision‐making, together with documentation 17. A person‐centered approach that focuses on the context, history, and resources of the individual has been suggested as particularly suitable for managing long‐term diseases 17.
Today >50% of patients with RA are ages >65 years 19, and their health care cost is increased 3–4‐fold over comparators in the general population 20. We hypothesized that a moderate‐to‐high intensity aerobic and resistance exercise with person‐centered guidance would decrease disability and improve physical fitness in older adults with RA.
Patients and Methods
Patients were recruited from the rheumatology clinics at the Sahlgrenska University Hospital, Gothenburg, and Skaraborg Hospital, Skövde, Sweden via the Swedish Rheumatology Quality Register. The recruitment, intervention, and data collection were performed between January 2015 and November 2016. The study complied with the Declaration of Helsinki and was approved by the Regional Ethics Review Board in Gothenburg (2014‐11‐24/790‐14). Informed, written consent was obtained from the patients before the baseline examinations.
The inclusion criteria were RA according to the American College of Rheumatology 1987/European League Against Rheumatism 2017 criteria 21, ages ≥65 years, disease duration >2 years, and low‐to‐moderate Disease Activity Score in 28 joints (DAS28 <5.1). The exclusion criteria were comorbidities such as unstable ischemic heart disease or arrhythmia that might preclude moderate intensity exercise, joint surgery within 6 months prior to inclusion, ongoing exercise of moderate‐to‐high intensity ≥2 times/week, inability to understand or speak Swedish, and inability to participate in physical testing that involved walking or bicycling.
A letter of invitation that contained comprehensive information on the study was sent out and was followed by a phone call, during which the patients could accept or decline the invitation (Figure 1). At the screening visit, a physical examination, resting electrocardiogram, and cardiopulmonary exercise testing (CPET) were performed to search for exclusion criteria. In total, 49 patients were included and examined at the Sahlgrenska University Hospital, Gothenburg, and 25 patients were included and examined at the Skaraborg Hospital, Skövde (Figure 1).
Figure 1.
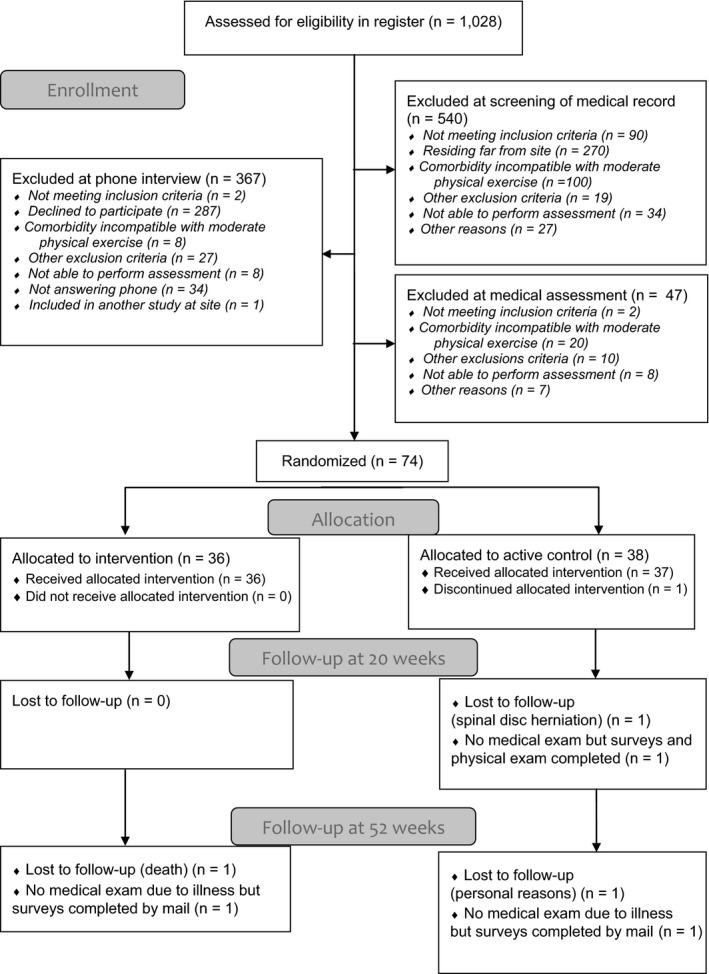
Consolidated Standards of Reporting Trials [CONSORT] diagram for the 2 groups in the randomized clinical trial.
Randomization
After screening and enrollment, the participants were randomized separately for each site to groups of 6 subjects by a person not involved in the examinations or intervention. Sealed opaque envelopes were used with a computer‐generated sequence of allocation, and the envelopes were divided by sex (men/women). The participants were informed of their group allocation by the physiotherapist leading the intervention (EL and GB).
Intervention
For the intervention group, the supervised exercise intervention consisted of gym‐based, moderate‐to‐high–intensity, aerobic and resistance exercise 3 times a week and home‐based exercise for 20 weeks (Figure 2). The person‐centered approach implied that the intervention started with an individual meeting, to create an understanding of the person establishing goals for exercise in a partnership and reaching an agreement on how the intervention should be performed. The gym‐based exercise was tailored based on the resources of the individual and consisted of warm‐up, 27 minutes of aerobic exercise at 70–89% of maximum heart rate in intervals of 3 minutes, and 5 resistance exercises at 70–80% of 1 repetition maximum (RM). Introduction to exercise began at a low level and slowly increased over 6 to 9 weeks. The physiotherapist was present at 2 of 3 sessions each week, and adjustments were made continuously. The patients performed exercise independently but attended the gym at approximately the same times and formed an informal group. In the control group, patients attended 1 individual meeting with the physiotherapist, where they were encouraged to perform home‐based exercise according to the same protocol as the intervention group, but with no gym‐based exercise, for 20 weeks (Figure 2).
Figure 2.
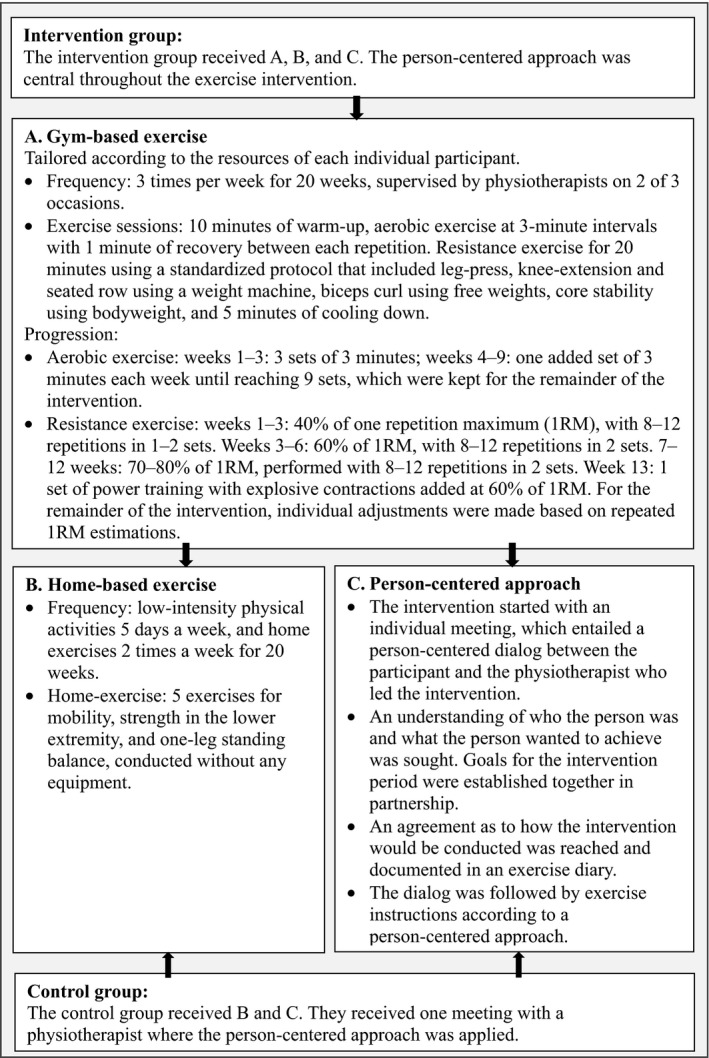
Intervention group and control group exercises.
Assessment
Background data and outcomes, comprising medical examination, questionnaire results, and 5 performance‐based tests, were assessed by blinded assessors (DK, SS, KS, and IG) at baseline, at postintervention (at 20 weeks), and at follow‐up (at 12 months). Follow‐up included medical examination, questionnaire results, and 4 performance‐based tests. The DAS28 was used to assess disease activity 22, 23.
Primary and secondary outcomes
The primary outcome, disability, was assessed using the HAQ DI 24, 25. The secondary outcome, physical fitness, was assessed by 5 performance‐based tests. Assessment of aerobic capacity through CPET was performed according to a protocol that was modified from the American Heart Association guidelines 26. A bicycle endurance test was performed on a cycle ergometer (Monark Ergometer 839 E, Monark Exercise AB) 27. After a 2‐minute warm‐up period at 50W, the patients cycled at a constant power of 70% or 75% of the maximum achieved power, which was based on the estimation from the CPET, and the total time was registered when the level of exertion was rated “very hard” on the Borg rating of perceived exertion 28. Functional balance was assessed with the timed up and go (TUG) test, in which the following series were timed: rise from an armchair, walk a distance of 3 meters as quickly as possible but still safely, walk back, and sit down 29. Leg muscle strength was assessed using the sit to stand (STS) test, in which the number of complete rises from a chair performed in 60 seconds was recorded 30. Isometric elbow flexion force was assessed with an electronic dynamometer 31. The patients were seated in a standardized position without back support and with legs stretched out. The forearm was supported by the trunk with the elbow at 90° flexion, and the maximum strength was measured over a period of 7 seconds. The Patient's Global Impression of Change (PGIC) 32 was measured at the postintervention examination and at the 12‐month follow‐up.
Measures of exercise load were performed using the Leisure Time Physical Activity Instrument (LTPAI), which assesses the amount of physical activity during a typical week, in terms of light, moderate, and vigorous activity. In this study, the sum of moderate and vigorous activity is given 33. Exercise load was registered by the physiotherapist leading the intervention (EL and GB). The patients were also asked to keep an exercise diary. During the follow‐up period, patients in the intervention group were contacted by phone 2–3 times and the reported exercise was registered.
Statistical analysis
Statistical analyses were performed using the SPSS, version 24.0 (IBM). Descriptive statistics were used to characterize the 2 groups. Comparisons between groups were performed with the Mann‐Whitney U test for ordinal variables and independent Student's t‐test for continuous variables, and the Mantel‐Haenszel test was used for ordinal categorical variables. For comparisons between baseline and postintervention examinations within a group, Wilcoxon's signed rank test was used for ordinal variables, and the paired‐sample t‐test was used for continuous variables. All significance tests were 2‐sided. Outcomes were analyzed according to intent‐to‐treat design, implying that all participants were invited to post‐treatment examination, whether they had participated in the intervention or not. Only measured values were included in the analyses of changes over time between the 2 groups and within the groups, implying that missing cases were not included in the analyses. To evaluate the effect size, Cohen's d coefficient was calculated for between‐group variables that showed a significant change 34. An effect size of 0.20 to <0.50 was regarded as small, 0.50 to <0.80 as medium, and >0.80 as large 34. To detect a clinically important difference of 0.2 on the HAQ DI score between groups, with an estimated SD of 0.5, 90% power, and 5% significance level using the Mann‐Whitney U test, 35 participants were needed in each group.
Results
Patients
The demographics and clinical characteristics of the participants are shown in Table 1. The groups were considered to be equivalent. A total of 73% of the patients were in remission (DAS28 <2.6) or had low disease activity (DAS28 <3.2) at baseline, and the disease activity was not significantly changed during the study.
Table 1.
Characteristics of the study population*
| Intervention (n = 36) | Control (n = 38) | |
|---|---|---|
| General information | ||
| Women | 27 (75) | 29 (76.3) |
| Age, mean ± SD years | 69.14 ± 2.61 | 70.11 ± 2.30 |
| Disease duration, mean ± SD years | 15.4 ± 10.7 | 17.4 ± 10.9 |
| Body measurements, mean ± SD | ||
| Body mass index | 25.58 ± 4.43 | 28.01 ± 4.53 |
| Length, cm | 168.9 ± 8.51 | 166.4 ± 8.04 |
| Weight, kg | 73.3 ± 16.34 | 77.4 ± 12.81 |
| Pain VAS current, mean ± SD mm | 20.67 ± 19.09 | 23.20 ± 15.68 |
| LTPAI, moderate + vigorous, mean ± SD hours | 3.46 ± 2.60 | 3.11 ± 2.30 |
| ESR, mean ± SD | 14.22 ± 12.07 | 12.71 ± 8.26 |
| CRP, mean ± SD | 6.89 ± 15.94 | 4.05 ± 4.75 |
| Disease activity by DAS28, mean ± SD | 2.33 ± 1.10 | 2.41 ± 0.90 |
| Disease activity by CDAI, mean ± SD | 5.35 ± 4.41 | 5.47 ± 3.35 |
| Education | ||
| ≤9 years | 13 (36.1) | 12 (31.6) |
| 10–12 years | 4 (11.1) | 8 (21.1) |
| >12 years | 14 (38.9) | 11 (28.9) |
| Missing | 5 (13.9) | 7 (18.4) |
| Marital status, living with an adult | 24 (66.7) | 24 (63.2) |
| Cigarette smoking | ||
| Current smoker | 3 (8.3) | 3 (7.9) |
| Former smoker | 20 (55.6) | 21 (55.3) |
| Never‐smoker | 13 (36.1) | 14 (36.8) |
| Autoantibodies | ||
| RF | 25 (69.4) | 26 (68.4) |
| Anti‐CCP | 26 (72.2) | 21 (55.3) |
| Erosive | 20 (55.6) | 21 (55.3) |
| Medication | ||
| No DMARD | 0 (0) | 4 (10.5) |
| Synthetic DMARD | 34 (94.4)† | 29 (76.3)† |
| Methotrexate | 31 (86.1) | 25 (65.8) |
| Other | 5 (13.9) | 5 (13.2) |
| Biologic DMARD | 14 (38.9)† | 17 (44.7)† |
| TNF inhibitors | 12 (33.3) | 9 (23.7) |
| Other DMARDs | 2 (5.6) | 8 (21.1) |
| Corticosteroids (oral) | 6 (16.7)† | 10 (26.3)† |
| NSAID | 17 (47.2)† | 22 (57.9)† |
| Paracetamol | 15 (41.7)† | 21 (55.3)† |
| Beta‐blocker | 5 (13.9)† | 12 (31.6)† |
Values are the number (%) unless indicated otherwise. VAS = visual analog scale; LTPAI = Leisure Time Physical Activity Instrument ESR = erythrocyte sedimentation rate; CRP = C‐reactive protein; DAS28 = Disease Activity Score in 28 joints; CDAI = Clinical Disease Activity Index; RF = rheumatoid factor; anti‐CCP = anti–cyclic citrullinated peptide; DMARD = disease‐modifying antirheumatic drug; TNF = tumor necrosis factor; NSAID = nonsteroidal antiinflammatory drug.
Significant.
In the intervention group, 50% of patients had a concomitant disease (from a total of 36 patients: cardiovascular disease 6, hypothyroidism 4, diabetes mellitus 2, pulmonary disease 2, previous cancers 8, and other diseases 3). Also in the intervention group, 19% of patients (n = 7) had a joint prosthesis. In the control group, 42% had a concomitant disease (from a total of 38 patients: cardiovascular disease 6, hypothyroidism 1, diabetes mellitus 3, pulmonary disease 1, previous cancers 5, and other diseases 3). In the control group, 26% of patients (n = 10) had a joint prosthesis.
Exercise attendance, level, and adverse effects
All patients in the intervention group completed the exercise intervention (Figure 1). Altogether, 72 patients (97%) completed the week 20 examinations. The mean attendance rate in the intervention group was 78%, with an average of 2.4 exercise sessions at the gym and 3.16 exercise sessions at home each week. The control group performed home exercise on average 2.84 times each week. The self‐reported hours of moderate‐to‐vigorous physical activity on the LTPAI were increased significantly (P = 0.001) in the intervention group (2.4‐hour increase) when the change was compared to that of the control group (0.3‐hour increase). The majority of the intervention group, at 78%, reached the targeted level of 70–80% of 1 RM. The other 8 patients reached approximately 60% of 1 RM in 1–3 of the exercises. One patient performed at a lower load level than that intended in the aerobic exercise. Adverse effects were defined as increased pain that could be related to exercise. For 4 patients in the intervention group, adverse effects led to persistent exercise modifications in 1 exercise throughout the intervention. Nineteen patients encountered temporarily increased pain, which was managed with temporary exercise modifications for approximately 1 week or was managed without modifications.
Disability
No significant differences between the 2 groups were found on the primary outcome, HAQ DI score (Table 2). In the intervention group there was a significant within‐group improvement (P = 0.022) of the HAQ DI score, corresponding to a 12% improvement of the scores. No such changes were found in the control group.
Table 2.
Between‐group analysis of the primary and secondary outcomes after 20 weeks*
| Measures | Intervention | Control | Between‐group | |||
|---|---|---|---|---|---|---|
| Baseline (n = 36)† | Post‐treatment: baseline (n = 36)‡ | Baseline (n = 38)† | Post‐treatment: baseline (n = 37)‡ | Analysis of change P | Effect size | |
| Primary outcome | ||||||
| HAQ DI, mean ± SD, median (range) | 0.52 ± 0.5, 0.38 (0, 1.75) | −0.063 ± 0.16, 0 (−0.38, 0.13)§ | 0.6 ± 0.48, 0.44 (0, 1.5) | −0.0097 ± 0.27, 0 (−0.75, 0.75) | 0.200 | 0.14 |
| Secondary outcomes | ||||||
| vo 2/kg/minute, ml | 18.6 ± 3.8 | 2.12 ± 1.93¶ | 17.8 ± 3.81 | −0.16 ± 1.57 | <0.001# | 1.30 |
| Endurance, minutes | 11.4 ± 6.53 | 6.97 ± 7.79¶ | 9.7 ± 5.12 | 1.00 ± 4.76 | <0.001# | 0.93 |
| TUG, seconds | 7.6 ± 1.6 | −0.68 ± 0.91¶ | 8.1 ± 1.7 | −0.14 ± 1.35 | 0.049# | 0.47 |
| STS, no. | 22.58 ± 4.2 | 3.11 ± 3.44¶ | 22.68 ± 5.49 | 0.49 ± 3.96 | 0.004# | 0.71 |
| Elbow flexion | 15.55 ± 5.6 | 0.58 ± 1.9 | 15.57 ± 6.32 | −0.12 ± 3.16 | 0.265 | 0.27 |
Missing values at baseline: intervention group: vo2/kg/minute (n = 3), endurance (n = 1); control group: vo2/kg/minute (n = 1). Missing delta values: intervention group: vo2/kg/minute (n = 4), endurance and elbow force (n = 1); control group: vo2/kg/minute (n = 8), Clinical Disease Activity Index (n = 2). HAQ DI = Health Assessment Questionnaire disability index; TUG = timed up and go; STS = sit to stand.
Mean ± SD.
Δ ± SD
Shown as mean ± SD as well as median (range).
P <0.05,
P <0.001.
Significant.
Physical fitness and global impression of change
Aerobic capacity (vo₂/kg/minute) was significantly improved in the intervention group compared to the control group (Table 2). This improvement was accompanied by a significantly increased endurance, measured by the bicycle endurance test, compared between the 2 groups. Functional balance, assessed by TUG, was significantly improved between the 2 groups. In addition, leg muscle strength assessed with the STS was significantly improved between the 2 groups, but the isometric elbow flexion force did not differ significantly between groups. The PGIC rating was significantly different between the 2 groups at the postintervention examinations, with much or very much improved health among 71.4% of the intervention group and 24.3% of the control group after 20 weeks (Figure 3A).
Figure 3.
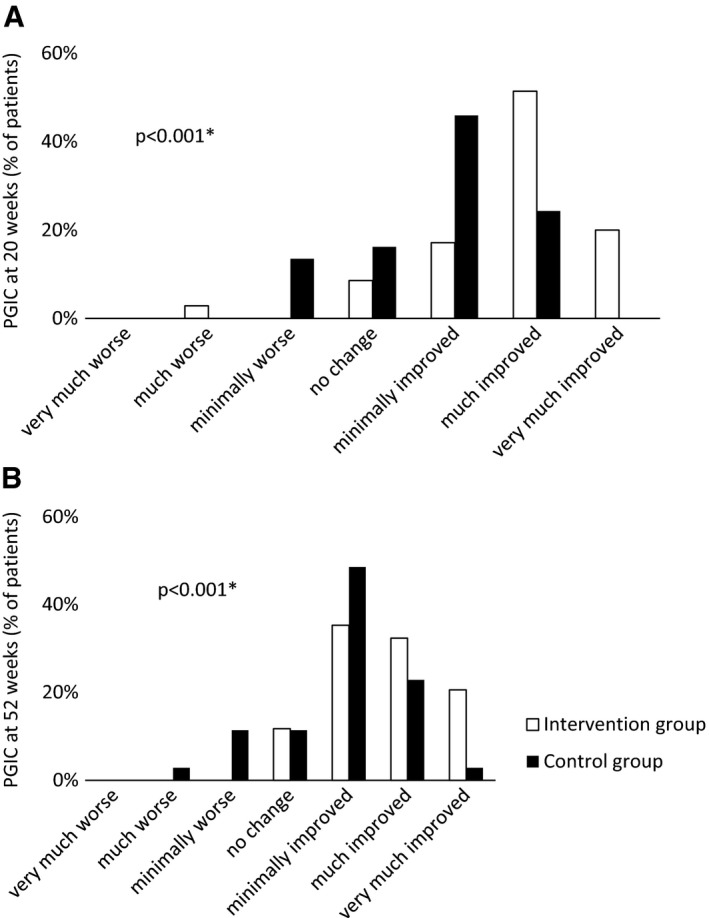
Rating of Patient's Global Impression of Change (PGIC) after (A) 20 weeks, and (B) 52 weeks. * = significant difference between groups.
Twelve‐month follow‐up
Altogether, 69 patients (93%) completed the entire 12‐month follow‐up examinations (Figure 1). Moderate‐to‐high–intensity activity, reported on the LTPAI, was increased compared to baseline, with 2.2 hours in the intervention group and 0.03 hours in the control group (P = 0.005). Based on phone calls and exercise diaries, 51% of patients in the intervention group (18 of 35) continued to exercise with the same intensity as during the intervention, and 34% (12 of 35) continued to exercise at a lower intensity. The members of the intervention group continued to perform home exercise on average 2.1 times/week and more strenuous exercise 1.4 times/week. The control group performed home exercise 1.9 times/week during the follow‐up period.
No significant between‐ or within‐group differences of change compared to baseline were found on HAQ DI score. There was a significant difference of change between groups on the endurance test (P = 0.022), with an increase of 4.7 minutes (P = 0.008) in the intervention group and 0.8 minutes (P = 0.104) in the control group compared to baseline. The STS score was significantly improved within both groups when compared to baseline (intervention group increased 2.5 [P = 0.021]; control group increased 1.5 [P = 0.043]), but no significant mean difference of change was found between groups. No significant differences were found on scores of TUG and isometric elbow flexion. The PGIC ratings were significantly different between the groups at the month 12 follow‐up, with much or very much improved health rated by 52.9% of the intervention group and 25.7% of the control group (Figure 3B).
Discussion
To the best of our knowledge, this is the first study to evaluate the effect of moderate‐to‐high intensity aerobic and resistance exercise for older adults with RA. The primary outcome, HAQ DI score, did not significantly improve when groups were compared. However, HAQ DI score showed a 12% within‐group improvement in the intervention group. HAQ DI has been acknowledged as insufficient in capturing effects of resistance exercise 35. A limitation of HAQ DI is the floor effect 36, which is the most likely reason for the lack of significant results, because the majority of the patients already scored below 0.5 on HAQ DI score at baseline. A reason for floor effects might be the nature of activities included in the HAQ DI score, covering domestic tasks with a requirement of overall mobility rather than physical fitness 25. Almost all study patients had low disease activity or were in remission both at baseline and throughout the study, which is in line with the advances made in the treatment of RA in recent years 37.
The intervention group significantly improved their aerobic capacity when compared to the control group. Furthermore, they achieved the level of aerobic capacity of middle‐aged to older adults with RA 38. Additionally, 3 of 4 other performance‐based tests, assessing endurance, functional balance, and leg muscle strength, significantly improved when compared to the control group. The positive results of this study show that older adults with RA can improve their physical fitness, which is important knowledge, because reductions of muscle mass, muscle strength, and walking speed are common both in patients with RA 8 of all ages and in older adults independent of diagnosis 39.
Physical fitness is a key factor in predicting maintained or increased physical independence over time 40, which is particularly important for patients with RA, since becoming dependent on others is one of the concerns of aging with RA 7. The intervention did not have any significant impact on isometric elbow flexion force, which could be related to the main focus of the exercise protocol being the lower limbs. Another potential reason could be the design of the test, since the electronic dynamometer that was used has commonly been used to study shoulder strength 41.
The PGIC was applied to study possible changes from the perspective of a patient. A total of 88.6% of the intervention group reported improvements in PGIC, and although the control group also scored improvements, the between‐group differences were significant, in favor of the intervention group. Physical activity has been found to have a positive impact on the experience of health 42, and increased physical activity and fitness improve health status 43. We believe that improved physical fitness, demonstrated by the performance‐based tests, conveyed to the patients a sense of improved health.
The self‐reported hours of exercise at a moderate‐to‐intense level increased by >2 hours per week in the intervention group, and the intensity of the performed exercise program appears to be crucial for achieving the effect of the exercise 44. Only a few drawbacks or adverse events were observed, leading to a minor, temporary modification of the protocol. This study showed that exercise with person‐centered guidance and a moderate‐to‐high intensity is possible for older adults with RA with a low‐to‐moderate disease activity. To be able to perform exercise at a moderate‐to‐high intensity at an older age is important to improve health outcomes and reduce mortality 45. A person‐centered approach, implying that the patients were actively involved in the tailoring of their own exercise 46, promoting empowerment 47 and the ability to manage symptoms while exercising, through individualization of load and progression, was assumed to have been a contributing factor for success. Personal goals were included in the individual exercise plans, which may also have contributed to the adherence over time 48. The adherence of the control group, which performed exercise at the level recommended as the minimum to obtain health benefits 11, was also good.
At the 12‐month follow‐up, there were no significant differences between groups on HAQ DI score or on most of the performance‐based tests. However, the endurance test was significantly improved in the intervention group compared to the control group, and leg‐muscle strength, assessed by the STS test, improved in both groups. In order to maintain positive outcomes of exercise, the intensity of the exercise must be maintained 49, and the diminishing results at the 12‐month follow‐up are assumed to be related to the reduction of total exercise in the group, commonly referred to as de‐training 50 and which occurs independently of exercise intensity 51. In the current study, approximately 50% of the patients in the intervention group at 12 months still reported exercising at an intensity in accordance with the intervention, which can be regarded as a high percentage when compared to a general Swedish RA population 13. Maintenance of exercise is a commonly known difficulty in patients with RA, who need to overcome several barriers, both general and diagnosis‐specific 52. In the current study, a contributing reason for the ability to continue exercising at a moderate‐to‐high–intensity level despite barriers might be found in the support from the physiotherapist on how to remain physically active 52. Barriers and facilitators will be further studied in a subsequent qualitative interview study.
A limitation to consider in this study is that as part of the screening and inclusion process, several potential participants were not included due to having a heart condition. This exclusion was a safety measure, because the exercise was performed outside the health care setting. A number of potential participants declined to participate due to reasons that were not always explicitly described but were possibly associated with health status. However, 46% of the patients had concomitant diseases or previous cancer, and 23% had prostheses and comorbidities that are negatively associated with physical functioning 53. An alternative for HAQ DI, showing floor effects in the current study, should be considered in future studies. Additionally, improvement of physical function in upper extremities appears to require changes in the exercise program or in an instrument to assess it.
Moderate‐to‐high intensity exercise with person‐centered guidance was found to effectively improve physical fitness in terms of aerobic capacity, endurance, strength, and dynamic balance in older adults with RA. The participants also rated their experienced health as improved. After 12 months, the positive effects of physical fitness partially persisted. The supervised exercise intervention is recommended for older adults with RA with a low disease activity.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Mrs. Lange had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design.
Svensson, Gjertsson, Mannerkorpi.
Acquisition of data.
Lange, Kucharski, Svedlund, Svensson, Bertholds, Gjertsson, Mannerkorpi.
Analysis and interpretation of data.
Lange, Gjertsson, Mannerkorpi.
Acknowledgments
The authors thank our colleagues Marie‐Louise Andersson, Anneli Lund, Lill‐Marie Knutsson, Christina Ljung, Anna Johansson, and Anette Thelander, who performed examinations in Gothenburg and Skövde, and we thank the exercise promotion organization Friskis & Svettis in Gothenburg for allowing us to use their facilities.
Clinicaltrials.gov identifier: NCT02397798.
Supported by the University of Gothenburg Center for Person‐Centered Care, the Health and Medical Care Committee of the Regional Executive Board (grant VGFOUREG‐66251), Region Västra Götaland, ALF/LUA at Sahlgrenska University Hospital (grant ALFGBG‐4636751), and the Swedish Rheumatism Association (grant R‐663361).
Drs. Gjertsson and Mannerkorpi contributed equally to this work.
References
- 1. Cevenini E, Monti D, Franceschi C. Inflamm‐ageing. Curr Opin Clin Nutr Metab Care 2013;16:14–20. [DOI] [PubMed] [Google Scholar]
- 2. Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non‐fatal falls among older adults. Inj Prev 2006;12:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stavropoulos‐Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72:1819–25. [DOI] [PubMed] [Google Scholar]
- 4. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti‐inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011;11:607–15. [DOI] [PubMed] [Google Scholar]
- 5. Hurkmans E, van der Giesen FJ, Vliet Vlieland TP, Schoones J, Van den Ende EC. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev 2009:CD006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rydwik E, Frandin K, Akner G. Effects of physical training on physical performance in institutionalised elderly patients (70+) with multiple diagnoses. Age Ageing 2004;33:13–23. [DOI] [PubMed] [Google Scholar]
- 7. Buitinga L, Braakman‐Jansen LM, Taal E, van de Laar MA. Future expectations and worst‐case future scenarios of patients with rheumatoid arthritis: a focus group study. Musculoskeletal Care 2012;10:240–7. [DOI] [PubMed] [Google Scholar]
- 8. Lemmey AB, Wilkinson TJ, Clayton RJ, Sheikh F, Whale J, Jones HS, et al. Tight control of disease activity fails to improve body composition or physical function in rheumatoid arthritis patients. Rheumatology (Oxford) 2016;55:1736–45. [DOI] [PubMed] [Google Scholar]
- 9. Giles JT, Bartlett SJ, Andersen RE, Fontaine KR, Bathon JM. Association of body composition with disability in rheumatoid arthritis: impact of appendicular fat and lean tissue mass. Arthritis Rheum 2008;59:1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conigliaro P, Triggianese P, Ippolito F, Lucchetti R, Chimenti MS, Perricone R. Insights on the role of physical activity in patients with rheumatoid arthritis. Drug Dev Res 2014;75 Suppl 1:S54–6. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization . Global recommendations on physical activity for health. WHO; 2010. [PubMed] [Google Scholar]
- 12. Tierney M, Fraser A, Kennedy N. Physical activity in rheumatoid arthritis: a systematic review. J Phys Act Health 2012;9:1036–48. [DOI] [PubMed] [Google Scholar]
- 13. Demmelmaier I, Bergman P, Nordgren B, Jensen I, Opava CH. Current and maintained health‐enhancing physical activity in rheumatoid arthritis: a cross‐sectional study. Arthritis Care Res (Hoboken) 2013;65:1166–76. [DOI] [PubMed] [Google Scholar]
- 14. Law RJ, Breslin A, Oliver EJ, Mawn L, Markland DA, Maddison P, et al. Perceptions of the effects of exercise on joint health in rheumatoid arthritis patients. Rheumatology (Oxford) 2010;49:2444–51. [DOI] [PubMed] [Google Scholar]
- 15. Wang M, Donovan‐Hall M, Hayward H, Adams J. People's perceptions and beliefs about their ability to exercise with rheumatoid arthritis: a qualitative study. Musculoskeletal Care 2015;13:112–5. [DOI] [PubMed] [Google Scholar]
- 16. De Jong Z, Munneke M, Kroon HM, van Schaardenburg D, Dijkmans BA, Hazes JM, et al. Long‐term follow‐up of a high‐intensity exercise program in patients with rheumatoid arthritis. Clin Rheumatol 2009;28:663–71. [DOI] [PubMed] [Google Scholar]
- 17. Ekman I, Swedberg K, Taft C, Lindseth A, Norberg A, Brink E, et al. Person‐centered care: ready for prime time. Eur J Cardiovasc Nurs 2011;10:248–51. [DOI] [PubMed] [Google Scholar]
- 18. Charon R. The patient‐physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA 2001;286:1897–902. [DOI] [PubMed] [Google Scholar]
- 19. Eriksson JK, Neovius M, Ernestam S, Lindblad S, Simard JF, Askling J. Incidence of rheumatoid arthritis in Sweden: a nationwide population‐based assessment of incidence, its determinants, and treatment penetration. Arthritis Care Res (Hoboken) 2013;65:870–8. [DOI] [PubMed] [Google Scholar]
- 20. Eriksson JK, Johansson K, Askling J, Neovius M. Costs for hospital care, drugs and lost work days in incident and prevalent rheumatoid arthritis: how large, and how are they distributed? Ann Rheum Dis 2015;74:648–54. [DOI] [PubMed] [Google Scholar]
- 21. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 22. Scott DL, Houssien DA. Joint assessment in rheumatoid arthritis. Br J Rheumatol 1996;35 Suppl 2:14–8. [DOI] [PubMed] [Google Scholar]
- 23. Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 2005;23 Suppl 39:S93–9. [PubMed] [Google Scholar]
- 24. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 25. Ekdahl C, Eberhardt K, Andersson SI, Svensson B. Assessing disability in patients with rheumatoid arthritis: use of a Swedish version of the Stanford Health Assessment Questionnaire. Scand J Rheumatol 1988;17:263–71. [DOI] [PubMed] [Google Scholar]
- 26. Pina IL, Balady GJ, Hanson P, Labovitz AJ, Madonna DW, Myers J. Guidelines for clinical exercise testing laboratories: a statement for healthcare professionals from the Committee on Exercise and Cardiac Rehabilitation, American Heart Association. Circulation 1995;91:912–21. [DOI] [PubMed] [Google Scholar]
- 27. Alemo Munters L, Dastmalchi M, Katz A, Esbjornsson M, Loell I, Hanna B, et al. Improved exercise performance and increased aerobic capacity after endurance training of patients with stable polymyositis and dermatomyositis. Arthritis Res Ther 2013;15:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borg G. Borg's perceived exertion and pain scales. Champaign (IL): Human Kinetics; 1998. [Google Scholar]
- 29. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- 30. Bohannon RW. Sit‐to‐stand test for measuring performance of lower extremity muscles. Percept Mot Skills 1995;80:163–6. [DOI] [PubMed] [Google Scholar]
- 31. Palstam A, Larsson A, Bjersing J, Lofgren M, Ernberg M, Bileviciute‐Ljungar I, et al. Perceived exertion at work in women with fibromyalgia: explanatory factors and comparison with healthy women. J Rehabil Med 2014;46:773–80. [DOI] [PubMed] [Google Scholar]
- 32. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther 2004;27:26–35. [DOI] [PubMed] [Google Scholar]
- 33. Mannerkorpi K, Hernelid C. Leisure Time Physical Activity Instrument and Physical Activity at Home and Work Instrument: development, face validity, construct validity and test‐retest reliability for subjects with fibromyalgia. Disabil Rehabil 2005;27:695–701. [DOI] [PubMed] [Google Scholar]
- 34. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Hillsdale (NJ): Lawrence Earlbaum; 1988. [Google Scholar]
- 35. Hakkinen A. Effectiveness and safety of strength training in rheumatoid arthritis. Curr Opin Rheumatol 2004;16:132–7. [DOI] [PubMed] [Google Scholar]
- 36. Maska L, Anderson J, Michaud K. Measures of functional status and quality of life in rheumatoid arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire II (HAQ‐II), Improved Health Assessment Questionnaire (Improved HAQ), and Rheumatoid Arthritis Quality of Life (RAQoL). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S4–13. [DOI] [PubMed] [Google Scholar]
- 37. Haugeberg G, Hansen IJ, Soldal DM, Sokka T. Ten years of change in clinical disease status and treatment in rheumatoid arthritis: results based on standardized monitoring of patients in an ordinary outpatient clinic in southern Norway. Arthritis Res Ther 2015;17:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neupert SD, Lachman ME, Whitbourne SB. Exercise self‐efficacy and control beliefs: effects on exercise behavior after an exercise intervention for older adults. J Aging Phys Act 2009;17:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Challal S, Minichiello E, Boissier MC, Semerano L. Cachexia and adiposity in rheumatoid arthritis: relevance for disease management and clinical outcomes. Joint Bone Spine 2016;83:127–33. [DOI] [PubMed] [Google Scholar]
- 40. Pereira C, Baptista F, Cruz‐Ferreira A. Role of physical activity, physical fitness, and chronic health conditions on the physical independence of community‐dwelling older adults over a 5‐year period. Arch Gerontol Geriatr 2016;65:45–53. [DOI] [PubMed] [Google Scholar]
- 41. Hirschmann MT, Wind B, Amsler F, Gross T. Reliability of shoulder abduction strength measure for the Constant‐Murley score. Clin Orthop Relat Res 2010;468:1565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Backman M, Browall M, Sundberg CJ, Wengström Y. Experiencing health: physical activity during adjuvant chemotherapy treatment for women with breast cancer. Eur J Oncol Nurs 2016;21 Suppl C:160–7. [DOI] [PubMed] [Google Scholar]
- 43. Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006;174:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada's Physical Activity Guidelines. Int J Behav Nutr Phys Act 2010;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor D. Physical activity is medicine for older adults. Postgrad Med J 2014;90:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alharbi TS, Carlström E, Ekman I, Jarneborn A, Olsson LE. Experiences of person‐centred care: patients’ perceptions. A qualitative study. BMC Nurs 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morgan S, Yoder LH. A concept analysis of person‐centered care. J Holist Nurs 2012;30:6–15. [DOI] [PubMed] [Google Scholar]
- 48. Withall J, Haase AM, Walsh NE, Young A, Cramp F. Physical activity engagement in early rheumatoid arthritis: a qualitative study to inform intervention development. Physiotherapy 2016;102:264–71. [DOI] [PubMed] [Google Scholar]
- 49. Lemmey AB, Williams SL, Marcora SM, Jones J, Maddison PJ. Are the benefits of a high‐intensity progressive resistance training program sustained in rheumatoid arthritis patients? A 3‐year followup study. Arthritis Care Res (Hoboken) 2012;64:71–5. [DOI] [PubMed] [Google Scholar]
- 50. Neufer PD. The effect of detraining and reduced training on the physiological adaptations to aerobic exercise training. Sports Med 1989;8:302–20. [DOI] [PubMed] [Google Scholar]
- 51. Sousa AC, Marinho DA, Gil MH, Izquierdo M, Rodriguez‐Rosell D, Neiva HP, et al. Concurrent training followed by detraining: does the resistance training intensity matter? J Strength Cond Res 2018;32:632–42. [DOI] [PubMed] [Google Scholar]
- 52. Veldhuijzen van Zanten JJ, Rouse PC, Hale ED, Ntoumanis N, Metsios GS, Duda JL, et al. Perceived barriers, facilitators and benefits for regular physical activity and exercise in patients with rheumatoid arthritis: a review of the literature. Sports Med 2015;45:1401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van den Hoek J, Roorda LD, Boshuizen HC, van Hees J, Rupp I, Tijhuis GJ, et al. Long‐term physical functioning and its association with somatic comorbidity and comorbid depression in patients with established rheumatoid arthritis: a longitudinal study. Arthritis Care Res (Hoboken) 2013;65:1157–65. [DOI] [PubMed] [Google Scholar]


