Abstract
The combination regimen of daclatasvir, asunaprevir, and beclabuvir has been developed for the treatment of hepatitis C virus infection. The objectives of this analysis were to characterize the relationship between the exposures of the daclatasvir, asunaprevir, and beclabuvir regimen and liver‐related laboratory elevations (Grade 3 or 4 alanine aminotransferase [ALT] and total bilirubin [Tbili]), and to evaluate the impact of selected covariates on the exposure‐response relationships. The exposure‐response analysis was performed with data from 1 phase 2 and 3 phase 3 studies in hepatitis C virus–infected subjects. The probability of liver‐related laboratory elevations were modeled using linear logistic regression. Selected covariates were tested using a forward‐addition and backward‐elimination approach. The final model for ALT elevation included Asian race, body weight in non‐Asian subjects, and asunaprevir exposure. The final model for Tbili elevation included Asian race, fibrosis score (F0‐F3 or F4) and asupanprevir exposure. Asian subjects had greater the Grade 3 or 4 ALT and Tbili elevation rates than non‐Asians. The Grade 3 or 4 ALT elevation rate increased with decreasing body weight in non‐Asian subjects. Subjects with F4 fibrosis score had a higher rate of Grade 3 or 4 Tbili elevation compared to subjects with F0 to F3 fibrosis score. Higher asunaprevir exposure was associated with increases in Grade 3 or 4 ALT and Tbili elevation rates; however, the impact on the ALT elevation was not clinically relevant and the effect on Tbili elevation was smaller than the other significant covariates.
Keywords: alanine aminotransferase, asunaprevir, beclabuvir, daclatasvir, exposure‐response, hepatitis C virus, total bilirubin
Approximately 80 to 185 million individuals are infected with hepatitis C virus (HCV) worldwide and the number of HCV‐infected patients is estimated to be approximately 2 million in Japan.1, 2, 3 It is estimated that 20% of patients with chronic HCV infection will develop cirrhosis.4 In recent years, HCV treatments have evolved rapidly from peg‐interferon (peg‐IFN) plus ribavirin to all‐oral combinations of direct‐acting antivirals (DAAs).5, 6 As the first DAA therapy without IFN, the combination of daclatasvir (pangenotypic NS5A inhibitor) and asunaprevir (NS3/4A protease inhibitor) therapy was approved in July 2014 as an indication for the treatment of chronic HCV GT‐1 infection (including compensated cirrhosis) in patients who are ineligible‐naïve or intolerant to IFN‐based therapy or who failed to respond to IFN‐based therapy, and in March 2015 for the remaining patient population with chronic HCV infection with/without compensated cirrhosis. The combination regimen of daclatasvir and asunaprevir improved effectiveness and safety profiles/tolerability of HCV therapy with limited adverse drug reactions and high treatment adherence. Sustained virologic response at posttreatment week 12 rates of a daclatasvir and asunaprevir regimen were 80.5% to 95.5%, and consistently high regardless of the presence of compensated cirrhosis.7, 8, 9 However, in the daclatasvir and asunaprevir combination regimen, sustained virologic response at posttreatment week 12 rates were 36.9% and 41.9% in subjects with baseline mutation of Y93H and L31F/I/M/V, respectively.10 Therefore, highly effective treatment without resistance variants was considered necessary. The fixed‐dose combination composed of daclatasvir, asunaprevir, and beclabuvir (3DAA regimen) was developed based on the dual therapy of daclatasvir plus asunaprevir by adding beclabuvir (nonnucleoside NS5B inhibitor). HCV NS5B is an RNA‐dependent RNA polymerase that is responsible for viral RNA synthesis and is therefore essential for viral replication. Beclabuvir was developed only for use in combination with daclatasvir and asunaprevir. There was no clinically meaningful drug‐drug interaction effect among daclatasvir, asunaprevir, and beclabuvir.11 Results of clinical studies showed a robust viral clearance of HCV in infected subjects treated with beclabuvir in combination with daclatasvir and asunaprevir.12, 13, 14 In the phase 2 and phase 3 clinical trials, 3DAA regimen showed a high safety profile with minimal serious adverse events (AEs) and AE‐related discontinuations, although the safety event rates were slightly higher in Japanese HCV patients compared to non‐Japanese HCV patients.12, 13, 14, 15, 16 Safety profiles of the 3DAA regimen and the combination regimen of daclatasvir and asunaprevir were generally comparable.14 The 3DAA regimen as a fixed combination tablet was approved in Japan in 2016.
The purpose of this analysis was to characterize the exposure‐response (E‐R) relationship of 3DAA regimen with treatment‐emergent liver‐related laboratory elevations (ALT and Tbili) in HCV‐infected subjects, and to assess the impact of covariates on these E‐R relationships. In the previous clinical study, no apparent relationship was reported between the observed daclatasvir exposure and safety events (ALT, aspartate aminotransferase, and Tbili elevations) in the daclatasvir and asunaprevir combination therapy.17 As for asunaprevir, greater and more frequent aminotransferase elevations were observed with the higher asunaprevir doses in a phase 2 study.18 The effect of beclabuvir exposure on safety event rates has not been clarified. Therefore, this E‐R analysis focused on assessing the effect of asunaprevir and beclabuvir exposure on Grade 3 or 4 liver related laboratory elevations as exposure metrics.
Methods
Clinical Studies and Patient Population
The safety E‐R analysis was performed with combined data from HCV subjects treated with 3DAA regimen in 1 phase 2 study (AI443014) and 3 phase 3 studies (AI443102, AI443113 and AI443117). These study protocols were approved by the institutional review board or independent ethics committee at each site. All participants provided written informed consent. Details for the studies are provided in Table S1.
Safety Exposure‐Response Analysis
Individual asunaprevir and beclabuvir exposure were calculated from the Bayesian post hoc parameters of the final population pharmacokinetic (PopPK) models of asunaprevir and beclabuvir.19 Asunaprevir and beclabuvir pharmacokinetics were adequately described by a 2‐compartment model and a 1‐compartment model, respectively. The asunaprevir PopPK model included cirrhosis (yes, no, or missing), baseline and time‐varying ALT, race (white, black, Asian, or others), age, coadministration (fixed‐dose combination tablet or all 3 drugs as separate tablets), asunaprevir formulation (tablet or capsule), and sex (male or female) as significant covariates on clearance or bioavailability. The significant covariates on clearance for beclabuvir PopPK model were race (white, black, Asian, or others), body weight, time‐varying ALT, age, and coadministration of proton pump inhibitor (yes or no). Cirrhosis, baseline and time‐varying ALT, and race had a larger impact on asunaprevir clearance than the other significant covariates. Race was the most significant covariate on beclabuvir clearance. The average steady‐state exposure (Cavg,ss) was selected as the exposure metric since the timing of onset of the laboratory abnormalities suggest that overall exposure, that is, Cavg,ss, was the most relevant exposure parameter rather than a single concentration at any given time, such as maximum plasma concentration or trough plasma concentration. This is supported by the fact that the transient rise and fall of plasma concentration is likely not reflected as significantly within the liver, where more consistent concentrations would be expected at steady state. Furthermore, area under the concentration versus time curve, which is an equivalent exposure measure to average plasma concentration, was also used to assess the relationship between drug exposure and safety event in other HCV compounds.20, 21
Missing baseline demographic and clinical laboratory covariates were imputed as the population median (continuous) or mode (categorical) of the nonmissing value, with no adjustment for study, race, or sex. There were only a few subjects (≤1%) with missing baseline body weight, baseline body mass index, fibrosis score, race, IL28B genotype (rs12979860), cirrhosis, and prior treatment type.
Model Development
Model development was conducted in 3 stages: the base model, covariate model, and final model. First, the base model was developed. The relationship between the probability of Grade 3 or 4 liver‐related laboratory elevations and the Cavg,ss for asunaprevir and beclabuvir was described using a logistic regression model, without consideration of any potential effect of covariates. The probability of AEs was given as equation 1.
| (1) |
where μ is the logit transform of P(AE). The logit (log‐odds) can be given as equation 2.
| (2) |
where β0 and βi are scalar and vector parameters that represent baseline odds and the effect of the predictor variable vector Xi on the log‐odds of having the events, where Xi consists of the covariate (predictor) values of subject i.
Prespecified covariates tested in the E‐R analyses of liver‐related laboratory elevations were listed in Table 1. Japanese subjects were belonged to Asian race in the covariate models. However, we referred to race as Japanese or non‐Japanese rather than Asian or non‐Asian when the impact of race was discussed on the Grade 3 or 4 ALT and Tbili elevation rates in the Japanese study.
Table 1.
Prespecified Covariates Tested in the Exposure‐Response Analysis of Liver‐Related Laboratory Elevations
| Category | Covariate |
|---|---|
| Demographic | Sex (female or male), age, race (Asian or non‐Asian), body weight, BMI |
| Baseline labs | Baseline liver enzymes (ALT or Tbili), baseline serum creatinine |
| Disease related | HCV RNA (>8 × 105 or ≤8 × 105), HCV genotype (GT‐1b, GT‐4 or other), IL28B genotype (rs12979860) (CC, CT, or TT alleles), prior treatment type (prior peg‐IFN failure, prior DAA failure, prior IFN treatment terminated due to safety issue or other), fibrosis score (F4 or F0‐F3), cirrhosis (yes or no/unknown), number of resistance mutationsa (≥1 or 0) |
| Treatment | Ribavirin usage (yes or no) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GT, genotype; HCV, hepatitis C virus; IFN, interferon; Tbili, total bilirubin.
NS5A polymorphism at M28, Q30, L31, or Y93.
Among the continuous covariates evaluated in the analysis data set, several highly correlated variables are expected. During model development, highly correlated covariates were evaluated but were not included in the same model without substantial evidence supporting their inclusion. For developing a covariate model, the effects of covariates of interest on the intercept of the base model were evaluated using a stepwise covariate model building approach in Perl‐speaks‐NONMEM, where covariates were tested in a forward addition (P < .05), followed by a backward elimination (P < .01) steps. Covariates were first tested on the intercept, then significant ones were further tested in the PK exposure slopes.
The final model was obtained by dropping the exposure of asunaprevir and/or beclabuvir if the slope was not significant and conducting more detailed inspections to understand the results, considering the potential correlation among the covariates, and correlation of covariate and PK exposures.
Model Evaluation
Model evaluation was conducted by comparing the observed values and rate of incidence of events with the final model simulations stratified by covariates of interest. Confidence intervals (CIs) of the simulation were obtained using bootstrapping methods (1000 runs). Model evaluation was also conducted using a visual predictive check of the final model and presented stratified by covariates of interest.22
Analysis Platforms
The E‐R safety analysis was performed using the NONMEM computer program (Version 7.2, level 2.0, ICON Development Solutions, Ellicott City, Maryland), compiled using Intel Fortran Compiler (Version 12.0.4, Intel Corp., Santa Clara, California). Perl‐speaks‐NONMEM (version 4.2, http://psn.sourceforge.net/) was used to aid the model development using NONMEM. Exploratory plots, postprocessing, and visualization of NONMEM output were performed using R (Version 3.0 or later, http://www.r-project.org/).
Results
Subject Characteristics
The analysis included 1153 (99.9%) of 1154 subjects whose safety and PK data treated with the 3DAA regimen were available. One subject in study AI443117 who had missing PK exposure was excluded. Of note, the majority of Asian subjects were Japanese, at 94.7% (216 of 228). An overview of the Grade 3 and 4 liver‐related laboratory elevations, key covariates, and PK exposure are presented in Table 2. Grade 3 or 4 ALT and Tbili elevations occurred in 30 (13.9%) and 12 (5.6%) of the 216 in the Japanese Phase 3 study (AI443117) who received the 3 DAA regimen and who had a PK exposure result. The event rates of ALT and Tbili elevations in Japanese subjects were numerically greater than those in the other studies with non‐Japanese subjects, which ranged from 0.6% to 4.6% and 0 % to 1.5 %, respectively. A higher number of female subjects was observed in the Japanese population (69% female in the Japanese population compared with 34%–42% in the non‐Japanese population). The Japanese population had a lower body weight compared to the non‐Japanese population (55 kg vs 78–82 kg). The asunaprevir exposure was approximately 2‐fold higher in the Japanese population compared to the non‐Japanese population in AI443014 and AI443102, whereas the asunaprevir exposure in non‐Japanese cirrhosis population in AI443113 was comparable to that in the Japanese population (221 ng/mL vs 224 ng/mL) because cirrhosis is one of the covariates influencing asunaprevir PK.23, 24
Table 2.
Overview of Liver Enzyme Adverse Events, Baseline Characteristics, and Exposure
| Category | Variable | AI443014 | AI443102 | AI443113 | AI443117 |
|---|---|---|---|---|---|
| No. of Subjects | N | 320 | 415 | 202 | 216 |
| Liver‐related laboratory elevations | ALT Grade 3,4, N (%) | 2 (0.6%) | 19 (4.6%) | 5 (2.5%) | 30 (13.9%) |
| Tbili Grade 3,4, N (%) | 1 (0.3%) | 0 (0%) | 3 (1.5%) | 12 (5.6%) | |
| Subject characteristics | Median baseline ALT, U/L | 60 | 54 | 79 | 47 |
| Median weight, kg | 81 | 78 | 82 | 55 | |
| Cirrhosis, N (%) | 20 (6%) | 0 (0%) | 200 (99%) | 46 (21%) | |
| Female, N (%) | 117 (37%) | 176 (42%) | 69 (34%) | 148 (69%) | |
| Asian race, N (%) | 3 (1%) | 5 (1%) | 4 (2%) | 216 (100%) | |
| Cavg,ss | DCV, ng/mL | 565 | 529 | 496 | 600 |
| ASV, ng/mL | 83 | 121 | 221 | 224 | |
| BCV, ng/mL | 959 | 713 | 738 | 1111 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ASV, asunaprevir; BCV, beclabuvir; Cavg,ss, average concentration at steady state; DCV, daclatasvir; Tbili, total bilirubin.
Values for Cavg,ss are median.
Alanine Aminotransferase Elevation
The base ALT model includes an intercept and prespecified linear effect of asunaprevir and beclabuvir concentration at steady state. The final model for Grade 3 or 4 ALT elevation included the effect of race (Asian or non‐Asian) and asunaprevir exposure and the effect of body weight in non‐Asian subjects.
The final ALT E‐R model was given as:
The final model parameters and their 95%CI are provided in Table 3.
Table 3.
Final Model Parameter Estimates
| Name | Estimate | Standard Error (RSE%)a | 95%CIb |
|---|---|---|---|
| Grade 3 or 4 alanine aminotransferase elevation | |||
| Intercept for non‐Asian (θ1) | –3.91 | 0.252 (6.44) | –4.54, –3.52 |
| Asian on intercept (θ3) | 1.53 | 0.311 (20.4) | 0.865, 2.20 |
| Slope of ASV (ng/mL, θ4) | 0.0017 | 0.00065 (38.3) | 0.00034, 0.00305 |
| BWT (kg) effect on intercept for non‐Asian(θ2) | –0.0475 | 0.0148 (31.2) | –0.0829, –0.0163 |
| Grade 3 or 4 total bilirubin elevation | |||
| Intercept for non‐Asian (θ1) | –6.79 | 0.658 (9.69) | –9.19, –6.1 |
| Slope of ASV (ng/mL, θ2), (ng/mL)−1 | 0.00321 | 0.000898 (28) | 0.00168, 0.00545 |
| Asian on intercept (θ3) | 2.01 | 0.624 (31) | 0.892, 3.81 |
| Fibrosis Grade 4 on intercept (θ4) | 1.64 | 0.563 (34.3) | 0.485, 2.97 |
ASV, asunaprevir; BWT, baseline body weight; CI, confidence interval; RSE, relative standard error.
RSE% is the relative standard error (standard error as a percentage of estimate).
Confidence interval values are taken from bootstrap calculations (1000 run, sampled stratified by study).
Grade 3 or 4 ALT elevation increased in Asian subjects and decreased with increasing weight in non‐Asian subjects. The final model indicated that higher asunaprevir exposure has a modest increase of the Grade 3 or 4 ALT elevation.
The impact of the isolated effect of covariates and asunaprevir exposure on ALT elevation is provided in Table 4. Grade 3 or 4 ALT elevation rates for a “typical” baseline weight of 75.9 kg and asunaprevir median exposure of 138 ng/mL was 2.5% (95%CI, 1.4–3.5) for non‐Asian subjects. For an Asian subject of the same weight and exposure, the Grade 3 or 4 ALT elevation rates would be 10.4%. Increase in ASV exposures from the 5th percentile (48 ng/mL Cavg,ss) to the 95th percentile (472 ng/mL) was predicted to increase the rate of Grade 3 or 4 ALT elevations by 2.2%, from 2.1% at the 5th percentile to 4.3% at the 95th percentile.
Table 4.
Impact of Covariate and Exposure
| Condition | Rate (%) | 95% CIa | |
|---|---|---|---|
| Grade 3 or 4 alanine aminotransferase elevation | |||
| Reference | Non‐Asian, median Cavg,ss exposure of ASV of 138 ng/mL, median weight of 75.9 kg | 2.5 | 1.4, 3.5 |
| Estimated event rate | Asian | 10.4 | 6.3, 15.3 |
| ASV 5%ile, 48 ng/mL | 2.1 | 1.2, 3.0 | |
| ASV 95%ile, 472 ng/mL | 4.3 | 2.1, 7.3 | |
| Weight 5%ile, 49 kg | 8.3 | 3.3, 16.1 | |
| Weight 95%ile, 104 kg | 0.7 | 0.2, 1.9 | |
| Asian + ASV exposure of 219 ng/mL | 11.8 | 7.6, 16.6 | |
| Grade 3 or 4 total bilirubin elevation | |||
| Reference | Non‐Asian, median Cavg,ss of ASV exposure of 138 ng/mL, fibrosis score 0–3 | 0.18 | 0.02, 0.33 |
| Estimated event rate | Asian | 1.3 | 0.20, 3.1 |
| ASV 5%ile, 48 ng/mL | 0.13 | 0.01, 0.25 | |
| ASV 95%ile, 472 ng/mL | 0.51 | 0.07, 1.2 | |
| Fibrosis Grade 4 | 0.90 | 0.11, 2.0 | |
| Asian + F4 fibrosis score | 6.4 | 1.9, 12.8 | |
| Asian + ASV exposure of 219 ng/mL | 1.7 | 0.29, 3.8 | |
ASV, asunaprevir; CI, confidence interval.
95%CI were calculated from bootstrap parameters (1000 run, sampled stratified by study).
The observed and model‐simulated Grade 3 or 4 ALT elevation stratified by race and body weight quartiles were compared to quantify the impact of significant covariates on Grade 3 or 4 ALT elevations (Table S2). The effect of body weight was a significant covariate in non‐Asian subjects. In non‐Asian subjects, simulated rate of Grade 3 or 4 ALT elevations was 6.1% for the lowest weight quartile and 0.9% for the highest weight quartile. The simulated results are consistent with the observed values.
Visual predictive checks of Grade 3 or 4 ALT elevation vs asunaprevir E‐R response by race are presented in Figure 1A. Visual predictive checks showed the asunaprevir E‐R response was generally described the data.
Figure 1.
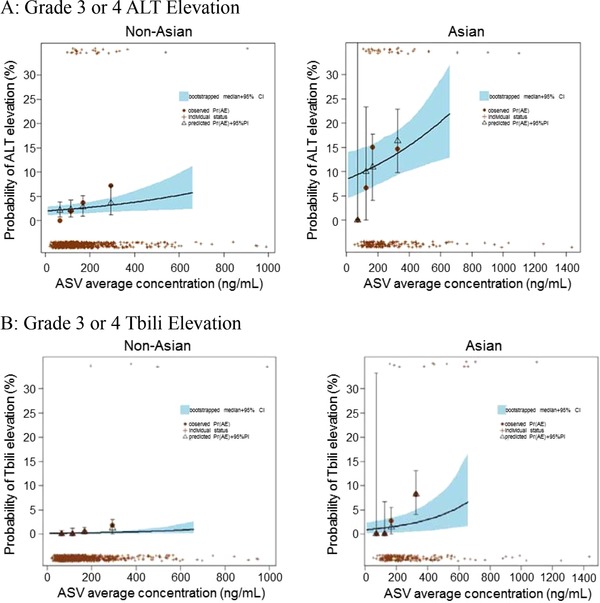
Visual predictive check of Grade 3 or 4 liver‐related laboratory elevations vs asunaprevir exposure and race. Predictions are for the reference condition of the population (median exposure and median weight of each group) except as indicated by the x‐axis labels. The individual “+” symbols at the top and bottom of the plot represent individual subjects with and without AE, respectively, in each category indicated by the x‐axis labels. The solid brown circles and the open black triangles indicate the observed and predicted AE, respectively, for each category. The vertical bars indicate the 95% prediction intervals and the blue shaded region indicates the 95% confidence interval for the bootstrapped model. The observed data include only subjects with categorical covariates consistent with the reference population, except as indicated by the x‐axis labels. ALT elevation rates were simulated up to 95th percentile of the ASV exposure in Asian subjects. AE, Grade 3 or 4 ALT or Tbili elevation event; ALT, alanine aminotransferase; ASV, asunaprevir; CI, confidence interval; PI, prediction interval; Pr(AE), probability of an Grade 3 or 4 ALT or Tbili elevation; Tbili, total bilirubin.
Total Bilirubin Elevation
The base Tbili model included an intercept and prespecified linear effect of asunaprevir and beclabuvir exposures. Significant covariates identified during stepwise covariate modeling were race (Asian or non‐Asian), fibrosis score (F4 fibrosis score or F0‐F3 fibrosis score), and asunaprevir exposure on the intercept. No covariates interacting on the drug effects were identified. The effect of beclabuvir exposure was not significant and dropped from the model. The final Tbili E‐R model was given as:
The final model parameters and their 95%CI are provided in Table 3. The final model for Grade 3 or 4 Tbili elevation indicated that subjects with F4 fibrosis score (Fibro Test score >0.75) had a higher rate of Grade 3 or 4 Tbili elevations compared to subjects with F0‐F3 fibrosis score; Asian subjects had a greater rate of Grade 3 or 4 Tbili elevations than non‐Asian subjects; and Grade 3 or 4 Tbili elevations increased with increasing asunaprevir exposure.
The impact of the isolated effect of covariates and asunaprevir exposure is provided in Table 4. The Grade 3 or 4 Tbili elevation rate for a non‐Asian with F0‐F3 fibrosis score and median asunaprevir exposure of 138 ng/mL was 0.18%. The Grade 3 or 4 Tbili elevation rate increased to 1.3% in Asian subjects. The combined effect of Asian race and F4 fibrosis score increased the event rate to 6.4% at asunaprevir exposure of median value of 219 ng/mL (estimated event rate of 1.7%) in Asian subjects, compared to the rate for Asian subjects alone of 1.3%.
The observed and model simulated Grade 3 or 4 Tbili elevation rate stratified by race, fibrosis score, and asunaprevir exposure were compared to quantify the impact of significant covariates on Grade 3 or 4 Tbili elevation (Table S2). The observed and estimated rates were both high in the subjects with F4 fibrosis score and higher asunaprevir exposure (>224 ng/mL), in particular, 4‐ to 9‐fold higher in Asian subjects compared with non‐Asian subjects (16.7% and 15.6% vs 4.4% and 1.7%).
Visual predictive checks of Tbili Grade 3 or 4 elevation vs asunaprevir exposure and race (Figure 1B) were performed and confirmed the model described the data.
Discussion
Grade 3 or 4 liver‐related laboratory elevations were modeled as the safety end points because they are more clinically relevant than Grade 1 or 2 elevations. The results from the previous clinical studies demonstrate that daclatasvir did not cause any liver enzyme elevations. In addition, daclatasvir exposure was similar whether administered as part of the daclatasvir and asunaprevir regimen or as part of the 3DAA regimen, indicating that beclabuvir did not alter the PK of daclatasvir.11 Therefore, the effects of asunaprevir and beclabuvir exposure on Grade 3 or 4 liver‐related laboratory elevations were evaluated in this E‐R analyses as exposure metrics. In the asunaprevir and beclabuvir PopPK analyses, the prediction‐corrected visual predictive checks showed that the models fit well to PK data of asunaprevir and beclabuvir. The shrinkage of apparent oral clearance in the final asunaprevir and beclabuvir models, which is the determinant for Cavg,ss, were relatively low (approximately 10%), suggesting that the Cavg,ss estimated using the Bayesian post hoc parameters were appropriate for the E‐R analyses. The final models for Grade 3 or 4 ALT and Tbili elevations included only the effect of asunaprevir exposure because beclabuvir exposure was not a significant predictor and the term for beclabuvir exposure was dropped during the backward elimination process (P < .01).
Asunaprevir exposure showed a modest impact in the final ALT model (only a 2% increase in rate of events from 5th percentile to 95th percentile in asunaprevir exposure). Asian race and lower body weight in non‐Asians were the most significant factors contributing to the increase of ALT elevations. As shown in the clinical studies, the Grade 3 or 4 ALT elevation rate was numerically higher in the Japanese study compared to other non‐Japanese studies (Table 2). The previous asunaprevir PopPK analysis showed that Japanese and non‐Japanese Asian subjects had higher asunaprevir exposure compared to white subjects.24 In addition, asunaprevir exposures would increase approximately 2‐fold in Asian subjects compared to non‐Asian subjects based on the PopPK analysis for 3DAA regimen.19 Although the reasons for the relatively higher exposure of asunaprevir in Japanese or Asian subjects are not entirely clear, the higher asunaprevir exposure might be considered as a factor of higher event rates in Japanese subjects. For further investigation, the Grade 3 or 4 ALT elevation of non‐Japanese subjects at the high quartile of the asunaprevir exposure (median asunaprevir exposure was 265 ng/mL) were compared to all Japanese subjects (Figure 2A). The rate of Grade 3 or 4 ALT elevation in non‐Japanese subjects with asunaprevir exposure in the highest quartile was significantly lower than that in Japanese subjects (6.0% vs 13.8%). The higher event rate in Japanese subjects was not fully explained by the higher asunaprevir exposure in Japanese subjects. This supports the interpretation of the E‐R analysis that impact of asunaprevir exposures is modest on the Grade 3 or 4 ALT elevation.
Figure 2.
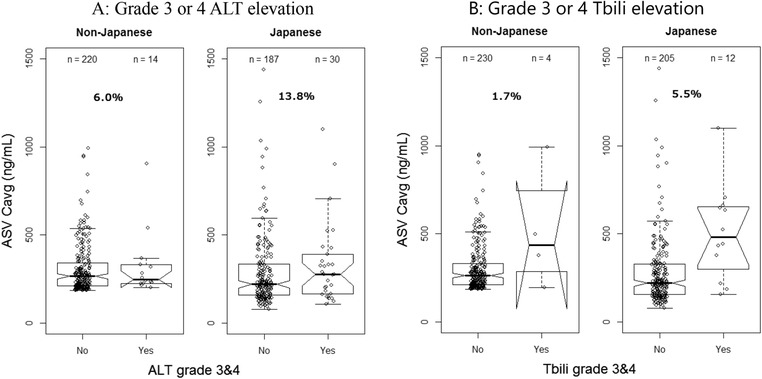
Comparison of Grade 3 or 4 liver‐related laboratory elevation rates in non‐Japanese with high asunaprevir exposure to Japanese. The line in the middle of the box is the median, the box is the interquartiles, and the whiskers are 1.5 times the interquartile range. ALT, alanine aminotransferase; ASV, asunaprevir; Cavg,ss, average concentration at steady state.
The impact of weight was estimated separately for Asian and non‐Asian subjects, since the Asian subjects were concentrated at the lower weight quartiles. As presented in Table S2, the results of the observed and predicted ALT rates stratified by body weight and race indicate that the difference between Asians and non‐Asians is not entirely explained by differences in body weight. In the lowest body weight quartile (34.1–61.8 kg), the observed rate of Grade 3 or 4 ALT elevation in non‐Asian and Asian subjects was 7.3% and 13.9%, respectively. The model was able to predict these reasonably with a predicted rate of 6.1% and 11.9% in non‐Asian and Asian subjects, respectively. In general, these data indicate that subjects with lower body weight are at a higher risk of events, which is further increased in Asian subjects with a trend toward a higher rate of events at higher asunaprevir exposures. The final model contained the body weight in the non‐Asian patients as a significant covariate. Few studies have been reported the relationship between body weight and ALT elevation. The demographics in the non‐Asian patients at each weight quantile were explored to evaluate the potential correlation with other covariates; however, there was no obvious trend to explain the higher Grade 3 or 4 ALT elevation rate in the lower weight group. The mechanism of increasing Grade 3 or 4 ALT elevation rate in the non‐Asian patients with lower body weight remains unclear, and further investigation would be needed.
Asunaprevir exposure was also included in the final E‐R model for Grade 3 or 4 Tbili elevation. Higher asunaprevir exposure was associated with increasing Grade 3 or 4 Tibli elevation. This finding was consistent with the E‐R model for the daclatasvir and asunaprevir regimen.17 The Grade 3 or 4 Tbili elevation increased in Asians and in subjects with F4 fibrosis score. Although fibrosis score was identified as a significant covariate in the final model, fibrosis score is correlated with cirrhosis status, and similar results were obtained with cirrhosis status as well, indicating that the rate of Grade 3 or 4 Tbili was higher in subjects with cirrhosis. This trend was observed in the clinical study.14
The Grade 3 or 4 Tbili elevation was higher in the Japanese phase 3 study compared with the non‐Japanese studies.13, 14, 16 Notably, asunaprevir exposure would be higher in Asian subjects compared to non‐Asian subjects. The higher asunaprevir exposure in Japanese subjects might be considered to contribute on higher event rate in Japanese subjects. For further investigation, the Grade 3 or 4 Tbili elevation of non‐Japanese subjects at the high quartile of the asunaprevir exposure was compared to all Japanese subjects (Figure 2B). Comparison of the asunaprevir exposures in Japanese and non‐Japanese subjects indicates that in non‐Japanese subjects with asunaprevir exposure at the highest quartile, the rate of Grade 3 or 4 Tbili elevation was significantly lower than that in Japanese subjects (1.7% vs 5.5%). These data indicate that although asunaprevir exposure had an effect on rate of Grade 3 or 4 Tbili events, the effect of comparable exposures may be higher in Asian subjects. Although asunaprevir exposure would increase in Japanese subjects compared to non‐Japanese subjects, the different event rate between Japanese and non‐Japanese subjects cannot be explained only by the difference of asunaprevir exposure.
Asunaprevir exposure was one of the factors that affect Grade 3 or 4 ALT elevation and Grade 3 or 4 Tbili elevation. It has been previously shown and also confirmed in the PopPK analysis that asunaprevir PK is very sensitive to changes in markers of hepatic function (ie, baseline and time‐varying ALT and cirrhosis status), and higher levels of ALT and the presence of cirrhosis resulted in higher plasma concentrations of asunaprevir.19, 23, 24 Therefore, it can be hypothesized that in some subjects certain events may cause higher levels of ALT or Tbili, which subsequently lead to higher plasma concentrations of asunaprevir.
Race is relevant for both asunaprevir PK and the response variables. Asian subjects had higher asunaprevir exposure and higher safety event rates than non‐Asian subjects. To assess the potential confounding effect of race and asunaprevir exposure, a sensitivity analysis was performed by excluding the non‐Asian subjects from the analysis data set and reestimating the effect of asunaprevir exposure. In Asian subjects, the parameter estimates of asunaprevir exposure on Grade 3 or 4 ALT and Tbili elevation were 0.000938 or 0.00263, respectively, which were within the 95%CIs of the final model parameters (Table 3). A confounding factor may compromise the E‐R analyses; however, the contribution of asunaprevir exposure on Grade 3 or 4 ALT and Tbili elevation was modest in the sensitivity analysis, which was consistent with the result from final E‐R models that included both race and asunaprevir exposure. The higher event rates in the Asian subjects were not fully explained by the difference in asunaprevir exposure; therefore, both Asian race and asunaprevir exposure were included in the final E‐R models.
In conclusion, higher asunaprevir exposure had a modest increase of the Grade 3 or 4 ALT and Tbili elevations. The magnitude of asunaprevir exposure was smaller than the other significant covariates. Asian subjects had a greater ALT elevation rate than non‐Asians, and Grade 3 or 4 ALT elevation decreased with increasing weight in non‐Asian subjects. Asian subjects had a greater rate of Grade 3 or 4 Tbili elevation than non‐Asian subjects, and subjects with F4 fibrosis score had a higher rate of Grade 3 or 4 Tbili elevation compared to subjects with F0‐F3 fibrosis score. Considering the predominance of Japanese in the Asian group (94.7%), the effect of Asian race is likely an effect of the Japanese ethnicity rather than that of Asian race in general.
Declaration of Conflicting Interests
M.O., T.U., and T.S. are employees of Bristol‐Myers Squibb K.K. T.G. is a former employee of Bristol‐Myers Squibb and was employed by Bristol‐Myers Squibb when the studies and analyses were conducted. H.L. is an employee of Certara and acted as a consultant for the analysis.
Supporting information
Table S1.
Table S2.
Acknowledgment
The authors acknowledge and thank all of the physicians, associated health care professionals, and patients who took part in the studies listed within this manuscript; Phyllis Chan, Cirincione Brenda, and LaCreta Frank for their scientific contributions in study concept, data analysis plan, and data interpretation for the study report; and Hiroki Ishikawa for his scientific contributions for the study report. The authors also acknowledge the scientific contributions on data analysis of Russ Wada and Michelle Green from Certara. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Data Sharing
Bristol‐Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- 1. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology (Baltimore, Md). 2013;57(4):1333–1342. [DOI] [PubMed] [Google Scholar]
- 2. Gower E, Estes C, Blach S, Razavi‐Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014;61(1 suppl):S45–S57. [DOI] [PubMed] [Google Scholar]
- 3. Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology. 2010;53(1):39–43. [DOI] [PubMed] [Google Scholar]
- 4. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology (Baltimore, Md). 2000;31(4):1014–1018. [DOI] [PubMed] [Google Scholar]
- 5. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C 2016. Hepatol. 2017;66(1):153–194. [DOI] [PubMed] [Google Scholar]
- 6. Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non‐A, non‐B hepatitis to hepatitis C virus cure. Hepatol. 2015;62(1 Suppl):S87–S99. [DOI] [PubMed] [Google Scholar]
- 7. Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology (Baltimore, Md). 2014;59(6):2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suda G, Kudo M, Nagasaka A, et al. Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. Gastroenterol. 2016;51(7):733–740. [DOI] [PubMed] [Google Scholar]
- 9. Wei L, Zhang M, Xu M, et al. A phase 3, open‐label study of daclatasvir plus asunaprevir in Asian patients with chronic hepatitis C virus genotype 1b infection who are ineligible for or intolerant to interferon alfa therapies with or without ribavirin. J Gastroenterol Hepatol. 2016;31(11):1860–1867. [DOI] [PubMed] [Google Scholar]
- 10. McPhee F, Suzuki Y, Toyota J, et al. High sustained virologic response to daclatasvir plus asunaprevir in elderly and cirrhotic patients with hepatitis C virus genotype 1b without baseline NS5A polymorphisms. Adv Ther. 2015;32(7):637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eley T LW, Huang S‐P, He B, et al. Evaluation of pharmacokinetic drug‐drug interactions (DDI) between BMS‐791325, an NS5b nonnucleoside polymerase inhibitor, daclatasvir, and asunaprevir in triple combination in hepatitis C virus (HCV) genotype 1–infected patients. 8th International Workshop on Clinical Pharmacology of Hepatitis Therapy. Cambridge, MA; 2013. [Google Scholar]
- 12. Everson GT, Sims KD, Rodriguez‐Torres M, et al. Efficacy of an interferon‐ and ribavirin‐free regimen of daclatasvir, asunaprevir, and BMS‐791325 in treatment‐naive patients with HCV genotype 1 infection. Gastroenterology. 2014;146(2):420–429. [DOI] [PubMed] [Google Scholar]
- 13. Muir AJ, Poordad F, Lalezari J, et al. Daclatasvir in combination with asunaprevir and beclabuvir for hepatitis C virus genotype 1 infection with compensated cirrhosis. JAMA. 2015;313(17):1736–1744. [DOI] [PubMed] [Google Scholar]
- 14. Toyota J, Karino Y, Suzuki F, et al. Daclatasvir/asunaprevir/beclabuvir fixed‐dose combination in Japanese patients with HCV genotype 1 infection. J Gastroenterol. 2017;52(3):385–395. [DOI] [PubMed] [Google Scholar]
- 15. Everson GT, Sims KD, Thuluvath PJ, et al. Daclatasvir + asunaprevir + beclabuvir +/– ribavirin for chronic HCV genotype 1–infected treatment‐naive patients. Liver Int. 2016;36(2):189–197. [DOI] [PubMed] [Google Scholar]
- 16. Kao JH, Yu ML, Peng CY, et al. Daclatasvir/asunaprevir/beclabuvir, all‐oral, fixed‐dose combination for patients with chronic hepatitis C virus genotype 1. J Gastroenterol Hepatol. 2017;32(12):1998–2005. [DOI] [PubMed] [Google Scholar]
- 17. Chan P, Zhu L, Eley T, et al. Exposure‐safety analysis for asunaprevir and daclatasvir in DUAL combination in Japanese and non‐Japanese subjects with hepatitis C virus infection. Presented at: Asian Pacific Association for the Study of the Liver; March 12–15, 2015; Istanbul.
- 18. Bronowicki JP, Pol S, Thuluvath PJ, et al. Randomized study of asunaprevir plus pegylated interferon‐alpha and ribavirin for previously untreated genotype 1 chronic hepatitis C. Antivir Ther. 2013;18(7):885–893. [DOI] [PubMed] [Google Scholar]
- 19. Osawa M, Ueno T, Shiozaki T, Ishikawa H, et al. Population pharmacokinetic analysis of daclatasvir, asunaprevir and beclabuvir in HCV‐infected non‐Japanese and Japanese subjects. World Conference of Pharmacometrics, Brisbane, Australia; 2016. [Google Scholar]
- 20. Hruska M, Wang X, Chan P, et al. Derivation of phase 3 dosing for peginterferon lambda‐1a in chronic hepatitis C, Part 2: exposure‐response analyses for efficacy and safety variables. J Clin Pharmacol. 2015;55(1):73–80. [DOI] [PubMed] [Google Scholar]
- 21. Lin CW, Menon R, Liu W, et al. Exposure‐safety response relationship for ombitasvir, paritaprevir/ritonavir, dasabuvir, and ribavirin in patients with chronic hepatitis C virus genotype 1 infection: analysis of data from five phase II and six phase III studies. Clin Drug Investig. 2017;37(7):647–657. [DOI] [PubMed] [Google Scholar]
- 22. Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction‐corrected visual predictive checks for diagnosing nonlinear mixed‐effects models. AAPS J. 2011;13(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Osawa M, Ueno T, Ishikawa H, Imai Y, Garimella T. Population pharmacokinetic analysis for daclatasvir and asunaprevir in Japanese subjects with chronic hepatitis C virus infection. J Clin Pharmacol. 2018;58(11):1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu L, Li H, Chan P, et al. Population pharmacokinetic analysis of asunaprevir in subjects with hepatitis C virus infection. Infect Dis Ther. 2018;7:261‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.


