Summary
Background
The importance of primary biliary cholangitis as an indication for liver transplantation has probably been influenced by the introduction of therapies, and changes in selection criteria and disease epidemiology.
Aims
To assess the time trends in liver transplantation for primary biliary cholangitis and to evaluate the characteristics of the patient population during the past three decades.
Methods
Patients undergoing liver transplantation from 1986 to 2015 in centres reporting to the European Liver Transplantation Registry were included. We excluded combined organ transplantations and patients <18 years. Trends were assessed using linear regression models.
Results
We included 112 874 patients, of whom 6029 (5.3%) had primary biliary cholangitis. After an initial increase in the first decade, the annual number of liver transplantation for primary biliary cholangitis remained stable at around 200. The proportion of liver transplantations for primary biliary cholangitis decreased from 20% in 1986 to 4% in 2015 (P < 0.001). Primary biliary cholangitis was the only indication showing a consistent proportional decrease throughout all decades. From the first to the third decade, the age at liver transplantation increased from 54 (IQR 47‐59) to 56 years (IQR 48‐62) and the proportion of males increased from 11% to 15% (both P < 0.001).
Conclusions
We have found a proportional decrease in primary biliary cholangitis as indication for liver transplantation. However, despite treatment with ursodeoxycholic acid and improved disease awareness, the absolute annual number of liver transplantations has stabilised.
1. INTRODUCTION
Primary biliary cholangitis (formerly called primary biliary cirrhosis, PBC) is a chronic cholestatic liver disease, characterised by progressive intrahepatic bile duct destruction which may eventually lead to fibrosis, cirrhosis, and death.1 In the 20 years following the first human liver transplantation in 1963, PBC was the leading indication for liver transplantation in Europe, accounting for 30%‐50% of all liver transplantations.2 However, despite increasing disease prevalence,3, 4 PBC is no longer a leading indication for liver transplantation. The recent change in nomenclature is figurative for the changed prognosis of patients over the past decades.5
Today, patients are usually diagnosed with PBC at an early stage, which allows for timely onset of therapy, often resulting in improvement of biochemical parameters and prognosis.6, 7, 8, 9, 10, 11, 12 However, patients who respond incompletely still have a significantly impaired prognosis compared to an age‐ and sex‐matched general population.13, 14 In case of liver failure, liver transplantation remains the only therapeutic option to prevent premature death. However, changes in selection criteria for liver transplantation and in the epidemiology, as well as the introduction of ursodeoxycholic acid (UDCA) as an effective treatment, may have impacted the relative importance of PBC as indication for transplantation. Previous European studies that have assessed trends in liver transplantation for PBC reported a decrease in transplantations. However, these studies were either single centre or single‐country studies, often covered relatively short study periods, or are now outdated.15, 16, 17 A long‐term European‐wide study is currently lacking, as is data on possible changes over time in the characteristics of the subgroup of patients with PBC who still require liver transplantation.
Thus, the primary aim of this study was to assess the time trends in the number of liver transplantations for PBC across Europe over the past three decades, both in absolute and proportional measures. Secondly, we aimed to evaluate the potential changes in characteristics of patients with PBC undergoing liver transplantation during this time period.
2. MATERIALS AND METHODS
2.1. Study design
Patient data were obtained through the European Liver Transplantation Registry (ELTR). ELTR data are available to all the members of the European Liver and Intestine Transplant Association (ELITA) for research purposes, once the study protocol is approved. All patients transplanted in ELTR‐associated centres from 1 January 1986 until 31 December 2015, were assessed. Patients who underwent combined organ transplantation and patients aged under 18 years at the time of liver transplantation were excluded. In order to more thoroughly assess patients’ characteristics, all patients with PBC who were listed for liver transplantation at any of the three liver transplantation centres in the Netherlands during the study period were included for in‐depth analyses.
This study was conducted in accordance with the principles of the Declaration of Helsinki. The protocol was approved by ELITA and the Dutch Organ Transplant Registry. The protocol was reviewed and approved by the institutional research board of the corresponding centre, and at each participating centre, in accordance with local regulations.
2.2. Data collection
Data collected for our primary analyses included date of birth, gender, date of listing for liver transplantation, date of liver transplantation, and the biochemical parameters used to calculate the model for end‐stage liver disease (MELD) score at the time of transplantation. 18 Indications for liver transplantation by primary and secondary aetiologies were classified using the ELTR aetiology codes. Patients with PBC were identified using these aetiology codes, including patients with PBC‐autoimmune hepatitis (PBC‐AIH) overlap syndrome (defined as interface hepatitis on liver histology combined with alanine aminotransferase (ALT) ≥5× upper limit of normal or IgG ≥2×)19 and patients with an additional diagnosis of hepatocellular carcinoma. For our in‐depth analyses, all patients with PBC listed for liver transplantation during the study period in the Netherlands were identified using the Dutch Organ Transplant Registry (NOTR) and the three local liver transplantation centre registrations. For these patients, the following additional data were extracted from medical records: date of PBC diagnosis, date of initiation of UDCA treatment when applicable, secondary indication for liver transplantation and biochemical parameters including total bilirubin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), albumin, creatinine, and INR at time of diagnosis, at time of initiation with UDCA treatment, at 1 year after treatment initiation, at time of listing for liver transplantation, and at time of liver transplantation. These measures were used to calculate the Barcelona, Paris I, and GLOBE response criteria for patients 1 year after the initial start of UDCA treatment.14, 20, 21 The secondary indication for liver transplantation was classified as either liver failure, an additional diagnosis of hepatocellular carcinoma, or quality of life (QOL) (defined as therapy‐refractory fatigue or pruritus after following the EASL guidelines for management of cholestatic liver diseases or a mean score of ≥4 in the fatigue or pruritus domain of the PBC‐40).22 The biochemical disease stage was determined based on serum albumin and bilirubin concentrations according to the Rotterdam criteria, classifying PBC into early (normal total bilirubin and normal albumin), moderately advanced (abnormal total bilirubin or abnormal albumin), or advanced disease (abnormal total bilirubin and abnormal albumin).10 UDCA treatment status was classified as “yes” when patients were treated with UDCA at time of listing, independent of treatment duration, dosage, and/or combination with other treatment.
2.3. Calculations
MELD scores provided in the registry data were used when available, also when this concerned patients with MELD exception points. If no MELD score was declared, we calculated it based on laboratory values using the following formula: 0.957 * Natural logarithm (ln) of (creatinine in mg/dL) + 0.378 * ln (bilirubin in mg/dL) + 1.120 * ln (INR) + 0.643.18 For our analyses, we preferred declared MELD scores over laboratory MELD scores when available, since laboratory MELD scores do not take into account possible MELD score exception points.
To exclude erroneous data, for all biochemical parameters included in the MELD score, clinically feasible minimum and maximum values were defined based on clinical expertise. Values exceeding these ranges were excluded from analyses and considered missing. The ranges were defined as follows: serum creatinine 0.01‐11.3 mg/dL (20‐1000 µmol/L), serum bilirubin 0.06‐58 mg/dL (1‐1000 µmol/L), INR 0.5‐10, and albumin 10‐60 g/L. The MELD score was considered to range from a minimum of 6 to a maximum of 40.23
2.4. Statistical analyses
Linear regression least‐square models were used to assess trends over time for our primary endpoints. For normally distributed continuous variables, the one‐way ANOVA was used for the comparison of more than two groups. In case of skewed distribution of continuous variables, the Mann‐Whitney U test was used for the comparison of two groups, and the Kruskal‐Wallis test for more than two groups. For categorical variables, differences between the three decades were compared using the chi‐squared test. For comparative purposes, the study period was divided into three groups according to the date of transplantation. Every group represents the time span of one decade (1 January 1986‐31 December 1995; 1 January 1996‐31 December 2005; 1 January 2006‐31 December 2015).
Statistical analysis was performed using SPSS version 21. A P‐value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Study population
Between 1 January 1986 and 31 December 2015, 128 802 patients underwent liver transplantation in 166 European Liver Transplantation Registry centres. We excluded 15 928 patients because of combined organ transplantation or age <18 years at time of liver transplantation. Thus, 112 874 patients were included in our study population. Of this total, 6029 (5.3%) were transplanted for PBC. In comparison, 26 861 (23.8%) were transplanted for viral hepatitis, 23 207 (20.6%) for alcoholic cirrhosis, 20 047 (17.8%) for cancers, and 9226 (8.2%) for autoimmune diseases of the liver other than PBC. Patient characteristics for those transplanted for PBC are presented in Table 1.
Table 1.
Characteristics of patients transplanted for PBC in ELTR centres from 1986 to 2016
| Decade of liver transplantation | P | |||
|---|---|---|---|---|
| 1986‐1996 | 1996‐2006 | 2006‐2016 | ||
| N = 1869 | N = 2157 | N = 2003 | ||
| Age | ||||
| Overall | 53.9 (47.3‐59.4) | 55.7 (49.0‐61.4) | 56.1 (48.4‐62.4) | <0.001 |
| Female | 53.6 (47.1‐59.1) | 55.6 (49.0‐61.4) | 55.9 (48.6‐62.3) | <0.001 |
| Male | 55.6 (47.3‐59.4) | 57.0 (48.1‐62.1) | 56.9 (48.4‐62.4) | 0.616 |
| Gender (%) | ||||
| Female | 1664 (89.0) | 1904 (88.3) | 1699 (84.8) | 0.532 |
| Male | 205 (11.0) | 253 (11.7) | 304 (15.2) | |
| PBC (%) | ||||
| PBC | 1866 (99.8) | 2123 (98.4) | 1918 (95.8) | <0.001 |
| PBC — AIH | 0 (0.0) | 10 (0.5) | 28 (1.4) | |
| PBC — HCC | 3 (0.2) | 24 (1.1) | 57 (2.8) | |
| MELD score | 17.0 (13.8‐20.5) | 15.3 (12.3‐19.1) | 16.8 (12.8‐21.7) | <0.001 |
| Creatinine (mg/dL) | 1.0 (0.81‐1.26) | 0.9 (0.8‐1.1) | 0.80 (0.6‐1.1) | <0.001 |
| Bilirubin (mg/dL) | 7.2 (3.5‐13.2) | 5.4 (2.7‐10.3) | 6.0 (2.6‐12.9) | 0.001 |
| INR | 1.2 (1.1‐1.5) | 1.2 (1.1‐1.4) | 1.3 (1.1‐1.6) | <0.001 |
PBC, primary biliary cholangitis; AIH, autoimmune hepatitis; HCC, hepatocellular carcinoma; MELD, model for end‐stage liver disease; INR, international normalised ratio.
Data are shown as median (IQR). Missing values: Age: 13 (0.2%), gender: 1 (0.02%), transplantation indication: 0 (0%), MELD score: 3280 (54.4%), creatinine: 2957 (49.0%), bilirubin: 3199 (53.0%), INR: 3534 (58.6%).
3.2. Primary Indications for liver transplantation—changes in absolute and proportional numbers
In 1986, 283 liver transplantations were performed, as compared to 5646 in 2015. The number of transplantations increased by 184 per year (95% CI 183‐184, P < 0.001) (Figure 1A). The absolute annual number of transplantations for PBC peaked to 279 in 1994. Thereafter, the annual transplant rate for PBC decreased to an average of 200 in the last decade (Figure 1B). These changes correspond with an average annual increase of 21.5 (95% CI 21.3‐21.7; P < 0.001) transplantations in the first decade, followed by a decrease of −1.9 (95% CI −2.1 to −1.7; P < 0.001) in the second decade, and a marginal annual decrease of −0.3 (95% CI −0.5 to −0.1; P = 0.002) in the last decade.
Figure 1.
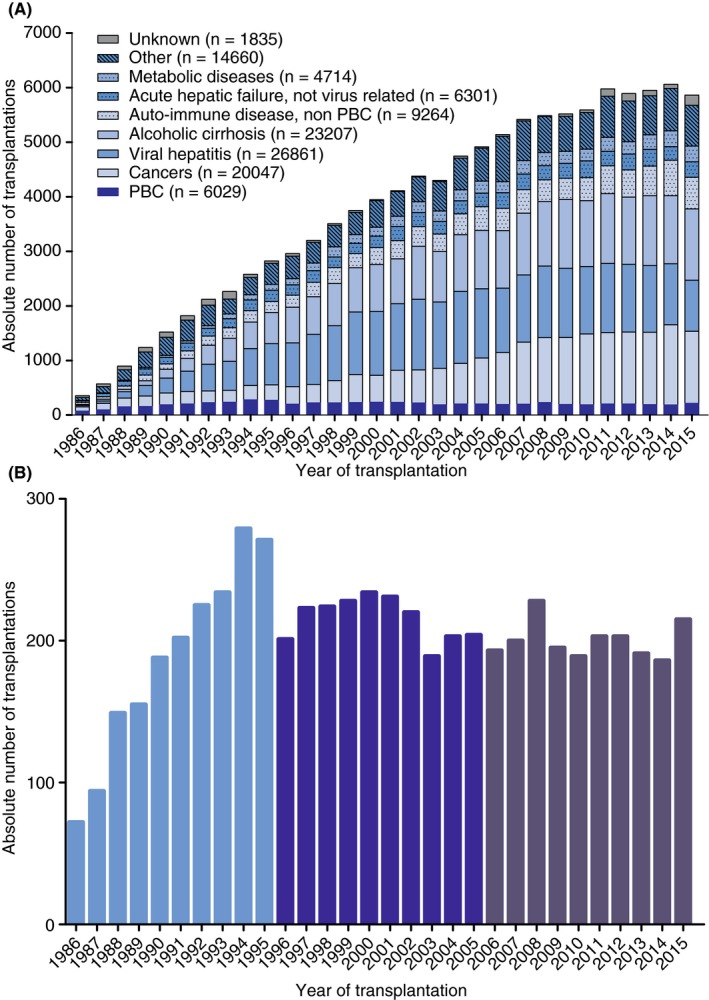
Annual absolute number of liver transplantations in ELTR centres from 1986‐2016 for (A) all primary disease aetiologies, and (B) PBC. ELTR, European Liver Transplant Registry; PBC, primary biliary cholangitis
The proportion of patients undergoing liver transplantation for PBC as compared to other indications decreased from 20.3% in 1986% to 3.7% in 2015 (Figure 2A). The greatest decrease was in the first decade, with an annual proportional decrease of 0.9% (95% CI −0.92 to −0.88; P < 0.001). Thereafter, the proportion of transplantations for PBC annually decreased with 0.3% (95% CI −0.343 to −0.337; P < 0.001) in the second decade and 0.1% (95% CI −0.064 to −0.057; P < 0.001) in the third decade. No other leading disease aetiology showed a persistent significant proportional decrease over all three decades (Figure 2B).
Figure 2.
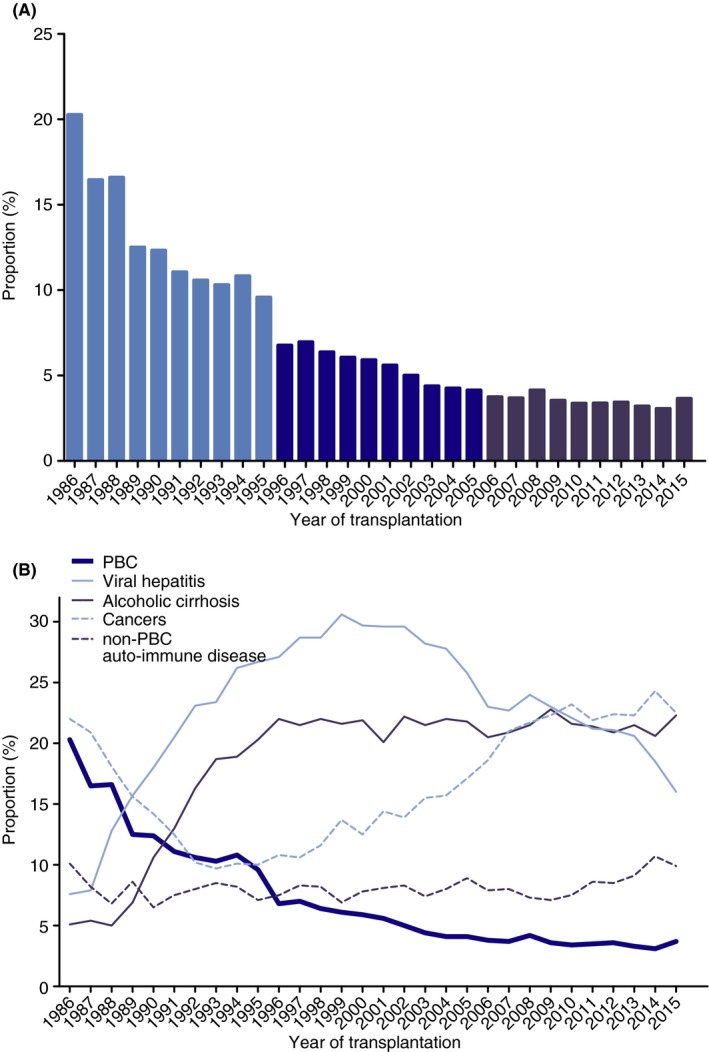
Proportion of liver transplantations in ELTR centres from 1986 to 2016 for (A) PBC and (B) PBC and other leading indications. ELTR, European Liver Transplant Registry; PBC, primary biliary cholangitis
3.3. Age
The overall median age at time of transplantation was 55 years (interquartile range [IQR] 48‐61) for patients with PBC, and 53 years (IQR 44‐59) for non‐PBC patients (P<.001). For PBC patients, the median age at time of transplantation increased significantly from 54 (IQR 47‐59) in the first decade to 56 (IQR 49‐61) in the second decade (P < 0.001), while no significant age difference was found thereafter (P = 0.255) (Figure 3). Furthermore, a change in distribution of patients’ age at time of transplantation was found (Figure 4). The proportion of patients aged >60 increased from 23% in the first decade to 35% in the last decade (P > 0.001), whereas the proportion of patients between 40‐49 and 50‐59 years decreased from 27% and 42% in the first decade, to 21% and 36% respectively in the last decade (both P < 0.001).
Figure 3.
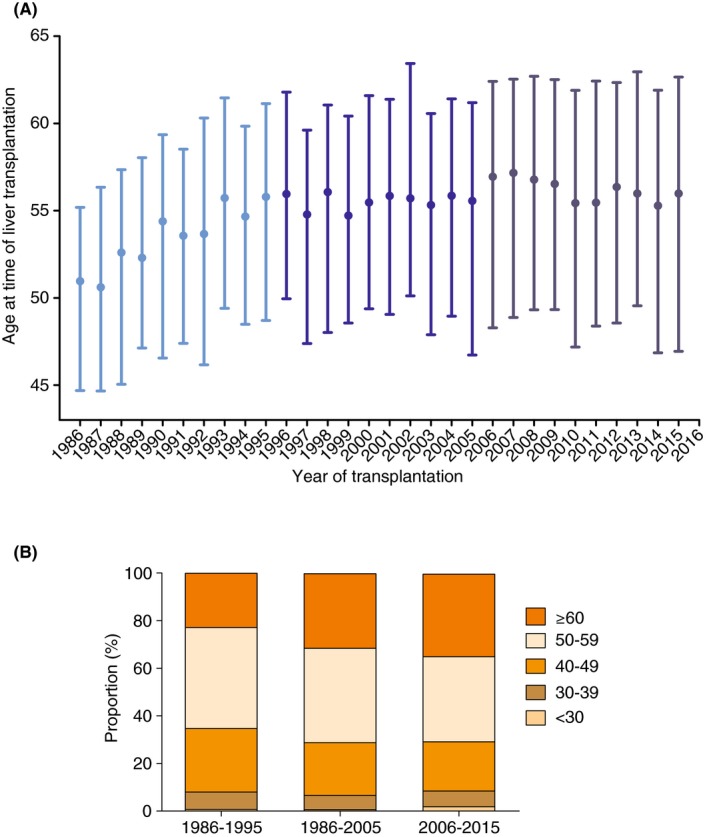
A, Age (median, interquartile range) at time of liver transplantation for PBC in Europe from 1986 to 2016, B, Proportional age distribution for transplanted PBC patients in Europe from 1986 to 2016. PBC, primary biliary cholangitis
Figure 4.
Percentage of male vs female patients transplanted for PBC in Europe from 1986 to 2016. PBC, primary biliary cholangitis
3.4. Gender
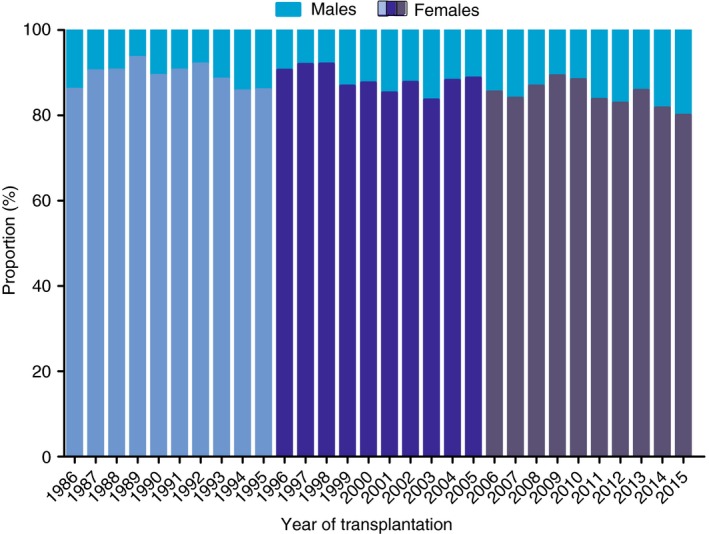
A total of 761 (12.6%) male and 5267 (87.4%) female patients with PBC were transplanted, corresponding to a male to female ratio of 1:6.9. For non‐PBC aetiologies, 74 484 (69.7%) males and 32 348 (30.3%) females underwent transplantation, corresponding to a female to male ratio of 1:0.43. In PBC patients, the proportion of transplantations for males increased significantly from 11.0% in the first decade to 15.1% in the third decade (P < 0.001) (Figure 5). In the first decade, males were significantly older than females with a median age of 56 years (IQR 49‐61) and 55 years (IQR 48‐60) respectively (P < 0.05), but no differences were found in the second (P = 0.755) and third decades (P = 0.695) (Figure S1A).
Figure 5.
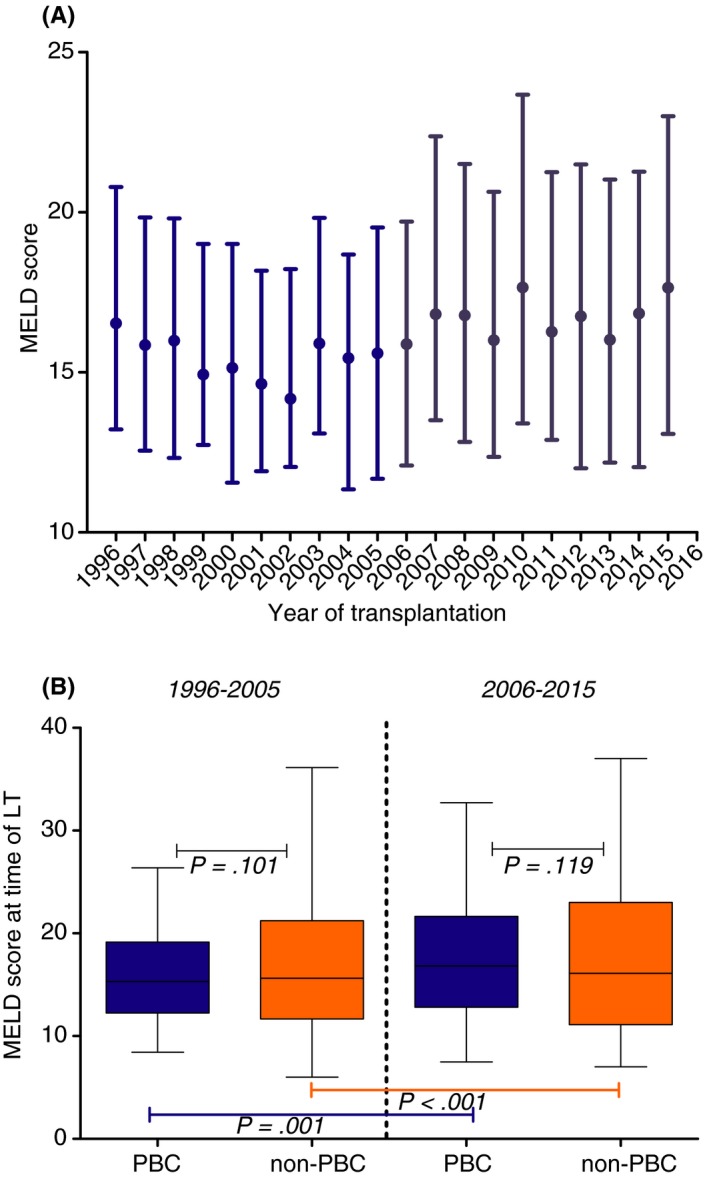
A, MELD scores at time of transplantation for patients with PBC in Europe from 1996 to 2015; B, MELD scores of patients transplanted for PBC vs non‐PBC aetiologies in 1996‐2005 vs 2006‐2015. MELD scores are reported as median with their interquartile ranges. As a result of a lack of biochemical data from the first decade of the study period, data are shown for 1996‐2015. In this period, MELD scores were available for 2749 (46%) of patients with PBC and 51 410 (48%) of non‐PBC patients
3.5. MELD scores
In patients with PBC, the median MELD score at time of transplantation increased from 15.3 (IQR 12.2‐19.2) in the second decade to 16.8 (IQR 12.8‐21.6) in the last decade (P < 0.001). An increase in MELD score between these decades was also found in non‐PBC liver transplantations, in which the median MELD score increased from 15.6 (IQR 11.7‐21.2) to 16.1 (IQR 11.1‐23.0) (P < 0.001) (Figure 5). There were no significant differences in MELD scores between PBC and non‐PBC patients in either the second or the third decade (P = 0.101 and P = 0.119 respectively). In PBC patients, no significant difference in MELD score was found between females (16.1, IQR 12.5‐20.8) and males (16.9, IQR 13.0‐21.0) (P = 0.160).
3.6. Characterisation of Nationwide Cohort
3.6.1. Mortality on the waiting list
From 1 January 1986 to 31 December 2015, 184 patients with PBC were placed on the waiting list for transplantation in the Netherlands. Of this total, one patient (0.5%) was alive without transplantation at the end of follow up. Twenty‐nine (15.8%) patients died on the waiting list or were removed from the waiting list due to clinical deterioration. Eight of these events occurred between 1986 and 1995, eight between 1996 and 2005, and 13 between 2006 and 2015, corresponding with a waiting list mortality of 12%, 16%, and 33% in the consecutive decades. In addition, three (1.6%) patients were removed at personal request or due to improved condition. The remaining 151 (82.1%) were transplanted.
3.6.2. Study population
Characteristics and biochemistry of the 151 patients who were transplanted for PBC in the Netherlands are presented in Table S1 . The median age at the time of transplantation was 54 years (IQR 48‐59). Transplanted patients were diagnosed with PBC at a median age of 46 (IQR 40‐51), and listed at a median age of 53 (IQR 47‐59). Males were significantly older than females at the time of transplantation, with a median age of 58 (IQR 53‐64) as compared to 53 (IQR 49‐58) (P = 0.006), while the MELD score at the time of liver transplantation did not differ between the sexes (P = 0.838).
3.6.3. Primary indications for liver transplantation: Absolute changes over time
The absolute number of transplantations for PBC nearly halved over time, from 65 in the first decade to 36 in the third decade (P < 0.001) (Figure S2). Of the 151 patients transplanted for PBC, 129 (85.4%) were listed for the primary indication PBC alone, 16 (10.6%) patients had a PBC‐AIH overlap syndrome, and six (4.0%) patients had an additional diagnosis of hepatocellular carcinoma. The proportion of patients with PBC transplanted due to poor quality of life significantly increased from 2% to 12% to 17% in the three consecutive decades (P = 0.004).
3.6.4. UDCA treatment and response for all patients with PBC
A total of 102 (67.5%) transplanted patients were treated with UDCA at the time of listing. The proportion of patients who were treated with UDCA increased significantly over time, from 37% in the first, to 92% in the last decade (P < 0.001). Table 2 shows the biochemical response for these UDCA‐treated patients according to the Barcelona, Paris I, and GLOBE response criteria.14, 20, 24, 25 In this cohort of transplanted patients with PBC, 6‐47% of patients showed a biochemical response to treatment, depending on the response criteria. The Paris I criteria identified the lowest percentage of complete biochemical responders after 12 months of UDCA treatment (6%). When classification by different response criteria was compared, this proportion was significantly lower than the percentage of complete responders as classified by the Barcelona criteria (30%) (P = 0.008) and by the GLOBE criteria (28%) (P = 0.004).
Table 2.
Biochemical response to 12 months of UDCA therapy in patients who underwent liver transplantation in the Netherlands between 1986 and 2016
| Decade of liver transplantation | |||
|---|---|---|---|
| 1986‐1996 | 1996‐2006 | 2006‐2016 | |
| N = 28 | N = 41 | N = 33 | |
| Barcelona criteria | |||
| Responder | 3 (20) | 10 (40) | 3 (21) |
| Incomplete responder | 12 (8) | 15 (60) | 11 (79) |
| Paris I criteria | |||
| Responder | 1 (5) | 1 (4) | 2 (10) |
| Incomplete responder | 19 (95) | 26 (96) | 18 (90) |
| GLOBE criteria | |||
| Responder | 0 (0) | 9 (39) | 7 (39) |
| Incomplete responder | 16 (100) | 14 (61) | 11 (61) |
Data are shown as n (%). Missing values: Barcelona criteria: 48 (47%), Paris I criteria: 37 (36%), GLOBE criteria: 45 (44%).
4. DISCUSSION
This study represents the largest European‐wide study on time trends in liver transplantations for PBC to date, covering the past 30 years. The percentage of transplantations for PBC as compared to other aetiologies decreased to less than one‐fifth of its original proportion of 20%. In contrast, the absolute number of transplantations for PBC has remained virtually stable over the last 10 years. Characteristics of patients undergoing transplantation for PBC have changed over time, whereby they are now older, have higher MELD scores, and are more likely to be male than 30 years ago. However, differences over time were quantitatively small.
The current study is the first to demonstrate a stable annual absolute number of transplantations for PBC over the past 10 years in Europe. This result confirms that today, in a minority of patients, we are still unable to prevent liver failure which underlines the necessity of additional therapeutic options for this group. A study by Lee et al that assessed transplantations trends for PBC in the United States showed an absolute annual decrease without reaching a steady state, but their study period only covered 11 years and ended in 2006.13 Our finding seems in contrast to the recent study by Webb et al reporting a decrease in the United Kingdom and the United States up until 2014. However, a true comparison is difficult, since the latter study only reported listings for transplantation and not actual liver transplantation, and measured numbers as a ratio against the total (increasing) general population. However, the steady absolute number of transplantations for PBC is also discordant with an overall increase in overall numbers of annual transplantations and with the increasing prevalence of PBC over the past decades.3, 4 Therefore, the stable absolute number of transplantations for PBC that we report could possibly be interpreted as a relative decrease.
Although we found an annual number of approximately 200 transplantations for PBC over the past 10 years the true number of PBC patients in need of liver transplantation may be higher. First, 16% of all listed patients with PBC in our secondary analysis in the Dutch cohort died while on the waiting list for transplantation or were removed due to deteriorating clinical condition. This result is in line with a recent study showing a waiting list mortality of 12% for PBC patients, which was higher than for most other aetiologies.26 Second, as compared to the number of Dutch patients who underwent transplantation for PBC reported in the ELTR database, we identified an additional 10% of patients after extensive review of medical records of all listed patients in the entire Dutch cohort. Evidently, not all transplanted patients with PBC had been reported as such, and were possibly labelled with more general aetiologies lacking further specification, such as cirrhosis or autoimmune disorders. Even more so, a minority of patients might have been incorrectly diagnosed when PBC was not recognised by treating physicians as the underlying cause of cirrhosis. Furthermore, within the top five primary indications for transplantation, PBC was the only one that showed a constant proportional decrease over three decades. We speculate that several factors may have contributed to this decrease. First is the possible influence of UDCA treatment. Recently, the Global PBC Study Group reported a strong association between UDCA treatment and improved transplant‐free survival.11 The number of transplantations for PBC peaked several years after UDCA was introduced in the early nineties, with a subsequent much more stable number of transplantations. Second, the introduction of urgency‐based allocation may have influenced the proportional decrease in transplantations for PBC. Since MELD‐based allocation in 2006, patients’ time on the waitlist is no longer a factor in allocation, possibly impeding the chance of receiving a transplantation for patients with a relatively slowly progressing disease such as PBC.1
We also identified several changes in the characteristics of patients undergoing transplantation for PBC over the past three decades. We found an increase in age at time of transplantation. This was in line with the results from a long‐term study from Birmingham and a recent larger study covering both the UK and the USA.16, 27 Most noticeable, however, was our finding of an increasing proportion of patients with PBC transplanted at age >60 years. This may be related to an overall improved medical care and physical condition, permitting transplantation at higher ages. The influence of possible changes in environmental triggers involved in the development of PBC should also be considered, especially since a recent large study showed that the age at PBC diagnosis has markedly increased over the years.28 However, with a median age at diagnosis of 46 years in patients with PBC listed for transplantation within our in‐depth population, this population undergoing liver transplantation for PBC was much younger at diagnosis than the large overall cohorts reported by the Global PBC Study Group (55 years) and the UK‐PBC Study Group (55 years).21, 29 This suggests that young patients may be more likely to develop end‐stage disease requiring liver transplantation, as has previously been reported.30 Furthermore, we found that an increasing number of males were transplanted for PBC over time, which was also reported in the United States.27 The increasing male to female ratio reported in epidemiological studies could contribute to these findings, as well as data that suggest males are less likely to respond well to treatment with UDCA.30, 31 The MELD score at time of transplantation was slightly but significantly higher after 2006, as compared to the period between 1996 and 2005. This might be explained by the introduction of MELD‐based allocation in 2006. This increase was found for both PBC and non‐PBC patients, although more pronounced in patients with PBC. We identified no differences in MELD scores between PBC and non‐PBC patients.
In our in‐depth analysis of the Dutch cohort, the patients' characteristics were comparable to the European‐wide cohort. Notably, we found that the proportion of patients with PBC who underwent transplantation for poor quality of life increased from 2% before 1995 to 17% in the last decade. Furthermore, we found that despite international guidelines recommending that all patients with PBC be treated with UDCA,32, 33 8% were not treated with UDCA during the last decade. Although we were unable to validate these findings in the European data, these factors might also have impacted the remaining number of liver transplantations for PBC over the last decade.
To the best of our knowledge, our study was the first to assess time trends in liver transplantation for PBC throughout Europe over a sizeable period of 30 years, during which UDCA was introduced as the standard of care. We were able to include all liver transplantations reported to the ELTR, resulting in a large population of 6029 transplanted PBC patients. The additional nationwide in‐depth analysis over the same study period enabled us to characterise the population in need for liver transplantation more extensively over time. However, some limitations should be taken into account. Biochemical data were incomplete in both the ELTR data and the Dutch in‐depth analysis. However, due to the large population and the similar patterns found in both analyses, we believe this has not introduced an important bias. Second, our in‐depth analyses are based on data of Dutch patients only and the extent to which findings can be generalised is uncertain. Nevertheless, since patient characteristics and MELD scores at transplantation were comparable to the PBC population in the ELTR database and similar transplantation patterns over time were observed, the results of our nationwide in‐depth analyses may well be representative of the European PBC population in need of liver transplantation.
In conclusion, we found that, despite a relative decrease, the absolute number of transplantations for PBC has reached a steady state. Still, over 200 European patients with PBC undergo liver transplantation annually. These patients are slightly older, have higher MELD scores, and are more likely to be male than 30 years ago. Effective second‐line therapies may further reduce the need for liver transplantation in patients with PBC in the future.
Supporting information
ACKNOWLEDGEMENTS
Declaration of personal interests: The following authors declared that they have no conflicts of interest: Q.P. Janssen, R. Adam, D. Mirza, E. Hidalgo, S.J. Wigmore, J. Klempnauer, J. Pratschke, W. Bennet, V. Karam. M.H. Harms reports a speaker's fee from Zambon Nederland B.V. C. Duvoux reports advisory arrangements for Astellas, Novartis and Sandoz, research grants for Astellas and Novartis, and travel grants of Astellas and Novartis. C. Watson reports being an Advisory board member for Glaxo Smith Kline 2017. M. Pinzani reports speaker´s fees from Echosens Paris, Abbvie, NeuroVive, and ChemoMab. Dr. Isoniemi reports a speaker´s fee Astellas, travel grants from Astellas and Novartis, and personal fees from Advisory board Sandoz. K. Zieniewicz reports a travel grant from Astellas. H.R. van Buuren is a consultant for Intercept Pharma Benelux and received an unrestricted research grant from Intercept Pharmaceuticals. B.E. Hansen reports grants from Intercept Pharmaceuticals and Zambon Nederland B.V. and consulting work for Intercept Pharmaceuticals and Novartis. H.J. Metselaar reports personnel fees from Chiesie and Astellas.
AUTHORSHIP
Guarantor of the article: MH Harms.
Author contributions: Study concept and design: Maren H. Harms, Quisette P. Janssen, Rene Adam, Christophe Duvoux, Darius Mirza4, Ernest Hidalgo, Christopher Watson, Stephen J. Wigmore, Massimo Pinzani, Helena Isoniemi, Johann Pratschke, Krzysztof Zieniewicz, Jurgen L. Klempnauer, William Bennet, Vincent Karam, Henk R. van Buuren, Bettina E. Hansen, Herold J. Metselaar. Acquisition of data: Maren H. Harms, Quisette P. Janssen, Rene Adam, Christophe Duvoux, Darius Mirza4, Ernest Hidalgo, Christopher Watson, Stephen J. Wigmore, Massimo Pinzani, Helena Isoniemi, Johann Pratschke, Krzysztof Zieniewicz, Jurgen L. Klempnauer, William Bennet, Vincent Karam, Henk R. van Buuren, Bettina E. Hansen, Herold J. Metselaar. Analysis and interpretation of data: Maren H. Harms, Quisette P. Janssen, Henk R. van Buuren, Bettina E. Hansen, Herold J. Metselaar. Drafting of the manuscript: Maren H. Harms, Quisette P. Janssen, Rene Adam, Christophe Duvoux, Henk R. van Buuren, Bettina E. Hansen, Herold J. Metselaar. Critical revision of the manuscript for important intellectual content: Maren H. Harms, Quisette P. Janssen, Rene Adam, Christophe Duvoux, Darius Mirza4, Ernest Hidalgo, Christopher Watson, Stephen J. Wigmore, Massimo Pinzani, Helena Isoniemi, Johann Pratschke, Krzysztof Zieniewicz, Jurgen L. Klempnauer, William Bennet, Vincent Karam, Henk R. van Buuren, Bettina E. Hansen, Herold J. Metselaar. Study supervision: Maren H. Harms, Quisette P. Janssen, Rene Adam, Christophe Duvoux, Darius Mirza4, Ernest Hidalgo, Christopher Watson, Stephen J. Wigmore, Massimo Pinzani, Helena Isoniemi, Johann Pratschke, Krzysztof Zieniewicz, Jurgen L. Klempnauer, William Bennet, Vincent Karam, Henk R. van Buuren, Bettina E. Hansen, Herold J. Metselaar.
All authors approved the final version of the manuscript.
APPENDIX A.
AUTHORS’ COMPLETE AFFILIATIONS
Maren H. Harms, Quisette P. Janssen, Henk R. van Buuren, Bettina E. Hansen and Herold J. Metselaar: Department of Gastroenterology and Hepatology, Erasmus University Medical Center, Rotterdam, the Netherlands. Rene Adam and Vincent Karam: Hepato‐Biliary Center, AP‐HP Paul‐Brousse Hospital, Paris Sud University, Villejuif, France. Christophe Duvoux: Department of Gastroenterology and Hepatology, Marne‐la‐Vallée University Hospital of Paris East, Paris, France. Darius Mirza: Queen Elisabeth Medical Center, The Queen Elizabeth Hospital, Birmingham, United Kingdom. Ernest Hidalgo: St. James & Seacroft University Hospital, Leeds, United Kingdom. Christopher Watson: Department of Surgery, Addenbrooke's Hospital, Cambridge, United Kingdom. Stephen J. Wigmore: Liver Transplantation Unit, University of Edinburgh Royal Infirmary, Edinburgh, United Kingdom. Massimo Pinzani: UCL Institute for Liver and Digestive Health –Royal Free Sheila Sherlock Liver Centre, London, UK. Helena Isoniemi: Transplantation and Liver Surgery Clinic, UCJ Helsingfors, Helsinki, Finland. Johann Pratschke: Klinik fur Allgemein Viszeral und Transplantationschirurgie, Charite Capmus Virchow Klinikum, Berlin, Germany. Krzysztof Zieniewicz: Department of General, Transplant & Liver Surgery, Medical University of Warsaw, Poland. Jurgen L. Klempnauer: Klinik fur Viszeral und Transplantationschirurgie, Medizinische Hochschule Hannover, Hannover, Germany. William Bennet: Transplantation and Liver Surgery Unit, Sahlgrenska University Hospital, Goteborg, Sweden. Bettina E. Hansen: Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada and Toronto Centre for Liver Disease, Toronto General Hospital, Toronto, Canada; for the European Liver and Intestine Transplant Association (ELITA).
Harms MH, Janssen QP, Adam R, et al. Trends in liver transplantation for primary biliary cholangitis in Europe over the past three decades. Aliment Pharmacol Ther. 2019;49:285–295. 10.1111/apt.15060
The Handling Editor for this article was Professor Grace Wong, and it was accepted for publication after full peer‐review.
Authors’ complete affiliations are listed in Appendix section.
Funding information
This study is endorsed by the European Liver and Intestine Transplant Association (ELITA). The authors acknowledge: the list of the 174 centers contributing to ELTR is available in the ELTR website www.eltr.org. The ELTR is supported by a grant from Astellas, Novartis, Institut Georges Lopez, Bridge to Life and logistic support from the Paul Brousse Hospital (Assistance Publique – Hôpitaux de Paris). The Organ Sharing Organizations: the French ABM (Sami Djabbour and Alain Jolly), the Dutch NTS (Cynthia Konijn), the Eurotransplant Foundation (Marieke Van Meel and Erwin de Vries), the Spanish ONT (Gloria de la Rosa), the UK‐Ireland NHSBT (Mike Chilton and Julia Micciche) are acknowledged for the data cross‐check and sharing with the ELTR.
REFERENCES
- 1. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565‐1575. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver . Electronic address eee. Results: Overall indications and results; 2017.
- 3. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181‐1188. [DOI] [PubMed] [Google Scholar]
- 4. Griffiths L, Dyson JK, Jones DE. The new epidemiology of primary biliary cirrhosis. Semin Liver Dis. 2014;34:318‐328. [DOI] [PubMed] [Google Scholar]
- 5. Beuers U, Gershwin ME, Gish RG, et al. Changing nomenclature for PBC: From 'cirrhosis' to 'cholangitis'. Clin Res Hepatol Gastroenterol. 2015;39:e57–e59. [DOI] [PubMed] [Google Scholar]
- 6. Angulo P, Batts KP, Therneau TM, Jorgensen RA, Dickson ER, Lindor KD. Long‐term ursodeoxycholic acid delays histological progression in primary biliary cirrhosis. Hepatology. 1999;29:644‐647. [DOI] [PubMed] [Google Scholar]
- 7. Corpechot C, Carrat F, Bahr A, Chretien Y, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297‐303. [DOI] [PubMed] [Google Scholar]
- 8. Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology. 2000;32:1196‐1199. [DOI] [PubMed] [Google Scholar]
- 9. Poupon RE, Lindor KD, Cauch‐Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884‐890. [DOI] [PubMed] [Google Scholar]
- 10. Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow‐up study. Gastroenterology. 2014;147:1338‐1349. [DOI] [PubMed] [Google Scholar]
- 11. Van derMeer AJ, Harms MH, Corpechot C, Thorburn D, Lammers WJ, Invernizzi P. Ursodeoxycholic Acid is Associated with a Prolonged Transplant‐free Survival in All Patients with Primary Biliary Cholangitis – There is no such thing as Non‐response. Poster presented at the 68th American Association for the Study of Liver Disease Liver Meeting, 2017.
- 12. Larghi A, Crosignani A, Battezzati PM, et al. Ursodeoxycholic and tauro‐ursodeoxycholic acids for the treatment of primary biliary cirrhosis: a pilot crossover study. Aliment Pharmacol Ther. 1997;11:409‐414. [DOI] [PubMed] [Google Scholar]
- 13. ter Borg PC, Schalm SW, et al. Prognosis of ursodeoxycholic Acid‐treated patients with primary biliary cirrhosis. Results of a 10‐yr cohort study involving 297 patients. Am J Gastroenterol. 2006;101:2044‐2050. [DOI] [PubMed] [Google Scholar]
- 14. Pares A, Caballeria L, Rodes J. Excellent long‐term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715‐720. [DOI] [PubMed] [Google Scholar]
- 15. Lee J, Belanger A, Doucette JT, Stanca C, Friedman S, Bach N. Transplantation trends in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:1313‐1315. [DOI] [PubMed] [Google Scholar]
- 16. Liermann Garcia RF, Evangelista Garcia C, McMaster P, Neuberger J. Transplantation for primary biliary cirrhosis: retrospective analysis of 400 patients in a single center. Hepatology. 2001;33:22‐27. [DOI] [PubMed] [Google Scholar]
- 17. Kuiper EM, Hansen BE, Metselaar HJ, et al. Trends in liver transplantation for primary biliary cirrhosis in the Netherlands 1988–2008. BMC Gastroenterol. 2010;10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864‐871. [DOI] [PubMed] [Google Scholar]
- 19. Chazouilleres O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis‐autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296‐301. [DOI] [PubMed] [Google Scholar]
- 20. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long‐term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871‐877. [DOI] [PubMed] [Google Scholar]
- 21. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804‐1812. [DOI] [PubMed] [Google Scholar]
- 22. Jacoby A, Rannard A, Buck D, et al. Development, validation, and evaluation of the PBC‐40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut. 2005;54:1622‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. European Association for the Study of the Liver . Electronic address eee. Eurotransplant Manual. Chapter 5: ET Liver Allocation System (ELAS); 2014.
- 24. Corpechot C, Chazouilleres O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long‐term outcome. J Hepatol. 2011;55:1361‐1367. [DOI] [PubMed] [Google Scholar]
- 25. Kuiper EM, Hansen BE, de Vries RA, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281‐1287. [DOI] [PubMed] [Google Scholar]
- 26. Singal AK, Fang X, Kaif M, et al. Primary biliary cirrhosis has high wait‐list mortality among patients listed for liver transplantation. Transpl Int. 2017;30:454‐462. [DOI] [PubMed] [Google Scholar]
- 27. Webb GJ, Rana A, Hodson J, et al. Twenty‐year comparative analysis of patients with autoimmune liver diseases on transplant waitlists. Clin Gastroenterol Hepatol. 2018;16:278. [DOI] [PubMed] [Google Scholar]
- 28. Murillo Perez F, Goet JC, Lammers WJ, et al. Milder disease stage in patients with primary biliary cholangitis over a 44‐year period: a changing natural history. Hepatology. 2017. [DOI] [PubMed] [Google Scholar]
- 29. Carbone M, Sharp SJ, Flack S, et al. The UK‐PBC risk scores: Derivation and validation of a scoring system for long‐term prediction of end‐stage liver disease in primary biliary cholangitis. Hepatology. 2016;63:930‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carbone M, Mells GF, Pells G, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144:560–569 e7; quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 31. Lleo A, Jepsen P, Morenghi E, et al. Evolving trends in female to male incidence and male mortality of primary biliary cholangitis. Sci Rep. 2016;6:25906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. [DOI] [PubMed] [Google Scholar]
- 33. European Association for the Study of the Liver . Electronic address eee, Hirschfield GM, Beuers U, et al. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


