Abstract
Aims
To identify factors associated with achievement of glycated haemoglobin A1c (HbA1c) target at 24 weeks after commencing basal insulin therapy in individuals with type 2 diabetes mellitus (T2DM).
Materials and methods
Post‐hoc pooled analysis of 16 randomized, treat‐to‐target trials involving individuals with T2DM inadequately controlled with oral anti‐hyperglycaemic drugs (n = 3415) initiated on once‐daily insulin glargine 100 U/mL (Gla‐100). Clinical outcomes were assessed by HbA1c response at 24 weeks and individuals were classified as “good responders” with HbA1c <7.0% (<53 mmol/mol) or as “poor responders” with HbA1c ≥7.0% (≥53 mmol/mol). Univariable and multivariable stepwise logistic regression analyses were performed to identify predictive factors for attaining HbA1c <7.0%.
Results
Lower levels of baseline HbA1c, fasting plasma glucose (FPG) and post‐prandial plasma glucose (PPG), higher body mass index (BMI), shorter diabetes duration and male sex were associated with a good glycaemic response, but not age or baseline C‐peptide levels. Gla‐100 dose (U/kg) was highest in the poor‐responder group, which had the fewest hypoglycaemia episodes. Univariable analysis for achievement of HbA1c <7.0% confirmed these observations. Multivariable analysis retained baseline HbA1c, body weight, BMI, sex, 2‐hours PPG and diabetes duration as predictors of a good response. Continued use of sulfonylureas, hypoglycaemia and change in body weight were indicative of poor response.
Conclusions
Baseline HbA1c was the strongest determinant for achieving target HbA1c <7.0% by supplementary Gla‐100 therapy, while sex and BMI were also useful indicators. However, age and C‐peptide levels at baseline did not predict glycaemic response to the introduction of basal insulin.
Keywords: basal insulin, glycaemic control, hypoglycaemia, meta‐analysis, type 2 diabetes
1. INTRODUCTION
Current treatment guidelines recommend a glycated haemoglobin A1c (HbA1c) level of less than 7.0% (<53 mmol/mol) as the goal for most individuals with type 2 diabetes mellitus (T2DM).1, 2 The American Diabetes Association (ADA)2 guidelines recommend a stepwise approach to therapies if glycaemic targets are not achieved, with basal insulin considered as second‐line or later therapy, with the exception of very poor glycaemic control (HbA1c >9.0%; >75 mmol/mol). Similarly, the recently updated ADA/European Association for the Study of Diabetes (EASD) consensus report recommends a stepwise approach to therapies in individuals with T2DM who are not at glycaemic target. However, with the exception of patients with HbA1c >11.0% (97 mmol/mol), addition of a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) as first injectable is recommended, with addition of basal insulin as second injectable.3
Currently, 24%‐54% of individuals with T2DM worldwide prescribed supplementary basal insulin do not achieve their therapeutic targets.4 Clinical inertia, defined as failure to adequately intensify treatment regimens sufficiently early, is an important contributing factor.5 Identifying biomedical predictors of treatment success may assist clinicians in the selection of therapeutic interventions. Therefore, a better understanding of the relationship between baseline characteristics and glycaemic outcomes may help to prevent unnecessary delay in optimizing the use of basal insulin therapy. Previous studies have investigated the relevance of baseline characteristics to determine effective outcomes in individuals with T2DM who are initiating insulin therapy.4, 6, 7, 8, 9 Those characteristics that have been shown to be associated with an inadequate glycaemic response, that is, not achieving HbA1c less than 7.0% (<53 mmol/mol), include the number of oral anti‐hyperglycaemic drugs (OADs) prescribed at baseline, in addition to HbA1c, fasting plasma glucose (FPG) levels, body mass index (BMI), diabetes duration and sex.4, 6, 7, 8, 9
The goal of this pooled participant‐level analysis of 16 randomized controlled clinical trials (RCTs) was to further investigate, in a larger cohort exposed to the same basal insulin treatment, which baseline and post‐baseline characteristics better predict the glycaemic response and achievement of HbA1c less than 7.0% at 24 weeks with the introduction and titration of basal insulin glargine 100 units (U)/mL (Gla‐100), administered once daily at bedtime in combination with existing OADs.
2. MATERIALS AND METHODS
2.1. Study and participant selection
Participant‐level data were collected and pooled for analysis from RCTs of up to 24 weeks, conducted by Sanofi, the manufacturer of Gla‐100 (Lantus, Paris, France) between 2000 and 2015. To fulfil the inclusion criteria for this analysis, all trials were required to enrol insulin‐naïve individuals whose T2DM was inadequately controlled (HbA1c >7.0%; >53 mmol/mol) while using OADs, with at least one study arm commencing once‐daily Gla‐100, and no other basal insulin, and using a titration algorithm, predominantly a once‐weekly dose adjustment of 2‐8 U/d, based on the patient's self‐measured plasma glucose (SMPG) to achieve an FPG of ≤5.6 mmol/L (≤100 mg/dL). Sixteen studies that met the inclusion criteria were identified and have been described elsewhere.9 All study participants (N = 3415) for whom data were analysed were mainly receiving one (metformin or sulfonylurea) or two OADs (metformin with a sulfonylurea); a few participants received other OADs alone or in combination (eg, gliptin, thiazolidinedione or glinide) with Gla‐100 (Figure S1).
2.2. Outcomes
All clinical outcomes were assessed over the 24‐week study period according to response, with participants stratified by HbA1c level at 24 weeks into “good responders” reaching HbA1c less than 7.0% (<53 mmol/mol) and “poor responders” with HbA1c ≥7.0% (≥53 mmol/mol). Outcomes in the poor responder group were further divided into “suboptimal” (7.0%‐8.0%; 53‐64 mmol/mol) and “minimal” (>8.0%; >64 mmol/mol) responder subgroups. Hypoglycaemia was defined as a confirmed plasma glucose (PG) level of <3.9 mmol/L (<70 mg/dL) or as severe hypoglycaemia requiring third‐party assistance. Baseline characteristics and treatment‐emergent events within the two responder groups were analysed primarily to identify factors that predicted achievement of an HbA1c target of <7.0% (<53 mmol/mol) at 24 weeks. Glycaemic outcomes per responder group were also sub‐analysed according to the frequency of hypoglycaemia events (ie, 0, 1‐3, and ≥ 4 events) during the 24‐week study. Average 2‐hour post‐prandial SMPG (2‐h SMPG) was derived from the mean of the 2‐hour post‐breakfast, lunch and evening meal, both before baseline and at the Week 24 visit.
2.3. Statistical analysis
Baseline parameters and clinical outcomes were presented descriptively up to 24 weeks. Univariable and multivariable analyses included all participants with a baseline HbA1c of ≥7.0% (≥53 mmol/mol) who received Gla‐100 and had no missing values for all covariates included in the analysis (n = 2626; 76.9%). Initially, univariable regression analysis was performed to measure associations between attaining HbA1c less than 7.0% (<53 mmol/mol) and baseline variables, including age, sex, diabetes duration, body weight, BMI, fasting C‐peptide, HbA1c, FPG and 2‐h SMPG. In addition, explanatory factors such as changes in FPG, 2‐h SMPG, body weight, final Gla‐100 dose, sulfonylurea use and hypoglycaemia during the 24‐week study period were incorporated in the statistical models using either continuous or categorical variables. The cut‐offs chosen for categorical variables such as baseline HbA1c (</≥8.5%; </≥69 mmol/mol), baseline FPG (</≥11.1 mmol/L) or baseline 2 h‐SMBG (</≥ 11.7 mmol/L) approximately referred to the corresponding means of the “good responder” group, whereas cut‐offs for body weight (</≥83 kg), BMI (</≥30 kg/m2) and diabetes duration (</≥10 years) were considered clinically useful by informal author review. Covariates with a P value of < 0.05 were then included in a stepwise logistic multivariable regression analysis using SAS software (version 9.4; SAS Institute, Cary, North Carolina) with an entry and retention threshold of P < 0.15. In addition, continuous predictors were standardized by subtracting the mean, and divided by the standard deviation (SD), allowing the comparison of odds ratios (ORs) of predictors to determine their importance within the model (Table S2). A Hosmer and Lemeshow Goodness‐of‐Fit Test was run on the logistic model to determine the significance between the observed and predicted probabilities, to confirm that there was no lack‐of‐fit problem and that the linear assumption was therefore reasonable. The final models consisted of the following covariates: sex, diabetes duration, baseline HbA1c, baseline FPG, baseline 2‐h SMPG, baseline body weight, BMI, sulfonylurea use during the study, change in body weight and hypoglycaemia during the study.
3. RESULTS
3.1. Participant characteristics and demographic variables
A total of 7386 individuals participated in the 16 RCTs, of whom 3415 were initiated on once‐daily Gla‐100 and were thus eligible for inclusion in the analysis. Baseline clinical characteristics according to HbA1c responder groups are presented in Table 1. At 24 weeks, 46.4% of participants achieved a good glycaemic response, as defined by an HbA1c level less than 7.0% (<53 mmol/mol) and 53.6% of participants had HbA1c levels ≥7.0% (53 mmol/mol). At 24 weeks, 43.6% and 27.7% of participants who achieved an HbA1c response <7.0% (53 mmol/mol) and ≥7.0% (53 mmol/mol), respectively, achieved an FPG target of ≤5.6 mmol/L (≤100 mg/dL) (Table 2). Age and fasting C‐peptide were similar across the two HbA1c responder groups, with a higher percentage of men than women in the good‐responder group.
Table 1.
Baseline characteristics of study participants according to Week 24 HbA1c responder groups
| Good responders | Poor responders | |||
|---|---|---|---|---|
| HbA1c <7.0% (<53 mmol/mol) | All HbA1c ≥7.0% (≥53 mmol/mol) | Sub‐optimal HbA1c 7.0%‐8.0% (53‐64 mmol/mol) | Minimal HbA1c >8.0% (>64 mmol/mol) | |
| n = 1584 | n = 1831 | n = 1262 | n = 569 | |
| Age (median, range), y | 57.9 (26.7‐82.4) | 57.8 (19.6‐86.2) | 58.1 (19.6‐84.7) | 57.1 (21.3‐86.2) |
| Men, n (%) | 901 (56.9) | 916 (50.0) | 641 (50.8) | 275 (48.3) |
| Body weight, kg | 87.6 (17.6) | 84.8 (18.7) | 85.6 (18.3) | 83.1 (19.5) |
| BMI, kg/m2 | 30.8 (5.0) | 30.3 (5.4) | 30.5 (5.4) | 29.9 (5.4) |
| Diabetes duration (median, range), y | 7.0 (0.0‐43) | 8.0 (0.4‐50.0) | 8.0 (0.4‐45.0) | 9.0 (0.6‐50.0) |
| Gla‐100 starting dose, U/d | 12.8 (5.1) | 13.9 (6.6) | 13.3 (5.6) | 15.3 (8.2) |
| Gla‐100 starting dose, U/kg | 0.15 (0.06) | 0.17 (0.09) | 0.16 (0.07) | 0.19 (0.11) |
| HbA1c, % | 8.41 (0.95) | 9.01 (1.01) | 8.82 (0.97) | 9.44 (0.98) |
| HbA1c, mmol/mol | 68.4 (10.4) | 75.0 (11.0) | 72.9 (10.6) | 79.7 (10.7) |
| n = 1567 | n = 1816 | n = 1251 | n = 565 | |
| FPG, mmol/L | 10.2 (2.7) | 11.1 (3.2) | 10.8 (3.0) | 11.8 (3.4) |
| FPG, mg/dL | 184.1 (49.2) | 199.7 (57.3) | 193.9 (54.2) | 213.2 (61.8) |
| n = 1232 | n = 1421 | n = 968 | n = 453 | |
| 2‐h SMPG overalla, mmol/L | 11.7 (3.1) | 13.3 (3.5) | 12.7 (3.3) | 14.4 (3.8) |
| 2‐h SMPG overalla, mg/dL | 210.5 (55.3) | 238.9 (63.9) | 229.6 (59.0) | 258.6 (69.4) |
| n = 883 | n = 1216 | n = 817 | n = 399 | |
| Fasting C‐peptide, nmol/L | 1.16 (0.57) | 1.14 (0.64) | 1.13 (0.57) | 1.16 (0.77) |
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; Gla‐100, glargine 100 U/mL; HbA1c, glycated haemoglobin A1c; SMPG, self‐monitored plasma glucose.
Values are given as mean (standard deviation) or as stated. Group numbers vary with missing values.
From 7‐point SMPG profiles: average 2 h post‐breakfast, lunch and evening meal.
Table 2.
Outcome measures at 24 weeks and change from baseline according to Week 24 HbA1c responder groups
| Good responders | Poor responders | |||
|---|---|---|---|---|
| HbA1c <7.0%; <53 mmol/mol | All HbA1c ≥7.0% (≥53 mmol/mol) | Sub‐optimal HbA1c 7.0%‐8.0% (53‐64 mmol/mol) | Minimal HbA1c >8.0% (>64 mmol/mol) | |
| Participants, n (% of total) | 1584 (46.4) | 1831 (53.6) | 1262 (37.0) | 569 (16.6) |
| HbA1c | n = 1584 | n = 1831 | n = 1262 | n = 569 |
| Week 24, % | 6.39 (0.41) | 7.88 (0.87) | 7.42 (0.30) | 8.91 (0.83) |
| Change from baseline, % | −2.02 (0.99) | −1.13 (1.09) | −1.40 (0.97) | −0.54 (1.10) |
| Week 24, mmol/mol | 46.4 (4.5) | 62.6 (9.5) | 57.6 (3.3) | 73.9 (9.1) |
| Change from baseline, mmol/mol | −22.1 (10.8) | −12.4 (11.9) | −15.3 (10.6) | −5.9 (12.0) |
| FPG, mmol/L | n = 1559 | n = 1799 | n = 1244 | n = 555 |
| Week 24 | 6.0 (1.5) | 7.0 (2.3) | 6.6 (1.9) | 7.8 (3.0) |
| Change from baseline | −4.2 (2.9) | −4.1 (3.6) | −4.1 (3.3) | −4.0 (4.2) |
| ≤5.6 mmol/L at Week 24, % | 43.6 | 27.7 | 30.4 | 21.7 |
| 2‐h SMPG overalla, mmol/L | n = 1221 | n = 1387 | n = 952 | n = 435 |
| Week 24 | 8.6 (1.9) | 10.2 (2.7) | 9.6 (2.2) | 11.4 (3.1) |
| Change from baseline | −3.1 (3.1) | −3.1 (3.5) | −3.1 (3.4) | −3.1 (3.8) |
| <7.8 mmol/L at Week 24, % | 39.1 | 17.7 | 26.3 | 7.1 |
| 2‐h SMPG breakfasta, mmol/L | n = 1201 | n = 1354 | n = 927 | n = 427 |
| Week 24 | 8.3 (2.6) | 9.7 (3.2) | 9.3 (2.9) | 10.7 (3.5) |
| Change from baseline | −4.0 (4.0) | −4.0 (4.3) | −4.0 (4.2) | −4.0 (4.6) |
| Gla‐100 dose, U/kg | n = 1584 | n = 1831 | n = 1262 | n = 569 |
| Week 24 | 0.42 (0.23) | 0.46 (0.26) | 0.44 (0.24) | 0.51 (0.29) |
| Change from baseline | 0.27 (0.23) | 0.29 (0.26) | 0.28 (0.25) | 0.32 (0.30) |
| Hypoglycaemiab | n = 1584 | n = 1831 | n = 1262 | n = 569 |
| Incidence, % (SE) | 53.7 (1.25) | 43.0 (1.16) | 46.5 | 35.2 |
| Events/person‐year, n | 6.5 (0.3) | 5.0 (0.3) | 5.3 (0.3) | 4.4 (0.5) |
| Body weight, kg | n = 1581 | n = 1811 | n = 1252 | n = 559 |
| Week 24 | 89.0 (17.9) | 87.2 (18.9) | 87.8 (18.6) | 85.7 (19.6) |
| Change from baseline | 1.4 (3.8) | 2.4 (3.4) | 2.3 (3.4) | 2.8 (3.5) |
Abbreviations: FPG, fasting plasma glucose; Gla‐100, glargine 100 U/mL; HbA1c, glycated haemoglobin A1c; PG, plasma glucose; SE, standard error; SMPG, self‐monitored plasma glucose.
Values are given as mean (standard deviation) or as stated. Group numbers vary with missing values.
From 7‐point SMPG profiles: 2‐h post‐prandial.
Overall hypoglycaemia: PG <70 mg/dL (<3.9 mmol/L).
3.2. Insulin dose and glycaemic control
Mean Gla‐100 starting doses ranged from 12.8 to 13.9 U/d (0.15 to 0.17 U/kg/d) in both responder groups (Table 1, Figure 1A). Mean final daily Gla‐100 doses at Week 24 were 38.1 U (0.42 U/kg) and 41.4 U (0.46 U/kg) in the HbA1c <7.0% and HbA1c ≥7.0% groups, respectively (Table 2, Figure 1A and Figure S2A). The corresponding mean Gla‐100 dose increments were 25.3 (0.27) and 27.5 U/d (0.29 U/kg/d) in the two HbA1c responder groups, respectively. The good‐responder group had a greater reduction in HbA1c from baseline to Week 24 of 2.02% (22.1 mmol/mol) compared with the poor‐responder group, in which HbA1c decreased by 1.13% (12.4 mmol/mol) (Table 2, Figure 1B and Figure S2A). The larger decrement in HbA1c in the good‐responder group was achieved despite starting with the lowest baseline HbA1c level (8.41% vs. 9.01%) and requiring the lowest final insulin dose (0.15 vs. 0.17 U/kg) (Table 2, Figure 1B and Figure S2B). Interestingly, in both responder groups, an inverse relationship was observed between insulin dose and frequency of hypoglycaemia (Table 2). Those participants who did not experience hypoglycaemia events had the highest insulin doses and dose increments at 24 weeks. The Gla‐100 dose and dose increments at 24 weeks according to frequency of hypoglycaemia are shown in Figure S3.
Figure 1.
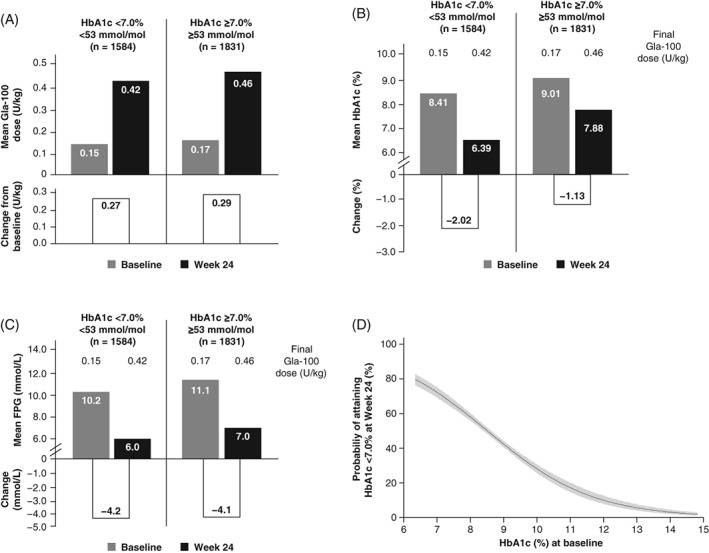
Mean daily Gla‐100 doses (A) HbA1c (B) FPG (C) and changes from baseline to Week 24 according to HbA1c responder groups; and probability of achieving <7.0% according to baseline HbA1c (D) based on univariable analysis. Grey bars indicate baseline values; black bars indicate values at 24 weeks; grey shading in D, indicates 95% confidence interval. FPG, fasting plasma glucose; Gla‐100, glargine 100 U/mL; HbA1c, glycated haemoglobin A1c
HbA1c change within each of the responder groups differed only slightly among the different OADs used during the study period (Table S1). However, a smaller percentage of individuals reached HbA1c target at 24 weeks with concomitant sulfonylurea compared with the other OAD groups (32% vs 49%‐57%).
FPG and 2‐h SMPG levels at baseline and at 24 weeks were higher in the poor responder group (Figure 1C, Figure S2C and Figure S4), although the reductions in these parameters during the course of the study were essentially similar in both responder groups: −4.1 to −4.2 mmol/L (−73.8 to −76.5 mg/dL) for FPG and −3.1 to −3.1 mmol/L (−56.0 to −56.7 mg/dL) for overall 2‐h SMPG (Table 2). The 2‐h SMPG levels at 24 weeks were higher in the poor‐responder group compared with the good‐responder group after each meal (breakfast, lunch and evening meal) (Figure S4).
3.3. Hypoglycaemia
The incidence and event rate of overall hypoglycaemia (PG <70 mg/dL; <3.9 mmol/L) or severe hypoglycaemia over 24 weeks was higher in the good‐responder group (53.7%; 6.5 ± 0.3 events/patient year) compared with the poor‐responder group (43.0%; 5.0 ± 0.3 events/patient year) (Table 2, Figure 2 and Figure S5). Similarly, the proportion of participants who experienced one to three or four or more episodes of hypoglycaemia were fewer in the poor‐responder group. Findings for nocturnal hypoglycaemia (PG <3.9 mmol/L) were similar to those for overall hypoglycaemia. The incidence of severe hypoglycaemia over 24 weeks was low at 2.0% in the responder groups.
Figure 2.
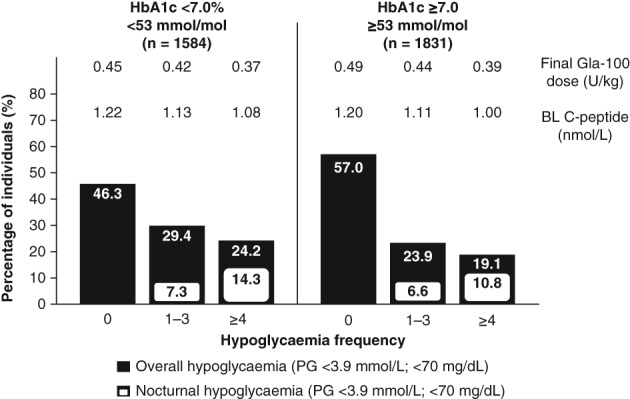
Incidences of confirmed overall and nocturnal hypoglycaemia among individuals with type 2 diabetes mellitus by number of events reported during a 24‐week period and by HbA1c responder group. Insets refer to mean final Gla‐100 dose and BL mean fasting C‐peptide levels per subgroup. BL, baseline; Gla‐100, glargine 100 U/mL; HbA1c, glycated haemoglobin A1c; PG, plasma glucose
In both responder groups, individuals who experienced the highest number of hypoglycaemia events had the lowest baseline fasting C‐peptide levels and the lowest final Gla‐100 dose (U/kg), a difference that was more evident in the poor‐responder group (Figure 2).
3.4. Body weight
Baseline and 24‐week mean body weights were lower in the poor‐responder group compared with the good‐responder group (Tables 1 and 2). Mean change in body weight was lower in the good‐responder group (+1.4 kg) than in the poor‐responder group (+2.4 kg) (Table 2). Within each responder group, participants with the lowest mean weight at baseline and at 24 weeks experienced a higher number of hypoglycaemia events. This was more notable in the poor‐responder group, in which those experiencing four or more events had the lowest body weight at baseline and at 24 weeks, despite the greater weight gain during the study period (Figure S6).
3.5. Factors associated with attainment of HbA1c <7.0% (<53 mmol/mol) at 24 weeks
Univariable analysis identified potential explanatory factors among baseline demographic characteristics, as well as clinical variables, at 24 weeks and during the study period that were related to the attainment of an HbA1c target of <7.0% (<53 mmol/mol) (Table 3). The relationship between baseline HbA1c values and the probability of reaching an end‐of‐trial target HbA1c of <7.0% (<53 mmol/mol) is illustrated in Figure 1D, corresponding to an OR of 0.53 (95% confidence interval [CI], 0.50‐0.58) per 1%‐unit (10.9 mmol/mol) (Table 3). Female sex, known duration of diabetes, sulfonylurea use, hypoglycaemia incidence during the study and change in body weight were all negatively associated with the probability of achieving an HbA1c of <7.0% (<53 mmol/mol) (Table 3). Age, baseline C‐peptide levels, change in FPG and 2‐h SMPG, as defined previously, over the 24‐week study period did not contribute to predicting whether an HbA1c target of <7.0% (<53 mmol/mol) would be achieved.
Table 3.
Univariable and multivariable analyses using potential prognostic and explanatory factors to estimate the probability of reaching HbA1c less than 7.0% (<53 mmol/mol) at Week 24 in participants with type 2 diabetes
| Variable | Categorical or continuous | Odds ratio (95% CI) | P value |
|---|---|---|---|
| Univariable analysis | |||
| Age at baseline | Per 10 y | 1.02 (0.95, 1.10) | 0.513 |
| Sex | Women vs men | 0.76 (0.66, 0.87) | <0.0001 |
| Body weight at baseline | ≥83.0 vs <83.0 kg | 1.38 (1.20, 1.58) | <0.0001 |
| Continuous (per 10 kg)a | 1.09 (1.05, 1.13) | <0.0001 | |
| BMI at baseline | ≥30.0 vs <30.0 kg/m2 | 1.21 (1.06, 1.38) | 0.006 |
| Continuous (per 5 kg/m2) | 1.09 (1.02, 1.16) | 0.008 | |
| Diabetes duration | ≥10 vs <10 y | 0.61 (0.53, 0.70) | <0.0001 |
| Continuous (per year)a | 0.96 (0.95, 0.97) | <0.0001 | |
| HbA1c at baseline | ≥8.5 vs <8.5%b | 0.35 (0.30, 0.40) | <0.0001 |
| Continuous (per 1 mmol/mol)a | 0.94 (0.94, 0.95) | <0.0001 | |
| Continuous (per 1%‐unit)a | 0.53 (0.50, 0.58) | <0.0001 | |
| FPG at baseline | ≥11.1 vs <11.1 mmol/Lc | 0.59 (0.51, 0.68) | <0.0001 |
| Continuous (per 1 mmol/L)a | 0.91 (0.89, 0.93) | <0.0001 | |
| Continuous (per 10 mg/dL)a | 1.00 (0.99, 1.00) | <0.0001 | |
| 2‐h SMPG at baseline (all meals) | ≥11.7 vs <11.7 mmol/Ld | 0.48 (0.41, 0.56) | <0.0001 |
| Continuous (per 1 mmol/L)a | 0.86 (0.84, 0.89) | <0.0001 | |
| Continuous (per 10 mg/dL)a | 0.99 (0.99, 0.99) | <0.0001 | |
| Baseline fasting C‐peptide | ≥1.2 vs <1.2 nmol/L | 1.00 (0.84, 1.20) | 0.97 |
| Continuous (per 0.2 nmol/L)a | 1.00 (1.00, 1.00) | 0.28 | |
| Sulfonylurea use during study | Yes vs no | 0.61 (0.52, 0.71) | <0.0001 |
| Hypoglycaemiaa during study | No vs yes | 0.65 (0.57, 0.75) | <0.0001 |
| Change in FPG | Continuous (per 10 mg/dL) | 0.99 (0.98, 1.00) | 0.19 0.19 |
| Continuous (per 1 mmol/L)a | 0.99 (0.97, 1.01) | ||
| Change in 2‐h SMPG (all meals) | Continuous (per 10 mg/dL) | 1.00 (0.99, 1.01) | 0.76 0.76 |
| Continuous (per 1 mmol/L)a | 1.00 (0.97, 1.02) | ||
| Change in body weight | Continuous (per 1.0 kg) | 0.92 (0.90, 0.94) | <0.0001 |
| Final Gla‐100 dose | Continuous (per 0.1 U/kg) | 0.93 (0.91, 0.96) | <0.0001 |
| Multivariable analysis model using categorical and continuous variables | |||
| Sex | Women vs men | 0.76 (0.64, 0.91) | 0.0021 |
| Diabetes duration | ≥10 vs <10 y | 0.63 (0.53, 0.75) | <0.0001 |
| HbA1c at baseline | ≥8.5 vs <8.5%b | 0.38 (0.32, 0.45) | <0.0001 |
| 2‐h SMPG at baseline (all meals) | ≥11.7 vs <11.7 mmol/Ld | 0.73 (0.61, 0.87) | 0.0005 |
| Body weight at baseline | ≥83.0 vs <83.0 kg | 1.29 (1.09, 1.54) | 0.0034 |
| Sulfonylurea use during study | Yes vs no | 0.59 (0.49, 0.71) | <0.0001 |
| Change in body weight | Continuous (per 1.0 kg) | 0.94 (0.91, 0.96) | <0.0001 |
| Hypoglycaemiaa during study | No vs yes | 0.62 (0.53, 0.74) | <0.0001 |
| Multivariable analysis model using continuous variables | |||
| Sex | Women vs men | 0.70 (0.60, 0.83) | <0.0001 |
| Diabetes duration | Per year | 0.96 (0.95, 0.98) | <0.0001 |
| HbA1c at baseline | Per 1 mmol/mol | 0.94 (0.94, 0.95) | <0.0001 |
| Per 1%‐unit | 0.53 (0.48, 0.60) | <0.0001 | |
| FPG at baseline | Per 1 mmol/L | 1.06 (1.02, 1.11) | 0.003 |
| Per 1 mg/dL | 1.00 (1.00, 1.01) | 0.0023 | |
| 2‐h SMPG at baseline (all meals) | Per 1 mmol/L | 0.93 (0.90, 0.96) | <0.0001 |
| Per 1 mg/dL | 1.00 (0.99, 1.00) | 0.0005 | |
| BMI at baseline | Per 5.0 kg/m2 | 1.19 (1.08, 1.31) | 0.0003 |
| Change in body weight | Per 1.0 kg | 0.94 (0.92, 0.97) | <0.0001 |
| Hypoglycaemiaa during study | No vs yes | 0.64 (0.54, 0.76) | <0.0001 |
Abbreviations: BMI, body mass index; CI, confidence interval; FPG, fasting plasma glucose; Gla‐100, glargine 100 U/mL; HbA1c, glycated haemoglobin A1c; PG, plasma glucose; SMPG, self‐monitored plasma glucose.
For multivariable analysis, n = 2626 patients were included. Outcomes of univariable and multivariable analysis using standardized predictors are shown in Table S2.
Confirmed with PG <3.9 mmol/L or severe hypoglycaemia.
≥69 vs <69 mmol/mol.
≥200 vs <200 mg/dL.
≥210 vs <210 mg/dL.
Covariates remaining in the final multivariable model analysis are represented in Table 3. Using categorical cut‐offs, participants with a baseline HbA1c of ≥8.5% (≥69 mmol/mol) were 0.35 times less likely to achieve an HbA1c target of <7.0% (<53 mmol/mol) than those with an HbA1c of <8.5% (<69 mmol/mol). Further predictive or explanatory factors of a good response in HbA1c within this model included higher baseline body weight, lower baseline 2‐h SMPG levels and hypoglycaemia occurring during treatment. Moreover, a longer duration of diabetes (≥10 vs <10 years), female sex, continued use of sulfonylurea and a greater change in weight were associated with a lower likelihood of reaching the target HbA1c level of <7.0%.
Using a multivariable analysis model with continuous variables, baseline HbA1c was the strongest predictor of reaching the target HbA1c level, with odds decreasing by a factor of 0.53 (95% CI, 0.48‐0.60) per 1%‐unit increase in HbA1c (OR, 0.94 [95% CI, 0.94‐0.95] per 1 mmol/mol increase) (Table 3). Although significant, the predictive power of diabetes duration and baseline 2‐h SMPG was less (Table 3). Sex and hypoglycaemia during the study were consistent with the findings used with categorical cut‐offs.
4. DISCUSSION
Recognition of the characteristics that influence treatment outcomes will enable clinicians to develop more efficient treatment strategies and provide better advice to individuals with T2DM. This post‐hoc analysis of pooled, participant‐level data concerning 3415 individuals with T2DM indicated that 54% did not reach the HbA1c goal of <7.0% (<53 mmol/mol) when given supplementary Gla‐100 over a period of 24 weeks. Several variables were identified with both univariable and multivariable analyses that appeared to be associated with achieving target HbA1c levels of <7.0%.
Baseline HbA1c was the most powerful predictor of response with observational, univariable and multivariable analyses, consistent with other studies involving supplementary basal and other insulins in individuals with T2DM.10, 11, 12, 13 The odds of achieving HbA1c <7.0% (<53 mmol/mol) decreased by 50% (OR 0.53 [95% CI, 0.48‐0.60]) per %‐unit after adjusting for other variables. However, only 26.5% and 17.7% of individuals reached a target HbA1c of <7.0% (<53 mmol/mol) at 24 weeks, with a baseline HbA1c of >8.0% (>64 mmol/mol) and >8.5% (>69 mmol/mol), respectively. These percentages were further reduced to 9%‐12% and 4%‐7%, with coexisting elevated 2‐h SMPG (>11.7 mmol/L) or diabetes duration ≥10 years at baseline, respectively (data not shown). Nonetheless, a decrease in HbA1c of 1.1% (12.4 mmol/mol) was achieved in the poor‐responder group, with end‐of‐treatment HbA1c ≥7.0% (≥53 mmol/mol), representing significant gains in glycaemic control despite failure to reach the target HbA1c level of <7.0% (<53 mmol/mol). The good‐ and sub‐optimal‐responder groups comprise 83% of the participants; however, a considerable proportion of individuals (17%) remained in poor control, with an end‐of‐treatment HbA1c of >8.0% (>64 mmol/mol).
We also observed that an increase in body weight over 24 weeks was modestly associated with failure to attain a good HbA1c response and was consistent with the premise that lifestyle modification, especially in conjunction with commencing basal insulin, is important in achieving glucose targets. Unexpectedly, higher baseline body weight or a higher BMI were modest and independent predictors of achieving a better response.6, 10 In addition, the poor‐responder group received more insulin as a consequence of greater dose titration, but had fewer hypoglycaemia events, suggesting the possibility of more insulin resistance.
Although the final insulin dose when weight adjusted was clearly different between the HbA1c responder groups, this could be related, in part, to the differences in body weight rather than dose. The final insulin dose was not retained as an explanatory variable in the multivariable analyses, suggesting that it was closely associated with another strong predictor that could only be baseline HbA1c.
Hypoglycaemia was a modestly powerful explanatory factor, with a higher incidence associated with attainment of target levels in accordance with other reports,14, 15, 16 with the exception of the ACCORD study.17 In our study population, hypoglycaemia occurrence was not a barrier to better control, even though, in short‐term, treat‐to‐target studies, the overall incidence is high. Within each responder group, a higher event rate was associated with lower insulin dose and lower baseline C‐peptide levels, as reported by others, suggesting the existence of sub‐populations with greater insulin deficiency and higher insulin sensitivity.2, 18, 19 Overall, the contribution of diabetes duration to predicting the achievement of glycaemic outcome is lower than that of HbA1c. This could be a consequence of the burden of disease, as those using two OADs during the study achieved less benefit overall, consistent with previous observations.6, 10, 11, 12 In addition, those using sulfonylureas during the study were less successful in achieving the HbA1c target, independently of other factors, such as hypoglycaemia and weight gain, with the odds changing only marginally between univariable and multivariable analyses (OR of 0.61 [95% CI, 0.52‐0.71] vs OR of 0.59 [95% CI, 0.49‐0.71], respectively).
HbA1c is associated with FPG and PPG; thus, the finding on univariable analysis that these measures predict achievement of a better response at 24 weeks was expected. Nonetheless, the odds per FPG at an OR of 0.91 (95% CI, 0.89‐0.93) are modest and less than an OR of 0.86 (95% CI, 0.84‐0.89) per 1 mmol/L for 2‐h SMPG. Knowledge of FPG does not supplement the ability to predict improvement in glycaemic control if the HbA1c level is known. In the prospective A1chieve study involving a variety of insulin types, lower FPG and PPG values predicted greater change in HbA1c.10
In the present study, individuals with higher HbA1c levels at 24 weeks recorded higher 2‐h SMPG values, both at baseline and at endpoint, for each meal. The absolute reduction in PPG between baseline and 24 weeks appears to be greatest with breakfast and least with dinner, possibly reflecting the waning biological activity of Gla‐100 toward the end of the 24‐hour period. Interestingly, change in FPG and, to a lesser extent, the 2‐h SMPG was only slightly greater in the good‐ than in the sub‐optimal‐ or minimal‐responder groups; although this was proportionately less in the latter group because baseline levels were much higher.
Female sex was a modest predictor of difficulty in attaining the HbA1c target on basal insulin, an association that survived into the multivariable models and is consistent with previous reports.6, 9, 10, 20
A limitation of this study is the focus on HbA1c goal attainment rather than using HbA1c as a continuous variable that might have identified additional factors important for improving glucose control rather than goal achievement.
While findings of the present analysis are consistent with those of other RCTs, pharmaco‐epidemiological and prospective cohort studies involving basal insulin and other insulin types,6, 8, 10, 11, 12, 20, 21 the analysis reflects only a subpopulation of those starting insulin in the real world.6
This study focused on whether patients with poorly controlled T2DM while using an OAD can achieve the recommended HbA1c goal of <7.0% with the introduction of basal insulin Gla‐100. This pooled analysis of individuals with T2DM with a mean HbA1c of 8.7% (72 mmol/mol) at baseline demonstrated that 54% of the study population did not achieve an HbA1c level of <7.0% (<53 mmol/mol) following the addition of basal insulin.
The results further suggest that, in individuals with a higher baseline HbA1c (>8.0%; >64 mmol/mol), basal insulin supplementation may need to be more aggressive, while also considering the risk of hypoglycaemia. Therefore, when an adequate response is not achieved, consideration should be given to other therapies to address excessive PPG excursions, such as prandial insulin, sodium glucose co‐transporter 2 inhibitors or a GLP‐1 RA in a free or fixed combination with insulin. Early introduction of basal insulin in combination with a GLP‐1 RA may be an option for individuals with T2DM with persistently poor glycaemic control despite the use of multiple OADs.22
CONFLICTS OF INTEREST
D. R. O. has received honoraria for lecturing and consulting from Boehringer Ingelheim, Eli Lilly, Roche Diagnostics, Sanofi and Takeda. W. L. is an employee of Sanofi, Germany and a shareholder in Sanofi. B. M. F. has served on advisory panels for Eli Lilly, Johnson & Johnson and Novo Nordisk; has served on the speakers' bureau for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi and Roche Diagnostics; and is a consultant for Locemia Solutions. M. Z. is an employee of Sanofi Inc., US and a shareholder in Sanofi. P. D. H. has received funding for research, advisory and lecturing activities from Antriabio, AstraZeneca, Biocon, GlaxoSmithKline, Janssen, Hanmi, Merck (MSD), Novo Nordisk, Roche Diagnostics and Sanofi. L. M. has received honoraria for consulting from Intarcia, Novo Nordisk and Sanofi. G. B. B. is a consultant for Eli Lilly and Sanofi; has received research support from Sanofi; and is on the speakers' bureau for Eli Lilly, Menarini and Sanofi.
Author contributions
D. R. O. was involved in designing the study, data analysis and interpretation, drafting the publication, critical revision and final approval of the manuscript. W. L. was involved in designing the study, data acquisition, data analysis and interpretation, drafting the publication, critical revision and final approval of the manuscript. B. M. F. was involved in designing the study, data analysis and interpretation, critical revision and final approval of the manuscript. M. Z. was involved in data acquisition, data analysis and interpretation, critical revision and final approval of the manuscript. P. D. H. was involved in data analysis and interpretation, drafting the publication, critical revision and final approval of the manuscript. L. M. was involved in data analysis and interpretation, critical revision and final approval of the manuscript. G. B. B. was involved in data analysis and interpretation, critical revision and final approval of the manuscript. All authors take responsibility for the accuracy and integrity of the data presented in this paper.
Supporting information
Table S1. Change in HbA1c from baseline to Week 24 by HbA1c‐responder group and concomitant oral antidiabetes drug used during the entire study period.
Table S2. Univariable and multivariable analyses using potential prognostic and explanatory factors to estimate the probability of reaching an HbA1c of <7.0% (<53 mmol/mol) at week 24 in participants with type 2 diabetes using standardized predictors for continuous variables in the multivariable analysis models.
Figure S1: Participant distribution and OAD use by Week 24 HbA1c‐responder group. *Includes all participants in the Gla‐100 and comparator arms who received metformin, sulfonylurea, or metformin plus sulfonylurea or plus other OADs (including thiazolidinediones, gliptins and glinides) as add‐on therapy. Gla‐100, glargine 100 units/mL; MET, metformin; OAD, oral anti‐hyperglycaemic drug; SU, sulfonylurea.
Figure S2. Mean daily Gla‐100 doses A, HbA1c B, and FPG C, Grey bars indicate baseline values; black bars indicate values at 24 weeks. FPG, fasting plasma glucose; Gla‐100, glargine 100 units/mL; HbA1c, glycated haemoglobin A1c.
Figure S3. Final daily Gla‐100 dose (units/kg) and increments from baseline to Week 24 by HbA1c‐responder groups and hypoglycaemia frequency (plasma glucose <3.9 mmol/L; <70 mg/dL) during the 24‐week study period. Gla‐100, glargine 100 units/mL; HbA1c, glycated haemoglobin A1c.
Figure S4. Two hours self‐measured post‐prandial glucose levels and changes from baseline according to HbA1c‐responder groups at Week 24. ALL, all meals combined; BRE, breakfast; DIN, dinner; LUN, lunch; 2‐h SMPG, 2‐hours self‐measured plasma glucose.
Figure S5. Incidences of confirmed overall and nocturnal hypoglycaemia among people with type 2 diabetes mellitus by number of events reported during 24 weeks and by HbA1c responder group. Insets refer to mean final Gla‐100 dose and BL mean fasting C‐peptide levels per subgroup. BL, baseline; Gla‐100, glargine 100 units/mL; HbA1c, glycated haemoglobin A1c; PG, plasma glucose.
Figure S6. Mean body weight and change according to hypoglycaemia event frequency during the study period and by HbA1c‐responder group at Week 24. HbA1c, glycated haemoglobin A1c.
ACKNOWLEDGMENTS
The authors would like to thank Jason Chao, Sanofi US Inc., for his contributions to the data analysis and interpretation for this publication. Editorial and writing support in the preparation of this manuscript was provided by Martina Fuchsberger, PhD, of Excerpta Medica, funded by Sanofi.
Owens DR, Landgraf W, Frier BM, et al. Commencing insulin glargine 100 U/mL therapy in individuals with type 2 diabetes: Determinants of achievement of HbA1c goal less than 7.0%. Diabetes Obes Metab. 2019;21:321–329. 10.1111/dom.13607
Funding information This study and underlying studies were funded by Sanofi.
REFERENCES
- 1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2018 executive summary. Endocr Pract. 2018;24:91‐120. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Standards of medical care in diabetes – 2018. Diabetes Care. 2018;41(suppl 1):S1‐S159.29222369 [Google Scholar]
- 3. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raccah D, Chou E, Colagiuri S, et al. A global study of the unmet need for glycemic control and predictor factors among patients with type 2 diabetes mellitus who have achieved optimal fasting plasma glucose control on basal insulin. Diabetes Metab Res Rev. 2017;33:e2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell‐Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balkau B, Calvi‐Gries F, Freemantle N, Vincent M, Pilorget V, Home PD. Predictors of HbA1c over 4 years in people with type 2 diabetes starting insulin therapies: the CREDIT study. Diabetes Res Clin Pract. 2015;108:432‐440. [DOI] [PubMed] [Google Scholar]
- 7. Khunti K, Damci T, Husemoen LL, Babu V, Liebl A. Exploring the characteristics of suboptimally controlled patients after 24 weeks of basal insulin treatment: an individualized approach to intensification. Diabetes Res Clin Pract. 2017;123:209‐217. [DOI] [PubMed] [Google Scholar]
- 8. Lin SD, Tsai ST, Tu ST, et al. Glycosylated hemoglobin level and number of oral antidiabetic drugs predict whether or not glycemic target is achieved in insulin‐requiring type 2 diabetes. Prim Care Diabetes. 2015;9:135‐141. [DOI] [PubMed] [Google Scholar]
- 9. Owens DR, Bolli GB, Charbonnel B, et al. Effects of age, gender, and body mass index on efficacy and hypoglycaemia outcomes across treat‐to‐target trials with insulin glargine 100 U/mL added to oral antidiabetes agents in type 2 diabetes. Diabetes Obes Metab. 2017;19:1546‐1554. [DOI] [PubMed] [Google Scholar]
- 10. Home PD, Shen C, Hasan MI, Latif ZA, Chen JW, González GG. Predictive and explanatory factors of change in HbA1c in a 24‐week observational study of 66,726 people with type 2 diabetes starting insulin analogs. Diabetes Care. 2014;37:1237‐1245. [DOI] [PubMed] [Google Scholar]
- 11. Khunti K, Caputo S, Damci T, et al.; SOLVE Study Group. The safety and efficacy of adding once‐daily insulin detemir to oral hypoglycaemic agents in patients with type 2 diabetes in a clinical practice setting in 10 countries. Diabetes Obes Metab. 2012;14:1129‐1136. [DOI] [PubMed] [Google Scholar]
- 12. Nichols GA, Kimes TM, Harp JB, Kou TD, Brodovicz KG. Glycemic response and attainment of A1C goals following newly initiated insulin therapy for type 2 diabetes. Diabetes Care. 2012;35:495‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cummings MH, Cao D, Hadjiyianni I, Ilag LL, Tan MH. Characteristics of insulin‐naïve people with type 2 diabetes who successfully respond to insulin glargine U100 after 24 weeks of treatment: a meta‐analysis of individual participant data from 3 randomized clinical trials. Clin Diabetes Endocrinol. 2018;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karl DM, Gill J, Zhou R, Riddle MC. Clinical predictors of risk of hypoglycaemia during addition and titration of insulin glargine for type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:622‐628. [DOI] [PubMed] [Google Scholar]
- 15. ORIGIN Trial Investigators . Predictors of nonsevere and severe hypoglycemia during glucose‐lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38:22‐28. [DOI] [PubMed] [Google Scholar]
- 16. Home P, Calvi‐Gries F, Blonde L, Pilorget V, Berlingieri J, Freemantle N. Clinical correlates of hypoglycaemia over 4 years in people with type 2 diabetes starting insulin: an analysis from the CREDIT study. Diabetes Obes Metab. 2018;20:921‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller ME, Bonds DE, Gerstein HC, et al.; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chow LS, Chen H, Miller ME, Marcovina SM, Seaquist ER. Biomarkers related to severe hypoglycaemia and lack of good glycaemic control in ACCORD. Diabetologia. 2015;58:1160‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Owens DR, Landgraf W, Zhang M, et al. Association of fasting C‐peptide levels with glycaemic efficacy and risk of hypoglycaemia in people with type 2 diabetes commencing insulin glargine 100 U/ml. Diabetologia. 2017;60:S35‐S36. [Google Scholar]
- 20. Wu N, Aagren M, Boulanger L, Friedman M, Wilkey K. Assessing achievement and maintenance of glycemic control by patients initiating basal insulin. Curr Med Res Opin. 2012;28:1647‐1656. [DOI] [PubMed] [Google Scholar]
- 21. Valensi P, Shaban J, Benroubi M, et al.; IMPROVE Study Expert Panel. Predictors of achieving HbA1c <7% and no hypoglycaemia 6 months after initiation of biphasic insulin aspart 30 in patients with type 2 diabetes in the IMPROVE study. Curr Med Res Opin. 2013;29:601‐609. [DOI] [PubMed] [Google Scholar]
- 22. Tong L, Pan C, Wang H, Bertolini M, Lew E, Meneghini LF. Impact of delaying treatment intensification with a glucagon‐like peptide‐1 receptor agonist in patients with type 2 diabetes uncontrolled on basal insulin: a longitudinal study of a US administrative claims database. Diabetes Obes Metab. 2018;20:831‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Change in HbA1c from baseline to Week 24 by HbA1c‐responder group and concomitant oral antidiabetes drug used during the entire study period.
Table S2. Univariable and multivariable analyses using potential prognostic and explanatory factors to estimate the probability of reaching an HbA1c of <7.0% (<53 mmol/mol) at week 24 in participants with type 2 diabetes using standardized predictors for continuous variables in the multivariable analysis models.
Figure S1: Participant distribution and OAD use by Week 24 HbA1c‐responder group. *Includes all participants in the Gla‐100 and comparator arms who received metformin, sulfonylurea, or metformin plus sulfonylurea or plus other OADs (including thiazolidinediones, gliptins and glinides) as add‐on therapy. Gla‐100, glargine 100 units/mL; MET, metformin; OAD, oral anti‐hyperglycaemic drug; SU, sulfonylurea.
Figure S2. Mean daily Gla‐100 doses A, HbA1c B, and FPG C, Grey bars indicate baseline values; black bars indicate values at 24 weeks. FPG, fasting plasma glucose; Gla‐100, glargine 100 units/mL; HbA1c, glycated haemoglobin A1c.
Figure S3. Final daily Gla‐100 dose (units/kg) and increments from baseline to Week 24 by HbA1c‐responder groups and hypoglycaemia frequency (plasma glucose <3.9 mmol/L; <70 mg/dL) during the 24‐week study period. Gla‐100, glargine 100 units/mL; HbA1c, glycated haemoglobin A1c.
Figure S4. Two hours self‐measured post‐prandial glucose levels and changes from baseline according to HbA1c‐responder groups at Week 24. ALL, all meals combined; BRE, breakfast; DIN, dinner; LUN, lunch; 2‐h SMPG, 2‐hours self‐measured plasma glucose.
Figure S5. Incidences of confirmed overall and nocturnal hypoglycaemia among people with type 2 diabetes mellitus by number of events reported during 24 weeks and by HbA1c responder group. Insets refer to mean final Gla‐100 dose and BL mean fasting C‐peptide levels per subgroup. BL, baseline; Gla‐100, glargine 100 units/mL; HbA1c, glycated haemoglobin A1c; PG, plasma glucose.
Figure S6. Mean body weight and change according to hypoglycaemia event frequency during the study period and by HbA1c‐responder group at Week 24. HbA1c, glycated haemoglobin A1c.


