Abstract
Host cell invasion by Trypanosoma cruzi metacyclic trypomastigote (MT) is mediated by MT‐specific surface molecule gp82, which binds to a still unidentified receptor, inducing lysosome spreading and exocytosis required for the parasitophorous vacuole formation. We examined the involvement of the major lysosome membrane‐associated LAMP proteins in MT invasion. First, human epithelial HeLa cells were incubated with MT in the presence of antibody to LAMP‐1 or LAMP‐2. Antibody to LAMP‐2, but not to LAMP‐1, significantly reduced MT invasion. Next, HeLa cells depleted in LAMP‐1 or LAMP‐2 were generated. Cells deficient in LAMP‐2, but not in LAMP‐1, were significantly more resistant to MT invasion than wild‐type controls. The possibility that LAMP‐2 might be the receptor for gp82 was examined by co‐immunoprecipitation assays. Protein A/G magnetic beads cross‐linked with antibody directed to LAMP‐1 or LAMP‐2 were incubated with HeLa cell and MT detergent extracts. Gp82 bound to LAMP‐2 but not to LAMP‐1. Binding of the recombinant gp82 protein to wild‐type and LAMP‐1‐deficient cells, which was dose dependent and saturable, had a similar profile and was much higher as compared with LAMP‐2‐depleted cells. These data indicate that MT invasion is accomplished through recognition of gp82 by its receptor LAMP‐2.
Keywords: cell invasion, Gp82 receptor, LAMP protein, Trypanosoma cruzi
1. INTRODUCTION
Many pathogenic microorganisms invade host cells as the first step for the establishment of infection. Among the mechanisms used by different pathogens for their internalisation is the binding of a surface protein to the target cell surface receptor, which triggers signalling cascades leading to events, such as the actin cytoskeleton remodelling (Cossart & Sansonetti, 2004). The outer membrane proteins internalin A and invasin, which interact with human E‐cadherin and integrin receptors, mediate the invasion of enteropathogenic bacteria Listeria and Yersinia, respectively (Cossart, Pizarro‐Cerdá, & Lecuit, 2003; Isberg & Barnes, 2001).
Trypanosoma cruzi, the protozoan parasite that causes Chagas disease, is thought to enter host cells in a receptor‐dependent manner. Surface glycoproteins belonging to the gp85/trans‐sialidase superfamily, such as gp82 and Tc85, have been identified as the main T. cruzi molecules implicated in cell invasion (Alves & Colli, 2007; Yoshida, 2006). The identification of target cell receptor for gp82 expressed specifically in metacyclic trypomastigotes (MTs), which correspond to the insect‐borne parasite forms, remains elusive. Prokineticin receptors, distributed in many different tissues, were described as potential receptor for the Tc85 glycoproteins expressed in tissue culture trypomastigotes (TCTs), which are equivalent to parasites circulating in the mammalian host bloodstream (Khusal et al., 2015). MT‐specific gp82 and Tc85 expressed in TCT are presumably recognised by different receptors, provided that they have distinct adhesion properties. Gp82 protein binds to gastric mucin, a property relevant for T. cruzi infection by the oral route (Staquicini et al., 2010), but its affinity for components such as laminin, heparan sulfate, and collagen is minimal (Cortez, Yoshida, Bahia, & Sobreira, 2012; Ramirez, Ruiz, Araya, Da Silveira, & Yoshida, 1993), whereas Tc85 glycoproteins bind to laminin and fibronectin, among other extracellular matrix factors (Giordano et al., 1999; Ouaissi, Cornette, & Capron, 1986). Binding of gp82 molecule to target cells induces lysosome spreading that culminates in exocytosis and MT internalisation in a vacuole containing lysosome‐associated membrane proteins (LAMPs; Cortez, Real, & Yoshida, 2016; Martins, Alves, Macedo, & Yoshida, 2011). TCT interaction with host cells has been associated with microfilament rearrangement and lysosome exocytosis triggered by a nonidentified soluble TCT factor (Rodríguez, Rioult, Ora, & Andrews, 1995; Rodríguez, Samoff, Rioult, Chung, & Andrews, 1996), the parasite being internalised in a vacuole expressing plasma membrane markers (Woolsey et al., 2003). Lysosome exocytosis contributes to TCT invasion by stimulating endocytosis associated with the delivery of acid sphingomyelinase to the outer leaflet of the plasma membrane (Fernandes et al., 2011).
Lysosomes play a critical role in gp82‐mediated MT invasion. Conditions that increase lysosome biogenesis and scattering were found to increase MT internalisation, whereas factors that induced lysosome accumulation in the perinuclear region had an opposite effect (Cortez et al., 2016). What remains to be determined is whether the major lysosome‐associated membrane proteins LAMP‐1 and LAMP‐2 are implicated in MT invasion. Here, we addressed that question and investigated the possibility that either LAMP‐1 or LAMP‐2 could be the receptor for MT surface molecule gp82.
2. RESULTS
2.1. T. cruzi MT invasion requires host cell LAMP‐2 and does not rely on plasma membrane repair mechanism
The requirement of lysosomes for gp82‐mediated MT invasion has been revealed using human epithelial HeLa cells. As a first approach to determine whether LAMP proteins are implicated, invasion assays using anti‐LAMP antibodies were performed. HeLa cells were incubated for 1 hr with MT in the absence or in the presence of antibody to human LAMP‐1 or LAMP‐2, at 7 μg ml−1, or in the presence of both antibodies. After fixation and Giemsa staining, the number of intracellular parasites was counted. Antibody directed to LAMP‐2, but not to LAMP‐1, significantly inhibited MT internalisation, and the presence of both antibodies did not have a higher inhibitory effect (Figure 1a). The effect of antibody to LAMP‐2 at different concentrations was also tested. Inhibition of MT invasion by anti‐LAMP2 antibody was dose dependent (Figure 1b). To further ascertain the involvement of LAMP‐2 in MT entry into HeLa cells, the strategy of generating cells depleted in LAMP‐1 or LAMP‐2 was contemplated. Although attempts in that direction were under way, we performed experiments with mouse fibroblast cell lines and their counterparts deficient in LAMP‐1, in LAMP‐2, or in both proteins, which were generated in Dr Paul Saftig's laboratory (Eskelinen et al., 2004), by incubating MT with the cells for 1 hr, followed by processing for intracellular parasite counting. MT invasion was significantly lower in LAMP‐2‐deficient and in double knockout cells, whereas no difference in invasion was observed in LAMP‐1‐deficient cells as compared with wild‐type controls (Figure 1c). As LAMP‐2 was reported to be required for TCT invasion by contributing for plasma membrane repair (Couto et al., 2017), experiments were performed to determine whether MT internalisation also relied on plasma membrane injury and repair mechanism used by TCT (Fernandes et al., 2011). One experiment consisted in incubating HeLa cells with MT in PBS++ medium (Cortez et al., 2016) with or without Ca2+. In contrast to TCT invasion, which in the absence of extracellular Ca2+ (condition nonpermissive for repair) is drastically reduced (Fernandes et al., 2011), the number of MT that entered cells was similar in the absence or in the presence of Ca2+ (Figure 1d). Another experiment consisted in incubating HeLa cells with MT in the presence of the pore‐forming bacterial toxin streptolysin O (SLO). As opposed to TCT invasion, which increased by ~60% in the presence of SLO at 50 ng ml−1 at repair‐permissive condition (Fernandes et al., 2011), MT invasion diminished by ~50% and ~70% at 50 and 100 ng ml−1 of SLO, respectively (Figure 1e), suggesting that SLO treatment affected MT–host cell interaction. When Hela cells were preincubated for 20 min with SLO at 50 or 100 ng ml−1, before incubation with parasites, no inhibitory effect on MT invasion was observed (Figure 1f), in contrast to the reported inhibition of TCT internalisation by more than 50% in cells pretreated with 50 ng ml−1 of SLO (Fernandes et al., 2011). The permeabilisation of HeLa cells by SLO was ascertained by treating cells with propidium iodide in the absence of Ca2+ (Figure S1). These data indicate that MT invasion is independent of plasma membrane injury and repair mechanism.
Figure 1.
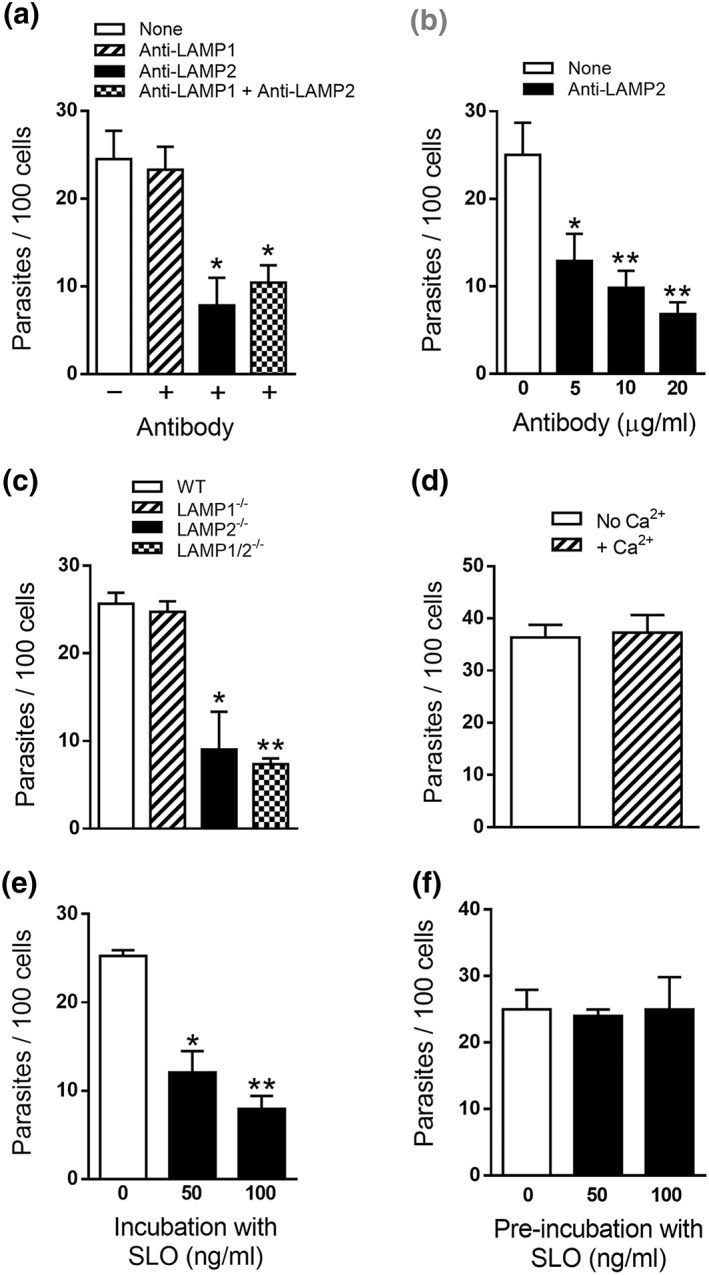
Involvement of LAMP‐2 in Trypanosoma cruzi metacyclic trypomastigote (MT) invasion. HeLa cells were incubated for 1 hr with MT in the absence or in the presence of anti‐LAMP1 or anti‐LAMP2 antibody or with both antibodies at 7 μg ml−1 (a) or with anti‐LAMP2 antibody at indicated concentrations (b), and the internalised parasites were quantified. Values are the means ± standard deviation of four (a) or three (b) independent assays performed in duplicate. MT invasion diminished significantly in the presence of anti‐LAMP2 antibody alone or when both antibodies were present (* P < 0.0005). (b) The inhibitory effect of anti‐LAMP2 antibody was dose dependent and significant (* P < 0.05 and ** P < 0.005). (c) Wild‐type (WT) mouse fibroblast cell lines and their counterparts deficient in LAMP‐1, in LAMP‐2, or in both proteins, were incubated with MT for 1 hr. Internalised parasites were counted in each duplicate from three independent assays. As compared with WT controls, MT invasion was significantly lower in LAMP‐2−/− and in double knockout cells (* P < 0.005 and ** P < 0.0001). (d) HeLa cells were incubated with MT for 1 hr in PBS++ medium with or without Ca2+. Internalised parasites were counted in each duplicate from three independent assays. The rate of MT invasion was similar in the absence or in the presence of Ca2+. HeLa cells were incubated for 1 hr with MT in the absence or in the presence of streptolysin O (SLO) at indicated concentrations (e) or were preincubated with SLO for 20 min and then discarded before addition of parasites (f). Values are the means ± standard deviation of three independent assays performed in duplicate. Incubation in the presence of SLO significantly inhibited MT invasion (* P < 0.001 and ** P < 0.0001), whereas pretreatment of cells had no effect
Lentiviral transduction methodology was employed to generate HeLa cells depleted in LAMP, using four different target sequences, two for LAMP‐1 and two for LAMP‐2. We produced four cell lines, which were analysed by Western blot using specific antibodies. LAMP‐1 was knocked down (kd) and was barely detectable in two cell lines (LAMP1‐kd1 and LAMP1‐kd2), LAMP‐2 was depleted in one cell line (LAMP2‐kd1), but in the other cell line (LAMP2‐kd2), it was not so clear (Figure 2a). Therefore, we performed an additional Western blot assay and confirmed that depletion was less effective in LAMP2‐kd2 cells (Figure S2). The different cell lines were analysed by confocal immunofluorescence, using anti‐LAMP antibodies. Control wild‐type cells reacted similarly with both antibodies (Figure 2b). Compatible with the Western blot profile, the LAMP‐deficient cells reacted accordingly with antibody to LAMP‐1 or LAMP‐2 (Figure 2c). Cells deficient in LAMP protein were examined for their susceptibility to MT invasion. MT internalisation decreased significantly in LAMP2‐kd1 cells, whereas the diminished susceptibility of LAMP2‐kd2 cells to MT invasion was not statistically significant, and LAMP‐1‐deficient cells were as susceptible to MT invasion as the wild‐type controls (Figure 3a). Confocal microscopy analysis was also performed with LAMP‐depleted cells incubated for 30 min with MT. In LAMP1‐kd1 populations, parasites were seen internalised or associated with cells (Figure 3b, left panel), regardless whether LAMP‐1 expression was high (red arrows), moderate (orange arrows), or negative (white arrows). On the other hand, in LAMP2‐kd1 populations, MT internalisation was restricted to a few LAMP‐2‐positive cells (Figure 3b, right panel, red arrows). Overall, these results support the notion that MT invasion is dependent on LAMP‐2.
Figure 2.
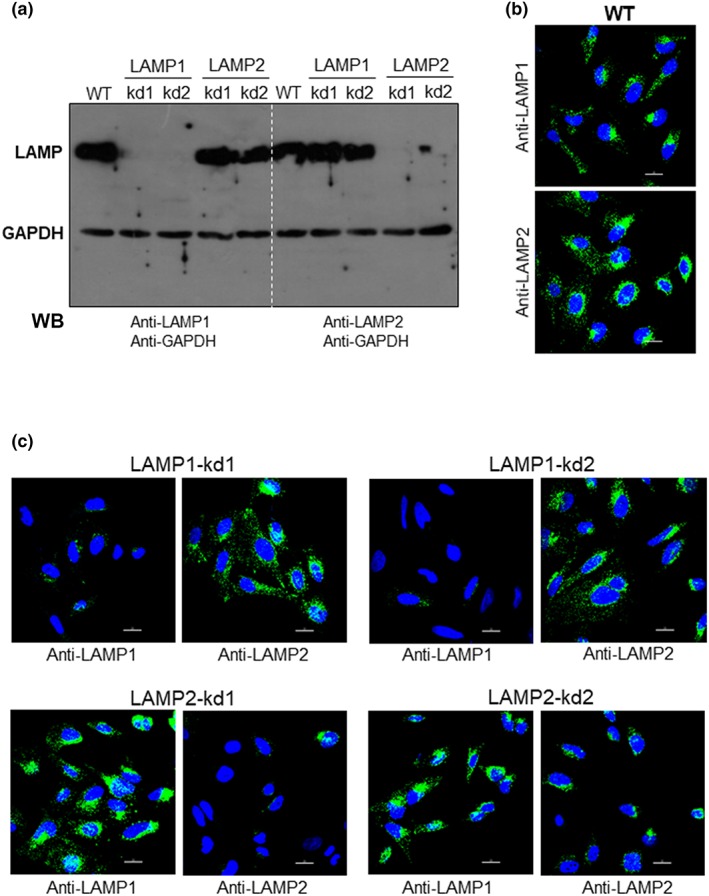
Depletion of LAMP proteins in HeLa cells. (a) HeLa cells were submitted to lentiviral transduction for LAMP‐1 or LAMP‐2 knock‐down (kd) and then analysed by Western blotting (WB) using the indicated antibodies. Note the depletion of LAMP‐1 in two independent cell lines and of LAMP‐2 in two other cell lines. (b) HeLa cells were analysed by confocal fluorescence microscopy using antibody to LAMP‐1 or LAMP‐2, Alexa Fluor 488‐conjugated anti‐mouse IgG (green), and DAPI (blue) for DNA, with 63× objective. Scale bar = 20 μm. (c) LAMP‐depleted HeLa cells were analysed by confocal fluorescence microscopy using antibody to LAMP‐1 or LAMP‐2, as in (b). WT: wild‐type
Figure 3.
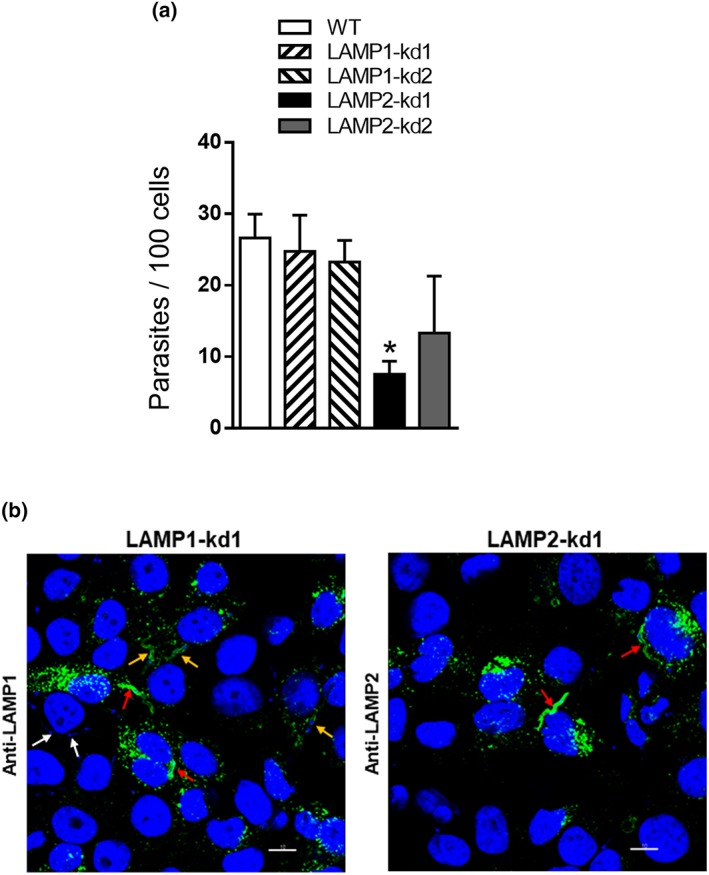
Reduced susceptibility of LAMP‐2‐deficient HeLa cells to metacyclic trypomastigote (MT) invasion. (a) HeLa cells depleted in either LAMP‐1 or LAMP‐2 as well as wild‐type (WT) control were incubated for 1 hr with MT. The amounts of intracellular parasites are shown as means ± standard deviation of three independent assays performed in duplicate. MT invasion was significantly diminished in cells deficient in LAMP‐2 (* P < 0.005). (b) HeLa cells depleted in LAMP‐1 or LAMP‐2 were incubated for 30 min with MT and visualised by confocal microscopy, upon reaction with antibody to LAMP‐1 or LAMP‐2 as in Figure 2b, with 100× objective. Scale bar = 10 μm. Note that in the left panel, parasites internalised in cells expressing LAMP‐1 at higher levels (red arrows) and lower levels (orange arrows) or in LAMP1‐negative cells (white arrows). In the right panel, parasites internalised in a few LAMP‐2‐positive cells (red arrows) are shown
2.2. MT gp82 binds to LAMP‐2 protein in a receptor‐mediated manner
As our data indicated that gp82‐mediated MT invasion requires LAMP‐2, we examined the possibility that gp82 binds to LAMP‐2 by performing co‐immunoprecipitation assays. Protein A/G magnetic beads cross‐linked with antibody to LAMP‐1 (bead L1) or LAMP‐2 (bead L2) were incubated with HeLa cell extract for 1 hr, washed, and then incubated for 1 hr with MT lysate, followed by washings and elution. The eluted samples, which correspond to the immunoprecipitates (IPs), were analysed by Western blotting using antibodies to LAMP proteins or to gp82, along with flowthrough samples (Figure 4a). In IP from L1 beads, LAMP‐1 appeared as a strong band and gp82 as a faint band, and in IP from LAMP2 beads, both LAMP‐2 and gp82 were detected as bands of high intensity, whereas no protein was detectable in IP from control empty (V) beads (Figure 4a). Analysis of flowthrough from LAMP1 and LAMP2 beads showed that the amounts of HeLa cell extracts or MT lysates applied to beads were equivalent (Figure 4a). Binding of native gp82 to LAMP‐2 was further confirmed by two additional experiments. In one of them, new batches of beads L1 and L2 were prepared and used as described above. LAMP‐2 and gp82 were revealed in IP from L2 beads as strong bands, whereas gp82 was not detected in IP from L1 beads (Figure 4b). Another experiment consisted in preparing beads cross‐linked to anti‐gp82 monoclonal antibody and incubating them first with MT lysates and then with HeLa cell extracts (Figure 4c). Both LAMP‐2 and gp82 were detected in IP from gp82‐beads (Figure 4c). IP from L1 beads was also analysed using anti‐LAMP1 and anti‐gp82 antibodies. We detected LAMP‐1 but not gp82 in IP from L1 beads (Figure 4d).
Figure 4.
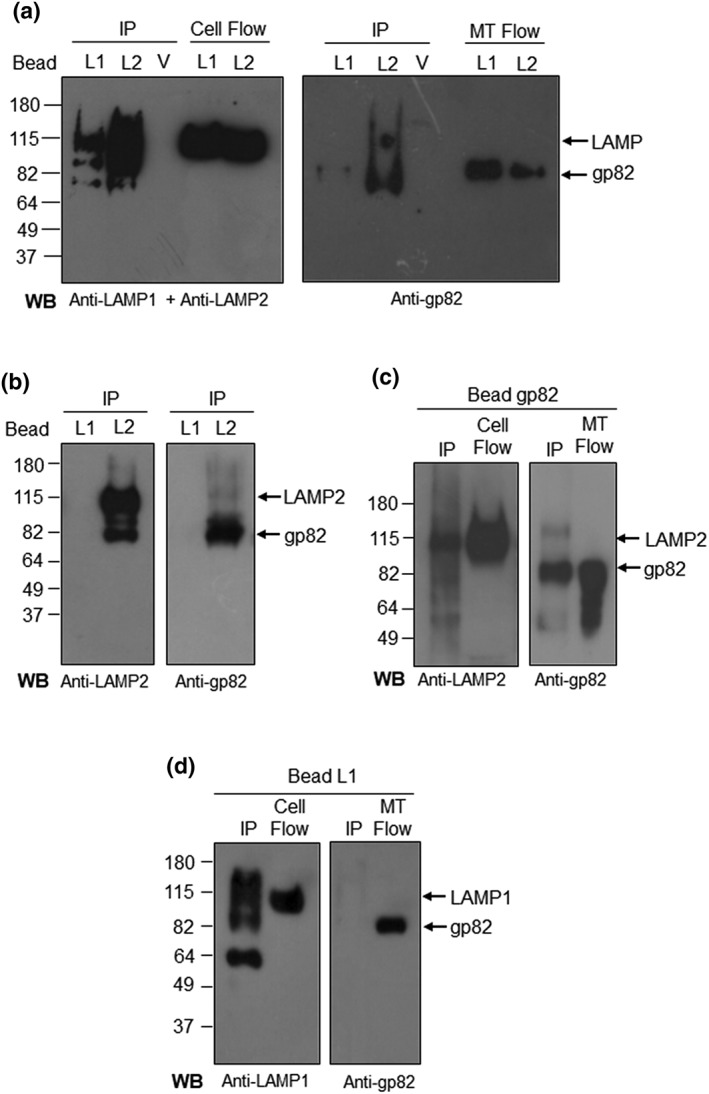
Binding of metacyclic trypomastigote (MT) gp82 to LAMP‐2. (a) Protein A/G magnetic beads, cross‐linked to antibody to LAMP‐1 or LAMP‐2, were incubated for 1 hr with HeLa cell extracts and afterwards with MT lysates for 1 hr. The eluates corresponding to immunoprecipitates (IPs) were analysed by Western blot (WB). Shown are the IP from LAMP1 beads (L1), LAMP2 beads (L2), control void beads (V), and the flowthrough samples from L1 and L2 beads corresponding to HeLa cell extract (cell flow) or MT lysate (MT flow). The blots were revealed with a mix of antibodies to LAMP‐1 and LAMP‐2 or with anti‐gp82 antibody. Note that both LAMP‐2 and gp82 were detected in IP from L2 beads. (b) The assay described in (a) was repeated with a new batch of protein A/G magnetic beads cross‐linked to antibody to LAMP‐1 or LAMP‐2. Note the presence of LAMP‐2 and gp82 in IP from L2 beads. No bands were detected in IP from L1 beads. (c) Protein A/G magnetic beads, cross‐linked to anti‐gp82 monoclonal antibody, were incubated with MT lysate for 1 hr, followed by 1‐hr incubation with HeLa cell extract. The WB of IP revealed LAMP‐2 and gp82. Shown also are the flowthorough samples corresponding to HeLa cell extract (cell flow) and MT lysate (MT flow). (d) LAMP1 beads were incubated with HeLa cell extract for 1 hr, followed by 1‐hr incubation with MT lysate. The WB of IP was revealed with antibody to LAMP‐1 and gp82. Also shown are the cell flow and MT flow, which served as control. Note that gp82 is absent in IP from L1 beads
To demonstrate that gp82 binds to LAMP‐2 in a receptor‐dependent manner, we performed an enzyme‐linked immunosorbent assay using microtitre plates coated with cells depleted in LAMP‐1 or LAMP‐2 and with wild‐type cells as control. The recombinant gp82 protein (r‐gp82) was added to cells, at varying concentrations, and after 1‐hr incubation, the binding was revealed as described in Section 4. Binding of r‐gp82 to cells was dose dependent and saturable and was much lower in LAMP‐2‐deficient cells than in wild‐type and LAMP‐1‐depleted cells (Figure 5a). Competition assay using anti‐LAMP antibody was also performed using wild‐type HeLa cells. Varying amounts of r‐gp82 were incubated for 1 hr with Hela cells in the presence of antibody to LAMP‐1 or LAMP‐2, at 10 μg ml−1 (Figure 5b). In the presence of anti‐LAMP2 antibody, r‐gp82 binding was much lower than in the presence of LAMP‐1 (Figure 5b), reinforcing the notion that LAMP‐2 is the receptor for gp82.
Figure 5.
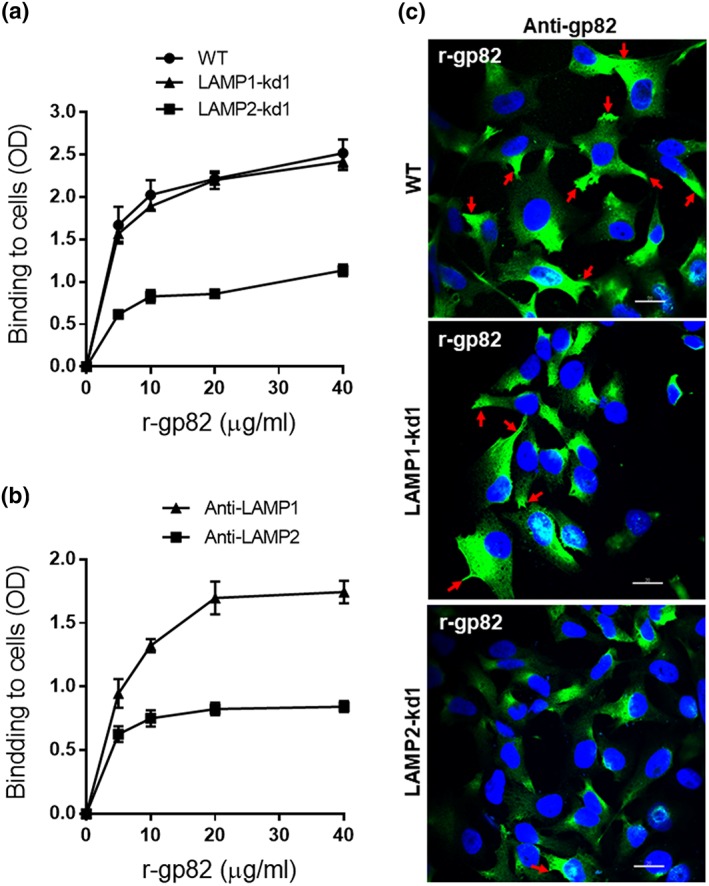
Binding of gp82 protein to wild‐type (WT) or LAMP‐deficient HeLa cells. (a) WT or LAMP‐kd cells were grown in enzyme‐linked immunosorbent assay microtitre plates, fixed, and incubated for 1 hr with r‐gp82 at indicated concentrations. Following washes in PBS and sequential incubation of cells for 1 hr at 37°C with anti‐gp82 antiserum and anti‐mouse IgG conjugated to peroxidase, the bound enzyme was revealed using o‐phenylenediamine. Cell binding is expressed as optical density (OD) value. A representative result of three independent assays performed in triplicate is shown. Note the lower binding of gp82 to LAMP2‐kd1 cells as compared with WT or LAMP1‐kd1 cells. (b) WT HeLa cells grown in enzyme‐linked immunosorbent assay microtitre plates were fixed and incubated for 1 hr with r‐gp82 at indicated concentrations, in the presence of antibody to LAMP‐1 or LAMP‐2. After washings, the reaction proceeded and was revealed as in (a). (c) WT and LAMP‐kd cells were incubated in the presence of r‐gp82, followed by reaction with anti‐gp82 monoclonal antibody and visualisation by confocal immunofluorescence, with 63× objective. Scale bar = 20 μm. Note the accumulation of r‐gp82 at the cell edges in WT and LAMP‐kd1 cells (red arrows)
Binding of gp82 to cells was also examined by confocal immunofluorescence. Cells were incubated for 1 hr with recombinant gp82 protein (r‐gp82) at 20 μg ml−1 and then processed for immunofluorescence using anti‐gp82 monoclonal antibody (Figure 5c). The r‐gp82 protein bound to wild‐type and LAMP1‐kd1 cells, the vast majority of cells showing a strong reaction with anti‐gp82 antibody, and noteworthy was the accumulation of r‐gp82 at the cell edges (Figure 5c, red arrows), which are the MT invasion sites (Mortara, 1991), suggesting a clustering of gp82 receptors at these sites. In LAMP2‐kd1 cells, fewer cells reacted with anti‐gp82 antibody, and r‐gp82 accumulation at the cell periphery was less evident (Figure 5c, lower panel).
An experiment was performed to ascertain that r‐gp82 induces lysosome scattering, as previously reported (Cortez et al., 2016). HeLa cells were incubated for 30 min with r‐gp82 at 20 μg ml−1, followed by reaction with anti‐LAMP2 antibody and confocal microscopy analysis. Lysosome spreading from the perinuclear region to the cell periphery was induced by r‐gp82, and accumulation of LAMP‐2 on the cell borders was clearly visualised (Figure S3, red arrows). We examined the localization of LAMP‐2 at the HeLa cell plasma membrane upon interaction with r‐gp82. HeLa cells were incubated in the absence or in the presence of 20 μg ml−1 of r‐gp82 for 30 min, followed by reaction with rabbit antibody to LAMP‐2 and mouse anti‐HeLa cell antibody that predominantly recognises the plasma membrane. The second antibody consisted of Alexa Fluor 555‐conjugated anti‐rabbit IgG and Alexa Fluor 488‐conjugated anti‐mouse IgG. Localization of LAMP‐2 at the plasma membrane increased upon interaction with r‐gp82 (Figure S4, white arrows).
2.3. Invasion of extracellular amastigote increases in LAMP‐depleted cells
Cell invasion experiment with LAMP‐depleted cells was also performed with extracellular amastigote (EA) derived from TCT. Differently from MT invasion, EA internalisation of HeLa cells is independent of lysosome mobilisation (Procópio, Da Silva, Cunningham, & Mortara, 1998). HeLa cells were incubated with EA for 2 hr, and the number of intracellular parasites was counted. As shown in Figure 6a, the number of parasites that entered cells deficient in LAMP‐1 or LAMP‐2 increased significantly as compared with that internalised by wild‐type cells. For confocal microscopy, wild‐type HeLa cells were incubated for 1 hr with EA, followed by reaction with anti‐LAMP antibody. There was no association of EA with lysosome marker during invasion (Figure 6b, red arrows), differently from MT internalisation (Figure 3b). This is compatible with the report that, during HeLa invasion of EA, which depends on the formation of plasma membrane expansions, the parasites at the cell periphery are weakly labelled with anti‐LAMP antibody (Procópio et al., 1998). The formation of cup‐like projections around the invading EA (Procópio, Barros, & Mortara, 1999) may be affected by lysosomes.
Figure 6.
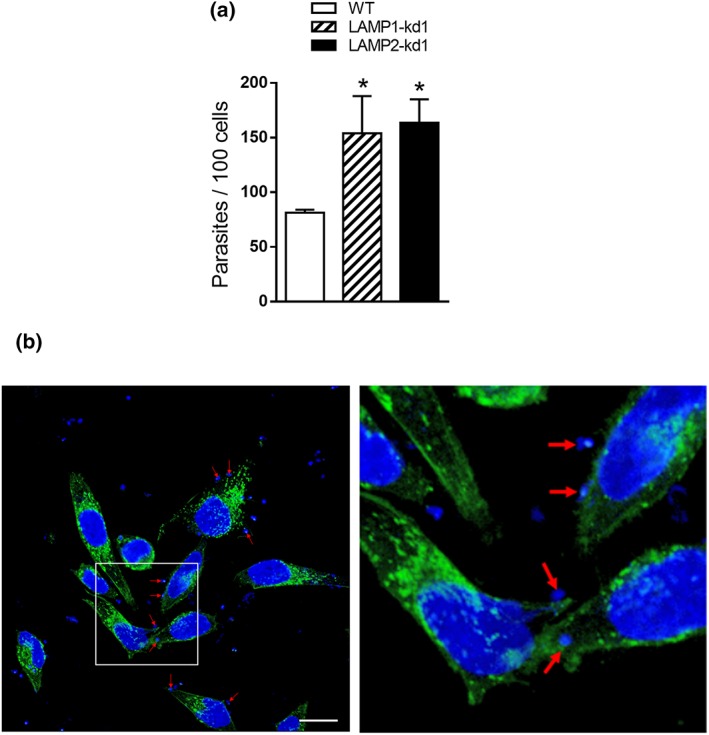
Increased extracellular amastigote (EA) internalisation in LAMP‐depleted HeLa cells. (a) HeLa cells depleted in either LAMP‐1 or LAMP‐2 as well as the wild‐type (WT) controls were incubated for 2 hr with EA. After fixation and Giemsa staining, the internalised parasites were quantified in at least 300 cells per replicate. Values are the means ± standard deviation of three independent assays performed in triplicate. EA invasion increased significantly in LAMP‐depleted cells (* P < 0.05). (b) Wild‐type HeLa cells were visualised by confocal microscopy after 1‐hr incubation with EA and reaction with anti‐LAMP2 antibody, as in Figure 2b, with 63× objective. Scale bar = 20 μm. Note the lack of association of EA with lysosome marker, more clearly visible in the magnified field (right panel), corresponding to the framed region (left panel)
3. DISCUSSION
The involvement of host cell LAMP‐2 in gp82‐mediated MT invasion was revealed in experiments using cells depleted in LAMP‐1 or LAMP‐2. Our results have also shown that the MT surface molecule gp82 binds to LAMP‐2, suggesting that LAMP‐2 is the long sought after receptor for gp82. In HeLa cells cultured in complete medium, lysosomes accumulate in the perinuclear region. Therefore, the levels of LAMP proteins on the cell surface are probably very low. LAMP proteins have been detected on the plasma membrane of other human cell lines, and their expression was shown to increase after exposure to a lysosomotropic reagent (Mane et al., 1989). In HeLa cells, a short‐term incubation in nutrient‐depleted medium, a condition that promotes lysosome scattering to the cell periphery and exocytosis, stimulates gp82‐mediated MT invasion (Cortez et al., 2016). Experiments with the recombinant gp82 protein have shown that it can induce lysosome spreading and accumulation of LAMP‐2 protein at the MT invasion sites. On the basis of these findings, we envision a scenario (Figure 7) in which MT attachment to host cells relies on gp82 recognition by LAMP‐2 present at low levels on the cell surface. This attachment triggers the lysosome mobilisation towards the cell periphery, further increasing the LAMP‐2 protein available at the plasma membrane.
Figure 7.
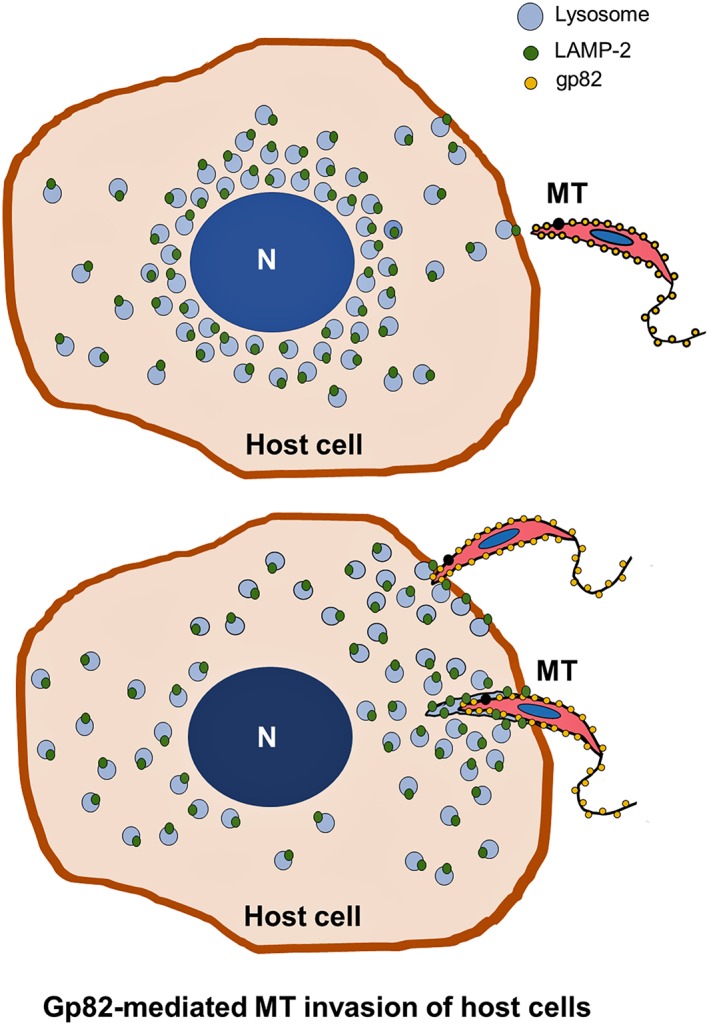
Model for host cell invasion by T. cruzi metacyclic trypomastigote (MT) mediated by gp82 binding to LAMP‐2 protein. MT gp82 binds to host cell LAMP‐2, present at low levels at the plasma membrane, and triggers lysosome scattering to the cell periphery. This increases the availability of LAMP‐2 as receptor for gp82 and further promotes MT internalisation within a vacuole resulting from the fusion of lysosomes with the plasma membrane
When the mode of MT invasion is compared with that of TCT, considerable differences emerge. In the case of TCT, only a minor proportion of parasites associate with lysosomes, the majority acquires plasma membrane markers at early times of infection (Woolsey et al., 2003). However, the involvement of LAMP proteins has been reported. Increased expression of LAMP‐1 on the plasma membrane of CHO cells was associated with higher susceptibility to TCT invasion (Kima, Burleigh, & Andrews, 2000). LAMP‐1/2 double‐deficient mouse fibroblasts were shown to be less susceptible to TCT invasion (Albertti, Macedo, Chiari, Andrews, & Andrade, 2010). Recently, it was reported that mouse fibroblasts deficient in LAMP‐2 were less permissive to invasion by TCT because LAMP‐2 absence influences the distribution of caveolin‐1 at the cell plasma membrane, which is crucial for plasma membrane repair (Couto et al., 2017). The mechanism of TCT internalisation relies on plasma membrane injury and repair that involves lysosome exocytosis (Fernandes et al., 2011). Our results have clearly indicated that MT invasion is independent of plasma membrane injury and repair mechanism. Invasion by MT and TCT also differs as concerns the lysosome mobilisation. MT induces spreading of lysosomes from the perinuclear region to the cell periphery (Cortez et al., 2016), whereas only the lysosomes proximal to TCT invasion site are mobilised (Rodríguez et al., 1996).
Through distinct mechanisms, MT and TCT enter host cells in a manner dependent on lysosome mobilisation and exocytosis, but invasion by EA is lysosome independent (Procópio et al., 1998). This may be related to the differential interaction of these parasite forms with the target cell membrane domains. Microvilli on the dorsal surface are the sites of EA invasion, whereas MT and TCT interaction with cells occurs at the cell edges (Mortara, 1991; Procópio et al., 1999; Schenkman, Andrews, Nussenzweig, & Robbins, 1988). Disruption of host cell actin microfilaments is induced by MT and TCT (Cortez, Atayde, & Yoshida, 2006; Rodríguez et al., 1995), and this may facilitate lysosome mobilisation. On the other hand, actin microfilaments are recruited during EA invasion, and actin‐enriched cup‐like membrane protrusions are seen around the parasite (Procópio et al., 1998).
From the present studies on MT invasion and from available data on EA internalisation, we infer that the different T. cruzi forms have developed specific strategies to enter target cells, making use of stage‐specific surface molecules to interact with host cells, such as MT gp82 and EA Ssp4. The amastigote‐specific glycoprotein Ssp4 (Andrews, Hong, Robbins, & Nussenzweig, 1987; Andrews, Robbins, Ley, Hong, & Nussenzweig, 1988) binds to target cells through its carbohydrate moiety (Silva, Luquetti, Rassi, & Mortara, 2006), and this leads to the parasite engulfment that resembles a phagocytic process (Bonfim‐Melo, Ferreira, & Mortara, 2018; Fernandes, Flannery, Andrews, & Mortara, 2013). It has been reported that galectin‐3, a member of β‐galactosidase‐binding lectin family, which accumulates around invading EA in macrophages (Machado et al., 2014), increased by about 20% in HeLa cells during the phagocytic cup formation and apparently bound to Ssp‐4 (Florentino et al., 2018). MT gp82 binds to HeLa cells through its peptide portion (Manque et al., 2000). Whether gp82 binds to a peptide sequence or to a carbohydrate portion of the highly glycosylated LAMP‐2 protein remains to be determined.
4. EXPERIMENTAL PROCEDURES
4.1. Parasites, cell lines, and cell invasion assay
T. cruzi strain CL was used for assays with MT. The parasites were maintained alternately in mice and in liver infusion tryptose medium containing 5% fetal bovine serum (FBS), following experimental protocols approved by the Committee on Ethics of Animal Experimentation of Universidade Federal de São Paulo (CEUA 9933271016). After one passage in Grace's medium (Invitrogen) to differentiate epimastigotes into MT, the parasites were purified as described (Teixeira & Yoshida, 1986). In addition to human epithelial HeLa cells, maintained as described (Rodrigues, Takahashi Sant'ana, Juliano, & Yoshida, 2017), mouse fibroblast cell lines deficient in LAMP‐1, LAMP‐2, or double knockout cells, generated in Dr Paul Saftig's laboratory at University of Kiel, Germany, were used. Cell invasion assays were performed in Dulbecco's modified Eagle's medium containing 10% FBS, unless stated otherwise, following a procedure described else (Rodrigues et al., 2017), by incubating HeLa cells for 1 hr with MT at multiplicity of infection = 10. Two hundred fifty Giemsa‐stained cells were counted to quantify internalised MT. T. cruzi G strain was used for assays with EA, generated as previously described (Bonfim‐Melo et al., 2015). Briefly, TCTs derived from Vero cells were incubated for 14 hr in liver infusion tryptose medium with 10% FBS, pH 5.8. Amastigotes were incubated in complete Roswell Park Memorial Institute medium at pH 7.3 for 1 hr and were then seeded onto each coverslip coated with 1.5 × 105 HeLa cells (multiplicity of infection = 10). After 2‐hr incubation, the coverslips were washed, fixed, and Giemsa stained. Internalised EA amastigotes were quantified in at least 300 cells per replicate.
4.2. Anti‐LAMP antibodies
Anti‐LAMP antibodies were from two sources: antibody produced in rabbit (Sigma‐Aldrich, now Merck) and anti‐human LAMP1 (H4A3) and LAMP2 (H4B4) antibodies from Developmental Studies Hybridoma Bank developed under the auspices of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biology, Iowa City, IA.
4.3. Lentiviral transduction and establishment of HeLa cell lines deficient in LAMP‐1 or LAMP‐2
To generate LAMP‐deficient cell lines, we used a strategy based on a previously described procedure (Bonfim‐Melo et al., 2015). Target sequences were acquired from Sigma‐Aldrich: LAMP‐1 (Cat Nos. TRCN0000285264 and TRCN0000029268) and LAMP‐2 (Cat Nos. TRCN0000029260 and TRCN0000029262). For lentivirus packaging and production, HEK293T cells were plated in six‐well plates (2.5 × 105 cells per well) and incubated for 24 hr in 2 ml of Dulbecco's modified Eagle's medium containing 10% FBS. Transfection solution was prepared as follows: 2.5 μl of FuGENE HD (Roche) was diluted in 97.5 μl of Opt‐MEM (Gibco™), mixed gently by swirling, and incubated for 5 min at room temperature. In another tube, 0.75 μg of pLKO.1 (vector containing shRNA target sequence) and 0.75 μg of lentiviral packaging (composed of equal parts of pCMV‐dR8.91 and pVSVG at 0.25 μg μl−1) were mixed. The lentiviral preparation and the transfection solution were mixed gently, incubated for 30 min at room temperature, and added to HEK293T. Twenty‐four hours later, the medium was discarded, and 3 ml of fresh medium was added. After 48 and 72 hr, the cell culture supernatant was harvested and filtered in a 0.45‐μm syringe filter to remove debris. For shRNAi transduction, 5 × 104 HeLa cells were plated in six‐well plates, and 2 ml of HEK293T culture medium enriched in lentivirus was added to each well in the presence of 8 μg ml−1 of hexadimethrine bromide (commercial brand name Polybrene; Sigma‐Aldrich). After 48 hr, HeLa cells were incubated with increasing concentrations of puromycin (0.2–2 μg ml−1) for 2 weeks in order to select transduced cells. To evaluate the transduction efficiency, the cells were washed with PBS and lysis buffer added (50 mM tris–HCl, 150 mM NaCl, 1 mM EDTA, 1% Igepal CA630, and 1× protease inhibitor cocktail, Thermo Scientific). After centrifugation at 16,000 × g for 5 min, the supernatant was collected and quantified by the Bradford method. Cell lysate (30 μg protein) was applied onto a 10% SDS‐PAGE gel and analysed by Western blot using antibody to LAMP‐1 or LAMP‐2 diluted 1:1,000 plus anti‐GAPDH as loading control diluted 1:5,000.
4.4. Visualisation of HeLa cell lysosomes by confocal microscopy
For confocal microscopy visualisation of lysosomes, HeLa cells were processed essentially as previously described, using anti‐human LAMP2 antibody and Alexa Fluor 488‐conjugated anti‐mouse IgG (Rodrigues et al., 2017). The coverslips were mounted in ProLong Gold (Invitrogen). Confocal images were acquired in a confocal microscope (Instituto de Farmacologia e Biologia Molecular, Universidade Federal de São Paulo), using 63× objective. Confocal images were processed and analysed using Leica LAS AF and Imaris (Bitplane) software.
4.5. Co‐immunoprecipitation and Western blotting
Protein A/G magnetic beads (Pierce Crosslink Magnetic IP/Co_IP Kit, Thermo Scientific) were cross‐linked with antibody directed to LAMP‐1 or LAMP‐2 according to the manufacturer's instructions. HeLa cell extracts were prepared by treating 3 × 106 cells with PBS plus 0.5% nonionic detergent Igepal CA630 (USB Corporation) and protease inhibitor cocktail. After centrifugation at 16,000 × g for 10 min, the supernatant was collected and used for co‐immunoprecipitation assays. MT extract, equivalent to 5 × 108 parasites, was prepared in the same manner as HeLa cell extract. Beads cross‐linked to anti‐LAMP antibody were incubated for 1 hr at room temperature with HeLa cell extracts, under agitation. After several washes in PBS containing 0.25% Igepal CA630 and 1‐hr incubation with MT extract, the bound proteins were eluted and analysed by Western blotting, using antibody to LAMP‐1, LAMP‐2, or anti‐gp82 monoclonal antibody. Protein G magnetic beads cross‐linked with monoclonal antibody to gp82 were also prepared; in this case, the beads were incubated first with MT extract and subsequently with HeLa cell lysates. The bound proteins were eluted and analysed by Western blotting.
4.6. Production and purification of recombinant protein gp82 (r‐gp82) and cell‐binding assay
The recombinant protein coded by the full‐length T. cruzi gp82 sequence in frame with glutathione S‐transferase (GenBank™ data base, Accession Number L14824) was produced and purified as detailed (Cortez et al., 2006). For cell‐binding assay, HeLa cells were seeded onto 96‐well microtitre plates at 4 × 104 cells per well and were grown overnight at 37°C. After fixation with 4% paraformaldehyde in PBS, washings with PBS, and blocking with PBS containing 2 mg ml−1 of BSA (PBS‐BSA) for 1 hr at room temperature, the cells were incubated for 1 hr at 37°C with r‐gp82 in PBS‐BSA. Following washes and 1‐hr incubation with anti‐gp82 polyclonal antiserum diluted in PBS‐FBS, the cells were incubated with anti‐mouse IgG conjugated to peroxidase. The bound enzyme was revealed using o‐phenylenediamine, and the absorbance at 490 nm was read in ELx800™ microplate reader (BioTek).
4.7. Statistical analysis
The Student's t‐test (GraphPad software Version 6.01) was employed.
CONFLICT OF INTEREST
We declare that we do not have any potential sources of conflict of interest.
Supporting information
Fig. S1. Incorporation of propidium iodide after treatment of HeLa cells with SLO. HeLa cells were treated for 20 min with 50 ng/ml SLO and 25 μg/ml propidium iodide in PBS++ without Ca 2+. After processing for immunofluorescence, the cells were visualized under Olympus BX51 microscope, with 40x objective and the images were acquired using Olympus DP71 camera and the software Image‐Pro Plus® Version 6.2.0.424 for Windows 2000/XP Professional.
Fig S2. Partial depletion of LAMP‐2 in HeLa cells. The indicated HeLa cells knocked down (kd) in LAMP‐1 or LAMP‐2 were analyzed by western blotting using anti‐LAMP2 antibody. Note the partial depletion of LAMP‐2 in LAMP2‐kd2 lineage.
Fig. S3. HeLa cell lysosome scattering induced by r‐gp82. A. Hela cells were incubated for 30 min in absence or in the presence of r‐gp82, followed by reaction with anti‐LAMP2 antibody and visualization by confocal immunofluorescence, with 63x objective. Scale bar = 30 μm. Note the spreading of lysosomes and accumulation in the cell periphery upon interaction with r‐gp82 (red arrows).
Fig. S4. Increased association of LAMP‐2 with HeLa cell plasma membrane upon interaction with r‐gp82. Hela cells were incubated for 30 min in absence or in the presence of r‐gp82, followed by reaction with rabbit antibody to LAMP‐2 and mouse anti‐HeLa cell antibody that predominantly recognizes the plasma membrane. After reaction with the second antibody, which consisted of Alexa Fluor 555‐conjugated anti‐rabbit IgG (red) and Alexa Fluor 488‐conjugated anti‐mouse IgG (green), the cells were visualized at the confocal microscope (Leica SP, with objective 63X. Scale bar = 20 nm. Note the increased localization of LAMP‐2 at the plasma membrane (white arrows) after interaction with r‐gp82.
ACKNOWLEDGEMENTS
This work was supported by the São Paulo Research Foundation (FAPESP) Grant 2016/15000‐4 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Grant 303825/2015‐4 and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) Finance Code 001. We thank Silene Macedo for help in preparing the policlonal antiserum to the gp82 protein.
Rodrigues JPF, Souza Onofre T, Barbosa BC, Ferreira ÉR, Bonfim‐Melo A, Yoshida N. Host cell protein LAMP‐2 is the receptor for Trypanosoma cruzi surface molecule gp82 that mediates invasion. Cellular Microbiology. 2019;21:e13003 10.1111/cmi.13003
REFERENCES
- Albertti, L. A. G. , Macedo, A. M. , Chiari, E. , Andrews, N. W. , & Andrade, L. O. (2010). Role of host lysosomal associated membrane protein (LAMP) in Trypanosoma cruzi invasion and intracellular development. Microbes and Infection, 12, 784–789. 10.1016/j.micinf.2010.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, M. J. M. , & Colli, W. (2007). Trypanosoma cruzi: Adhesion to the host cell and intracellular survival. IUBMB Life, 59, 274–279. 10.1080/15216540701200084 [DOI] [PubMed] [Google Scholar]
- Andrews, N. W. , Hong, K. S. , Robbins, E. S. , & Nussenzweig, V. (1987). Stage‐specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi . Experimental Parasitology, 64, 474–484. 10.1016/0014-4894(87)90062-2 [DOI] [PubMed] [Google Scholar]
- Andrews, N. W. , Robbins, E. S. , Ley, V. , Hong, K. S. , & Nussenzweig, V. (1988). Developmentally regulated, phospholipase C‐mediated release of the major surface glycoprotein of amastigotes of Trypanosoma cruzi . Journal of Experimental Medicine, 167, 300–314. 10.1084/jem.167.2.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfim‐Melo, A. , Ferreira, É. R. , & Mortara, R. A. (2018). Rac1/WAVE2 and Cdc42/N‐WASP participation in actin‐dependent host cell invasion by extracellular amastigotes of Trypanosoma cruzi . Frontiers in Microbiology, 9, 360 10.3389/fmicb.2018.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfim‐Melo, A. , Zanetti, B. F. , Ferreira, E. R. , Vandoninck, S. , Han, S. W. , Van Lint, J. , … Bahia, D. (2015). Trypanosoma cruzi extracellular amastigotes trigger the protein kinase D1–cortactin–actin pathway during cell invasion. Cellular Microbiology, 17, 1797–1810. 10.1111/cmi.12472 [DOI] [PubMed] [Google Scholar]
- Cortez, C. , Real, F. , & Yoshida, N. (2016). Lysosome biogenesis/scattering increases host cell susceptibility to invasion by Trypanosoma cruzi metacyclic forms and resistance to tissue culture trypomastigotes. Cellular Microbiology, 18, 748–760. 10.1111/cmi.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez, C. , Yoshida, N. , Bahia, D. , & Sobreira, T. J. P. (2012). Structural basis of the interaction of a Trypanosoma cruzi surface molecule implicated in oral infection with host cells and gastric mucin. PLoS One, 7, e42153 10.1371/journal.pone.0042153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez, M. , Atayde, V. , & Yoshida, N. (2006). Host cell invasion mediated by Trypanosoma cruzi surface molecule gp82 is associated with F‐actin disassembly and is inhibited by enteroinvasive Escherichia coli . Microbes and Infection, 8, 1502–1512. 10.1016/j.micinf.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Cossart, P. , Pizarro‐Cerdá, J. , & Lecuit, M. (2003). Invasion of mammalian cells by Listeria monocytogenes: Functional mimicry to subvert cellular functions. Trends in Cell Biology, 13, 23–31. 10.1016/S0962-8924(02)00006-5 [DOI] [PubMed] [Google Scholar]
- Cossart, P. , & Sansonetti, P. J. (2004). Bacterial invasion: The paradigms of enteroinvasive pathogens. Science, 304, 242–248. 10.1126/science.1090124 [DOI] [PubMed] [Google Scholar]
- Couto, N. F. , Pedersane, D. , Rezende, L. , Dias, P. P. , Corbani, T. L. , Bentini, L. C. , … Andrade, L. O. (2017). LAMP‐2 absence interferes with plasma membrane repair and decreases T. cruzi host cell invasion. PLoS Neglected Tropical Diseases, 11, e0005657 10.1371/journal.pntd.0005657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen, E.‐L. , Schmidt, C. K. , Neu, S. , Willenborg, M. , Fuertes, G. , Salvador, N. , … Saftig, P. (2004). Disturbed cholesterol traffic but normal proteolytic function in LAMP‐1/LAMP‐2 double‐deficient fibroblasts. Molecular Biology of the Cell, 15, 3132–3145. 10.1091/mbc.E04-02-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, M. C. , Cortez, M. , Flannery, A. R. , Tam, C. , Mortara, R. a. , & Andrews, N. W. (2011). Trypanosoma cruzi subverts the sphingomyelinase‐mediated plasma membrane repair pathway for cell invasion. The Journal of Experimental Medicine, 208, 909–921. 10.1084/jem.20102518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, M. C. , Flannery, A. R. , Andrews, N. , & Mortara, R. A. (2013). Extracellular amastigotes of Trypanosoma cruzi are potent inducers of phagocytosis in mammalian cells. Cellular Microbiology, 15, 977–991. 10.1111/cmi.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentino, P. T. V. , Real, F. , Orikaza, C. M. , da Cunha, J. P. C. , Vitorino, F. N. L. , Cordero, E. M. , … Mortara, R. A. (2018). A carbohydrate moiety of secreted stage‐specific glycoprotein 4 participates in host cell invasion by Trypanosoma cruzi extracellular amastigotes. Frontiers in Microbiology, 9, 693 10.3389/fmicb.2018.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano, R. , Fouts, D. L. , Tewari, D. , Colli, W. , Manning, J. E. , & Alves, M. J. (1999). Cloning of a surface membrane glycoprotein specific for the infective form of Trypanosoma cruzi having adhesive properties to laminin. The Journal of Biological Chemistry, 274, 3461–3468. 10.1074/jbc.274.6.3461 [DOI] [PubMed] [Google Scholar]
- Isberg, R. R. , & Barnes, P. (2001). Subversion of integrins by enteropathogenic Yersinia . Journal of Cell Science, 114, 21–28. [DOI] [PubMed] [Google Scholar]
- Khusal, K. G. , Tonelli, R. R. , Mattos, E. C. , Soares, C. O. , Di Genova, B. M. , Juliano, M. A. , … Alves, M. J. (2015). Prokineticin receptor identified by phage display is an entry receptor for Trypanosoma cruzi into mammalian cells. Parasitology Research, 114, 155–165. 10.1007/s00436-014-4172-6 [DOI] [PubMed] [Google Scholar]
- Kima, P. E. , Burleigh, B. , & Andrews, N. W. (2000). Surface‐targeted lysosomal membrane glycoprotein‐1 (Lamp‐1) enhances lysosome exocytosis and cell invasion by Trypanosoma cruzi . Cellular Microbiology, 2, 477–486. 10.1046/j.1462-5822.2000.00071.x [DOI] [PubMed] [Google Scholar]
- Machado, F. C. , Cruz, L. , da Silva, A. A. , Cruz, M. C. , Mortara, R. A. , Roque‐Barreira, M. C. , & da Silva, C. V. (2014). Recruitment of galectin‐3 during cell invasion and intracellular trafficking of Trypanosoma cruzi extracellular amastigotes. Glycobiology, 24, 179–184. 10.1093/glycob/cwt097 [DOI] [PubMed] [Google Scholar]
- Mane, S. M. , Marzella, L. , Bainton, D. F. , Holt, V. K. , Cha, Y. , Hildreth, J. E. , & August, J. T. (1989). Purification and characterization of human lysosomal membrane glycoproteins. Archives of Biochemistry and Biophysics, 268, 360–378. 10.1016/0003-9861(89)90597-3 [DOI] [PubMed] [Google Scholar]
- Manque, P. M. , Eichinger, D. , Juliano, M. a. , Juliano, L. , Araya, J. E. , & Yoshida, N. (2000). Characterization of the cell adhesion site of Trypanosoma cruzi metacyclic stage surface glycoprotein gp82. Infection and Immunity, 68, 478–484. 10.1128/IAI.68.2.478-484.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, R. M. , Alves, R. M. , Macedo, S. , & Yoshida, N. (2011). Starvation and rapamycin differentially regulate host cell lysosome exocytosis and invasion by Trypanosoma cruzi metacyclic forms. Cellular Microbiology, 13, 943–954. 10.1111/j.1462-5822.2011.01590.x [DOI] [PubMed] [Google Scholar]
- Mortara, R. A. (1991). Trypanosoma cruzi: Amastigotes and trypomastigotes interact with different structures on the surface of HeLa cells. Experimental Parasitology, 73, 1–14. 10.1016/0014-4894(91)90002-E [DOI] [PubMed] [Google Scholar]
- Ouaissi, M. A. , Cornette, J. , & Capron, A. (1986). Identification and isolation of Trypanosoma cruzi trypomastigote cell surface protein with properties expected of a fibronectin receptor. Molecular and Biochemical Parasitology, 19, 201–211. 10.1016/0166-6851(86)90002-2 [DOI] [PubMed] [Google Scholar]
- Procópio, D. O. , Barros, H. C. , & Mortara, R. A. (1999). Actin‐rich structures formed during the invasion of cultured cells by infective forms of Trypanosoma cruzi . European Journal of Cell Biology, 78, 911–924. 10.1016/S0171-9335(99)80093-4 [DOI] [PubMed] [Google Scholar]
- Procópio, D. O. , Da Silva, S. , Cunningham, C. C. C. , & Mortara, R. A. (1998). Trypanosoma cruzi: Effect of protein kinase inhibitors and cytoskeletal protein organization and expression on host cell invasion by amastigotes and metacyclic trypomastigotes. Experimental Parasitology, 90, 1–13. 10.1006/expr.1998.4314 [DOI] [PubMed] [Google Scholar]
- Ramirez, M. I. , de C Ruiz, R. , Araya, J. E. , Da Silveira, J. F. , & Yoshida, N. (1993). Involvement of the stage‐specific 82‐kilodalton adhesion molecule of Trypanosoma cruzi metacyclic trypomastigotes in host cell invasion. Infection and Immunity, 61, 3636–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, J. P. F. , Takahashi Sant'ana, G. H. , Juliano, M. A. , & Yoshida, N. (2017). Inhibition of host cell lysosome spreading by Trypanosoma cruzi metacyclic stage‐specific surface molecule gp90 downregulates parasite invasion. Infection and Immunity, 85(9), e00302‐17 10.1128/IAI.00302-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, A. , Rioult, M. G. , Ora, A. , & Andrews, N. W. (1995). A trypanosome‐soluble factor induces IP3 formation, intracellular Ca2+ mobilization and microfilament rearrangement in host cells. The Journal of Cell Biology, 129, 1263–1273. 10.1083/jcb.129.5.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, A. , Samoff, E. , Rioult, M. G. , Chung, A. , & Andrews, N. W. (1996). Host cell invasion by trypanosomes requires lysosomes and microtubule/kinesin‐mediated transport. The Journal of Cell Biology, 134, 349–362. 10.1083/jcb.134.2.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman, S. , Andrews, N. W. , Nussenzweig, V. , & Robbins, E. S. (1988). Trypanosoma cruzi invade a mammalian epithelial cell in a polarized manner. Cell, 55, 157–165. 10.1016/0092-8674(88)90018-9 [DOI] [PubMed] [Google Scholar]
- Silva, C. V. , Luquetti, A. O. , Rassi, A. , & Mortara, R. A. (2006). Involvement of Ssp‐4‐related carbohydrate epitopes in mammalian cell invasion by Trypanosoma cruzi amastigotes. Microbes and Infection, 8, 2120–2129. [DOI] [PubMed] [Google Scholar]
- Staquicini, D. I. , Martins, R. M. , Macedo, S. , Sasso, G. R. S. , Atayde, V. D. , Juliano, M. A. , & Yoshida, N. (2010). Role of GP82 in the selective binding to gastric mucin during oral infection with Trypanosoma cruzi . PLoS Neglected Tropical Diseases, 4, e613 10.1371/journal.pntd.0000613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira, M. M. , & Yoshida, N. (1986). Stage‐specific surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi identified by monoclonal antibodies. Molecular and Biochemical Parasitology, 18, 271–282. 10.1016/0166-6851(86)90085-X [DOI] [PubMed] [Google Scholar]
- Woolsey, A. M. , Sunwoo, L. , Petersen, C. A. , Brachmann, S. M. , Cantley, L. C. , & Burleigh, B. A. (2003). Novel PI 3‐kinase‐dependent mechanisms of trypanosome invasion and vacuole maturation. Journal of Cell Science, 116, 3611–3622. 10.1242/jcs.00666 [DOI] [PubMed] [Google Scholar]
- Yoshida, N. (2006). Molecular basis of mammalian cell invasion by Trypanosoma cruzi . Anais da Academia Brasileira de Ciências, 78, 87–111. 10.1590/S0001-37652006000100010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Incorporation of propidium iodide after treatment of HeLa cells with SLO. HeLa cells were treated for 20 min with 50 ng/ml SLO and 25 μg/ml propidium iodide in PBS++ without Ca 2+. After processing for immunofluorescence, the cells were visualized under Olympus BX51 microscope, with 40x objective and the images were acquired using Olympus DP71 camera and the software Image‐Pro Plus® Version 6.2.0.424 for Windows 2000/XP Professional.
Fig S2. Partial depletion of LAMP‐2 in HeLa cells. The indicated HeLa cells knocked down (kd) in LAMP‐1 or LAMP‐2 were analyzed by western blotting using anti‐LAMP2 antibody. Note the partial depletion of LAMP‐2 in LAMP2‐kd2 lineage.
Fig. S3. HeLa cell lysosome scattering induced by r‐gp82. A. Hela cells were incubated for 30 min in absence or in the presence of r‐gp82, followed by reaction with anti‐LAMP2 antibody and visualization by confocal immunofluorescence, with 63x objective. Scale bar = 30 μm. Note the spreading of lysosomes and accumulation in the cell periphery upon interaction with r‐gp82 (red arrows).
Fig. S4. Increased association of LAMP‐2 with HeLa cell plasma membrane upon interaction with r‐gp82. Hela cells were incubated for 30 min in absence or in the presence of r‐gp82, followed by reaction with rabbit antibody to LAMP‐2 and mouse anti‐HeLa cell antibody that predominantly recognizes the plasma membrane. After reaction with the second antibody, which consisted of Alexa Fluor 555‐conjugated anti‐rabbit IgG (red) and Alexa Fluor 488‐conjugated anti‐mouse IgG (green), the cells were visualized at the confocal microscope (Leica SP, with objective 63X. Scale bar = 20 nm. Note the increased localization of LAMP‐2 at the plasma membrane (white arrows) after interaction with r‐gp82.


