Abstract
Objectives
Older age and major depressive disorder (MDD) are both risk factors for the development of cardiovascular diseases. Testosterone has been associated with MDD and metabolic syndrome (MetS) in men, although associations in women are less clear. Therefore, we investigated whether testosterone is associated with MetS and whether this association is different for depressed and non‐depressed older men and women.
Methods
In this prospective cohort study, 478 participants (349 patients with MDD and 129 controls) aged between 60 and 93 years from the Netherlands Study of Depression in Older Persons were included. Total testosterone (TT) and sex‐hormone binding globulin levels were measured using a second‐generation radioimmune assay. Free testosterone (FT) was calculated based on TT. MetS was defined according to the National Cholesterol Education Program Adult Treatment Panel III criteria.
Results
A higher risk for MetS was found in men with low FT and TT (odds ratio [OR]: 0.67, 95% confidence interval [95%CI]: 0.47‐0.95 and OR: 0.51, 95%CI: 0.34‐0.75), and in women with high FT (OR: 1.41, 95%CI: 1.08‐1.82). Strong associations in the same direction were found with adiposity, glucose, and plasma lipid MetS components at baseline, but not with changes in these components at 2‐year follow‐up. The associations did not significantly differ between MDD patients and controls.
Conclusions
Independently of having MDD, low testosterone levels in men and, in contrast, high testosterone levels in women were significantly associated with MetS and its components.
Keywords: depressive disorder, major, metabolic syndrome, older adults, testosterone
Key points.
Low free and total testosterone levels in men and in contrast high free testosterone levels in women are associated with the metabolic syndrome and its components in older adults.
Depression does not alter the association between testosterone and metabolic syndrome.
1. INTRODUCTION
The older population shows the highest prevalence of the metabolic syndrome (MetS)1 which puts this group at increased risk for cardiovascular diseases (CVD) and cardiovascular‐related mortality.2 Among the proposed mechanisms to explain the association between age and MetS is the influence of testosterone. Testosterone levels decline during aging, especially in men, who have 10 times higher levels than women in younger adulthood.3
Low testosterone levels in men and high levels in women are thought to alter adipose tissue function, with effects on lipid metabolism, insulin resistance, and fat mass expansion which may induce metabolic disadvantages.4 However, this association is complex, as testosterone levels have both detrimental and beneficial effects depending on the type of tissue and sex.4 Earlier research in men has shown that low testosterone levels may contribute to the development of MetS but that the magnitude of this effect is dependent on several other factors and that the relation may also be bidirectional.5, 6 In contrast to the findings in men, higher testosterone levels in women have been associated with MetS.7 That said, prospective analyses are lacking and not all studies have found this association.8
In spite of a range of studies on this issue, many aspects of the complex relationship between testosterone and MetS remain unclear. This warrants further investigation into possible underlying pathophysiological processes, especially in the older population who are at the highest CVD mortality risk.9 Influencing factors may include major depressive disorder (MDD), which has been associated with 80% to 90% elevated risk for CVD onset.10 Additionally, the presence of MetS in depressed older adults has been associated with greater symptom severity of depression.11 Dysregulations in the immune response and the hypothalamic‐pituitary‐adrenal axis seen in MDD12 closely respond to alterations in the hypothalamic‐pituitary‐gonadal axis. This not only alters testosterone levels but also impacts its function.4, 13, 14 Indeed, low testosterone levels have been associated with MDD in men,15, 16, 17, 18 although associations in women are inconsistent.8, 17, 19, 20
Given these associations, we hypothesized that MDD or the use of antidepressants increases the vulnerability for testosterone level alterations. Therefore, we aimed to determine the associations between testosterone and MetS in older adults and test whether this association is stronger in depressed or antidepressant‐using patients.
2. MATERIALS AND METHODS
2.1. Study population
Data for this study were obtained from Netherlands Study of Depression in Older Persons (NESDO). For methodological details, we refer to previously published work.21, 22 In short, this multicentre prospective cohort aims to study the course, predictors, and consequences of late‐life depression. In total, 510 participants, aged 60 to 93 years, were recruited from 2007 to 2010. A group of 378 older persons with MDD, dysthymia, or minor depression was recruited from both general practitioners and mental health care facilities to cover a wide range of severities. The 132 controls without a history of depression were recruited via general practitioners. After baseline assessment, participants were invited for a second face‐to‐face interview with repeated measures of psychological, social, and physical functioning after 2 years.23 During this period, 28 people died. Of the 482 participants who were still available for the study, 401 (83.4%) took part in the follow‐up assessment. All participants gave their written and oral consent. The study protocol was approved by the Ethical Review Board of the VU Medical Center in Amsterdam, and subsequently by the local ethical review boards.
The presence or absence of depression was assessed by the Composite Interview Diagnostic Instrument24 at baseline and after 2 years of follow‐up and was administered in face‐to‐face interviews. Depression diagnosis was present when participants met the criteria with a 6‐month recency.
For the current study, we analysed MDD patients (N = 359) and healthy controls (N = 132). Thirteen participants were excluded due to missing data on plasma testosterone or sex‐hormone binding globulin (SHBG). In total, 349 MDD patients (119 men and 230 women) and 129 controls (50 men and 79 women) were available for the baseline analyses. Ninety‐nine participants (36 men and 63 women) dropped out between the baseline and the 2‐year follow‐up assessment (20.7% of baseline sample). Female non‐responders suffered more often from MDD, and male non‐responders were older compared with responders. MetS rates did not differ between responders and non‐responders. In total, 266 MDD patients (91 men and 175 women) and 113 control participants (42 men and 71 women) were available for longitudinal analysis.
2.2. Measurements
Predictors included testosterone (TT), free testosterone (FT), and SHBG. Blood was taken after an overnight fast at baseline (mean time of sampling 9:04 AM; range 8:10 to 10:45). EDTA plasma was stored at −80°C until determination of the androgenic markers. TT was measured using a second‐generation testosterone assay without extraction (Architect i2000 analyzer; Abbott Diagnostics, Abbott Park, IL). In contrast to earlier radioimmune assays, second‐generation testosterone assays suffer less from cross‐reactivity with other steroid hormones and are less influenced by SHBG levels.25, 26 Strong correlation coefficients are reported between our radioimmunoassay and the gold standard ID‐LC‐MS/MS measurement in women (R = 0.95).26 Intra‐ and inter‐assay variations were between 4% and 10% at concentrations of 0.02, 2, and 20 nmol/L. The lower limit of quantification was 0.45 nmol/L. In 23 of 318 women (7.2%), the TT level was below the threshold and therefore imputed as 0.44 nmol/L as done before.19 SHBG was measured using an automated immunometric assay (Architect i2000 analyzer). Intra‐ and inter‐assay variations were between 4% and 6% over the whole range. The lower limit of detection for SHBG was 2 nmol/L. FT was calculated from TT, SHBG, and albumin levels using the formula published by Vermeulen.27
Outcome measures were MetS (as a dichotomous variable) and its individual components (as continuous variables) at baseline and after 2 years of follow‐up. MetS was defined according to the adjusted National Cholesterol Education Program Adult Treatment Panel III (ATP III) criteria.2 To fulfil the diagnosis of MetS, at least three of the following criteria had to be met: blood pressure >130 mmHg systolic or >85 mmHg diastolic or antihypertensive medication; waist circumference >102 cm for men and >88 cm for women; fasting glucose levels >5.6 mmol/L (100 mg/dL) or anti‐diabetic medication; high‐density‐lipoprotein (HDL) cholesterol levels <1.0 mmol/L (40 mg/dL) for men and <1.3 mmol/L (50 mg/dL) for women or medication for reduced HDL cholesterol; and fasting triglycerides levels >1.7 mmol/L (150 mg/dL) or medication for hypertriglyceridemia.
Waist circumference was determined by measuring tape, measuring between the lower rib margin and the iliac crest following normal expiration, over light clothing. HDL cholesterol, triglycerides, and glucose levels were measured by standard laboratory methods after overnight fasting. Nineteen patients were set as missing value because they did not fast before the assessment. Blood pressure was measured in a supine position on the right arm by an electronic Omron sphygmomanometer and was averaged over two readings. HDL cholesterol levels, triglyceride levels, and fasting glucose levels were adjusted for medication effects.28, 29, 30 When participants were using antihypertensive medication, 10 mmHg was added to the systolic blood pressure and 5 mmHg was added to the diastolic blood pressure.31 When participants were using fibrates, 0.10 mmol/L [3.8 mg/dL] was subtracted from HDL cholesterol and 0.67 mmol/L [60 mg/dL] was added to triglycerides.28, 29 When participants were using anti‐diabetic medication, when the glucose level was below 7 mmol/L [126 mg/dlL], a value of 7 mmol/L was given.30 Body mass index (BMI) is not a MetS component but is often used as a weight measurement. Therefore, we included this variable as an outcome measure as well, to see whether both assessments are comparable. In general, waist circumference and BMI are highly correlated, independent of sex (r = 0.93, range 0.87‐0.95).32
2.3. Statistical analyses
All analyses were performed in strata separately for men and women. Data were analysed using IBM SPSS Statistics 24. A two‐tailed P value <0.05 was considered statistically significant.
Characteristics were determined at baseline, using percentages, by chi‐squared tests or means with SD by analysis of variance, dependent on the type of variable.
The first aim was to test the association between TT and FT with MetS diagnosis (dichotomous) and its individual components (continuous) at baseline and after 2‐year follow‐up. In the cross‐sectional analyses, these associations were examined using logistic and linear multivariable regression analyses, when appropriate. All analyses were adjusted for age and for education, smoking status, alcohol use, number of chronic diseases, and testosterone‐affecting medication in a second adjustment. Level of education was specified in years of education. Smoking status was dichotomized into non‐ and current smoker. Alcohol use was defined as daily alcohol intake in units. The number of chronic diseases was counted (including hypertension, range 0 through 8). Use of medication was considered as present when using Anatomical Therapeutic Chemical Classification (ATC) codes at least 50% of the days.33 Out of the 478 participants, 33 were taking medications that could influence their testosterone level; seven participants were taking sex hormones (ATC code G03), six participants were taking selective estrogen receptor modifiers (SERM) or aromatase inhibitors (ATC code L02B), and 21 participants were taking glucocorticoids (ATC code H02AB). All users of sex hormones, SERM's, or aromatase inhibitors were female. As none of them was using (anti‐) androgens and their testosterone levels were within the reference intervals, we decided not to exclude these women. However, the main analyses were repeated in a sensitivity analysis with exclusion of this group. Nonlinear associations were analysed by adding squared TT and FT, respectively, to the model. FT was logarithmically transformed to reduce their positively skewed distributions. For interpretation purposes only, TT and FT were standardized (calculated z‐scores) before entering the regression model calculating odds ratios (ORs) for MetS. In the prospective analyses, associations between standardized TT and FT with the change in MetS components over 2 years were examined using linear regression analyses. Two‐year follow‐up values of MetS components were subtracted from baseline values. Therefore, a positive delta score means a lowering of MetS components over 2 years. For waist circumference, triglycerides, blood pressure, glucose, and BMI, this would, in most cases, be associated with an improved metabolic profile. In contrast, a positive delta score for HDL would in most cases mean a worse metabolic profile. These delta scores were entered as outcomes in the regression model. Again, all analyses were adjusted for age first and adjusted for the other covariates afterwards as mentioned before.
To test whether the association between testosterone levels and MetS was similar for persons with and without MDD, interaction terms (ie, testosterone * MDD) were entered in the cross‐sectional models. Next, the interaction term testosterone*antidepressant use was added to the regression models. When interactions were significant (P < .05), tests were run separately for MDD subgroups. All analyses were adjusted according to the previously mentioned models.
3. RESULTS
3.1. Findings in older men
The characteristics of the 169 men are shown in Table 1. Of our sample of men, 70% was currently depressed, and 46% was diagnosed with MetS. The mean plasma TT and FT levels were 16.7 ± (SD) 6.3 nmol/L and 307.1 ± 100.0 pmol/L.
Table 1.
Baseline socio‐demographic, lifestyle, and clinical characteristics of 169 men and 309 women
|
Men (N = 169) |
Women (N = 309) |
|
|---|---|---|
| Age (years) | 69.6 ± 7.4 | 70.9 ± 7.3 |
| Northern European ancestry | 94.1% | 94.5% |
| Education (years) | 11.9 ± 3.8 | 10.5 ± 3.2 |
| BMI (kg/m2) | 26.5 ± 3.7 | 26.4 ± 4.5 |
| Smoking | 19.5% | 23.2% |
| Alcohol use (no. drinks/day) | 0.9 ± 1.2 | 0.6 ± .8 |
| Chronic disease | 2.1 ± 1.5 | 2.5 ± 1.6 |
| Testosterone affecting medication | 4.1% | 6.5% |
| Androgenic features | ||
| Testosterone (nmol/L) | 16.7 ± 6.3 | 0.96 ± 0.40 |
| Free testosterone (pmol/L) | 307.1 ± 100.0 | 14.1 ± 7.62 |
| Outcome measures | ||
| Metabolic syndrome diagnosis (adj. ATPIII) | 45.6% | 43.4% |
| Waist circumference (cm) | 99.6 ± 11.4 | 91.8 ± 12.8 |
| Triglyceridesa (mmol/L) | 1.5 ± 0.8 | 1.4 ± 0.7 |
| HDL cholesterolb (mmol/L) | 1.3 ± 0.3 | 1.7 ± 0.4 |
| Systolic blood pressure (mmHg) | 155.8 ± 20.8 | 151.3 ± 22.4 |
| Diastolic blood pressure (mmHg) | 86.3 ± 10.9 | 82.8 ± 11.3 |
| Fasting glucosec (mmol/L) | 6.1 ± 1.3 | 5.8 ± 1.3 |
| Interactions | ||
| Diagnosis of major depressive disorder | 70.4% | 74.4% |
| Use of antidepressants | 49.7% | 55.0% |
Data are percentages or mean ± standard deviation (SD). P‐values are based on chi‐squared tests (for dichotomous and categorical variables) or t‐tests for independent samples (for continuous variables). Triglyceride, HDL cholesterol, blood pressure, and fasting glucose values were adjusted for medication use (see measurements).
To convert the values of triglycerides to milligrams per decilitre, divide by 0.01129.
To convert the values for HDL cholesterol to milligrams per decilitre, divide by 0.02586.
To convert the values of glucose to milligrams per decilitre, divide by 0.05551.
3.1.1. Cross‐sectional associations between testosterone and MetS
Associations between TT and FT with MetS (components) and BMI are shown in Table 2 and Figures 1 and 2. Overall, both low TT and FT levels were associated with a higher risk for MetS (standardized OR: 0.51 CI95%; 0.34; 0.75 and OR: 0.67 CI95%; 0.47; 0.95). Low androgen levels were linearly associated with adverse MetS components except for blood pressure, with the strongest associations displayed for TT levels. After adjustment for all covariates, a 1SD (ie, 6.3 nmol/L) lower TT level was associated with a mean 3.5 cm higher waist circumference (P < .001), a mean 0.18 mmol/L higher triglycerides (P = .006), a mean 0.04 mmol/L lower HDL (P = .01), a mean 0.23 mmol/L higher fasting glucose (P = .03), and a mean 0.96 kg/m2 higher BMI (P < .001). SHBG showed ORs and standardized betas in same direction as for TT and FT (data not shown).
Table 2.
Associations of TT and FT levels with MetS diagnosis and components in 169 men and 309 women
| MetS diagnosis | Waist | Triglycerides | HDL | Systolic BP | Diastolic BP | Glucose | BMI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | |
| Men | ||||||||||||||||
| Testosterone | ||||||||||||||||
| Age adjusted | 0.52 (0.36–0.76) | <.001 | −0.34 | <.001 | −0.21 | .005 | 0.23 | .002 | −0.13 | .08 | −0.09 | .22 | −0.18 | .02 | −0.29 | <.001 |
| Fully adjusteda | 0.51 (0.34–0.75) | <.001 | −0.33 | <.001 | −0.22 | .006 | 0.19 | .01 | −0.11 | .13 | −0.09 | .22 | −0.18 | .03 | −0.26 | <.001 |
| Free testosterone | ||||||||||||||||
| Age adjusted | 0.66 (0.46–0.92) | .02 | −0.31 | <.001 | −0.15 | .06 | 0.12 | .13 | −0.03 | .67 | −0.04 | .65 | −0.16 | .05 | −0.29 | <.001 |
| Fully adjusteda | 0.67 (0.47–0.95) | .02 | −0.30 | <.001 | −0.15 | .06 | 0.09 | .21 | −0.02 | .79 | −0.04 | .58 | −0.14 | .09 | −0.27 | <.001 |
| Women | ||||||||||||||||
| Testosterone | ||||||||||||||||
| Age adjusted | 0.89 (0.70–1.12) | .33 | −0.03 | .66 | 0.02 | .67 | 0.06 | .34 | 0.07 | .23 | 0.08 | .18 | −0.02 | .77 | 0.02 | .69 |
| Fully adjustedc | 0.92 (0.71–1.19) | .53 | −0.01 | .92 | 0.02 | .69 | 0.08 | .19 | 0.08 | .19 | 0.10 | .08 | −0.01 | .86 | 0.06 | .28 |
| Free testosterone | ||||||||||||||||
| Age adjusted | 1.31 (1.04–1.66) | .02 | 0.19 | .002 | 0.25 | <.001 | −0.12 | .04 | 0.09 | .11 | 0.09 | .11 | 0.16 | .01 | 0.22 | <.001 |
| Fully adjusteda | 1.41 (1.08–1.82) | .01 | 0.19 | .001 | 0.25 | <.001 | −0.13 | .02 | 0.11 | .07 | 0.10 | .08 | 0.14 | .02 | 0.23 | <.001 |
Data are standardized odds ratios (OR) with 95% CI or standardized betas determined by (log) linear regression. As the independent variables were naturally log‐transformed in the logistic regression analyses, a one‐unit change in FT or TT will not be a constant effect in the odds but will vary with FT and TT itself. The dependent and independent variables were therefore standardized, in order for the effect sizes of the ORs to become comparable among the different models. The standardized ORs and beta coefficients refer to how many SD the dependent variable will change per SD increase in the independent variable.
Adjusted for age, education, smoking status, alcohol use, number of chronic diseases, and testosterone affecting medication.
Abbreviation: BP, blood pressure.
Figure 1.
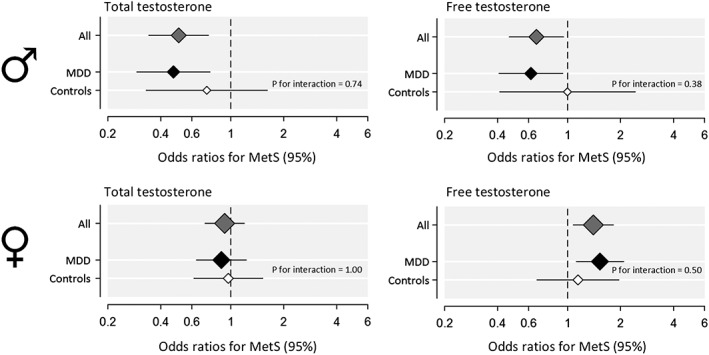
Relationship between plasma total and free testosterone and metabolic syndrome in 169 men and 309 women. Adjusted odds ratios for metabolic syndrome are shown for all participants and separately for depressed and controls on a logarithmic scale. Interaction terms for free testosterone and total testosterone with major depressive disorder are shown as well, but were not significant. Total testosterone and free testosterone levels were standardized before analysis. The size of the diamonds is proportional to the number of participants. Error bars represent standard errors (SE)
Figure 2.
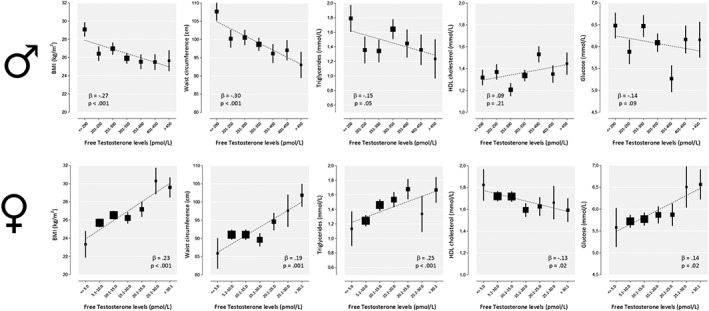
Relationship between plasma free testosterone and metabolic syndrome components in 169 men and 309 women. Free testosterone levels were categorized for visualization purposes only, as all analyses were carried out with continuous data. Free testosterone levels were loge‐transformed before analysis, and back‐transformed geometric mean levels are presented on logarithmic scales. Error bars represent standard errors (SE). The sizes of the boxes are proportional to the number of participants
3.1.2. Longitudinal associations between testosterone and MetS
Associations between TT and FT with 2‐year changes in MetS components and BMI are shown in Table 3. In contrast to the cross‐sectional analyses, neither baseline TT nor FT levels were associated with changes in MetS components over 2 years, except for fasting glucose. A higher FT level was associated with lower fasting glucose levels after 2 years (P = .02).
Table 3.
Associations of TT and FT levels with delta changes in MetS components in 133 men and 246 women over 2 years
| ΔWaist | ΔTriglycerides | ΔHDL | ΔSystolic BP | ΔDiastolic BP | ΔGlucose | ΔBMI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | β | β | p | β | p | β | p | |
| Men | ||||||||||||||
| Testosterone | ||||||||||||||
| Age adjusted | −0.09 | .31 | 0.06 | .53 | 0.03 | .76 | 0.06 | .53 | 0.00 | .99 | 0.15 | .11 | −0.08 | .36 |
| Fully adjusteda | −0.10 | .26 | 0.07 | .47 | −0.01 | .90 | 0.05 | .63 | 0.01 | .94 | 0.15 | .10 | −0.12 | .20 |
| Free testosterone | ||||||||||||||
| Age adjusted | −0.05 | .57 | 0.12 | .21 | −0.03 | .74 | 0.07 | .46 | 0.04 | .70 | 0.22 | .02 | −0.09 | .30 |
| Fully adjusteda | −0.07 | .48 | 0.13 | .17 | −0.06 | .49 | 0.07 | .44 | 0.05 | .62 | 0.22 | .02 | −0.14 | .14 |
| Women | ||||||||||||||
| Testosterone | ||||||||||||||
| Age adjusted | −0.05 | .50 | 0.01 | .86 | 0.11 | .14 | −0.05 | .43 | −0.00 | .97 | −0.01 | .84 | −0.03 | .61 |
| Fully adjusted a | −0.05 | .47 | 0.02 | .81 | 0.10 | .14 | −0.05 | .47 | 0.00 | .99 | −0.02 | .81 | −0.03 | .70 |
| Free testosterone | ||||||||||||||
| Age adjusted | 0.01 | .84 | 0.12 | .09 | 0.07 | .36 | −0.03 | .72 | 0.05 | .61 | 0.00 | .96 | −0.06 | .35 |
| Fully adjusted a | −0.02 | .76 | 0.12 | .10 | 0.05 | .48 | −0.03 | .68 | 0.04 | .61 | −0.01 | .88 | −0.05 | .48 |
Data are standardized betas determined by linear regression. As the independent variables were naturally log‐transformed in the logistic regression analyses, a one‐unit change in FT or TT will not be a constant effect in the odds but will vary with FT and TT itself. The dependent and independent variables were therefore standardized, in order for the effect sizes of the beta's to become comparable among the different models. The standardized beta coefficients refer to how many SD the dependent variable will change per SD increase in the independent variable.
Adjusted for age, education, smoking status, alcohol use, number of chronic diseases, and testosterone affecting medication.
Abbreviation: BP, blood pressure.
3.1.3. Moderating effects of MDD
Although associations were slightly stronger in the MDD group than in controls (Figure 1), they were not statistically significantly different. For the MetS components, there was only one exception. The adverse effects of lower TT levels on systolic blood pressure were stronger in depressed patients compared with controls (standardized β for interaction −0.46 (P = .04), for MDD patients −0.20 (P = .02) and for controls 0.14 (P = .43).
3.2. Findings in older women
The characteristics of the 309 women are shown in Table 1. Of our female sample, 72% was currently depressed, and 43% was diagnosed with MetS. The mean plasma TT and FT levels were 0.96 nmol/L ± 0.40 nmol/L and 14.1 pmol/L ± 7.62 pmol/L (mean ± SD).
3.2.1. Associations between testosterone and MetS
Association between TT and FT with MetS (components) and BMI are shown in Table 2 and Figures 1 and 2. Overall, high FT levels were associated with a higher risk for MetS (standardized OR: 1.41; 95%CI: 1.08‐1.82; P = .01). After adjustment for all covariates, high FT, but not TT, levels were linearly associated with MetS as well as adverse MetS components, except for systolic and diastolic blood pressure. In contrast to men, a 1‐SD (7.62 pmol/L) higher FT level was associated with a mean 2.4 cm higher waist circumference (P < .001), a mean 0.18 mmol/L higher triglycerides (P < .001), a mean 0.05 mmol/L higher HDL (P = .02), a mean 0.18 mmol/L higher fasting glucose (P = .02), and a mean 1.0 kg/m2 higher BMI (P < .001). SHBG showed standardized betas in opposite direction as for FT. The exclusion of 13 women using aromatase inhibitors, SERM, estrogens, or progestagens did not remarkably change the results (standardized OR: 1.40; 95%CI: 1.07‐1.82; P = .01).
3.2.2. Longitudinal associations between testosterone and MetS
Associations between TT and FT with a 2‐year change in MetS components and BMI are shown in Table 3. As in men, neither TT nor FT were associated with changes in MetS components over 2 years.
3.2.3. Moderating effects of MDD
Similar to older men, associations were slightly stronger in the MDD group than in controls (Figure 1), although not statistically significantly different. Only women with MDD and high levels of FT showed a higher mean waist circumference and BMI compared with controls (standardized β for interaction 1.13 [P = .03] and 1.05 [P = .045]), for MDD participants 0.24 (P < .001) and 0.29 (P < .001) and for controls −.04 (P = .77) and.02 (P = .88).
4. DISCUSSION
In this study, we aimed to investigate whether plasma testosterone is associated with MetS and whether this association is different for depressed and non‐depressed older men and women. Strong associations between lower levels of TT and FT in men and, in contrast, higher levels of FT in women with MetS and its components were found. However, the association in depressed older people did not differ significantly from healthy controls.
Our findings in men of an inverse association between testosterone and MetS are in line with earlier findings in men aged older than 608, 34, 35 as were the relationships with MetS components in one study,35 but not in the other study.8 In contrast, relationships of largely the opposite direction were found in our sample of older women. Higher levels of FT (but not of TT) were associated with MetS and its components, which is also in line with previous studies of postmenopausal women.7, 36, 37, 38 Similarly, in younger women with polycystic ovarian syndrome (PCOS), high FT and TT levels have also been linked to MetS,13 whereas such data are largely lacking in postmenopausal women with PCOS.13 Caution should be taken when interpreting findings with calculated FT in epidemiological research, as the formula is importantly dependent on age.39
Sex‐hormone binding globulin may explain why women, in contrast to men, are particularly sensitive to FT and not to TT levels. In our study, the associations for SHBG with MetS were in the same direction for both sexes, which is remarkable given the contrasting results for FT and TT. SHBG binds to androgens and determines for a large part the amount of FT. However, it also has metabolic effects and helps to protect women against exposure to excessively high testosterone levels. The occupancy of SHBG steroid‐binding sites by androgens is sex dependent with an occupation rate of only 18% in women and up to 56% in men. This possibly leads to a more swift response to increased or decreased androgen levels in women,40 which might explain our strong findings with FT but not with TT.
In line with other studies, we found no longitudinal associations between TT and FT and the incidence of MetS in older people.41, 42, 43 Any changes in (components of) the MetS may have already taken place years before our baseline assessment, as a high or low androgen status may have been present since puberty—exerting its metabolic effects over decades. Moreover, a single measurement of testosterone could have been insufficient to detect associations. However, as suggested before,43 the case of reverse causation might be true as well supposing TT and FT can be considered as a marker of MetS, but any potential causal role should be investigated during longer follow‐up periods and/or in experimental designs. In supplementation studies, testosterone may help to improve lipid profiles, body composition, and insulin sensitivity in older and hypogonadal men, whereas anti‐androgenic treatment had similar effects in women with PCOS.4 Unfortunately, experimental studies in older women are scarce.44
Despite being the subject of many studies, the underlying mechanisms of the relationship between MDD and MetS remain largely unclear. Several mechanisms, like unhealthy lifestyle habits and immune‐inflammatory dysregulation, may be involved, which all influence androgen levels.4, 13 We could not confirm, however, that the relationship between androgenic markers and MetS is moderated by MDD or antidepressant use in older people, since associations were similar in depressed and non‐depressed older people. Given the complexity of the relationship between androgenic markers and MetS and the heterogeneity of MDD, it is possible that the interaction terms that were tested were too crude to be informative. Regarding antidepressants, effects on immune‐inflammatory dysregulation but also obesity might be medication‐type specific.45 Moreover, certain subtypes of MDD might be more vulnerable for testosterone alterations. In addition, the hypothalamic‐pituitary‐adrenal axis and hypothalamic‐pituitary‐gonadal axis are closely linked, as the end‐products steroid hormones of both systems have the same backbone, being derived from cholesterol. Furthermore, these two systems crosstalk, with stimulating and inhibiting effects from both sides.46
Some limitations of our study need to be discussed. TT was measured at one time point, which limits its merit to reflect the androgenic status of the participants. However, TT gradually declines with age and may be too small to be measurable and of clinical importance over a period of 2 years for both men and women.43, 47 Previous studies generally also measured the androgenic status at one time point. In addition, a radioimmune assay was used to determine testosterone levels, which is less reliable than liquid chromatography with tandem mass spectrometry (LC‐MS/MS) analyses, in particular at the lower concentrations generally measured in women (<5 nmol/L).48, 49 However, as stated in the method section, good correlations have been reported between this radioimmunoassay we used and the ID‐LC‐MS/MS measurement of testosterone.26 A strength of our study is its design with measurements of MetS components at two time points, which enabled us to study testosterone not only as a marker but also as a predictor for changes in MetS components. Finally, we studied the association in a well‐characterized sample of older subjects with standardized assessment of psychiatric disorders.
In conclusion, our study showed that lower TT and FT levels in older men and higher levels of FT in older women were associated with MetS. Remarkably, testosterone had opposite adverse effects on the metabolic phenotype among the sexes. However, this association was not moderated by MDD or antidepressant use. Therefore, TT and FT could not explain the previously reported relationship between MDD and MetS in older people.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
A.W., E.G., M.B., F.B., and R.S. conceived the research question. A.W. did the data analysis with help from E.G. All authors contributed to the interpretation of the results. A.W. wrote the first draft of the paper, which was further refined by all other authors. All authors revised the article critically and gave their approval for the version to be published. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
SPONSOR'S ROLE
Funding sources had no role in the study design, data acquisition, or analyses or in the decision to submit the article for publication.
FUNDING
The infrastructure for the NESDO study (http://nesdo.amstad.nl) is funded through the Fonds NutsOhra (project 0701‐065), Stichting tot Steun VCVGZ, NARSAD The Brain and Behaviour Research Fund (grant ID 41080), and the participating universities and mental health care organizations (VU University Medical Center, Leiden University Medical Center, University Medical Center Groningen, UMC St Radboud, and GGZ inGeest, GG Net, GGZ Nijmegen, GGZ Rivierduinen, Lentis, and Parnassia).
de Wit AE, Giltay EJ, de Boer MK, et al. Associations between testosterone and metabolic syndrome in depressed and non‐depressed older men and women. Int J Geriatr Psychiatry. 2019;34:463–471. 10.1002/gps.5040
REFERENCES
- 1. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356‐359. [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735‐2752. [DOI] [PubMed] [Google Scholar]
- 3. Durdiakova J, Ostatnikova D, Celec P. Testosterone and its metabolites—modulators of brain functions. Acta Neurobiol Exp. 2011;71(4):434‐454. [DOI] [PubMed] [Google Scholar]
- 4. Schiffer L, Kempegowda P, Arlt W, O'Reilly MW. Mechanisms in endocrinology: the sexually dimorphic role of androgens in human metabolic disease. Eur J Endocrinol. 2017;177(3):R125‐R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone‐binding globulin predict the metabolic syndrome and diabetes in middle‐aged men. Diabetes Care. 2004;27(5):1036‐1041. [DOI] [PubMed] [Google Scholar]
- 6. Brand JS, Rovers MM, Yeap BB, et al. Testosterone, Sex Hormone‐Binding Globulin and the Metabolic Syndrome in Men: An Individual Participant Data Meta-Analysis of Observational Studies. PLoS ONE. 2014;9(7):e100409 10.1371/journal.pone.0100409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guducu N, Gormus U, Kutay SS, Kavak ZN, Telatar B. Endogenous sex hormones and their associations with cardiovascular risk factors in post‐menopausal women. J Endocrinol Invest. 2013;36(8):588‐592. [DOI] [PubMed] [Google Scholar]
- 8. Chrysohoou C, Panagiotakos D, Pitsavos C, et al. Low total testosterone levels are associated with the metabolic syndrome in elderly men: the role of body weight, lipids, insulin resistance, and inflammation; the Ikaria study. Rev Diabet Stud. 2013;10(1):27‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kochanek KD, Murphy SL, Xu J, Tejada‐Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65(4):1‐122. [PubMed] [Google Scholar]
- 10. Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta‐analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763‐2774. [DOI] [PubMed] [Google Scholar]
- 11. Mulvahill JS, Nicol GE, Dixon D, et al. Effect of metabolic syndrome on late‐life depression: associations with disease severity and treatment resistance. (1532–5415 (Electronic)). [DOI] [PMC free article] [PubMed]
- 12. Penninx BWJH. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2016. [DOI] [PubMed] [Google Scholar]
- 13. Delitala AP, Capobianco G, Delitala G, Cherchi PL, Dessole S. Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Arch Gynecol Obstet. 2017;296(3):405‐419. [DOI] [PubMed] [Google Scholar]
- 14. McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol. 2014;35(1):42‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008;65(3):283‐289. [DOI] [PubMed] [Google Scholar]
- 16. Chung S‐D, Kao L‐T, Lin H‐C, Xirasagar S, Huang C‐C, Lee H‐C. Patients receiving androgen deprivation therapy for prostate cancer have an increased risk of depressive disorder. PLoS One. 2017;12(3):e0173266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McIntyre RS, Mancini D, Eisfeld BS, et al. Calculated bioavailable testosterone levels and depression in middle‐aged men. Psychoneuroendocrinology. 2006;31(9):1029‐1035. [DOI] [PubMed] [Google Scholar]
- 18. Shores MM, Moceri VM, Sloan KL, Matsumoto AM, Kivlahan DR. Low testosterone levels predict incident depressive illness in older men: effects of age and medical morbidity. J Clin Psychiatry. 2005;66(1):7‐14. [DOI] [PubMed] [Google Scholar]
- 19. Giltay EJ, van der Mast RC, Lauwen E, Heijboer AC, de Waal MWM, Comijs HC. Plasma testosterone and the course of major depressive disorder in older men and women. Am J Geriatr Psychiatry. 2017;25(4):425‐437. [DOI] [PubMed] [Google Scholar]
- 20. Rohr UD. The impact of testosterone imbalance on depression and women's health. Maturitas. 2002;41:25‐46. [DOI] [PubMed] [Google Scholar]
- 21. Comijs HC, van Marwijk HW, van der Mast RC, et al. The Netherlands study of depression in older persons (NESDO); a prospective cohort study. BMC Res Notes. 2011;4(1):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post‐traumatic stress disorder. Acta Psychiatr Scand. 2006;114(3):187‐193. [DOI] [PubMed] [Google Scholar]
- 23. Comijs HC, Nieuwesteeg J, Kok R, et al. The two‐year course of late‐life depression; results from the Netherlands study of depression in older persons. BMC Psychiatry. 2015;15(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wittchen HU. Reliability and validity studies of the WHO—composite international diagnostic interview (CIDI): a critical review. J Psychiatr Res. 1994;28(1):57‐84. [DOI] [PubMed] [Google Scholar]
- 25. Groenestege WM, Bui HN, ten Kate J, et al. Accuracy of first and second generation testosterone assays and improvement through sample extraction. Clin Chem. 2012;58(7):1154‐1156. [DOI] [PubMed] [Google Scholar]
- 26. Heijboer AC, Savelkoul E, Kruit A, Endert E, Blankenstein MA. Inaccurate first‐generation testosterone assays are influenced by sex hormone‐binding globulin concentrations. J Appl Lab Med. 2016;1(2):194‐201. [DOI] [PubMed] [Google Scholar]
- 27. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666‐3672. [DOI] [PubMed] [Google Scholar]
- 28. Bays HE, Dujovne CA, McGovern ME, et al. Comparison of once‐daily, niacin extended‐release/lovastatin with standard doses of atorvastatin and simvastatin (the ADvicor versus other cholesterol‐modulating agents trial evaluation [ADVOCATE]). Am J Cardiol. 2003;91(6):667‐672. [DOI] [PubMed] [Google Scholar]
- 29. Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial). Am J Cardiol. 2005;95(4):462‐468. [DOI] [PubMed] [Google Scholar]
- 30. Vogelzangs N, Suthers K, Ferrucci L, et al. Hypercortisolemic depression is associated with the metabolic syndrome in late‐life. Psychoneuroendocrinology. 2007;32(2):151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Group SCR . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). JAMA. 1991;265(24):3255‐3264. [PubMed] [Google Scholar]
- 32. Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the ‘beef’. Int J Obes (Lond). 2007;31(10):1552‐1553. [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization Collaborarting Centre for Drug Statistic M . Anatomical Therapeutic Chemical (ATC) Classification. Geneva: World Health Organization; 2007. [Google Scholar]
- 34. Brand JS, van der Tweel I, Grobbee DE, Emmelot‐Vonk MH, van der Schouw YT. Testosterone, sex hormone‐binding globulin and the metabolic syndrome: a systematic review and meta‐analysis of observational studies. Int J Epidemiol. 2014;40(1):189‐207. [DOI] [PubMed] [Google Scholar]
- 35. Chubb SA, Hyde Z, Almeida OP, et al. Lower sex hormone‐binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study. Eur J Endocrinol. 2008;158(6):785‐792. [DOI] [PubMed] [Google Scholar]
- 36. Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A. Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the atherosclerosis risk in communities study. Am J Epidemiol. 2004;160(6):540‐548. [DOI] [PubMed] [Google Scholar]
- 37. Sutton‐Tyrrell K, Wildman RP, Matthews KA, et al. Sex‐hormone‐binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the study of women across the nation (SWAN). Circulation. 2005;111(10):1242‐1249. [DOI] [PubMed] [Google Scholar]
- 38. Weinberg ME, Manson JE, Buring JE, et al. Low sex hormone–binding globulin is associated with the metabolic syndrome in postmenopausal women. Metabolism. 2006;55(11):1473‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Handelsman DJ. Free testosterone: pumping up the tires or ending the free ride? Endocr Rev. 2017;38(4):297‐301. [DOI] [PubMed] [Google Scholar]
- 40. Hammond GL. Diverse roles for sex hormone‐binding globulin in reproduction. Biol Reprod. 2011;85(3):431‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fenske B, Kische H, Gross S, et al. Endogenous androgens and sex hormone–binding globulin in women and risk of metabolic syndrome and type 2 diabetes. J Clin Endocrinol Metabol. 2015;100(12):4595‐4603. [DOI] [PubMed] [Google Scholar]
- 42. Haring R, Völzke H, Spielhagen C, Nauck M, Wallaschofski H. The role of sex hormone‐binding globulin and testosterone in the risk of incident metabolic syndrome. Eur J Prev Cardiol. 2013;20(6):1061‐1068. [DOI] [PubMed] [Google Scholar]
- 43. Haring R, Teumer A, Volker U, et al. Mendelian randomization suggests non‐causal associations of testosterone with cardiometabolic risk factors and mortality. Andrology. 2013;1(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 44. Davis SR, Wahlin‐Jacobsen S. Testosterone in women—the clinical significance. Lancet Diabetes Endocrinol. 2015;3(12):980‐992. [DOI] [PubMed] [Google Scholar]
- 45. Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11(1):129 129–7015–7011‐7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toufexis D, Rivarola MA, Lara H, Viau V. Stress and the reproductive axis. J Neuroendocrinol. 2014;26(9):573‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yeap BB, Alfonso H, Chubb SAP, et al. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography‐tandem mass spectrometry in a population‐based cohort of older men. J Clin Endocrinol Metabol. 2012;97(11):4030‐4039. [DOI] [PubMed] [Google Scholar]
- 48. Rautenberg MW, Lentjes EGWM. Bepaling fertiliteitshormonen anno 2006. Ned Tijdschr Klin Chem Labgeneesk. 2007;32(1):42‐46. [Google Scholar]
- 49. Thijssen JHH. Bepalingen van geslachtshormonen in bloed anno 2000: juistheid en standaardisatie. Ned Tijdschr Klin Chem Labgeneesk. 2000;25(6):346‐351. [Google Scholar]


