Abstract
Upadacitinib is a novel selective Janus kinase 1 inhibitor developed for treatment of rheumatoid arthritis and other autoimmune diseases. The objective of this study was to assess the pharmacokinetics and safety of a single upadacitinib dose in subjects with normal renal function and in subjects with renal impairment. A total of 24 subjects between the ages of 18 and 75 years were assigned to 1 of 4 renal function groups based on estimated glomerular filtration rate (normal, mild, moderate, severe; N = 6/group). A single 15‐mg dose of upadacitinib extended‐release formulation was administered under fasting conditions. Serial plasma and urine samples were assayed to evaluate the effect of renal impairment on upadacitinib exposure through regression analysis and analysis of covariance. The primary analysis was the regression analysis of upadacitinib exposures versus estimated glomerular filtration rate. The point estimates for upadacitinib plasma exposure ratios (90% confidence interval [CI]) in subjects with mild, moderate, and severe renal impairment were 1.18 (90%CI, 1.06–1.32), 1.33 (90%CI, 1.11–1.59), and 1.44 (90%CI, 1.14–1.82) for area under the plasma concentration–time curve and 1.06 (90%CI, 0.92–1.23), 1.11 (90%CI, 0.88–1.40), and 1.14 (90%CI, 0.84–1.56) for maximum observed plasma concentration, respectively, relative to subjects with normal renal function based on the regression analysis. The analysis of covariance categorical analysis provided consistent results. Upadacitinib was well tolerated by all subjects, and no safety issues were identified in subjects with renal impairment. Renal impairment has a limited effect on upadacitinib pharmacokinetics. This is in agreement with the known limited role of urinary excretion in upadacitinib elimination. Based on the limited impact on exposure, no dose adjustment is necessary for upadacitinib in subjects with impaired renal function.
Keywords: Janus kinase inhibitor, pharmacokinetics, renal impairment, upadacitinib
Upadacitinib (ABT‐494) is a novel, Janus kinase (JAK) inhibitor with preferential selectivity toward JAK1. Upadacitinib potently inhibits JAK1 but is less potent against the other isoforms, JAK2, JAK3, and tyrosine kinase 2.1 The enhanced selectivity of upadacitinib against JAK1 may offer an improved benefit‐risk profile in patients with inflammatory disease, including limiting the adverse effects on immune function and erythropoietin signaling.2, 3 Upadacitinib is being developed for the treatment of rheumatoid arthritis (RA) as well as for other immune‐mediated inflammatory diseases. In subjects with moderate to severe RA, upadacitinib demonstrated favorable efficacy and acceptable safety profiles, including in 2 phase 2 studies and in 5 phase 3 studies.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Additionally, upadacitinib recently demonstrated favorable efficacy in subjects with ulcerative colitis, Crohn disease, and atopic dermatitis in phase 2 studies.13, 14, 15 Phase 3 studies are under way in psoriatic arthritis, Crohn disease, ulcerative colitis, atopic dermatitis, and ankylosing spondylitis (phase 2/3).16, 17, 18, 19, 20, 21, 22, 23, 24
Upadacitinib pharmacokinetics were characterized in healthy subjects following the administration of a range of immediate‐release (1 mg to 48 mg) and extended‐release (7.5 mg to 45 mg [including data on file, AbbVie]) doses.3, 12 Upadacitinib plasma exposures were approximately dose‐proportional over the range of immediate‐release or extended‐release doses evaluated in clinical studies.3, 12, 25 Approximately 20% of upadacitinib immediate‐release dose is eliminated in urine as unchanged upadacitinib.3 Upadacitinib was evaluated in the phase 3 RA studies at doses of 15 mg and 30 mg once daily using the extended‐release formulation. The bioavailability of the extended‐release formulation used in this study as well as in phase 3 studies is estimated to be 70% to 80% relative to the immediate‐release formulation.12 Upadacitinib is a nonsensitive substrate for cytochrome P450 3A; coadministration of the strong cytochrome P450 3A inhibitor ketoconazole increased upadacitinib plasma maximum observed plasma concentration (Cmax) and area under the plasma concentration–time curve (AUC) by 70% and 75%, respectively, relative to administration of upadacitinib alone.26
Although renal elimination plays a minor role in upadacitinib clearance, renal dysfunction is common among RA patients as well as patients with other immune‐mediated disorders.27, 28, 29, 30, 31, 32, 33, 34 Therefore, it is important to characterize the effect of different degrees of renal impairment on upadacitinib plasma exposures to inform whether dose adjustment is needed in patients with renal impairment.
The objective of the study reported herein was to assess the pharmacokinetics and safety of a single upadacitinib dose in subjects with mild, moderate, and severe renal impairment relative to subjects with normal renal function.
Methods
The study was conducted in accordance with the protocol, International Conference on Harmonisation Good Clinical Practice guidelines, applicable regulations, and guidelines governing clinical study conduct and ethical principles that have their origin in the Declaration of Helsinki. The study protocol was approved by the institutional review boards of the study sites (Orlando Clinical Research Center, Orlando, Florida; University of Miami, Miami, Florida; Clinical Pharmacology of Miami, LLC, Miami, Florida), and all participants gave written informed consent before participation in the study.
Study Design and Participants
The study was a single‐dose, open‐label study conducted at 3 sites in the United States. Male and female subjects between 18 and 75 years of age with a body mass index ≥18.0 and ≤38.0 kg/m2 were eligible to enroll. Subjects were assigned to 1 of 4 groups (6 subjects per group) according to the estimated glomerular filtration rate (eGFR) as calculated by the Modification of Diet in Renal Disease equation: normal renal function (eGFR ≥90 mL/min/1.73 m2), mild renal impairment (60–89 mL/min/1.73 m2), moderate renal impairment (30–59 mL/min/1.73 m2), and severe renal impairment (15–29 mL/min/1.73 m2).35 Subjects with normal renal function were enrolled in a manner that ensured they were similar to subjects with renal impairment with respect to age, weight, sex, and ethnicity/race. In addition to calculating eGFR by using the Modification of Diet in Renal Disease equation, creatinine clearance was also assessed by using the Cockcroft Gault equation.36 Based on medical history, physical examination, vital sign assessment, laboratory profile, and a 12‐lead electrocardiogram, subjects with normal renal function were required to be in general good health and subjects with renal impairment were required to be in stable condition. Use of known inhibitors or inducers of drug‐metabolizing enzymes was prohibited within 30 days of study drug administration and through the end of the study, except for weak inhibitors, which are needed to control the underlying renal impairment and/or related disorders in the renal impairment groups.
Subjects received a single 15‐mg dose of upadacitinib extended‐release formulation after an approximate 10‐hour fast and 4 hours before a meal. Subjects were confined to the study site 1 day prior to dosing and remained at the site until study procedures were completed on day 6.
Pharmacokinetic Sampling and Bioanalytical Methods
Blood samples for assay of upadacitinib in plasma were collected into dipotassium ethylenediaminetetraacetic acid–containing collection tubes before dosing (0 hour) and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48, 72, 96, and 120 hours after dosing. Urine samples for assay of upadacitinib were collected in containers without preservatives over the following intervals: 0 to 12, 12 to 24, 24 to 48, 48 to 72, 72 to 96, and 96 to 120 hours after dosing. Plasma and urine concentrations of upadacitinib were determined using a validated liquid chromatography method with tandem mass spectrometric detection as previously described.3 The lower limit of quantitation for upadacitinib in plasma and urine was established at 0.0543 ng/mL and 1.06 ng/mL, respectively. The assay coefficient of variation was ≤5.6% for plasma and ≤3.3% for urine, and the mean absolute bias was ≤5.7% for both plasma and urine.
Pharmacokinetic and Statistical Analyses
Pharmacokinetic parameters of upadacitinib were estimated by noncompartmental methods using SAS Version 9.3 (SAS Institute, Inc., Cary, North Carolina) and included the Cmax and the time to Cmax (peak time, Tmax), terminal phase elimination half‐life (t1/2), AUC from time 0 to the time of the last measurable concentration (AUCt) or to infinity (AUC∞), and apparent oral clearance value (CL/F, where F is the bioavailability). The percentage of upadacitinib dose eliminated unchanged in urine was calculated as the amount of upadacitinib recovered in urine divided by the administered dose and multiplied by 100. Renal clearance was calculated as the amount of upadacitinib eliminated in urine divided by AUC∞.
To assess the effect of renal impairment on upadacitinib plasma exposures, a regression analysis was performed on the logarithms of Cmax, AUCt, and AUCinf against the eGFR and creatinine clearance, with eGFR as the parameter of primary interest. The 90%CIs were provided for the ratios of the predicted Cmax, AUCt, and AUCinf values by using the mean value from each impaired group to that from the normal group. In addition, an analysis of covariance (ANCOVA) was performed for Cmax, AUCt, and AUCinf, CL/F, Tmax, and β. Body weight, sex, and age were considered as possible covariates (P < .10) in both the regression and ANCOVA analyses. Within the framework of the ANCOVA, the effect of renal impairment was estimated for each group and compared to the normal group (P < .05). For AUCt, AUCinf, and Cmax, 90%CIs were provided for the ratio of the central value of each impaired group to that of the normal group.
Safety Assessments
Safety and tolerability were evaluated during the study through monitoring of treatment‐emergent adverse events (defined as adverse events reported from the time of study drug administration through day 30), physical examinations, vital sign measurements, 12‐lead electrocardiograms, and clinical laboratory tests.
Results
Participants
A total of 24 subjects (16 males, 8 females) were enrolled and completed the study. The characteristics of the study participants were generally similar across the groups (Table 1). The demographic characteristics of the subjects in the normal renal function group were similar to subjects with renal impairment, as required by the study protocol.
Table 1.
Demographic and Baseline Characteristics
| Degree of Renal Impairment | ||||
|---|---|---|---|---|
| Characteristic | Normal (n = 6) | Mild (n = 6) | Moderate (n = 6) | Severe (n = 6) |
| Age (y)a | 63.2 ± 6.79 | 62.2 ± 8.52 | 68.2 ± 10.5 | 59.7 ± 8.19 |
| (53–71) | (48–70) | (47–75) | (47–70) | |
| Weight (kg)a | 81.2 ± 17.0 | 87.9 ± 21.9 | 81.6 ± 11.2 | 84.5 ± 16.9 |
| (54.5–102) | (55.6–120) | (66.0–95.2) | (53.4–105) | |
| Height (cm)a | 169 ± 9.95 | 170 ± 9.32 | 169 ± 5.04 | 165 ± 5.84 |
| (150–177) | (158–182) | (163–178) | (154–170) | |
| BMI (kg/m2)a | 28.3 ± 3.34 | 30.2 ± 6.53 | 28.7 ± 3.96 | 31.1 ± 6.61 |
| (24.3–33.2) | (22.3–37.5) | (24.0–33.7) | (20.0–37.9) | |
| Sex | ||||
| Male | 4 (67%) | 4 (67%) | 4 (67%) | 4 (67%) |
| Female | 2 (33%) | 2 (33%) | 2 (33%) | 2 (33%) |
| Race | ||||
| White | 5 (83%) | 3 (50%) | 5 (83%) | 6 (100%) |
| Black | 1 (17%) | 3 (50%) | 0 | 0 |
| Asian | 0 | 0 | 1 (17%) | 0 |
| eGFR | 110 ± 15.6 | 61.3 ± 7.94 | 43.5 ± 8.17 | 21.2 ± 5.12 |
| (mL/min/1.73 m2)a | (94–133) | (50–70) | (31–52) | (15–30) |
| CLcr | 119 ± 23 | 74.9 ± 22.3 | 52.6 ± 13.3 | 32 ± 9.15 |
| (mL/min)a | (84.8–141) | (53.4–116) | (39.2–76.8) | (16.2–41.1) |
BMI, body mass index; CLcr, creatinine clearance; eGFR, estimated glomerular filtration rate.
Mean ± standard deviation.
Pharmacokinetics
The mean upadacitinib plasma concentration versus time profiles by group are presented in Figure 1. A summary of the pharmacokinetic parameters of upadacitinib after administration in each of the groups is shown in Table 2.
Figure 1.
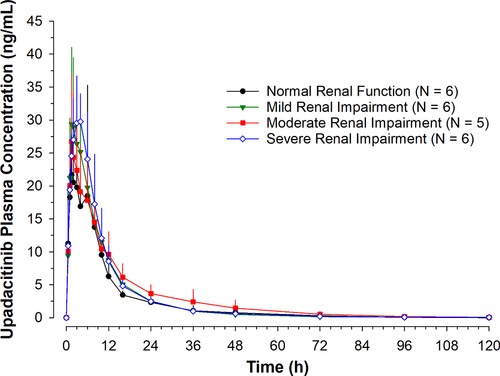
Mean + SD upadacitinib plasma concentrations vs time profiles. Sensitivity analysis excluding subject with moderate renal impairment who had distinctively low upadacitinib exposure.
Table 2.
Pharmacokinetic Parameters (Geometric Mean; Arithmetic Mean ± SD) of Upadacitinib in Subjects With Renal Impairment
| Degree of Renal Impairment | ||||
|---|---|---|---|---|
| Parameter | Normal (N = 6) | Mild (N = 6) | Moderate (N = 5)a | Severe (N = 6) |
| Cmax (ng/mL) | 29.4; 31.1 ± 11.8 | 30.8; 32.5 ± 10.2 | 27.3; 28.2 ± 8.05 | 33.2; 33.7 ± 5.96 |
| Tmax b (h) | 1.8 (1.0–6.0) | 2.5 (1.5–6.0) | 1.5 (1.0–6.0) | 3.5 (2.0–6.0) |
| AUCt (ng • h/mL) | 256; 265 ± 75.5 | 302; 314 ± 87.9 | 350; 358 ± 85.8 | 333; 337 ± 63.6 |
| AUCinf (ng • h/mL) | 260; 270 ± 77.7 | 310; 323 ± 90.7 | 352; 361 ± 86.9 | 337; 341 ± 63.2 |
| t1/2 c (h) | 11.0 ± 5.51 | 10.5 ± 7.00 | 10.4 ± 11.2 | 8.63 ± 4.43 |
| fe (%) | 9.24; 9.91 ± 4.05 | 6.51; 7.03 ± 3.05 | 4.60; 4.76 ± 1.34 | 2.10; 2.48 ± 1.62 |
| CLR (L/h) | 5.32; 5.64 ± 2.13 | 3.15; 3.42 ± 1.53 | 1.96; 2.14 ± 0.930 | 0.94; 1.06 ± 0.547 |
AUCinf, area under the plasma concentration–time curve from time zero to infinite time; AUCt, area under the plasma concentration–time curve from time zero to last measurable concentration; CLR, renal clearance; Cmax, maximum observed plasma concentration; fe, the fraction of upadacitinib dose excreted unchanged in urine; t1/2, terminal elimination half‐life; Tmax, time to Cmax.
Sensitivity analysis excluding subject who had distinctively low upadacitinib exposure.
Median.
Harmonic mean (pseudo‐standard deviation); evaluations of t1/2 were based on statistical tests for β.
Regression analyses showed upadacitinib AUCinf central values were 18%, 33%, and 44% higher in subjects with mild, moderate, and severe renal impairment, respectively, compared to subjects with normal renal function. In this analysis, one subject in the moderate renal impairment group had 77% lower upadacitinib AUC than subjects with normal renal function. The subject's Cmax and AUC were also notably lower than all other subjects with moderate renal impairment. This subject was excluded from the moderate renal impairment group analyses to ensure a conservative estimate for the effect of renal impairment on upadacitinib plasma exposures.
Upadacitinib Cmax central values were similar in subjects with renal impairment compared to subjects with normal renal function (Table 2 and Figure 2). Regression analyses of creatinine clearance showed similar results for Cmax and AUCinf. In ANCOVA analyses, there was no statistically significant difference in central values for upadacitinib Tmax, Cmax, AUCt, AUCinf, or β (as a measure for terminal phase elimination half‐ life) in subjects with renal impairment compared to subjects with normal renal function. The point estimates from the ANCOVA analyses were consistent with those from the regression analyses (Figure 2).
Figure 2.
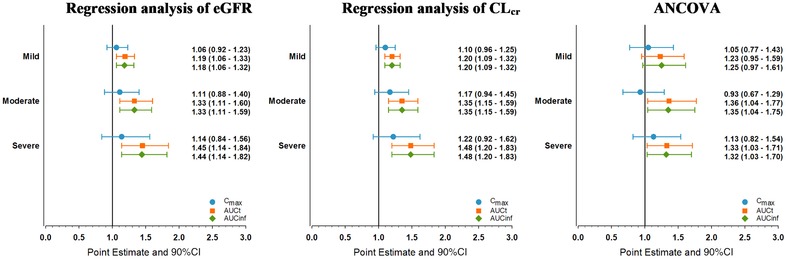
Point estimates and 90% confidence intervals of the effect of renal impairment on upadacitinib maximum concentration and area under the plasma concentration–time curve. Sensitivity analysis excluding subject with moderate renal impairment who had distinctively low upadacitinib exposure. ANCOVA, analysis of covariance; CI, confidence interval; CLcr, creatinine clearance; eGFR, estimated glomerular filtration rate.
Safety
Upadacitinib 15 mg was well tolerated by subjects in the study. One subject in the mild renal impairment group had adverse events of diarrhea and upper respiratory infection, considered by the investigator as mild and having a reasonable possibility of being related to the study drug. No other adverse events were reported. There were no clinically significant physical exam findings, vital sign measurements, electrocardiogram abnormalities, or laboratory test abnormalities.
Discussion
The purpose of the study was to evaluate the effects of mild, moderate, and severe renal impairment on the pharmacokinetics of upadacitinib, a JAK1 inhibitor that has demonstrated efficacy in RA and other inflammatory diseases. The results suggest that renal impairment has only a limited effect on upadacitinib pharmacokinetics, which is in line with the minor contribution of renal elimination to upadacitinib clearance.3
There was no statistically significant difference in central values for upadacitinib Tmax, Cmax, AUCt, AUCinf, or β (as a measure for half‐life) in subjects with mild, moderate, or severe renal impairment compared to subjects with normal renal function. Based on a conservative analysis excluding one outlier with low exposures in the moderate renal impairment group, upadacitinib AUCinf central values are modestly (<50%) higher and Cmax central values (with single dosing) were similar in subjects with renal impairment compared to subjects with normal renal function. A sensitivity analysis was conducted including the outlier subject (data not shown); exclusion of the outlier subject had no meaningful impact on the results from the regression analyses, and this exclusion provided a more conservative estimate from the ANCOVA analyses for the moderate impairment group. Of note, the renal clearance of upadacitinib decreased in order of increasing severity of renal impairment (5.6 L/hr in subjects with normal renal function and 1.06 L/hr in subjects with severe renal impairment). However, due to the overall minor contribution of renal clearance to upadacitinib elimination, this decrease in renal clearance translated into a limited impact on upadacitinib exposure (<50% increase in AUC).
Population pharmacokinetic analyses of upadacitinib pharmacokinetics in healthy subjects and subjects with RA (with creatinine clearance ≥40 mL/min) demonstrated that RA patients with mild or moderate renal impairment (with creatinine clearance ≥40 mL/min) are estimated to have 16% and 32% higher AUC compared to subjects with normal renal function.25 These estimates from the population pharmacokinetic analyses including subjects with RA are very consistent with the results from this dedicated renal impairment study in non‐RA patients, further confirming the lack of need for dose adjustments in RA patients with renal impairment.
This study did not evaluate the effect of hemodialysis on upadacitinib exposures. However, given that renal elimination plays a minor role in upadacitinib elimination, hemodialysis is not expected to have a clinically relevant effect on upadacitinib plasma exposures.37 In subjects with RA, upadacitinib CL/F for the immediate‐release formulation is estimated to be 30.2 L/h (503 mL/min).25 Based on a percentage of upadacitinib immediate‐release dose eliminated in the urine of approximately 20%,3 upadacitinib nonrenal clearance is estimated to be approximately 400 mL/min (assuming 100% bioavailability for the immediate‐release formulation). Therefore, 3 hours of high‐flux hemodialysis with dialysis clearance of 200 mL/min administered every 2 days would contribute <10% to upadacitinib overall elimination. Given that the bioavailability of the extended‐release formulation is 70% to 80% relative to the immediate‐release formulation, these conclusions will also be applicable to the extended‐release formulation.
In summary, this study characterized the effect of mild, moderate, and severe renal impairment on upadacitinib pharmacokinetics. As expected by the minor role of renal elimination in upadacitinib clearance, renal impairment has only a limited effect on upadacitinib exposures, resulting in a 44% increase in AUC in subjects with severe renal impairment compared to subjects with normal renal function and no effect on upadacitinib Cmax. Based on the limited impact of renal impairment on upadacitinib exposure and supported by exposure‐response analyses of efficacy and safety of upadacitinib in subjects with RA based on phase 2 and phase 3 trials (detailed reports of these analyses are forthcoming), adjustment of upadacitinib dose in subjects with renal impairment is not warranted.
Acknowledgments
The authors thank the study participants and the investigators and site personnel who conducted the study. Medical writing support was provided by Therese Stickler, a freelance writer under contract with AbbVie.
Funding
This work was supported by AbbVie.
Declaration of Conflicting Interests
AbbVie contributed to the study design, research, and interpretation of the data and the writing, review, and approval of the manuscript. Mohamed‐Eslam F. Mohamed, Sheryl Trueman, Tian Feng, Jaclyn Anderson, and Ahmed A. Othman are employees of AbbVie and may hold AbbVie stock or stock options. Thomas Marbury is an employee and equity owner of Orlando Clinical Research Center and declares no other conflicts of interest.
Data Sharing
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (eg, protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html
Dr. Ahmed A. Othman is a Fellow of the American College of Clinical Pharmacology.
References
- 1. Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT‐494). BMC Rheumatol. 2018;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs. 2014;23(8):1067–1077. [DOI] [PubMed] [Google Scholar]
- 3. Mohamed MF, Camp HS, Jiang P, Padley RJ, Asatryan A, Othman AA. Pharmacokinetics, safety and tolerability of ABT‐494, a novel selective JAK 1 inhibitor, in healthy volunteers and subjects with rheumatoid arthritis. Clin Pharmacokinet. 2016;55(12):1547–1558. [DOI] [PubMed] [Google Scholar]
- 4. Kremer JM, Emery P, Camp HS, et al. A phase IIb study of ABT‐494, a selective JAK‐1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti‐tumor necrosis factor therapy. Arthritis Rheumatol. 2016;68(12): 2867–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and safety of ABT‐494, a selective JAK‐1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68(12):2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease‐modifying anti‐rheumatic drugs (SELECT‐NEXT): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018;391(10139):2503–2512. [DOI] [PubMed] [Google Scholar]
- 7. Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease‐modifying anti‐rheumatic drugs (SELECT‐BEYOND): a double‐blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139): 2513–2524. [DOI] [PubMed] [Google Scholar]
- 8. Smolen J, Cohen S, Emery P, et al. Upadacitinib as monotherapy: a phase 3 randomized controlled double‐blind study in patients with active rheumatoid arthritis and inadequate response to methotrexate. Ann Rheum Dis. 2018;77(suppl 2):67–68. [Google Scholar]
- 9. Fleischmann R, Pangan A, Mysler E, et al. A phase 3, randomized, double‐blind study comparing upadacitinib to placebo and to adalimumab, in patients with active rheumatoid arthritis with inadequate response to methotrexate [abstract]. Arthritis Rheumatol 2018;70(suppl 10). https://acrabstracts.org/abstract/a-phase-3-randomized-double-blind-study-comparing-upadacitinib-to-placebo-and-to-adalimumab-in-patients-with-active-rheumatoid-arthritis-with-inadequate-response-to-methotrexate/. Accessed September 13, 2018. [Google Scholar]
- 10. van Vollenhoven R Takeuchi T, Pangan AL, et al. A phase 3, randomized, controlled trial comparing upadacitinib monotherapy to MTX monotherapy in MTX‐naïve patients with active rheumatoid arthritis [abstract]. Arthritis Rheumatol. 2018;70(suppl 10). https://acrabstracts.org/abstract/a-phase-3-randomized-controlled-trial-comparing-upadacitinib-monotherapy-to-mtx-monotherapy-in-mtx-naive-patients-with-active-rheumatoid-arthritis/. Accessed September 13, 2018. [Google Scholar]
- 11. Genovese MC Fleischmann R, Combe B, et al. Upadacitinib (ABT‐494) in patients with active rheumatoid arthritis and inadequate response or intolerance to biological DMARDs: a phase 3 randomized, placebo‐controlled, double‐blind study of a selective JAK‐1 inhibitor [abstract]. Arthritis Rheumatol. 2017;69(suppl 10). https://acrabstracts.org/abstract/upadacitinib-abt-494-in-patients-with-active-rheumatoid-arthritis-and-inadequate-response-or-intolerance-to-biological-dmards-a-phase-3-randomized-placebo-controlled-double-blind-study-of-a-selec/. Accessed January 2, 2019. [Google Scholar]
- 12. Mohamed MF, Zeng J, Marroum PJ, Song IH, Othman AA. Pharmacokinetics of upadacitinib with the clinical regimens of the extended‐release formulation utilized in rheumatoid arthritis phase 3 trials [published online ahead of print April 24, 2018]. Clin Pharmacol Drug Dev. 10.1002/cpdd.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Ghosh S, Panes J, et al. Efficacy and safety of upadacitinib as an induction therapy for patients with moderately‐to‐severely active ulcerative colitis: data from the phase 2b study u‐achieve. United European Gastroenterology Week, Austria Center Vienna, Vienna, Austria. October 23, 2018. OP195.
- 14. Sandborn WJ, Feagan BG, Panes J, et al. Safety and efficacy of ABT‐494 (upadacitinib), an oral JAK1 inhibitor, as induction therapy in patients with Crohn's disease: results from Celest. Gastroenterology. 2017;152(5):S1308–S1309. [Google Scholar]
- 15. Guttman‐Yassky E, Silverberg JI, Thaci D, et al. Primary results from a phase 2b, randomized, placebo‐controlled trial of upadacitinib for patients with atopic dermatitis [Abstract 6533]. American Academy of Dermatology Annual Meeting, San Diego, CA, USA. February 16–20, 2018.
- 16. AbbVie . A study comparing ABT‐494 to placebo and to adalimumab in participants with psoriatic arthritis who have an inadequate response to at least one non‐biologic disease modifying anti‐rheumatic drug (SELECT ‐ PsA 1) [ClinicalTrials.gov identifier NCT03104400]. https://clinicaltrials.gov/ct2/show/NCT03104400?term=NCT03104400&rank=1. Accessed October 15, 2018.
- 17. AbbVie . A study comparing ABT‐494 to placebo in participants with active psoriatic arthritis who have a history of inadequate response to at least one biologic disease modifying anti‐rheumatic drug (SELECT ‐ PsA 2) [ClinicalTrials.gov identifier NCT03104374]. https://clinicaltrials.gov/ct2/show/NCT03104374?term=NCT03104374&rank=1. Accessed October 15, 2018.
- 18. AbbVie . A study of the efficacy and safety of upadacitinib (ABT‐494) in subjects with moderately to severely active Crohn's disease who have inadequately responded to or are intolerant to biologic therapy [ClinicalTrials.gov identifier NCT03345836]. https://clinicaltrials.gov/ct2/show/NCT03345836?term=NCT03345836&rank=1. Accessed October 15, 2018.
- 19. AbbVie . A study of the efficacy and safety of upadacitinib (ABT‐494) in subjects with moderately to severely active Crohn's disease who have inadequately responded to or are intolerant to conventional therapies but have not failed biologic therapy. [ClinicalTrials.gov identifier NCT03345849]. https://clinicaltrials.gov/ct2/show/NCT03345849?term=NCT03345849&rank=1. Accessed October 15, 2018.
- 20. AbbVie . A study to evaluate the safety and efficacy of ABT‐494 for induction and maintenance therapy in subjects with moderately to severely active ulcerative colitis [ClinicalTrials.gov identifier NCT02819635]. https://clinicaltrials.gov/ct2/show/NCT02819635?term=NCT02819635&rank=1. Accessed October 19, 2018.
- 21. AbbVie . A study to evaluate upadacitinib in combination with topical corticosteroids in adolescent and adult participants with moderate to severe atopic dermatitis (AD Up) [ClinicalTrials.gov Identifier: NCT03568318]. https://clinicaltrials.gov/ct2/show/NCT03568318?term=NCT03568318&rank=1. Accessed October 19, 2018.
- 22. AbbVie . Evaluation of upadacitinib in adolescent and adult patients with moderate to severe atopic dermatitis (eczema)‐ measure up 1 (Measure Up 1). [ClinicalTrials.gov Identifier: NCT03569293]. https://clinicaltrials.gov/ct2/show/NCT03569293?term=NCT03569293&rank=1. Accessed October 19, 2018.
- 23. AbbVie . A study to evaluate upadacitinib in adolescent and adult subjects with moderate to severe atopic dermatitis. [ClinicalTrials.gov Identifier: NCT03607422]. https://clinicaltrials.gov/ct2/show/NCT03607422?term=NCT03607422&rank=1. Accessed October 19, 2018.
- 24. AbbVie . A study evaluating the safety and efficacy of upadacitinib in subjects with active ankylosing spondylitis (SELECT Axis 1) [Clinicaltrials.gov identifier: NCT03178487]. https://clinicaltrials.gov/ct2/show/NCT03178487?term=NCT03178487&rank=1. Accessed October 15, 2018..
- 25. Klunder B, Mohamed MF, Othman AA. Population pharmacokinetics of upadacitinib in healthy subjects and subjects with rheumatoid arthritis: analyses of phase I and II clinical trials. Clin Pharmacokinet. 2018;57(8):977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohamed MF, Jungerwirth S, Asatryan A, Jiang P, Othman AA. Assessment of effect of CYP3A inhibition, CYP induction, OATP1B inhibition, and high‐fat meal on pharmacokinetics of the JAK1 inhibitor upadacitinib. Br J Clin Pharmacol. 2017;83(10):2242–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodrigues JC, Bargman JM. Antimalarial drugs for the prevention of chronic kidney disease in patients with rheumatoid arthritis: the importance of controlling chronic inflammation? Clin J Am Soc Nephrol. 2018;13:679–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karie S, Gandjbakhch F, Janus N, et al. Kidney disease in RA patients: prevalence and implication on RA‐related drugs management: the MATRIX study. Rheumatology (Oxford). 2008;47(3):350–354. [DOI] [PubMed] [Google Scholar]
- 29. Karstila K, Korpela M, Sihvonen S, Mustonen J. Prognosis of clinical renal disease and incidence of new renal findings in patients with rheumatoid arthritis: follow‐up of a population‐based study. Clin Rheumatol. 2007;26(12):2089–2095. [DOI] [PubMed] [Google Scholar]
- 30. Hickson LJ, Crowson CS, Gabriel SE, McCarthy JT, Matteson EL. Development of reduced kidney function in rheumatoid arthritis. Am J Kidney Dis. 2014;63(2):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lewis B, Mukewar S, Lopez R, Brzezinski A, Hall P, Shen B. Frequency and risk factors of renal insufficiency in inflammatory bowel disease inpatients. Inflamm Bowel Dis. 2013;19(9):1846–1851. [DOI] [PubMed] [Google Scholar]
- 32. Oikonomou K, Kapsoritakis A, Eleftheriadis T, Stefanidis I, Potamianos S. Renal manifestations and complications of inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(4):1034–1045. [DOI] [PubMed] [Google Scholar]
- 33. Primas C, Novacek G, Schweiger K, et al. Renal insufficiency in IBD—prevalence and possible pathogenetic aspects. J Crohns Colitis. 2013;7(12):e630–634. [DOI] [PubMed] [Google Scholar]
- 34. Chi CC, Wang J, Chen YF, Wang SH, Chen FL, Tung TH. Risk of incident chronic kidney disease and end‐stage renal disease in patients with psoriasis: a nationwide population‐based cohort study. J Dermatol Sci. 2015;78(3):232–238. [DOI] [PubMed] [Google Scholar]
- 35. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 36. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]
- 37. US Department of Health and Human Services . Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling, 2010. http://www.fda.gov/downloads/Drugs/Guidances/UCM204959.pdf. Accessed September 5, 2018.


