Summary
We investigated microcirculatory perfusion disturbances following cardiopulmonary bypass in the early postoperative period and whether the course of these disturbances mirrored restoration of endothelial glycocalyx integrity. We performed sublingual sidestream dark field imaging of the microcirculation during the first three postoperative days in patients who had undergone on‐pump coronary artery bypass graft surgery. We calculated the perfused vessel density, proportion of perfused vessels and perfused boundary region. Plasma was obtained to measure heparan sulphate and syndecan‐1 levels as glycocalyx shedding markers. We recruited 17 patients; the mean (SD) duration of non‐pulsatile cardiopulmonary bypass was 103 (18) min, following which 491 (29) ml autologous blood was transfused through cell salvage. Cardiopulmonary bypass immediately decreased both microcirculatory perfused vessel density; 11 (3) vs. 16 (4) mm.mm−2, p = 0.052 and the proportion of perfused vessels; 92 (5) vs. 69 (9) %, p < 0.0001. The proportion of perfused vessels did not increase after transfusion of autologous salvaged blood following cardiopulmonary bypass; 72 (7) %, p = 0.19 or during the first three postoperative days; 71 (5) %, p < 0.0001. The perfused boundary region increased after cardiopulmonary bypass; 2.2 (0.3) vs. 1.9 (0.3) μm, p = 0.037 and during the first three postoperative days; 2.4 (0.3) vs. 1.9 (0.3) μm, p = 0.003. Increased plasma heparan sulphate levels were inversely associated with the proportion of perfused vessels during cardiopulmonary bypass; R = −0.49, p = 0.02. Plasma syndecan‐1 levels were inversely associated with the proportion of perfused vessels during the entire study period; R = −0.51, p < 0.0001. Our study shows that cardiopulmonary bypass‐induced acute microcirculatory perfusion disturbances persist in the first three postoperative days, and are associated with prolonged endothelial glycocalyx shedding. This suggests prolonged impairment and delayed recovery of both microcirculatory perfusion and function after on‐pump cardiac surgery.
Keywords: blood oxygen transport, oxygen delivery to tissues: factors impacting; cardiopulmonary bypass management; endothelial glycocalyx; microcirculation
Introduction
Cardiac surgery with cardiopulmonary bypass is associated with microcirculatory perfusion disturbances that persist for the first few hours following surgery 1, 2, 3, 4. Although these microcirculatory alterations may play a role in the development of postoperative organ dysfunction and prolonged hospital stay 4, 5, 6, little is known about the course and recovery of these disturbances in the early postoperative period 7, 8. The onset of bypass is associated with an immediate decrease in microcirculatory perfusion due to an acute reduction in capillary density 1, mainly caused by systemic inflammation and endothelial dysfunction 4, 5, 9. Moreover, bypass‐associated haemodilution causes a decrease in haemoglobin and blood viscosity, which may result in redistribution of red blood cell flow 10. We further showed that the haemodilution component of bypass only accounts for a part of microcirculatory perfusion disturbances as observed in a rat model with extracorporeal circulation 11. In addition, the bypass‐induced pro‐inflammatory response caused microvascular leakage, which may contribute to impaired microcirculatory perfusion 12, 13.
Degradation of the endothelial glycocalyx layer and subsequent shedding of its constituents is seen as an early marker of endothelial injury, and may influence microcirculatory perfusion patterns 2, 14, 15. Cardiopulmonary bypass‐induced degradation of the glycocalyx allows flowing red blood cells to increase their contact with endothelial cells, thereby enhancing vascular flow resistance 16, 17, 18, 19. Consequently, loss of glycocalyx integrity may contribute to perfusion heterogeneity, a reduction in functional capillaries and microvascular shunt following bypass 3. Although glycocalyx dimensions appear to be restored when mimicking physiological shear conditions using bypass with pulsatile flow 2, the restoration capacity of the glycocalyx following non‐pulsatile flow during bypass remains uncertain.
In this study, we investigated whether acute perfusion disturbances following bypass are restored in the early postoperative period, and whether this course mirrors the recovery of endothelial glycocalyx dimensions. We hypothesised that disturbances in microcirculatory perfusion continue after bypass and are accompanied by prolonged glycocalyx shedding and decreased glycocalyx dimensions.
Methods
This prospective, single‐centre, observational study was approved by the Human Subjects Committee of the VU University Medical Centre (Amsterdam, the Netherlands). We obtained written informed consent from all subjects before inclusion. We included patients aged 18–85 years scheduled for elective coronary artery bypass graft surgery with bypass. Exclusion criteria were: re‐do surgery; emergency surgery; type‐1 diabetes mellitus; body mass index over 35 kg.m−2; and those with haematological, hepatic or renal disease (estimated glomerular filtration rate < 50 ml.min−1).
All procedures were performed as previously described by our group 2. We induced anaesthesia using intravenous sufentanil (1–3 μg.kg−1) and midazolam (0.1 mg.kg−1), combined with rocuronium (0.5–1.0 mg.kg−1) and maintained by continuous propofol infusion (200–400 mg.h−1). Patients received tranexamic acid (1 g) during induction of anaesthesia, dexamethasone (1 mg.kg−1) and cefazolin (1 g) after induction. We inserted a radial artery catheter for haemodynamic monitoring and blood sampling during surgery, and measured blood gas values using a blood gas analyser.
A Sorin Stockert C5 or a S5 heart‐lung machine with a centrifugal blood pump and a heater‐cooler device (Sorin Stockert Instrumente GMBH, Munich, Germany) was used for bypass with a phosphorylcholine‐coated extracorporeal circuit (P.h.i.s.i.o., The Sorin Group, Mirandola, Italy). The circuit was primed with 1000 ml modified fluid gelatine (Braun Melsungen AG, Melsungen, Germany), 500 ml lactated Ringer's solution (Baxter BV, Utrecht, the Netherlands), 100 ml 20% mannitol (Baxter BV, Utrecht, the Netherlands), 50 ml sodium bicarbonate 8.4% (Braun Melsungen AG, Germany), 5000 IU bovine heparin and 1 g cefazolin. Cardiopulmonary bypass was initiated after heparin administration (3–5 mg.kg−1) when target activated clotting time (ACT) exceeded 480 s, and supplemental doses were administered if necessary. Myocardial protection was accomplished using 4°C crystalloid cardioplegia solution (St. Thomas cardioplegic solution). Blood flow during bypass was non‐pulsatile and was kept between 2.2 l.min−1.m−2 and 2.6 l.min−1.m−2 with mild hypothermia (34–36°C). At the end of surgery, anticoagulation with heparin was reversed using protamine in a 1:1 ratio to achieve normal activated clotting time and a further 2 g of tranexamic acid was given.
During the surgical procedure, shed blood from the thoracic cavity and residual blood from the extracorporeal circuit after weaning from bypass were collected into a cell saver reservoir (Autolog, Medtronic, MN, USA). After plasma removal, red blood cells were washed using 2000‐ml bags of sodium chloride 0.9% solution. Subsequently, autologous cell salvaged and washed blood was transfused into the patient using a blood transfusion filter (PALL SQ40SE, Pall Medical, Oss, the Netherlands) 20.
We performed sublingual microcirculatory measurements using non‐invasive sidestream dark field video microscopy (GlycoCheck Glycocalyx Measurement Software) for visualisation of flowing red blood cells 21. Video frames were combined using ImageJ (National Institutes of Health, Bethesda, MD, USA) to acquire video clips of around 5 s per measurement site in vessels ranging from 5 to 25 μm in diameter. We used automatic vascular analysis software (AVA 3.0, Microvision Medical, Amsterdam, the Netherlands) to analyse stabilised video clips from three different sublingual sites per time‐point according to microvascular scoring recommendations by De Backer et al. 22. Briefly, we manually identified vessels and scored the flow in each vessel by eye using the following classification: no flow; intermittent flow (at least 50% of the time absent flow); and continuous flow. We considered vessels scored with absent or intermittent flow to be non‐perfused. Subsequently, the proportion of perfused vessels (PPV) was automatically calculated as the proportion of perfused micro‐vessels from the total number of identified micro vessels. Analyses were performed by one researcher and one‐third of the obtained videos were randomly reviewed by another researcher to determine inter‐observer agreement. Pearson coefficients of the intra‐ and inter‐observer correlation of the perfused vessel density were 0.97 (p < 0.0001) and 0.95 (p < 0.0001), respectively.
Median red blood cell flow width was automatically calculated (Glycocheck, Microvascular Health Solutions, Orem, Utah, USA), as was the average perfused boundary region (red blood cell permeable lumen), which is the difference between the median and extreme outer lumens of the flowing red blood cell column (Fig. 1). Calculations were considered complete when at least 3000 vascular segments (300 measurement sites × 10 movies) per time‐point were measured. Capillary red blood cell concentration was calculated as the percentage of microcirculatory filling with haemoglobin carrying cells within the identified microvessels 21.
Figure 1.
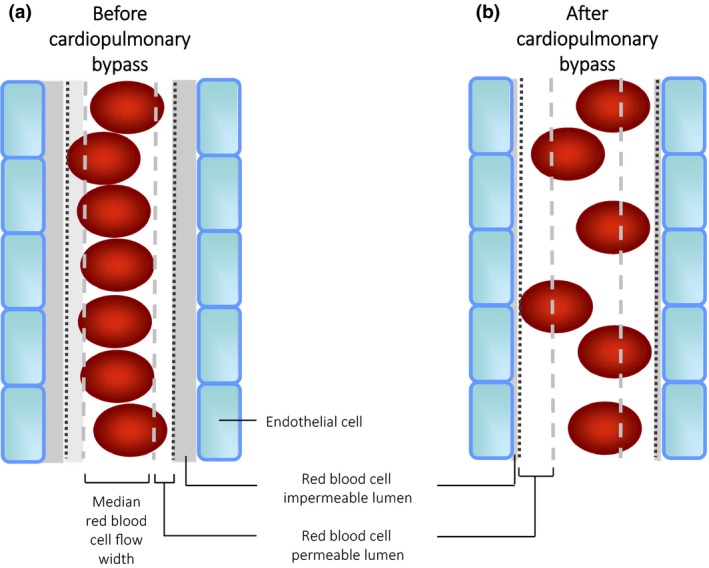
Red blood cell (RBC) flow patterns under normal conditions before cardiopulmonary bypass (left panel) and after bypass (right panel). Under normal conditions, red blood cells preferentially flow within the centre of microvessels, creating a RBC‐free permeable luminal layer. This minimises the contact between RBCs and endothelial cells, and decreases flow resistance. After bypass, the thickness of the RBC‐free luminal layer decreases, which might theoretically result in altered flow resistance. RBC, red blood cell.
We collected arterial blood in citrate tubes and centrifuged samples twice to obtain platelet free plasma, which was stored at −80°C. Levels of heparan sulphate and syndecan‐1 were measured using ELISA (SEA565hu and SEB966hu, Cloud‐clone Corporation, Hubei, China) in accordance to manufacturer's instructions and corrected for corresponding haematocrit levels.
We performed microcirculatory measurements and sampled blood at several peri‐operative time points: a day before surgery (pre‐op); after induction of anaesthesia (pre‐bypass); after going on to bypass 0.9%; after infusion of cell salvaged and washed concentrated red blood cells after weaning from bypass (RBC infusion); and 24 (24 h Postop) and 72 h (72 h Postop) following surgery.
We performed statistical analyses using the SPSS statistical software package (SPSS Statistics 22.0, IBM, New York, NY, USA) and GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA). We calculated the sample size based on previous microcirculatory perfusion measurements in patients undergoing cardiac surgery in which bypass induced a decrease in perfused vessel density from 19 to 14 (2.0) mm.mm−2 perfused vessels per video recording, and an increase in perfused boundary region (red blood cell permeable lumen) from 2.2 to 2.5 (0.2) 2, 23. Using an alpha of 0.05 and a power of 0.9, a sample size of 14 was required for microcirculatory perfusion measurements, and a sample size of 10 was required for perfused boundary region measurements. We assessed normality of distribution using the Shapiro–Wilk test. We evaluated peri‐operative changes in microvascular perfusion and red blood cell‐permeable lumen of the sublingual microvasculature by a repeated measures (RM) ANOVA, with a correction for sphericity using the Greenhouse–Geisser correction for the following variables: the perfused vessel density; PPV; perfused boundary region; and capillary RBC filling. Within‐group differences were tested with paired t‐tests or Wilcoxon signed‐rank tests for non‐continuous data. We analysed correlations between microcirculatory parameters and glycocalyx markers using a Pearson correlation test. We considered a p value of < 0.05 to be statistically significant.
Results
Six patients were not studied due to protocol violation or technical issues, and 17 were included in the final analysis. Patient characteristics are listed in Table 1. Lactate increased, 1.9 (0.7) mmol.l−1 vs. 1.1 (0.5) mmol.l−1, p < 0.001, and base excess decreased following bypass when compared with pre‐bypass values; −0.8 (2.3) vs. 0.4 (1.3) mmol.l−1, p = 0.009. The lactate rise and base excess fall were not restored during the first three postoperative days; 1.9 (0.9) mmol.l−1 vs. 1.1 (0.5) mmol.l−1, p < 0.001; and −0.6 (1.9) vs. 0.4 (1.3) mmol.l−1, p = 0.009, respectively.
Table 1.
Characteristics of 17 patients undergoing cardiopulmonary bypass. Values are median (IQR[range]), number (proportion) or mean (SD)
| Age; y | 69 (63–74 [53–79]) |
| Men | 15 (88%) |
| Body mass index; kg.m−2 | 29 (4%) |
| Diabetes mellitus II | 2 (12%) |
| Hypertension | 5 (29%) |
| Surgery time; min | 239 (38) |
| Bypass time; min | 103 (18) |
| Cross‐clamp time; min | 70 (14) |
| Number of anastomoses; n | 3 (3–4 [2–5]) |
| Packed RBC transfusion | 2 (12%) |
| FFP transfusion | 0 (0%) |
| Platelet transfusion | 3 (18%) |
| Cell saver transfusion; ml | 491 (119) |
| Intensive care length of stay; d | 1 (1–1 [1–1]) |
| Hospital stay; d | 6 (5–8 [5–13]) |
RBC, red blood cell; FFP, fresh frozen plasma.
Figure 2 shows representative images of changes in the microcirculatory network during cardiac surgery for one patient. A dense network of capillaries filled with red blood cells was seen before bypass (panel a), which disappeared during bypass (panel b). Transfusion of autologous blood resulted in a transient filling of the larger vessels (panel c), whereas capillary density remained decreased in the days following surgery (panel d).
Figure 2.
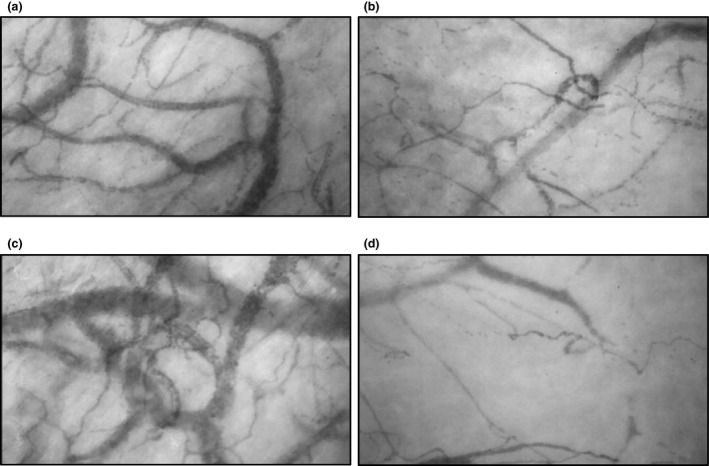
Examples of microcirculatory perfusion recordings in a patient before cardiopulmonary bypass (panel a); during bypass (panel b); after infusion of cell salvaged red blood cells after weaning from bypass (panel c) and 72 h after surgery (panel d).
Figure 3 shows changes in red blood cell characteristics and microcirculatory perfusion before surgery, during bypass and up to 72 h following surgery. Haemoglobin and haematocrit decreased during bypass, and were partially restored after transfusion of autologous blood (panel a and b, respectively; RM p < 0.001 for both parameters). Despite the changes in haemoglobin and haematocrit levels, capillary red blood cell concentration remained relatively stable over time (panel b).
Figure 3.
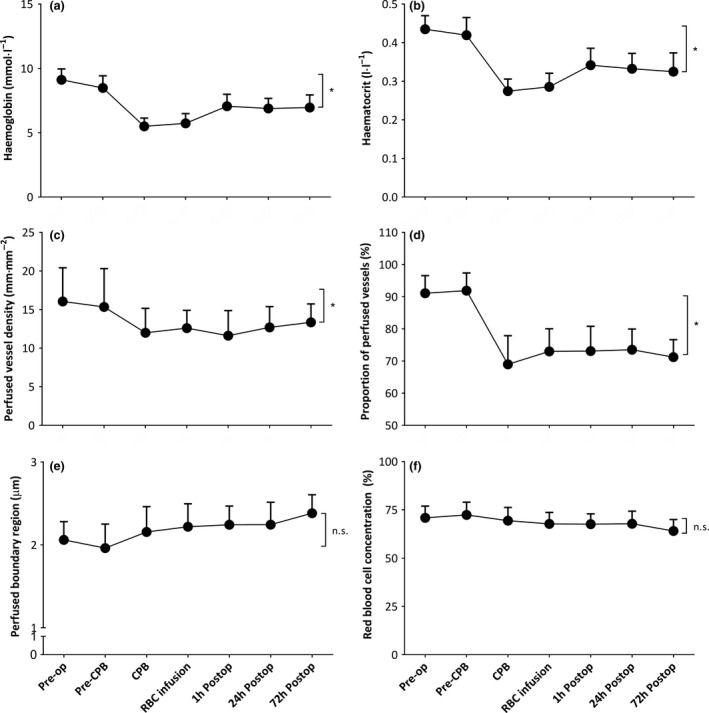
Haemoglobin concentrations (panel a), RM p < 0.001; haematocrit levels (panel b), RM p < 0.001; perfused vessel density (panel c), RM with Greenhouse–Geiser correction, p = 0.05; proportion of perfused vessels (panel d), RM with Greenhouse–Geiser correction, p < 0.0001; perfused boundary region (panel e), RM with Greenhouse–Geiser correction, ns; and capillary red blood cell concentration (panel f), RM with Greenhouse–Geiser correction, ns: before CPB; during CPB; after infusion of cell salvaged red blood cells after weaning from CPB and 24 h and 72 h following surgery. Values are mean (SD). *p < 0.05, #p < 0.05 vs. Pre‐op. PVD, perfused vessel density; PPV, proportion of perfused vessels; PBR, perfused boundary region; CPB, cardiopulmonary bypass; RM, RM ANOVA. RM, repeated measures; Pre‐op, pre‐operative; Postop, postoperative; CPB, cardiopulmonary bypass.
The onset of bypass was associated with an immediate decrease in perfused vessel density (panel c; p = 0.052) and the PPV (panel d; p < 0.0001). Microcirculatory perfused vessel density and PPV did not increase after weaning from bypass (p = 0.19 vs. during bypass, respectively) despite transfusion of autologous cell salvaged blood at the end of surgery. Moreover, microcirculatory perfused vessel density and the PPV remained altered up to 72 h postoperatively (RM p = 0.05 and RM p < 0.0001, respectively).
In parallel, the perfused boundary region (red blood cell permeable lumen; Fig. 1) increased after weaning from bypass compared with pre‐bypass values (Fig. 3, panel e; p = 0.037 vs. pre‐bypass). In particular, the perfused boundary region further increased at 72 h following surgery (p = 0.003 vs. pre‐bypass). Increased perfused boundary region (red blood cell permeable lumen) was associated with decreased capillary red blood cell concentration (R = −0.80; p < 0.001).
Figure 4 demonstrates the changes in plasma concentrations of glycocalyx shedding products, heparan sulphate (panel a) and syndecan‐1 (panel b) before and during bypass and at several time‐points after bypass up to 72 h. The onset of bypass was associated with a two‐fold increase in plasma levels of heparan sulphate (p < 0.0001), followed by a six‐fold increase in plasma levels of syndecan‐1 after weaning from bypass (p < 0.0001) compared with pre‐operative values. Heparan sulphate levels were restored to pre‐bypass levels after weaning from bypass, whereas syndecan‐1 levels remained increased in the first three days following surgery (RM p < 0.0001).
Figure 4.
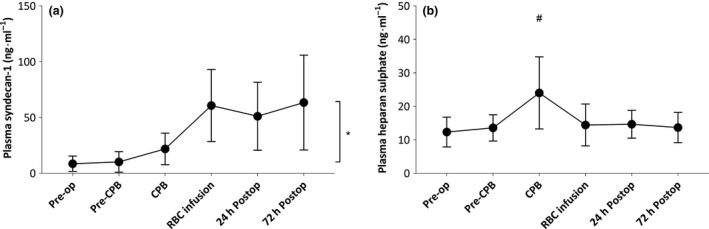
Plasma concentrations of heparan sulphate (panel a) and syndecan‐1 (panel b) before CPB; during CPB; after infusion of cell salvaged red blood cells and 24 h and 72 h following surgery. Values are mean (SD). *p < 0.05, #p < 0.05 vs. Pre‐op; Pre‐op, pre‐operative; postop, postoperative; CPB, cardiopulmonary bypass.
Figure 5 demonstrates the association between plasma glycocalyx degradation products and the perfused boundary region (red blood cell permeable lumen; panel a + c), as well as microcirculatory perfusion (PPV; panel b + d) and ratio of capillary to systemic haematocrit (panel e + f). During bypass, increased levels of heparan sulphate were moderately associated with increased perfused boundary region dimensions (panel a; R = 0.50; p = 0.03) and inversely associated with the PPV (panel b; R = −0.49; p = 0.02). Plasma syndecan‐1 levels were weakly associated with increased perfused boundary region dimensions (panel c; R = 0.36; p = 0.02) and showed a moderate inverse association with the PPV during the entire study period (panel d; R = −0.51; p < 0.0001). In addition, increased ratios of capillary to systemic haematocrit were moderately associated with plasma heparan sulphate levels (panel e; R = 0.47; p = 0.01) and weakly associated with increased plasma syndecan‐1 levels (panel f; R = 0.31; p = 0.03).
Figure 5.
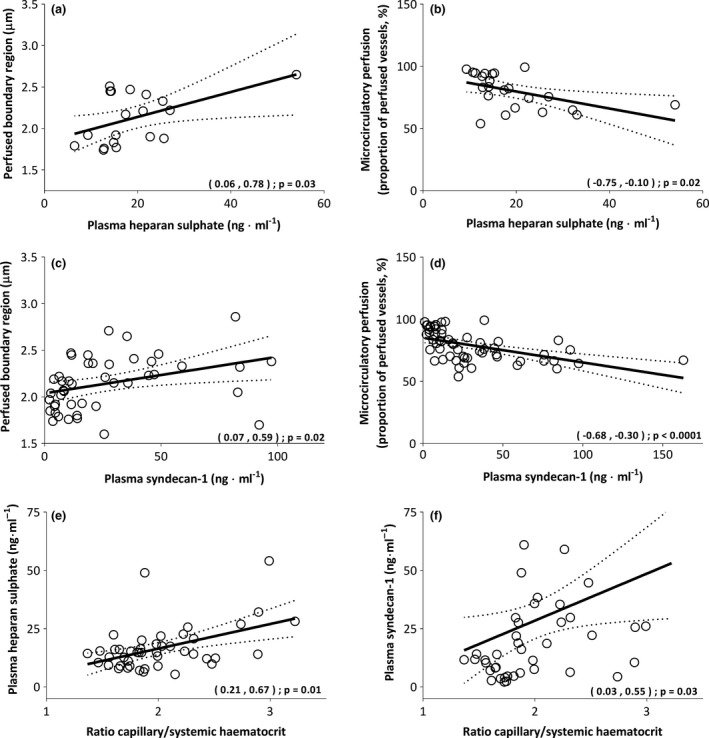
Associations between plasma glycocalyx shedding molecules, heparan sulphate and syndecan‐1, and perfused boundary region (PBR, panel a + c); microcirculatory perfusion (PPV, panel b + d); and the ratio of capillary to systemic haematocrit (panel e + f). Values are presented with 95%CI. PBR, perfused boundary region; PPV, proportion of perfused vessels.
Discussion
This is the first study to show the persistence of bypass‐associated disturbances in microcirculatory perfusion and glycocalyx dimensions in the first three postoperative days. Moreover, disturbances in microcirculatory perfusion and glycocalyx dimensions were associated with increased circulating levels of glycocalyx shedding markers. Autologous red blood cell transfusion at the end of surgery did not restore microcirculatory perfusion, despite partially restoring haemoglobin levels. These findings are suggestive of a prolonged impairment of both microcirculatory perfusion and function after bypass.
Although it was commonly speculated that alterations in microcirculatory perfusion would continue in the postoperative period 1, 3, 6, there are limited data available concerning the postoperative course of microcirculatory parameters 7, 8. Our results are in line with previous findings that bypass‐associated microcirculatory perfusion disturbances are not restored immediately after weaning from bypass, and continue in the first postoperative hours 1, 2, 7, 8. In addition, we showed that these disturbances persist for the first three postoperative days, underlining the fragility of the microvascular network and delayed restoration capacity following acute injury.
Secondly, we showed that the onset of bypass immediately increased plasma levels of the glycocalyx side chain molecule heparan sulphate, followed by shedding of the transmembrane core protein syndecan‐1 in the postoperative period. These results are indicative of a step‐by‐step degradation of the endothelial glycocalyx layer, where shedding of heparan sulphate side chains may facilitate accessibility of heparanase to accelerate metalloproteases to further cleave glycocalyx core proteins 24, 25, 26. We also showed that the perfused boundary region increased after onset of bypass, which has previously been confirmed by other studies on patients undergoing bypass 2, 27.
Our study demonstrated that microcirculatory perfusion disturbances were paralleled by decreased glycocalyx dimensions and associated with increased glycocalyx shedding markers, suggesting a close relationship between glycocalyx integrity and microcirculatory perfusion. During bypass, microcirculatory perfusion is affected by acute local alterations in vascular flow resistance, reduced functional capillary density and increased heterogeneity of microcirculatory flow 1, 3. The endothelial glycocalyx holds a central position between circulating blood components and the vascular endothelium, adapting its permeability to facilitate changes in microcirculatory red blood cell deformation and thereby compensating for increased perfusion heterogeneity 3, 18, 19. Degradation of the endothelial glycocalyx layer following bypass may therefore attenuate the adaptive ability of the microvasculature to the sudden changes in vascular resistance and flow 2, 3, 14. In addition, bypass‐induced shedding of the glycocalyx layer promotes endothelial activation, leading to increased vascular permeability and oedema formation which may subsequently impair endothelial function and microcirculatory perfusion 3, 6, 15. Recent studies have shown that both endothelial glycocalyx and microcirculatory parameters were able to be restored after weaning from bypass when pulsatile flow was applied, indicating the interconnectivity and importance of the integrity of the endothelial glycocalyx for restoring microcirculatory perfusion and function 2, 7, 28.
Besides loss of glycocalyx volume, bypass‐related haemodilution affects blood flow resistance by decreasing haematocrit levels, and therefore also contributes to microcirculatory perfusion disturbances 11, 29. Cardiopulmonary bypass‐associated systemic haemodilution did not seem to affect capillary red blood cell concentration, and microcirculatory perfusion was not affected by infusion of autologous blood at the end of surgery. However, bypass‐associated increased ratios of capillary to systemic haematocrit were inversely associated with increased glycocalyx shedding, suggesting that loss of glycocalyx integrity may influence microcirculatory perfusion profiles by changing the Fahraeus effect 30. Microcirculatory flow is highly dependent on red blood cell rheological‐mechanical properties, especially deformability, which is facilitated by the endothelial glycocalyx layer 31. Based on these findings, decreased microcirculatory perfusion during bypass may be caused by a combination of systemic inflammation and haemodilution that initiates a vicious circle of glycocalyx damage, endothelial cell dysfunction and altered microcirculatory flow patterns. Partial systemic correction of red blood cell concentration alone may therefore not sufficiently restore microcirculatory oxygen delivery and extraction 9, 32, 33. Future therapies aimed at protecting endothelial function during bypass could therefore be of importance to preserve microcirculatory perfusion following bypass 12, 13.
A limitation of our study is that all microcirculatory parameters were measured in the sublingual microcirculatory network, and may not be representative of other vital organs. It should also be mentioned that glycocalyx dimensions were deduced from perfused boundary region measurements, an inverse and indirect measure of glycocalyx thickness. However, changes in perfused boundary regions repeatedly showed moderate correlation with markers of circulating glycocalyx shedding, and are therefore indicative of glycocalyx degradation 34, 35, 36, 37, 38. Sublingual microcirculatory monitoring is widely accepted and used as a measure of the systemic microcirculation, and provides easy non‐invasive monitoring. In addition, a growing number of studies underline the predictive value of decreased sublingual microcirculatory perfusion and glycocalyx dimensions in the development of postoperative organ injury and morbidity in multiple patient populations 6, 7, 34, 35, 36, 37.
To our knowledge, our study is the first to show that microcirculatory perfusion disturbances persist in the first three postoperative days in adults undergoing cardiac surgery with bypass. The prolonged impairment of microcirculatory perfusion was accompanied by decreased glycocalyx dimensions, and was associated with glycocalyx shedding. These results increase our understanding of the delayed ability for postoperative restoration following disturbances in acute microcirculatory perfusion and glycocalyx integrity after bypass, and underline the potentially important role of microcirculatory alterations in the development of postoperative organ dysfunction.
Acknowledgements
ND is financially supported by the Dutch Heart Foundation, the Netherlands (grant number 2016T064). C.v.d.B. is financially supported by the European Society of Anaesthesiology (Research Project Grant 2016), European Society of Intensive Care Medicine [Levi‐Montalcini Award 2017] and Dutch Society of Anaesthesiologists [YIG 2017]. The remaining authors are financially supported by their department. No financial support was provided by industry. No conflicts of interest are declared by the authors.
Presented in part at Euroanaesthesia, Copenhagen, Denmark, June 2018 and at the 20th International Vascular Biology Meeting, Helsinki, Finland, June 2018.
You can respond to this article at http://www.anaesthesiacorrespondence.com
References
- 1. Koning NJ, Atasever B, Vonk AB, Boer C. Changes in microcirculatory perfusion and oxygenation during cardiac surgery with or without cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 2014; 28: 1331–40. [DOI] [PubMed] [Google Scholar]
- 2. Koning NJ, Vonk AB, Vink H, Boer C. Side‐by‐Side alterations in glycocalyx thickness and perfused microvascular density during acute microcirculatory alterations in cardiac surgery. Microcirculation 2016; 23: 69–74. [DOI] [PubMed] [Google Scholar]
- 3. Koning NJ, Simon LE, Asfar P, Baufreton C, Boer C. Systemic microvascular shunting through hyperdynamic capillaries after acute physiological disturbances following cardiopulmonary bypass. American Journal of Physiology ‐ Heart and Circulatory Physiology 2014; 307: H967–75. [DOI] [PubMed] [Google Scholar]
- 4. De Backer D, Dubois MJ, Schmartz D, et al. Microcirculatory alterations in cardiac surgery: effects of cardiopulmonary bypass and anesthesia. Annals of Thoracic Surgery 2009; 88: 1396–403. [DOI] [PubMed] [Google Scholar]
- 5. Jongman RM, Zijlstra JG, Kok WF, et al. Off‐pump CABG surgery reduces systemic inflammation compared with on‐pump surgery but does not change systemic endothelial responses: a prospective randomized study. Shock 2014; 42: 121–8. [DOI] [PubMed] [Google Scholar]
- 6. Brudney CS, Gosling P, Manji M. Pulmonary and renal function following cardiopulmonary bypass is associated with systemic capillary leak. Journal of Cardiothoracic and Vascular Anesthesia 2005; 19: 188–219. [DOI] [PubMed] [Google Scholar]
- 7. O'Neil MP, Fleming JC, Badhwar A, Guo LR. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: microcirculatory and systemic effects. Annals of Thoracic Surgery 2012; 94: 2046–53. [DOI] [PubMed] [Google Scholar]
- 8. Bienz M, Drullinsky D, Stevens LM, Bracco D, Noiseux N. Microcirculatory response during on‐pump versus off‐pump coronary artery bypass graft surgery. Perfusion 2016; 31: 207–15. [DOI] [PubMed] [Google Scholar]
- 9. Boyle EM Jr, Pohlman TH, Johnson MC, Verrier ED. Endothelial cell injury in cardiovascular surgery: the systemic inflammatory response. Annals of Thoracic Surgery 1997; 63: 277–84. [DOI] [PubMed] [Google Scholar]
- 10. Pries AR, Fritzsche A, Ley K, Gaehtgens P. Redistribution of red blood cell flow in microcirculatory networks by hemodilution. Circulation Research 1992; 70: 1113–21. [DOI] [PubMed] [Google Scholar]
- 11. Koning NJ, de Lange F, Vonk AB, et al. Impaired microcirculatory perfusion in a rat model of cardiopulmonary bypass: the role of hemodilution. American Journal of Physiology – Heart and Circulatory Physiology 2016; 310: H550–8. [DOI] [PubMed] [Google Scholar]
- 12. Koning NJ, de Lange F, van Meurs M, et al. Reduction of vascular leakage by imatinib is associated with preserved microcirculatory perfusion and reduced renal injury markers in a rat model of cardiopulmonary bypass. British Journal of Anaesthesia 2018; 120: 1165–75. [DOI] [PubMed] [Google Scholar]
- 13. Dekker NA, van Meurs M, van Leeuwen AL, et al. Vasculotide, an angiopoietin‐1 mimetic, reduces pulmonary vascular leakage and preserves microcirculatory perfusion during cardiopulmonary bypass in rats. British Journal of Anaesthesia 2018; 121: 1041–51. [DOI] [PubMed] [Google Scholar]
- 14. Cabrales P, Vázquez BY, Tsai AG, Intaglietta M. Microvascular and capillary perfusion following glycocalyx degradation. Journal of Applied Physiology 2007; 102: 2251–9. [DOI] [PubMed] [Google Scholar]
- 15. Marechal X, Favory R, Joulin O, et al. Endothelial glycocalyx is associated with impaired microvascular perfusion. Shock 2008; 29: 572–6. [DOI] [PubMed] [Google Scholar]
- 16. Bruegger D, Rehm M, Abicht J, et al. Shedding of the endothelial glycocalyx during cardiac surgery: on‐pump versus off‐pump coronary artery bypass graft surgery. Journal of Thoracic and Cardiovascular Surgery 2009; 138: 1445–7. [DOI] [PubMed] [Google Scholar]
- 17. Lee DH, Dane MJ, van den Berg BM, et al. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE 2014; 9: 96477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McClatchey PM, Schafer M, Hunter KS, Reusch JEB. The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. American Journal of Physiology – Heart and Circulatory Physiology 2016; 311: H168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gouveneur M, van den Berg BM, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. Journal of Internal Medicine 2006; 259: 393–400. [DOI] [PubMed] [Google Scholar]
- 20. Vonk AB, Meesters MI, Garnier RP, et al. Intraoperative cell salvage is associated with reduced postoperative blood loss and transfusion requirements in cardiac surgery: a cohort study. Transfusion 2013; 53: 2782–9. [DOI] [PubMed] [Google Scholar]
- 21. Nieuwdorp M, Meuwese MC, Mooij HL, et al. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. Journal of Applied Physiology 2008; 104: 845–52. [DOI] [PubMed] [Google Scholar]
- 22. De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Critical Care 2007; 11: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koning NJ, Vonk AB, Barneveld LJ, et al. Pulsatile flow during cardiopulmonary bypass preserves postoperative microcirculatory perfusion irrespective of systemic hemodynamics. Journal of Applied Physiology 2012; 112: 1727–34. [DOI] [PubMed] [Google Scholar]
- 24. Nikolova V, Koo CY, Ibrahim SA, et al. Differential roles for membrane‐bound and soluble syndecan‐1 (CD138) in breast cancer progression. Carcinogenesis 2009; 30: 397–407. [DOI] [PubMed] [Google Scholar]
- 25. Jalkanen M, Rapraeger A, Saunders S, Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix‐binding ectodomain from its membrane‐associated domain. Journal of Cell Biology 1987; 105: 3087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramani VC, Pruett PS, Thompson CA, DeLucas LD, Sanderson RD. Heparan sulfate chains of syndecan‐1 regulate ectodomain shedding. Journal of Biological Chemistry 2012; 287: 9952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nussbaum C, Haberer A, Tiefenthaller A, et al. Pertubation of the microvascular glycocalyx and perfusion in infants after cardiopulmonary bypass. Journal of Thoracic and Cardiovascular Surgery 2015; 150: 1474–81. [DOI] [PubMed] [Google Scholar]
- 28. Elbers PW, Wijbenga J, Solinger F, et al. Direct observation of the human microcirculation during cardiopulmonary bypass: effects of pulsatile perfusion. Journal of Cardiothoracic and Vascular Anesthesia 2011; 25: 250–5. [DOI] [PubMed] [Google Scholar]
- 29. Pries AR, Secomb TW, Sperandio M, Gaehtgens P. Blood flow resistance during hemodilution: effect of plasma composition. Cardiovascular Research 1998; 37: 225–35. [DOI] [PubMed] [Google Scholar]
- 30. Yalzin O, Jani VP, Johnson PC, Cabrales P. Implications of enzymatic degradation of the endothelial glycocalyx on the microvascular hemodynamics and the arteriolar red cell free layer of the rat cremaster muscle. Frontiers in Physiology 2018; 9: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barshtein G, Pries AR, Goldschmidt N, et al. Deformability of transfused red blood cells is a potent determinant of transfusion‐induced change in recipient's blood flow. Microcirculation 2016; 23: 479–86. [DOI] [PubMed] [Google Scholar]
- 32. Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme haemodilution with low‐ and high‐viscosity dextran and a low‐viscosity Hb‐based O2 carrier. American Journal of Physiology – Heart and Circulatory Physiology 2004; 287: H363–73. [DOI] [PubMed] [Google Scholar]
- 33. Nielsen ND, Martin‐Loeches I, Wentowski C. The effects of red blood cell transfusion on tissue oxygenation and the microcirculation in the intensive care unit: a Systematic Review. Transfusion Medicine Reviews 2017; 4: 205–22. [DOI] [PubMed] [Google Scholar]
- 34. Dane MJC, Khairoun M, Lee DH, et al. Association of kidney function with changes in the endothelial surface layer. Clinical Journal of the American Society of Nephrology 2014; 9: 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng J, Selke F. Microvascular dysfunction in patients with diabetes after cardioplegic arrest and cardiopulmonary bypass. Current Opinion in Cardiology 2016; 31: 618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. Clinical Journal of the American Society of Nephrology 2012; 23: 1900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nieuwdorp M, Mooij HL, Kroon J, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006; 55: 1127–32. [DOI] [PubMed] [Google Scholar]
- 38. Torres Filho IP, Torres LN, Salgado C, Dubick MA. Plasma syndecan‐1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. American Journal of Physiology – Heart and Circulatory Physiology 2016; 310: H1468–78. [DOI] [PubMed] [Google Scholar]


