Abstract
Background
Hodgkin lymphoma (HL) survivors treated with abdominal radiotherapy and/or alkylating chemotherapy have an increased risk of colorectal cancer (CRC). This study was aimed at evaluating the prevalence of colorectal neoplasia in HL survivors.
Methods
This multicenter cohort study assessed the diagnostic yield of advanced colorectal neoplasia detected by a first surveillance colonoscopy among HL survivors treated with abdominal radiotherapy and/or procarbazine. Advanced colorectal neoplasia included advanced adenomas (high‐grade dysplasia, ≥25% villous component, or ≥10‐mm diameter), advanced serrated lesions (dysplasia or ≥10‐mm diameter), and CRC. The results were compared with those for a Dutch general population cohort that underwent a primary screening colonoscopy (1426 asymptomatic individuals 50‐75 years old). This study demonstrated the results of a predefined interim analysis.
Results
A colonoscopy was performed in 101 HL survivors, who were significantly younger (median, 51 years; interquartile range [IQR], 45‐57 years) than the general population controls (median, 60 years; IQR, 55‐65 years; P < .001). The prevalence of advanced neoplasia was higher in HL survivors than controls (25 of 101 [25%] vs 171 of 1426 [12%]; P < .001). Advanced adenomas were detected in 14 of 101 HL survivors (14%) and in 124 of 1426 controls (9%; P = .08). The prevalence of advanced serrated lesions was higher in HL survivors than controls (12 of 101 [12%] vs 55 of 1426 [4%]; P < .001). Serrated polyposis syndrome was present in 6% of HL survivors and absent in controls (P < .001).
Conclusions
HL survivors treated with abdominal radiotherapy and/or procarbazine have a high prevalence of advanced colorectal neoplasia. The implementation of a colonoscopy surveillance program should be considered.
Keywords: colorectal cancer (CRC) screening, epidemiology, polyps/adenomas
Short abstract
Hodgkin lymphoma survivors treated with abdominal radiotherapy and/or procarbazine have a high prevalence of advanced colorectal neoplasia. The implementation of a colonoscopy surveillance program should be considered.
Introduction
The risk of colorectal cancer (CRC) is increased in many cancer survivors, including survivors of Hodgkin lymphoma (HL), testicular cancer, Wilms tumors, central nervous system malignancies, and bone cancer.1, 2, 3, 4, 5, 6, 7, 8 In HL survivors specifically, the reported relative risks of CRC range from 2 to 7 in comparison with the general population.3, 4, 5, 6, 7, 8, 9 This increased risk continues up to 40 years after HL treatment and is strongly related to treatment with abdominal radiotherapy and/or alkylating agents, mainly procarbazine.3, 5, 9 A recent population‐based study has demonstrated that childhood cancer survivors have an excess risk of developing colorectal adenomas in addition to CRC.10 Risk factors for adenomas in this cancer survivor population also include abdominopelvic irradiation and procarbazine.
In Dutch clinical practice, colonoscopy surveillance programs exist for several high‐risk populations, such as patients with familial CRC, Lynch syndrome, and inflammatory bowel disease. The efficacy of colonoscopy surveillance in these high‐risk populations results from the early detection of CRC and the removal of premalignant lesions; this leads to reduced CRC incidence and improved CRC‐related survival rates.11, 12
Only 1 recent report has evaluated the yield of colonoscopy in childhood cancer survivors since the recommendation of the US Children’s Oncology Group.13 After a median interval of 19 years since treatment with abdominal radiotherapy, adenomas or serrated lesions of any size were detected in 15 of 54 patients (28%).14 However, in that study, no comparison with the general population was made.
Colonoscopy surveillance is not incorporated into Dutch guidelines on the management of lymphoma or childhood cancer survivors. This is related to the lack of knowledge of the natural history and presence of precursor lesions, which may influence surveillance efficacy.15 Here we report on a predefined interim analysis of a study aimed at comparing the diagnostic yields of a first surveillance colonoscopy in HL survivors and a first screening colonoscopy in the general population.
Materials and Methods
Study Aims
The primary aim of this multicenter cohort study was to assess the diagnostic yield of advanced colorectal neoplasia detected by a first surveillance colonoscopy among HL survivors at increased risk for CRC. Advanced colorectal neoplasia was defined as an advanced adenoma (high‐grade dysplasia, ≥25% villous component, or ≥10‐mm diameter), an advanced serrated lesion (hyperplastic polyp/sessile serrated adenoma with dysplasia or ≥10‐mm diameter), or CRC. This definition of advanced colorectal neoplasia slightly differs from our original study protocol developed in 201316 because recent literature and guidelines acknowledge advanced serrated lesions as high‐risk lesions for CRC development and have changed the definition of advanced neoplasia to also include advanced serrated lesions.14, 17, 18, 19
The results were compared with those for a Dutch cohort of 1426 asymptomatic individuals aged 50 to 75 years who underwent a primary screening colonoscopy between 2009 and 2010 before implementation of the Dutch national fecal immunochemical test–based screening program.20, 21 This cohort was chosen because it contained the best comparable data available, representing the Dutch general population and a fairly recent time period of colonoscopies. Data on the colonoscopy results of the Dutch national screening program are for a fecal immunochemical test–positive population, and this reduces comparability. In addition, data from other countries lacked detailed information on neoplastic lesions or lacked comparability of CRC incidence.
The medical ethics committee of the Netherlands Cancer Institute approved this study protocol. All participating patients provided written informed consent. The study was registered at the Dutch Trial Registry (ID NTR4961). All authors had access to the study data and reviewed and approved the final manuscript.
Study Population
HL survivors were invited for study participation at 4 Dutch study centers (the Netherlands Cancer Institute in Amsterdam, the Erasmus MC Cancer Institute in Rotterdam, the University Medical Center Utrecht, and the Radboud University Medical Center in Nijmegen). The inclusion criterion was the treatment of primary or recurrent HL by means of any of the following regimens:
Abdominal radiotherapy consisting of at least para‐aortic and iliac fields.
A cumulative procarbazine dose ≥ 2.8 g/m2 (eg, ≥2 mechlorethamine, vincristine, procarbazine, and prednisone [MOPP] cycles or ≥4 mechlorethamine, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, and vinblastine [MOPP/ABV] cycles).
Abdominal radiotherapy (any field or fields) and chemotherapy (any regimen).
Additional inclusion criteria were an HL diagnosis at the age of 16 to 50 years, survival for at least 8 years after HL treatment, a current age of 25 years or older, and a life expectancy of 5 years or longer. Patients who met 1 of the following criteria were excluded: proctocolectomy, colonoscopy surveillance for other indications, ongoing cytotoxic treatment or radiotherapy for malignant disease, coagulopathy (prothrombin time > 50% of control and partial thromboplastin time > 50 seconds) or anticoagulants (vitamin K antagonists or direct oral anticoagulants) that could not be stopped, comorbidity leading to an impaired physical performance (World Health Organization [WHO] performance status of 3‐4), and mental retardation. Patients who underwent a colonoscopy in the past 5 years were also excluded, whereas patients who underwent a colonoscopy more than 5 years ago were not excluded. These patients did not have follow‐up colonoscopies and thus did not have relevant neoplasia for surveillance (eg, a negative colonoscopy).
Sample Size Calculation
On the basis of a 9% prevalence of advanced adenomas or CRC in the asymptomatic general population, an increase to 15% or more in HL survivors was defined as a significant change. To detect such a difference with 80% power, we needed to include at least 259 study participants (according to a 2‐sided test for 2 independent proportions with a 5% significance level). A predefined interim analysis was performed after the inclusion of 100 participants. Here we report the results of this interim analysis.
Study Procedures
All patients were invited for participation by their hematologist or radiation oncologist, as previously described, and received oral and standard written information about the preparation and the colonoscopy.16 Details on the colonoscopy procedure (including preparation, conscious sedation, quality measure reporting, and data registration) were published previously.16 In the presence of colorectal neoplasia (adenoma, serrated lesion, or CRC), polypectomy was performed or biopsies were taken according to the standard protocol, regardless of the endoscopic appearance or size. A routine histological evaluation of all colorectal neoplasias was performed by experienced gastrointestinal pathologists. The endoscopic size of the neoplastic lesion was used for analyses except if the microscopic measurement of the pathologist was larger than the endoscopic measurement. Follow‐up was performed according to standard clinical care.
Statistical Analyses
All data were stored and analyzed with SPSS Statistics 22. Dichotomous or categorical data were compared between groups with chi‐square tests or Fisher exact tests, whereas continuous data were compared with Mann‐Whitney U tests. Univariate and multivariate logistic regression was performed to assess odds ratios for colorectal neoplasia associated with patient characteristics, including HL treatment.22 The significance level was defined as a 2‐sided P value ≤ .05.
Results
Colonoscopy Participants
A total of 246 HL survivors were invited for participation (Fig. 1). Between February 2015 and February 2017, 101 HL survivors (41%) underwent a colonoscopy. Patients had been diagnosed with HL between 1975 and 2004 at a median age of 25 years (interquartile range [IQR], 20‐32 years; Table 1). HL treatment included both abdominal radiotherapy and procarbazine in 35% of patients, procarbazine without abdominal radiotherapy in 50%, and abdominal radiotherapy without procarbazine in 15%. The median interval between HL diagnosis and colonoscopy was 22 years (IQR, 19‐28 years). Colonoscopy quality measures are reported in Supporting Table 1. The cecal intubation rate was 100%, and only 1 adverse event was reported (ie, hospitalization for 1 day due to abdominal pain after colonoscopy).
Figure 1.
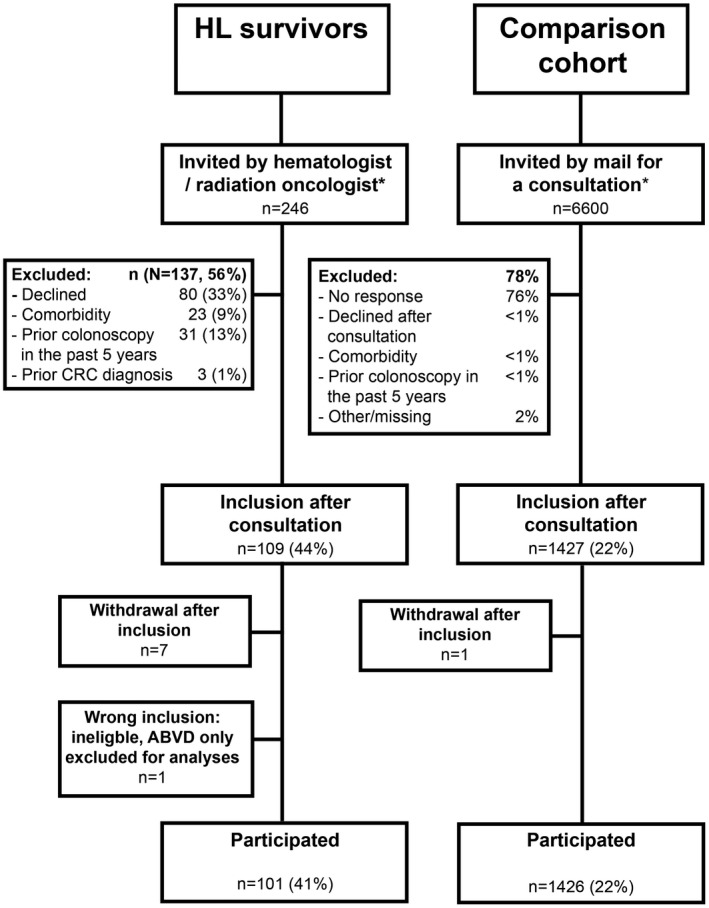
Flow diagram of the study invitations and participants. *Invitations were accompanied by an information leaflet, which included information about CRC in general and the advantages and possible risks of colonoscopy. ABVD indicates doxorubicin, bleomycin, vinblastine, and dacarbazine; CRC, colorectal cancer; HL, Hodgkin lymphoma.
Table 1.
HL Survivor Characteristics (n = 101)
| Characteristic | Value |
|---|---|
| Age at HL treatment, median (IQR), y | 25 (20‐32) |
| Age at HL treatment, % | |
| 16‐25 y | 51 |
| 26‐35 y> | 36 |
| 36‐48 y | 13 |
| Time since HL treatment, median (IQR), y | 22 (19‐28) |
| Time since HL treatment, % | |
| 12‐19 y | 29 |
| 20‐29 y | 55 |
| 30‐40 y | 17 |
| Year of HL treatment, % | |
| 1975‐1984 | 15 |
| 1985‐1994 | 50 |
| 1995‐2004 | 35 |
| HL stage, % | |
| I | 11 |
| II | 50 |
| III | 21 |
| IV | 17 |
| Unknown | 2 |
| HL treatment category, % | |
| Abdominal RT + procarbazine | 35 |
| Procarbazine | 50 |
| Abdominal RT | 15 |
| HL radiotherapy, % | |
| No RT | 12 |
| Cervical RT only | 1 |
| Mantle field only | 38 |
| Abdominal RT, % | |
| Para‐aortic + iliac + spleen | 15 |
| Para‐aortic + iliac | 4 |
| Para‐aortic + spleen | 22 |
| Para‐aortic only | 5 |
| Iliac only | 3 |
| Lumbal region only | 1 |
| HL chemotherapy, % | |
| No CT | 9 |
| MOPP a | 20 |
| MOPP/ABV | 58 |
| Other with procarbazine b | 7 |
| Other without procarbazine | 6 |
Abbreviations: BCVPP, carmustine, cyclophosphamide, vinblastine, procarbazine, and prednisone; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; CT, chemotherapy; HL, Hodgkin lymphoma; IQR, interquartile range; MOPP, mechlorethamine, vincristine, procarbazine, and prednisone; MOPP/ABV, mechlorethamine, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, and vinblastine; MOPP/ABVD, mechlorethamine, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine, and dacarbazine; RT, radiotherapy.
Including 2 patients who also received MOPP/ABV.
Including BEACOPP, MOPP/ABVD, and BCVPP.
Some numbers do not add up to 100% due to rounding.
HL survivors underwent a colonoscopy at a median age of 51 years (IQR, 45‐57 years), whereas the median age was 60 years (IQR, 55‐65 years) for the general population cohort, which hereafter is called the controls (P < .001; Table 2). The HL survivors were 56% male and 44% female, and these proportions were comparable to the distribution in the controls.
Table 2.
Patient and Colonoscopy Characteristics of HL Survivors and General Population Controls
| Characteristic | HL Survivors (n = 101) | Controls (n = 1426) | P |
|---|---|---|---|
| Age, median (IQR), y | 51 (45‐57) | 60 (55‐65) | <.001 |
| Age, % | |||
| <40 y | 9 | 0 | |
| 40‐49 y | 37 | 0 | |
| 50‐59 y | 37 | 47 | |
| 60‐69 y | 17 | 47 | |
| ≥70 y | 1 | 7 | |
| Sex, % | |||
| Male | 56 | 51 | .28 |
| Female | 44 | 49 | |
| Neoplastic lesions in total groups a | |||
| No. per patient, median | 2 | 0 | <.001 |
| No. per patient, % | |||
| 0 | 28 | 55 | |
| ≥1 | 72 | 45 | <.001 |
| Neoplasia detection per patient, % | |||
| Adenomas | |||
| ≥1 adenoma | 55 | 29 | <.001 |
| ≥1 advanced adenoma b | 14 | 9 | .08 |
| Serrated lesions | |||
| ≥1 serrated lesion | 47 | 27 | <.001 |
| ≥1 advanced serrated lesion c | 12 | 4 | <.001 |
| Serrated polyposis syndrome d | 6 | 0 | <.001 |
| WHO 1 | 5 | 0 | |
| WHO 3 | 1 | 0 | |
| Colorectal cancer | 0 | 0.6 | .42 |
| Advanced neoplasia e | 25 | 12 | <.001 |
| Neoplastic lesions in patients aged 50‐70 y f | |||
| ≥1 advanced adenoma b | 15 | 8 | .09 |
| ≥1 advanced serrated lesion c | 20 | 4 | <.001 |
| Serrated polyposis syndrome d | 11 | 0 | <.001 |
| Advanced neoplasia e | 33 | 11 | <.001 |
Abbreviations: HL, Hodgkin lymphoma; IQR, interquartile range; WHO, World Health Organization.
Chi‐square tests or Fisher exact tests were used.
Defined as adenomas, serrated lesions, or cancer.
Defined as a conventional adenoma with a ≥10‐mm diameter, a ≥25% villous component, or high‐grade dysplasia.
Defined as a serrated lesion with a ≥10‐mm diameter or dysplasia.
WHO 1 is diagnosed in the presence of 5 serrated lesions proximal to the sigmoid, with at least 2 having a ≥10‐mm diameter; WHO 3 is diagnosed in the presence of 20 serrated lesions in the colorectum, regardless of size.
Defined as an advanced adenoma, an advanced serrated lesion, or colorectal cancer.
The n values were 54 for HL survivors and 1331 for controls.
Some numbers do not add up to 100% due to rounding.
Prevalence of Colorectal Neoplasia in HL Survivors and the General Population Cohort
A total of 350 neoplastic lesions of any type (adenomas, serrated lesions, or CRC) were detected in 101 HL survivors, whereas 1529 were detected in 1426 controls (mean, 3.5 per HL survivor [standard deviation, 4.9] vs 1.1 per control [standard deviation, 1.8]; P < .001). In HL survivors, neoplastic lesions were more frequently located in the proximal colon (73% vs 40% in controls) and less frequently located in the distal colon (21% vs 31%) and in the rectum (7% vs 28%; overall P < .001).
Any type of neoplastic lesion was detected in 72% of HL survivors and in 45% of controls (P < .001). The prevalence of advanced adenomas was 14% in HL survivors and 9% in controls (P = .08; Table 2). The prevalence of advanced serrated lesions was higher in HL survivors than controls (12% vs 4%; P < .001). Six HL survivors were diagnosed with serrated polyposis syndrome: 5 had at least 5 serrated lesions proximal to the sigmoid colon, 2 of which were ≥10 mm in diameter (WHO criterion 1), and 1 had 20 serrated lesions or more, regardless of size, but they were located throughout the colorectum (WHO criterion 3).23 In contrast, serrated polyposis syndrome was not observed in controls (P < .001).
None of the HL survivors were diagnosed with CRC, whereas 0.6% of the controls were (P = .42). The prevalence of advanced neoplasia, which was defined as an advanced adenoma, an advanced serrated lesion, or CRC, was 25% in HL survivors and 12% in controls (P < .001 [uncorrected for the younger age of HL survivors]).
Difference in Colorectal Neoplasia Prevalence in Male and Female HL Survivors
The prevalence of advanced adenomas was higher in male HL survivors than male controls (23% vs 10%; P = .002), whereas the prevalence of advanced serrated lesions was similar in the 2 groups (5% in HL survivors vs 4% in controls; P = .64; Fig. 2).
Figure 2.
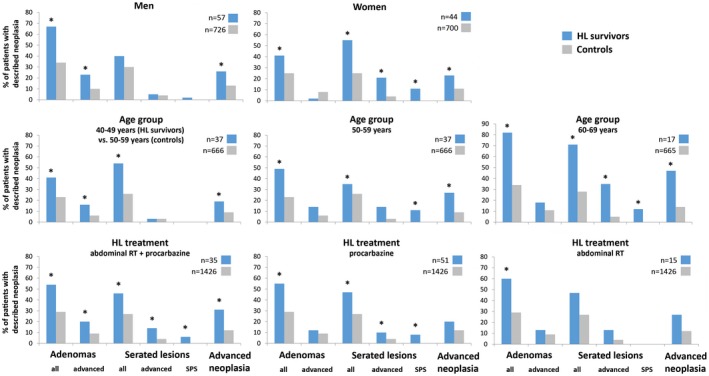
Frequencies of neoplasia detection in subgroups of HL survivors and general population controls. An advanced adenoma is defined as an adenoma with a ≥10‐mm diameter, a ≥25% villous component, or high‐grade dysplasia; an advanced serrated lesion is defined as a serrated lesion with a ≥10‐mm diameter or dysplasia; and advanced neoplasia is defined as an advanced adenoma, an advanced serrated lesion, or colorectal cancer. *There was a significant difference between groups (P < .05). HL indicates Hodgkin lymphoma; RT, radiotherapy; SPS, serrated polyposis syndrome.
In women, the prevalence of advanced adenomas was not significantly different in HL survivors and controls (2% vs 8%; P = .24), whereas the prevalence of advanced serrated lesions was evidently higher (21% in HL survivors vs 4% in controls; P < .001). Serrated polyposis syndrome was present in 11% of female HL survivors (vs 0% of controls; P < .001).
Prevalence of Colorectal Neoplasia in Different Age Groups
When we evaluated study participants aged 50 to 70 years, advanced neoplasia was present in 33% of HL survivors and in 11% of controls (median age, 56 years [IQR, 52‐60 years] vs 59 years [IQR, 55‐64 years]; P < .001).
Because the youngest age of the controls was 50 years, we compared 37 HL survivors aged 40 to 49 years with 666 controls aged 50 to 59 years (Fig. 2). The prevalence of advanced neoplasia was higher in younger HL survivors than older controls (19% vs 9%; P = .045). In 37 HL survivors and 666 controls aged 50 to 59 years, the prevalence of advanced neoplasia was 27% and 9%, respectively (P < .001). Advanced neoplasia was more common in 17 HL survivors aged 60 to 69 years (47% vs 14% in 665 controls; P < .001).
Prevalence of Colorectal Neoplasia After Exposure to Abdominal Radiotherapy and/or Procarbazine
The prevalence of advanced neoplasia was more common in the HL survivors treated with both abdominal radiotherapy and procarbazine (n = 35) than controls (31% vs 12%; P = .001). In the HL survivors with procarbazine treatment without abdominal radiotherapy (n = 51), the prevalence of advanced adenomas was similar to the prevalence in controls (12% vs 9%; P = .45). The prevalence of advanced serrated lesions, however, was higher in these HL survivors than controls (10% vs 4%; P = .04). Advanced neoplasia was slightly but not significantly more common in these HL survivors than controls (20% vs 12%; P = .10). In the 15 HL survivors who were treated with abdominal radiotherapy without procarbazine, the prevalences of advanced adenomas, advanced serrated lesions, and advanced neoplasia were also high but not significantly different from those of controls (13% vs 9% [P = .38], 13% vs 4% [P = .12], and 27% vs 12% [P = .10], respectively).
Logistic Regression Models for Colorectal Neoplasia Development in HL Survivors and Controls
Other potential risk factors for colorectal neoplasia, including the frequency of first‐degree relatives with CRC, smoking, body mass index, and alcohol use, were similarly present or less frequently present in HL survivors versus controls (Supporting Table 2).
A univariate logistic regression model showed that HL survivors treated with either abdominal radiotherapy or procarbazine had an odds ratio for advanced neoplasia of 2.0 (95% confidence interval [CI], 1.1‐3.6; P = .03) in comparison with controls, whereas HL survivors treated with both abdominal radiotherapy and procarbazine had an odds ratio for advanced neoplasia of 3.4 (95% CI, 1.6‐7.0; P = .001; Table 3). In a multivariate model (adjusted for age, sex, family history, smoking, and body mass index), the odds ratio of advanced neoplasia was 3.3 (95% CI, 1.6‐6.6; P = .001) for HL survivors treated with either abdominal radiotherapy or procarbazine and 5.7 (95% CI, 2.6‐12.6; P < .001) for HL survivors treated with both abdominal radiotherapy and procarbazine.
Table 3.
Logistic Regression Models for the Prevalence of Neoplasia in Hodgkin Lymphoma Treatment Groups Versus General Population Controls
| OR (95% CI) | |||
|---|---|---|---|
| Advanced Adenoma a | Advanced Serrated Lesion b | Advanced Neoplasia c | |
| Univariate | |||
| Treatment category | |||
| Control population (n = 1426) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Abdominal RT or procarbazine (n = 66) | 1.2 (0.6‐2.8) | 2.9 (1.2‐6.5) | 2.0 (1.1‐3.6) |
| Abdominal RT and procarbazine (n = 35) | 2.6 (1.1‐6.1) | 4.0 (1.5‐10.7) | 3.4 (1.6‐7.0) |
| Multivariate | |||
| Treatment category | |||
| Control population | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Abdominal RT or procarbazine | 2.5 (1.0‐5.9) | 3.7 (1.4‐9.8) | 3.3 (1.6‐6.6) |
| Abdominal RT and procarbazine | 4.5 (1.8‐11.2) | 6.2 (2.1‐18.2) | 5.7 (2.6‐12.6) |
| Age (reference <55 y) | |||
| 55‐59 y | 2.0 (1.1‐4.0) | 1.3 (0.6‐3.1) | 1.7 (1.0‐2.9) |
| 60‐64 y | 2.4 (1.3‐4.6) | 2.7 (1.3‐5.7) | 2.2 (1.3‐3.7) |
| 65‐69 y | 2.9 (1.5‐5.8) | 1.4 (0.5‐3.7) | 2.1 (1.2‐3.8) |
| ≥70 y | 3.8 (1.7‐8.4) | 2.7 (0.9‐7.8) | 3.5 (1.8‐6.7) |
| Sex (reference male) | 1.4 (0.9‐2.1) | 0.9 (0.5‐1.5) | 1.2 (0.8‐1.6) |
| Family history d | 1.6 (1.0‐2.7) | 1.4 (0.7‐2.8) | 1.7 (1.1‐2.6) |
| Smoking e | 2.1 (1.3‐3.4) | 3.3 (1.9‐5.9) | 2.5 (1.7‐3.7) |
| BMI, kg/m2 | 1.0 (1.0‐1.1) | 1.1 (1.0‐1.1) | 1.0 (1.0‐1.1) |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio; RT, radiotherapy.
Defined as a conventional adenoma with a ≥10‐mm diameter, a ≥25% villous component, or high‐grade dysplasia.
Defined as a serrated lesion with a ≥10‐mm diameter or dysplasia.
Defined as an advanced adenoma, an advanced serrated lesion, or colorectal cancer.
First‐degree relative(s) with colorectal cancer versus no first‐degree relative (reference).
Current smoker versus former/nonsmoker (reference).
Discussion
This is the first study to demonstrate that HL survivors have a higher prevalence of advanced colorectal neoplasia, particularly advanced serrated lesions and serrated polyposis syndrome, than the general population. These results, in combination with the existing evidence for an increased risk of CRC associated with abdominal radiotherapy and/or procarbazine, indicate that colonoscopy surveillance should be considered for HL survivors who have received these treatments.3, 4, 5, 9
We demonstrated an increased prevalence of advanced colorectal neoplasia in HL survivors between 40 and 70 years old. True differences in the prevalence of neoplasia were underestimated in this study because HL survivors had a much lower median age than the general population comparison group. Unfortunately, no statistical adjustment could be performed to account for this age difference because of the lack of data on primary colonoscopy yields in a young group of average‐risk individuals.
HL treatment consisting of either abdominal radiotherapy or procarbazine was associated with an increased prevalence of advanced colorectal neoplasia. The largest odds ratio for the prevalence of advanced colorectal neoplasia was seen in HL survivors whose treatment consisted of both abdominal radiotherapy and procarbazine; however, the difference with patients who had either procarbazine or abdominal radiotherapy was not statistically significant. These associations remained after adjustments for common risk factors of advanced neoplasia in the general population, such as smoking and a family history of CRC.22, 24
Recent literature and recent guidelines of both the European Society of Gastrointestinal Endoscopy and the US Multi‐Society Task Force on Colorectal Cancer acknowledge that similarly to advanced adenomas, serrated lesions that have dysplasia and/or are at least 10 mm in size should be approached as high‐risk lesions.14, 17, 18, 19 We, therefore, now include advanced serrated lesions within the definition of advanced colorectal neoplasia.16, 20
The current Dutch CRC screening program with biennial fecal immunochemical testing has insufficient sensitivity for advanced adenomas and especially for advanced serrated lesions for this high‐risk group.25, 26 Based on the results of this interim analysis, sufficient evidence is provided to currently consider a colonoscopy surveillance program for HL survivors. Detailed eligibility criteria for a surveillance program cannot be derived from our results; however, some suggestions, based on upper and lower limits of increased risks of advanced neoplasia, may aid implementation in the clinic. Previous studies from our own group and others suggest that there is no evidence for starting surveillance sooner than 8 to 10 years after HL treatment because the increase in CRC risk starts approximately 10 to 15 years after treatment.3, 4, 5, 9 Because HL survivors have an increased risk of advanced colorectal neoplasia from the age of 40 years and CRC risk is especially increased in HL patients treated before the age of 35 years, we recommend that surveillance start not later than the age of 40 years.9 In the current study, we did not have sufficient patient numbers to evaluate the prevalence of advanced colorectal neoplasia before the age of 40 years. Similarly to the recommendations for other high‐risk groups such as individuals with familial CRC, we suggest a 5‐year interval after a negative colonoscopy because we do not have any evidence yet to suggest that a shorter interval is indicated.26 In the presence of neoplasia, guidelines for colonoscopy surveillance after polypectomy should be followed. However, further research is necessary to evaluate the preventive effect of surveillance colonoscopies. On the basis of these results, the details of the surveillance program, such as the interval and the starting age, should be optimized. The efficacy of a surveillance program in HL survivors, including the burden and cost‐effectiveness of such a program, should be evaluated in future studies. Future studies should also evaluate the potential benefits of a colonoscopy surveillance program for other cancer survivors who have been similarly treated.1, 2, 3
In the current study, comorbidities and prior colonoscopies were reasons for exclusion in 9% and 13%, respectively, and 33% declined participation. When surveillance colonoscopies are being implemented, increasing the participation rate will be important, and this should start with increasing awareness among hematologists and radiation oncologists by the incorporation of this recommendation into the guideline for HL survivors.
In addition to the higher prevalence of colorectal neoplasia in HL survivors, the pattern of development may be different from that in the general population. First, neoplastic lesions were more frequently located in the proximal colon in HL survivors in comparison with controls. This includes the transverse colon, which is the colonic region that receives a significant irradiation dose during para‐aortic radiotherapy and that develops a high frequency of CRCs in HL survivors.15, 27 Second, especially in female HL survivors, we detected a high prevalence of serrated lesions and serrated polyposis syndrome. The reported prevalence of serrated polyposis syndrome in the general population ranges from 0% to 0.09%.28 Therefore, prior anticancer treatment may be a predisposing factor for the development of serrated polyposis syndrome. The explanation for why different types of neoplastic lesions were observed between men and women is unclear and deserves more study.
Finally, our group previously demonstrated heterogeneous molecular characteristics of therapy‐related CRCs with a high frequency of somatic mismatch repair gene mutations.15 These mutations could not be associated with a colonic region, sex, or a specific HL treatment. We aim to evaluate the molecular characteristics of the neoplastic lesions detected in the current study to gain more insight into therapy‐related CRC pathogenesis.
A limitation of our study concerns the comparability of our study results with the comparison population because colonoscopies were performed in different time periods (2015‐2017 for HL survivors vs 2009‐2010 for controls). The Dutch national screening program is not a suitable comparison population because colonoscopies are performed in the fecal immunochemical test–positive population only. In other countries, either the CRC incidence is not comparable with the incidence in the Netherlands, or detailed data are lacking on neoplastic lesions. Therefore, the current comparison cohort gives the best comparison with the Dutch general population. In both the current study and the comparison population, expert endoscopists of (partially overlapping) participating centers performed high‐quality colonoscopies with high‐definition scopes with narrow‐band imaging. Both studies demonstrated adequate cecal intubation rates, withdrawal times, and bowel preparation scores. Nonetheless, the endoscopic identification of serrated lesions can be difficult for expert and nonexpert endoscopists, and this may have affected our results.29 Endoscopists in both studies were instructed to detect and remove all colonic polyps, regardless of their endoscopic appearance or size.21 In addition, the prevalence of serrated lesions in the control population was similar to the prevalence in other recent primary colonoscopy and fecal immunochemical test–based screening cohorts.30 These cohorts originated from 4 other European countries and included patients who underwent a colonoscopy between 2009 and 2015.
Because of the relatively small number of HL survivors, the evidence provided by this study is insufficient for small subgroups (eg, patients treated with abdominal radiotherapy alone). Another limitation of our study is that we may have overestimated the overall benefit of colonoscopy surveillance in HL survivors because they have high morbidity and mortality from other diseases, including cardiovascular disease and other second malignancies.31, 32, 33
Our results are not representative for current HL patients because HL treatment regimens have seen substantial changes over the past decades, including reductions of radiation target volumes and doses and improvements in techniques. In addition, chemotherapy regimens and doses have been adapted. Despite these adaptations, a recent study showed that the risk of gastrointestinal malignancies did not appear to decrease over the treatment period of 1965‐2000.5 Thus, a large population of survivors is currently at risk of developing second gastrointestinal malignancies. Future studies should evaluate risks for more recently treated HL survivors.
In conclusion, HL survivors who have been treated with abdominal radiotherapy and/or procarbazine have a high prevalence of advanced colorectal neoplasia and serrated polyposis syndrome. In addition to the increased risk of CRC, the evidence provided by this interim analysis indicates that colonoscopy surveillance should be considered. Although the sample size of this study precludes the definition of precise eligibility criteria for such a surveillance program, we recommend that surveillance start at least 8 to 10 years after HL treatment.
Funding Support
Monique E. van Leerdam obtained funding from the Dutch Digestive Disease Foundation (Maag Lever Darm Stichting) through funding project FP 14‐04. The Dutch Digestive Disease Foundation had no role in the study design or in the collection, analysis, and interpretation of data.
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Lisanne S. Rigter: Conceptualization, writing–original draft, formal analysis, data curation, investigation, project administration, methodology, and writing–review and editing. Manon C. W. Spaander: Conceptualization, data curation, investigation, project administration, methodology, and writing–review and editing. Berthe M. P. Aleman: Conceptualization, data curation, investigation, supervision, methodology, and writing–review and editing. Tanya M. Bisseling: Conceptualization, data curation, investigation, project administration, methodology, and writing–review and editing. Leon M. Moons: Conceptualization, data curation, investigation, project administration, methodology, and writing–review and editing. Annemieke Cats: Methodology and writing–review and editing. Pieternella J. Lugtenburg: Conceptualization, data curation, investigation, methodology, and writing–review and editing. Cecile P. M. Janus: Conceptualization, data curation, investigation, methodology, and writing–review and editing. Eefke J. Petersen: Conceptualization, data curation, investigation, methodology, and writing–review and editing. Judith M. Roesink: Conceptualization, data curation, investigation, methodology, and writing–review and editing. Richard W. M. van der Maazen: Conceptualization, data curation, investigation, methodology, and writing–review and editing. Petur Snaebjornsson: Conceptualization, resources, investigation, methodology, and writing–review and editing. Ernst J. Kuipers: Data curation, investigation, methodology, and writing–review and editing. Marco J. Bruno: Data curation, investigation, supervision, methodology, and writing–review and editing. Evelien Dekker: Data curation, investigation, supervision, methodology, and writing–review and editing. Gerrit A. Meijer: Conceptualization, resources, investigation, supervision, methodology, and writing–review and editing. Jan Paul de Boer: Conceptualization, data curation, investigation, methodology, and writing–review and editing. Flora E. van Leeuwen: Conceptualization, formal analysis, data curation, investigation, supervision, methodology, and writing–review and editing. Monique E. van Leerdam: Conceptualization, funding acquisition, writing–original draft, formal analysis, data curation, investigation, project administration, supervision, methodology, and writing–review and editing.
Supporting information
First, we thank all participating patients. We also thank Els Wieten, Agnes Reijm, Maria van Vugt, Dorien van der Biessen, Mariette van Kouwen, Denise Buter, Klaartje Nijssen, and Roel de Weijer for the invitations to and care of the participating patients. In addition, we thank Pauline van Mulligen and Margriet Lemmens for the logistics and analyses of the stool tests, Katarzyna Jóźwiak for the statistical support, and Michael Schaapveld for the epidemiologic insights.
References
- 1. Reulen RC, Frobisher C, Winter DL, et al. Long‐term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305:2311‐2319. [DOI] [PubMed] [Google Scholar]
- 2. Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long‐term survivors. J Natl Cancer Inst. 2005;97:1354‐1365. [DOI] [PubMed] [Google Scholar]
- 3. Teepen JC, de Vroom SL, van Leeuwen FE, et al. Risk of subsequent gastrointestinal cancer among childhood cancer survivors: a systematic review. Cancer Treat Rev. 2016;43:92‐103. [DOI] [PubMed] [Google Scholar]
- 4. Hodgson DC, Gilbert ES, Dores GM, et al. Long‐term solid cancer risk among 5‐year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25:1489‐1497. [DOI] [PubMed] [Google Scholar]
- 5. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373:2499‐2511. [DOI] [PubMed] [Google Scholar]
- 6. Henderson TO, Oeffinger KC, Whitton J, et al. Secondary gastrointestinal cancer in childhood cancer survivors: a cohort study. Ann Intern Med. 2012;156:757‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nottage K, McFarlane J, Krasin MJ, et al. Secondary colorectal carcinoma after childhood cancer. J Clin Oncol. 2012;30:2552‐2558. [DOI] [PubMed] [Google Scholar]
- 8. Tukenova M, Diallo I, Anderson H, et al. Second malignant neoplasms in digestive organs after childhood cancer: a cohort‐nested case‐control study. Int J Radiat Oncol Biol Phys. 2012;82:e383‐e390. [DOI] [PubMed] [Google Scholar]
- 9. van Eggermond AM, Schaapveld M, Janus CP, et al. Infradiaphragmatic irradiation and high procarbazine doses increase colorectal cancer risk in Hodgkin lymphoma survivors. Br J Cancer. 2017;117:306‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teepen JC, Kok JL, van Leeuwen FE, et al. Colorectal adenomas and cancers after childhood cancer treatment: a DCOG‐LATER record linkage study. J Natl Cancer Inst. 2018;110:758‐767. [DOI] [PubMed] [Google Scholar]
- 11. Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta‐analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15‐year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829‐834. [DOI] [PubMed] [Google Scholar]
- 13. Daly PE, Samiee S, Cino M, et al. High prevalence of adenomatous colorectal polyps in young cancer survivors treated with abdominal radiation therapy: results of a prospective trial. Gut. 2017;66:1797‐1801. [DOI] [PubMed] [Google Scholar]
- 14. Hassan C, Quintero E, Dumonceau JM, et al. Post‐polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013;45:842‐851. [DOI] [PubMed] [Google Scholar]
- 15. Rigter LS, Snaebjornsson P, Rosenberg EH, et al. Double somatic mutations in mismatch repair genes are frequent in colorectal cancer after Hodgkin’s lymphoma treatment. Gut. 2018;67:447‐455. [DOI] [PubMed] [Google Scholar]
- 16. Rigter LS, Spaander MC, Moons LM, et al. Colorectal cancer surveillance in Hodgkin lymphoma survivors at increased risk of therapy‐related colorectal cancer: study design. BMC Cancer. 2017;17:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi‐Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844‐857. [DOI] [PubMed] [Google Scholar]
- 18. Ponugoti PL, Rex DK. Yield of a second screening colonoscopy 10 years after an initial negative examination in average‐risk individuals. Gastrointest Endosc. 2017;85:221‐224. [DOI] [PubMed] [Google Scholar]
- 19. Regge D, Iussich G, Segnan N, et al. Comparing CT colonography and flexible sigmoidoscopy: a randomised trial within a population‐based screening programme. Gut. 2017;66:1434‐1440. [DOI] [PubMed] [Google Scholar]
- 20. Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non‐cathartic CT colonography in population‐based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13:55‐64. [DOI] [PubMed] [Google Scholar]
- 21. Hazewinkel Y, de Wijkerslooth TR, Stoop EM, et al. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 2014;46:219‐224. [DOI] [PubMed] [Google Scholar]
- 22. IJspeert JE, Bossuyt PM, Kuipers EJ, et al. Smoking status informs about the risk of advanced serrated polyps in a screening population. Endosc Int Open. 2016;4:E73‐E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Snover DC, Ahnen DJ, Burt RW, et al. Serrated polyps of the colon and rectum and serrated polyposis In: Bosman T, Carneiro F, Hruban R, et al, eds. WHO Classification of Tumours of the Digestive System. Lyon, France: International Agency for Research on Cancer; 2010:160‐165. [Google Scholar]
- 24. Davenport JR, Su T, Zhao Z, et al. Modifiable lifestyle factors associated with risk of sessile serrated polyps, conventional adenomas and hyperplastic polyps. Gut. 2018;67:456‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang LC, Shun CT, Hsu WF, et al. Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clin Gastroenterol Hepatol. 2017;15:872‐879.e1. [DOI] [PubMed] [Google Scholar]
- 26. CBO . Hereditary Colorectal Cancer, National Guideline, 2015. https://www.mdl.nl/sites/www.mdl.nl/files/richlijnen/Erfelijke_darmkanker_-_december_2015_def.pdf. [Google Scholar]
- 27. Youn P, Li H, Milano MT, et al. Long‐term survival among Hodgkin’s lymphoma patients with gastrointestinal cancer: a population‐based study. Ann Oncol. 2013;24:202‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Herwaarden YJ, Verstegen MH, Dura P, et al. Low prevalence of serrated polyposis syndrome in screening populations: a systematic review. Endoscopy. 2015;47:1043‐1049. [DOI] [PubMed] [Google Scholar]
- 29. Bouwens MW, van Herwaarden YJ, Winkens B, et al. Endoscopic characterization of sessile serrated adenomas/polyps with and without dysplasia. Endoscopy. 2014;46:225‐235. [DOI] [PubMed] [Google Scholar]
- 30. IJspeert JE, Bevan R, Senore C, et al. Detection rate of serrated polyps and serrated polyposis syndrome in colorectal cancer screening cohorts: a European overview. Gut. 2017;66:1225‐1232. [DOI] [PubMed] [Google Scholar]
- 31. Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016;17:1325‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Eggermond AM, Schaapveld M, Lugtenburg PJ, et al. Risk of multiple primary malignancies following treatment of Hodgkin lymphoma. Blood. 2014;124:319‐327. [DOI] [PubMed] [Google Scholar]
- 33. van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40‐year disease risk. JAMA Intern Med. 2015;175:1007‐1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


