1.
To the Editor:
Recently, a real‐world study of primarily Medicare‐insured patients compared the risk of recurrent venous thromboembolism (VTE) and major bleeding associated with rivaroxaban, warfarin, and low‐molecular weight heparin (LMWH) following a first‐episode of VTE among patients with cancer.1 Results suggested that rivaroxaban treatment is associated with a lower risk of recurrent VTE versus LMWH or warfarin, and that the rate of major bleeding does not significantly differ across treatments.1 As this original study was conducted among an elderly population (age ≥65 years), we sought to assess the risk of recurrent VTE and major bleeding associated with rivaroxaban, LMWH, and warfarin treatment following a first‐episode of VTE in commercially‐insured—and younger—patients.
We have previously detailed our methodology.1 A retrospective cohort study design was employed whereby cancer patients diagnosed with a first VTE between January 1, 2013 and September 30, 2016 (ie, the index date) were identified in Truven Health MarketScan Research Databases. Only patients with lower extremity deep vein thrombosis and pulmonary embolism were included. Patients were required to have initiated anticoagulation <7 days post‐index and cohorts were formed based on the index anticoagulant (ie, warfarin, rivaroxaban, or LMWH). VTE and major bleeding events were monitored during the observation period. For VTE, the observation period spanned from the index date until end of eligibility or data availability. For major bleeding, the observation period spanned from the index date until discontinuation of the index anticoagulant treatment. Recurrent VTE was defined as a hospitalization with a primary diagnosis of VTE ≥7 days after the first VTE and was identified using International Classification of Diseases, Ninth and Tenth revisions, Clinical Modification. Major bleeding was identified using the Cunningham algorithm.2 Study outcomes were assessed using Cox proportional hazards models with hazard ratios (HRs) and Kaplan‐Meier rates. Inverse probability of treatment weights (IPTW) based on propensity scores were used to adjust for baseline confounding.
A total of 13 804 commercially‐insured patients were included. Of these, 3370 were initiated on rivaroxaban, 4774 on warfarin, and 4313 on LMWH. IPTW resulted in generally well‐balanced cohorts (ie, standardized difference <10%). Mean ages among the rivaroxaban, warfarin, and LMWH cohorts were 62.6, 63.9, and 60.2 years, respectively, which is ~10 years younger than patients in our previous study.1 Similarly, patients had fewer comorbidities relative to those included in Streiff et al.'s study (Quan‐Charlson comorbidity index 3.7 vs 4.7). Other baseline characteristics were generally similar between the two studies (see Table 1).
Table 1.
Patient characteristics of weighted cohortsa
| Rivaroxaban vs warfarin | Rivaroxaban vs LMWH | Warfarin vs LMWH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rivaroxaban cohort | Warfarin cohort | Std.diff. (%)b | Rivaroxaban cohort | LMWH cohort | Std.diff. (%)b | Warfarin cohort | LMWH cohort | Std. diff. (%)b | |
| Characteristics | (N = 3370) | (N = 4774) | (N = 3370) | (N = 4313) | (N = 4774) | (N = 4313) | |||
| Demographicsc | |||||||||
| Age, mean ± SD [median] | 63.6 ± 13.2 [62.8] | 63.4 ± 13.4 [62.6] | 1.3 | 61.3 ± 12.7 [61.0] | 61.3 ± 12.7 [61.3] | 0.2 | 62.0 ± 13.2 [61.6] | 62.2 ± 13.1 [62.0] | 1.4 |
| Gender, female, n (%) | 1673 (49.7) | 2394 (50.1) | 1.0 | 1771 (52.5) | 2261 (52.4) | 0.2 | 2405 (50.4) | 2177 (50.5) | 0.2 |
| Type of index VTEc, n (%) | |||||||||
| PE | 839 (24.9) | 1191 (24.9) | 0.1 | 955 (28.3) | 1216 (28.2) | 0.3 | 1254 (26.3) | 1139 (26.4) | 0.3 |
| DVT | 1608 (47.7) | 2286 (47.9) | 0.4 | 1607 (47.7) | 2041 (47.3) | 0.8 | 2226 (46.6) | 2020 (46.8) | 0.4 |
| PE and DVT | 923 (27.4) | 1297 (27.2) | 0.5 | 808 (24.0) | 1056 (24.5) | 1.2 | 1294 (27.1) | 1153 (26.7) | 0.8 |
| Regionc, n (%) | |||||||||
| South | 1184 (35.1) | 1694 (35.5) | 0.7 | 1237 (36.7) | 1581 (36.7) | 0.1 | 1506 (31.6) | 1316 (30.5) | 2.2 |
| Midwest | 974 (28.9) | 1390 (29.1) | 0.5 | 916 (27.2) | 1137 (26.4) | 1.8 | 1443 (30.2) | 1313 (30.5) | 0.5 |
| Northeast | 629 (18.7) | 891 (18.7) | 0.0 | 711 (21.1) | 962 (22.3) | 2.9 | 987 (20.7) | 929 (21.5) | 2.1 |
| West | 512 (15.2) | 698 (14.6) | 1.6 | 438 (13.0) | 541 (12.5) | 1.4 | 728 (15.3) | 660 (15.3) | 0.2 |
| Unknown | 71 (2.1) | 101 (2.1) | 0.0 | 68 (2.0) | 91 (2.1) | 0.7 | 110 (2.3) | 94 (2.2) | 0.8 |
| Type of cancer with higher risk of VTEd, e based on Khorona's score, n (%) | |||||||||
| Very high risk | 306 (9.1) | 424 (8.9) | 0.8 | 577 (17.1) | 837 (19.4) | 5.9 | 679 (14.2) | 791 (18.3) | 11.2 |
| High risk | 919 (27.3) | 1306 (27.4) | 0.2 | 1108 (32.9) | 1634 (37.9) | 10.5 | 1585 (33.2) | 1654 (38.3) | 10.8 |
| Quan‐Charlson comorbidity indexe | |||||||||
| Mean ± SD [median] | 3.4 ± 2.9 [3.0] | 3.4 ± 3.0 [3.0] | 1.6 | 3.9 ± 3.2 [3.0] | 3.9 ± 3.2 [3.0] | 1.5 | 4.2 ± 3.1 [4.0] | 4.2 ± 3.1 [4.0] | 0.4 |
| Selected baseline comorbidities, n (%) | |||||||||
| Hypertension | 1954 (58.0) | 2822 (59.1) | 2.3 | 1836 (54.5) | 2254 (52.3) | 4.5 | 2785 (58.3) | 2285 (53.0) | 10.8 |
| COPD | 765 (22.7) | 1109 (23.2) | 1.3 | 757 (22.5) | 947 (22.0) | 1.2 | 1133 (23.7) | 962 (22.3) | 3.4 |
| Diabetes | 483 (14.3) | 687 (14.4) | 0.2 | 586 (17.4) | 940 (21.8) | 11.1 | 847 (17.7) | 927 (21.5) | 9.4 |
| Congestive heart failure | 474 (14.1) | 715 (15.0) | 2.7 | 488 (14.5) | 512 (11.9) | 7.7 | 737 (15.4) | 477 (11.1) | 12.9 |
| Liver disease | 337 (10.0) | 482 (10.1) | 0.4 | 342 (10.1) | 377 (8.7) | 4.8 | 509 (10.7) | 383 (8.9) | 6.0 |
| Obesity | 374 (11.1) | 565 (11.8) | 2.3 | 302 (9.0) | 345 (8.0) | 3.5 | 556 (11.6) | 406 (9.4) | 7.3 |
| Renal disease | 286 (8.5) | 575 (12.0) | 11.7 | 256 (7.6) | 259 (6.0) | 6.3 | 573 (12.0) | 284 (6.6) | 18.7 |
| Atrial fibrillation/flutter | 231 (6.9) | 321 (6.7) | 0.5 | 198 (5.9) | 201 (4.7) | 5.4 | 332 (7.0) | 255 (5.9) | 4.3 |
| Stroke/TIA | 204 (6.1) | 290 (6.1) | 0.1 | 182 (5.4) | 234 (5.4) | 0.1 | 338 (7.1) | 251 (5.8) | 5.2 |
Abbreviations: DVT, deep vein thrombosis; LMWH, low‐molecular‐weight heparin; PE, pulmonary embolism; SD, standard deviation; Std. diff., standardized difference; TIA, transient ischemic attack; VTE, venous thromboembolism.
Baseline characteristics for the weighted cohorts were obtained by using inverse probability of treatment weights; weighted on the following variables: age, sex, cancer type, region, time from first cancer to initial VTE, time to anticoagulant initiation, year of first VTE, setting in which the VTE was diagnosed (inpatient, outpatient, or emergency room), type of VTE (DVT, PE, or both), treatment with an antineoplastic agent, insurance type, Charlson comorbidity index, health care costs, and health care resource used during the 6‐month period before the first VTE.
For continuous variables, the standardized difference is calculated by dividing the absolute difference in means of the treated A and treated B cohorts by the pooled SD of both groups. The pooled SD is the square root of the average of the squared SDs. For categorical variables with two levels, the standardized difference is calculated using the following equation where P is the respective proportion of participants in each group: (PtreatedA‐PtreatedB)/√[p(1‐p)], where p = (PtreatedA + PtreatedB)/2.
Evaluated at the index date.
Not mutually exclusive.
Evaluated during the 6‐month baseline period.
The mean (median) durations of treatment were 5.5 (3.6), 3.5 (2.0), and 5.8 (4.0) months for patients initiated on rivaroxaban, LMWH, and warfarin, respectively. Relative to patients initiated on LMWH, the rate of recurrent VTE was 17% lower for patients initiated on rivaroxaban (P = 0.010; Figure 1A). Rates of recurrent VTE were not significantly different between the rivaroxaban versus warfarin cohorts (P = 0.456; Figure 1B) and warfarin versus LMWH cohorts (P = 0.103; Figure 1C).
Figure 1.
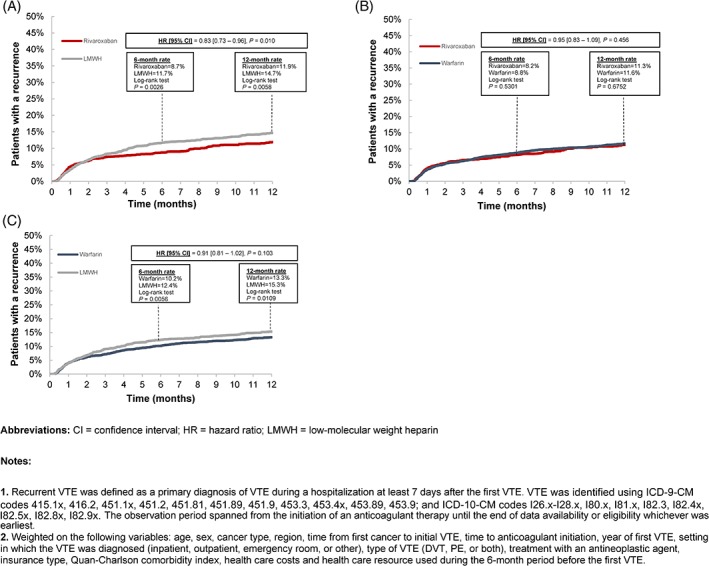
Cumulative incidence of recurrent VTE1 with pairwise comparisons of all cohorts2
Major bleeding was not significantly different between patients initiated on rivaroxaban versus LMWH (HR [95% CI] = 0.91 [0.71; 1.17], P = 0.455; see Supporting Information Figure S1). Similarly, there was no difference in the risk of major bleeding between warfarin‐ versus rivaroxaban‐initiated patients (HR [95% CI] = 1.08 [0.86; 1.37], P = 0.500) and LMWH‐ versus warfarin‐initiated patients (HR [95% CI] = 0.86 [0.68; 1.08], P = 0.187; see Supporting Information Figure S1).
Two of the principal differences between this study and our previous study are that patients included here were younger and the median duration of treatment was consistently higher across all cohorts (rivaroxaban: 3.6 vs 3.0 months; LMWH: 2.0 vs 1.0 months; warfarin: 4.0 vs 3.5 months). Furthermore, using the rates reported by Streiff et al. as a reference, VTE recurrence rates were 25%‐50% lower in the current study across all evaluated treatments, comparisons, and time points (ie, 6‐ or 12‐month rate).
When comparing the results of the current study with those of clinical trials, the 6‐month VTE recurrence rates (Figure 1) are in line with those noted in the CATCH (LMWH: 7.2%; warfarin: 10.5%), CLOT (LMWH: 9.0%; warfarin 17.0%), and SELECT‐D trials (LMWH: 11.0%; rivaroxaban: 4.0%).3, 4, 5 It is likely that the similar findings noted in this study and previous clinical trials could be explained by age. More precisely, the mean age of the commercial population evaluated in our study was ~62 years old, while the mean age of patients in the CATCH and CLOT trials were ~59 and ~62 years old, respectively; and the median age in the SELECT‐D trial was 67 years old. With respect to safety outcomes, the rate of major bleeding events at 6 months (LMWH: ~5%; warfarin: ~4%; rivaroxaban: ~4%) are very similar to those reported in the CATCH (LMWH: ~3%; warfarin: ~3%), CLOT (LMWH: 6.0%; warfarin: 4.0%), and SELECT‐D trials (LMWH: 4.0%; rivaroxaban: 6.0%). Despite these similarities, it is possible that the number of recurrent VTE was underestimated in the current study as the definition of recurrence was restricted to VTE documented during a hospitalization.
In this real‐world analysis, patients with cancer who initiated standard‐of‐care LMWH had a 17% and 9% higher risk of recurrent VTE compared to rivaroxaban and warfarin, respectively, but a similar risk of major bleeding. These conclusions, which are based on a commercially‐insured population, are similar to those of Streiff et al.1 in an older population, except younger patients had a lower risk of VTE. This may reflect patients' age since older patients have more comorbid conditions and/or the longer duration of anticoagulation observed in younger patients. The results presented here further support that the real‐world efficacy of LMWH is reduced by suboptimal treatment duration.6 This may explain the lower efficacy of LMWH in a real‐world setting versus that previously observed in the controlled environments of randomized controlled trials. Additional trials assessing the prevention of VTE with direct oral anticoagulants in patients with cancer are underway. They should shed more light on potentially avoiding morbidity and mortality associated with VTE with anticoagulant treatment.
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Figure S1 Cumulative incidence of major bleeding events
REFERENCES
- 1. Streiff MB, Milentijevic D, McCrae K, et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018;93(5):664‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee AYY, Levine MN, Baker RI, et al. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146‐153. [DOI] [PubMed] [Google Scholar]
- 4. Lee AY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677‐686. [DOI] [PubMed] [Google Scholar]
- 5. Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT‐D). J Clin Oncol. 2018;36(20):2017‐2023. [DOI] [PubMed] [Google Scholar]
- 6. Khorana AA, McCrae KR, Milentijevic D, et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer‐associated thrombosis. Res Pract Thromb Haemost. 2017;1(1):14‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Cumulative incidence of major bleeding events


