Abstract
Purpose
The main objective of this study was to analyze validated cases of drug‐induced anaphylactic reactions in children with regard to incriminated drugs, clinical characteristics, and associated factors. A further objective was to compare differences in incriminated drugs and characteristics between validated cases and a reference excluding anaphylactic reaction cases (basic dataset).
Methods
Spontaneous reports of anaphylactic reactions in children (0‐17 years) registered between January 2000 to December 2016 were extracted from the adverse drug reaction database of the German Federal Institute for Drugs and Medical Devices. These reports were restricted to drugs for which at least four cases were found. After case validation, 159 reports remained (validated dataset) and were compared with the basic dataset (n = 12.168 reports) using inferential statistics.
Results
Estimated yearly increase of reports (36.8 vs 0.1), most frequently incriminated drugs (antibiotics 30.2% vs 11%, analgesics/antipyretics 22.0% vs 5.6%; P values less than 0.001) and route of administration (38.4% vs 6.7%) differed between the validated dataset and the basic dataset. Validated cases differed in severity (higher with atracurium), reported symptoms (urticaria leading with analgesics), and associated factors (atopy/allergy rarely reported with antibiotics) depending on the incriminated drug class. In 13.8% (11.3% if excluding repeated readministration in one person) of the cases, the drug had not been tolerated before.
Conclusions
A heterogeneous clinical phenotype with differences in associated factors was observed, suggesting different underlying mechanisms triggered by the different drug groups. Occurrence of serious drug‐induced anaphylactic reactions in children could be reduced by carefully considering patient history.
Keywords: adverse drug reaction, anaphylactic reaction, anaphylaxis, atopy, pharmacoepidemiology, spontaneous reports
KEY POINTS.
Only a few studies have investigated drug‐induced anaphylactic reactions in children.
The adverse drug reaction (ADR) database of the German Federal Institute for Drugs and Medical Devices provided the opportunity to examine this rare ADR on a larger scale.
Intravenous administration was noted for 38% of incriminated drugs. In 13.8% of cases (11.3% if excluding repeated readministration in one person), previous hypersensitivity to the drug had been reported, and these cases appeared to be more severe than cases designated as “drug never used before.”
Antibiotics, analgesics, and MRI contrast media were most frequently suspected of having induced the anaphylactic reaction in validated cases.
Cefaclor accounted for 27% and amoxicillin for 8.3% of cases induced by antibiotics, although exposure to amoxicillin seems to outweigh cefaclor exposure.
1. INTRODUCTION
According to the allergy for global use nomenclature, anaphylaxis is defined as a severe, life‐threatening generalized or systemic hypersensitivity reaction1 resembling an immediate‐type reaction.2, 3
The distal pathophysiological pathway in immune‐mediated and non–immune‐mediated anaphylaxis involves the release of mediators such as histamine, tryptase, and other bioactive mediators from basophils and mast cells.4
Drugs rank either second5, 6 or third7, 8, 9 behind food and insect venoms as elicitors of anaphylaxis in children. One study reported an incidence of 0.5/100 000 person‐years based on the clinical evaluation of these cases.10
Antibiotics, particularly beta‐lactams, and nonsteroidal antiinflammatory drugs (NSAIDs) are reported as frequent elicitors of drug‐induced anaphylaxis in children.11, 12, 13, 14, 15 However, these observations are based on a limited number of anaphylaxis cases in children (less than 100).
Some publications have reported atopy and allergy as risk factors for severe courses of anaphylaxis16, 17 whereas others have not.12, 14, 15, 18 However, risk factors and cofactors may differ between age groups or according to the underlying pathophysiology and are not sufficiently studied in children.19
This paucity of data prompted us to further investigate drug‐induced anaphylaxis in children on a larger scale and over a longer period of time (ie, 159 validated cases in 16 years) by exploring the adverse drug reaction (ADR) database of the German Federal Institute for Drugs and Medical Devices (BfArM).
The main objective was to analyze validated cases with regard to incriminated drugs, clinical phenotype, and associated factors. One limitation of spontaneous ADR data is the lack of control groups. A further objective was thus to compare differences in incriminated drugs and characteristics between validated cases and a reference excluding anaphylactic reaction cases (basic dataset).
2. MATERIAL AND METHODS
2.1. BfArM's ADR database
As described earlier,20, 21 physicians in Germany are obliged by their professional conduct code to report ADRs to their professional councils, which forward these reports to either BfArM (responsible for chemically defined drugs)22 or Paul‐Ehrlich‐Institut (PEI) (responsible for monoclonal antibodies, vaccines, etc).23, 24 These reports can also be reported directly to BfArM, PEI, or marketing authorization holders who then forward the reports to the authorities.
In BfArM's ADR database, drugs are coded according to the World Health Organization (WHO) Drug Dictionary and the Anatomical Therapeutic Chemical (ATC) classification system.25 ADRs are coded using Medical Dictionary for Regulatory Activities (MedDRA) terminology.26
The data lock point of the present analysis was December 2016.
2.2. Case identification
We identified all spontaneous ADR reports (no study reports) referring to children (0‐17 years), registered between January 2000 and December 2016 and originating from Germany (n = 14 508). Subsequently, we selected all anaphylactic reaction cases (n = 505) by application of the Standardized MedDRA Query (SMQ) “anaphylactic reaction” (version 19.1 as of September 2016).26 The 505 cases were restricted to reports where the “suspected/interacting” drug was reported more than three times in order to exclude influence by single reports. This resulted in 242 reports. All ADR reports coded as medication errors or with evidence of ADRs due to intentional suicide/self‐injury were excluded by application of respective SMQs (pertains to each of the three datasets).
2.3. Validation of cases with anaphylactic reactions
The 242 reports were assessed by one of two (either B.S. or W.F‐B) board‐certified specialists in dermatology and allergology. Only cases in which (a) the correctness of the diagnosis “anaphylactic reaction” according to a national guideline3 and (b) the causal relationship with the incriminated drug according to WHO criteria27 was at least possible were considered for further analysis. Reports with only few symptoms or reports where symptoms were already transformed into the diagnosis “anaphylaxis” were also considered if
-
‐
respective treatment or treatment in an intensive/emergency care unit was reported,
-
‐
the patient had to be hospitalized,
-
‐
the event occurred under medical surveillance (eg, during anesthesia),
-
‐
the case was reported as life‐threatening, or
-
‐
the physician already had classified the anaphylactic reaction suggesting medical expertise concerning anaphylactic reactions.
For quality assurance, the final dataset was reviewed by a pharmacist. Eventually, the validated dataset consisted of 159 cases including 164 incriminated drugs (equal causal probability for two drugs in five cases). The analysis of the incriminated drugs and routes of administration referred to the 164 drugs, whereas all other analyses referred to the 159 cases (see Figure 1).
Figure 1.
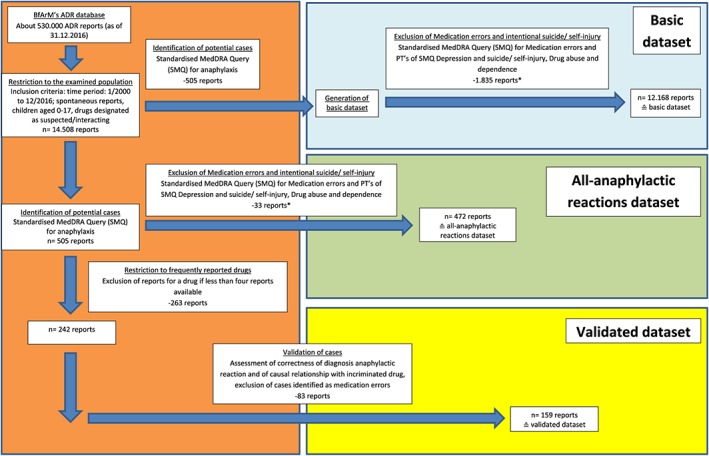
Flow chart depicting the process of identification, selection, and validation of spontaneous reports of anaphylactic reactions contained in the Federal Institute for Drugs and Medical Devices (BfArM's) adverse drug reaction (ADR) database and description of the three datasets (*since cases in which the ADR resulted from a medication error had been deleted from the validated cases, such reports [medication errors or intentional overdose, eg, suicide] were also excluded in the other two datasets by applying respective SMQs. The reasoning for this approach was that, usually, in these cases, inappropriate doses are administered, resulting in a higher risk for ADRs) [Colour figure can be viewed at wileyonlinelibrary.com]
2.4. Quality of validated cases
The completeness of data in the validated cases was assessed according to a published score.28 Calculation of the score was modified as it was not computed for every reported drug‐ADR pair (in case more than one ADR had been reported) and then aggregated to an average, to yield an overall score for the corresponding report. Instead, since our analysis focused on anaphylactic reactions, the calculation of the score referred only to the diagnosis anaphylactic reaction. A completeness score of 0.89 [0.81‐0.95] was calculated (greater than 0.8, well‐documented according to Bergvall et al28). Most data in the variable dose (30.8% of reports) was missing.
2.5. Generation and comparison of additional datasets
In order to address the lack of a control group, we generated a reference group (“basic dataset”) containing all other ADR reports on children 0 to 17 years excluding the 505 cases identified by the SMQ “anaphylactic reaction” (n = 12 168 reports). In addition, we created the “all‐anaphylactic reactions” dataset in order to examine whether differences between the basic dataset and the validated dataset might have resulted from the validation process or from restriction to reports with drugs reported in more than three cases. This dataset was based on the 505 identified anaphylactic reaction cases and finally resulted in 472 reports. The same predefined inclusion and exclusion criteria of cases were applied for both datasets.
The three datasets were compared with regard to basic characteristics, incriminated drugs, and the seriousness criteria based on the legal (not clinical) definition, ie, outcome of the ADR is fatal, life‐threatening or leads to (prolonged) hospitalization, persistent or significant disabilities, or congenital anomalies/birth defects.29
2.6. Analysis of the validated cases
Any analysis was based on the information provided in the complete report including narrative and follow‐ups.
Cases were classified with regard to increasing severity (grade I‐IV) according to a national guideline.3 Grade I reactions, for example, are characterized by cutaneous and subjectively perceived general symptoms only, whereas grade IV refers to cardiovascular and/or respiratory arrest (unclassifiable cases are denoted as NOS).
Cases were also analyzed concerning reported symptoms by analyzing assigned preferred terms26 and associated factors like atopy/allergy. Atopy is an individual susceptibility usually occurring in childhood to become sensitized and produce immunoglobulin E (IgE) antibodies in response to ordinary exposures to allergens. These individuals can develop allergic asthma, allergic rhinoconjunctivitis, or atopic dermatitis.1 No published algorithm to diagnose atopy was found. Hence, an individual was designated as atopic if either atopy or one of the following conditions was reported: atopic dermatitis/asthma/pollinosis, a total IgE greater than 100 kU/L, or IgE slightly elevated. A patient was designated as allergic if allergy (NOS or specified) was reported.
The classification “drug administered before” referred to the previous administration of drugs with the same active ingredient except in cases where excipients were also cosuspected (eg, coloring agents or flavors). The classification “drug not tolerated before” referred to the occurrence of hypersensitivity‐like symptoms after previous administration.
2.7. Statistical analysis
The descriptive analysis was carried out with means (±SD) (for age, estimated yearly increase, drugs per report) and frequency distributions with percentages (all other results). Because of unequal variances, Welch t test was performed to compare mean ages between drug subgroups and the remaining validated cases. For differences in frequency distributions between the two anaphylactic reactions datasets and the basic dataset and in the validated dataset between drug subgroups and the remaining cases (without the respective drug subgroup), the chi‐square test was applied (in case of less than six cases: Fisher exact test).
3. RESULTS
3.1. Comparison of datasets
Table 1 shows the characteristics of the three datasets. The number of reports in the basic dataset increased by an average of 36 reports per year, whereas the annual number of validated cases remained stable with an average proportion of 1.4% (range: 0.7‐2.2%) per year. The validated cases in comparison with the basic dataset more often reported the seriousness criteria life‐threatening (23.3% vs 5.8%) or hospitalization (45.3% vs 30.0 %) but less often death (0.6% vs 3.5%).
Table 1.
Characterization of the three datasetsa
| Spontaneous Reports from 2000 to 2016 Without Medication Errors and Intentional Overdose; Age: 0 to 17 years | |||
|---|---|---|---|
| Criteria | Basic datasetb (without anaphylactic reaction cases) (n = 12 168 cases) | All‐anaphylactic reactions dataset (determined by SMQc) (n = 472 cases) | Validated dataset (n = 159 cases) |
| Estimated yearly increase (in cases ±SD) | y = 36.875 (±110.9) | y = 0.0625 (±7.7) | y = 0.0625 (±5.4) |
| Number of suspected/interacting drugsd | 16 777 | 576 | 164 |
| Drugs per report (±SD) | 1.4 (0.4‐2.4) | 1.2 (0.5‐1.9) | 1.0 (0.8‐1.2) |
| Primary source | |||
| Physician | 61.1% (n = 7437) | 67.4% (n = 318) | 71.1% (n = 113) |
| Consumer/non‐HCPe | 8.9% (n = 1084) | 8.7% (n = 41) | 5.7% (n = 9) |
| Seriousf | 82.5% (n = 10 041) | 87.5% (n = 413) | 88.0% (n = 140) |
| Hospitalization | 30.0% (n = 3647) | 41.9% (n = 198) | 45.3% (n = 72) |
| Life‐threatening | 5.8% (n = 710) | 22.0% (n = 104) | 23.3% (n = 37) |
| Death | 3.5% (n = 426) | 6.1% (n = 29)g | 0.6% (n = 1) |
| Mean age (years ± SD) | 8.2 (2.0‐14.4) | 10.0 (4.4‐15.6) | 8.9 (3.5‐14.3) |
| Male | 50.2% (n = 6106) | 48.7% (n = 230) | 48.4% (n = 77) |
| Female | 43.4% (n = 5278) | 50.0% (n = 236) | 51.6% (n = 82) |
| Unknown | 6.4% (n = 784) | 1.3% (n = 6) | |
| Administration routeh | |||
| Intravenous | 6.7% (n = 1121) | 25.0% (n = 144)* | 38.4% (n = 63)* |
| Oral | 38.9% (n = 6519) | 39.9% (n = 230) | 39.6% (n = 65) |
| Rectal | 0.8% (n = 139) | 3.3% (n = 19) | 4.3% (n = 7) |
| Unknown | 21.2% (n = 3555) | 19.4% (n = 112) | 12.8% (n = 21) |
| Analgesics (N02)i and ibuprofenj | 687 cases (5.6%) | 56 cases (11.9%)* | 35 cases (22.0%)* |
| Mean age (years ± SD) | 6.9 (0.7‐13.1) | 9.1 (4.2‐14.0) | 7.9 (3.2‐12.6) |
| Female | 40.8% (n = 280) | 33.9% (n = 19) | 34.3% (n = 12) |
| Male | 52.0% (n = 357) | 66.1% (n = 37) | 65,7% (n=23) |
| Unknown | 7.3% (n = 50) | ||
| Antibiotics (J01)i | 1336 cases (11.0%) | 89 cases (18.9%)* | 48 cases (30.2%)* |
| Mean age (years ± SD) | 8.2 (2.2‐14.2) | 9.7 (4.0‐15.4) | 8.8 (3.4‐14.2) |
| Female | 48.1% (n = 643) | 52.8% (n = 47) | 54.2% (n = 26) |
| Male | 48.1% (n = 643) | 47.2% (n = 42) | 45.8% (n = 22) |
| Unknown | 3.7% (n = 50) | ||
| Iron | 40 cases (0.3%) | 9 cases (1.9%)* | 7 cases (4.4%)* |
| Mean age (years ± SD) | 8.2 (1.6‐14.8) | 15.1 (11.3‐18.9) | 14.7 (10.4‐19.0) |
| Female | 60.0% (n = 24) | 77.8% (n = 7) | 71.4% (n = 5) |
| Male | 25.0% (n = 10) | 22.2% (n = 2) | 28.6% (n = 2) |
| Unknown | 15.0% (n = 6) | ||
| Alglucosidase | 35 cases (0.3%) | 12 cases (2.5%)* | 12 cases (7.5%)* |
| Mean age (years ± SD) | 2.7 (−1.9‐7.3) | 3.3 (0.4‐6.2) | 3.3 (0.4‐6.2) |
| Female | 51.4% (n = 18) | 33.3% (n = 4) | 33.3% (n = 4) |
| Male | 37.1% (n = 13) | 66.7% (n = 8) | 66.7% (n = 8) |
| Unknown | 11.4% (n = 4) | ||
| MRI (V08C)i | 57 cases (0.5%) | 25 cases (5.3%)* | 19 cases (11.9%)* |
| Mean age (years ± SD) | 12.0 (7.7‐16.3) | 12.1 (7.3‐16.9) | 11.5 (6.4‐16.6) |
| Female | 49.1% (n = 28) | 72.0% (n = 18) | 73.7% (n = 14) |
| Male | 47.4% (n = 27) | 28.0% (n = 7) | 26.3% (n = 5) |
| Unknown | 3.5% (n = 2) | ||
| Atracurium | 3 cases (0.02%) | 5 cases (1.1%)* | 5 cases (3.1%)* |
| Mean age (years ± SD) | 11.7 (9.4‐14.0) | 9.4 (3.0‐15.8) | 9.4 (3.0‐15.8) |
| Female | 0% | 20.0% (n = 1) | 20.0% (n = 1) |
| Male | 100.0% (n = 3) | 80.0% (n = 4) | 80.0% (n = 4) |
In this table, the three generated datasets with their basic characteristics (eg, yearly increase, number of drugs, and primary sources), their number of reports, and their proportional ratio in the respective dataset are depicted.
Serving as a reference.
Standardized MedDRA Query (SMQ). The dataset “all‐anaphylactic reactions” includes all identified anaphylactic reactions by application of the respective SMQ. The 159 validated cases (validated dataset) are also included in this dataset.
In some cases, more than one drug is reported as suspected. Therefore, the number of reported drugs exceeds the number of reports.
There are also other primary sources besides physicians or consumer/non–health care personnel (HCPs). Thus the percentages do not yield 100%.
The “seriousness” assessment may not reflect the clinical severity of the reaction since they refer to the legal definition of the Medicinal Products Act: An adverse drug reaction (ADR) is considered serious when the ADR results in death, is life‐threatening, requires in‐patient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or is a congenital anomaly/birth defect. One case may contain more than one of these criteria.
Twenty‐nine cases with the seriousness criterion “death” were determined; 14 of these cases were assessed within the validation process, leading to the exclusion of 13 cases. The remaining 15 cases were excluded because of the criterion “drug was not reported more than three times.”
Frequency distributions of administration routes refer to the total number of drugs per dataset.
First, the reported suspected/interacting drug subgroups of the validated dataset were identified. Then, respective ATC codes were assigned to the identified drug subgroups. Subsequently, their ATC codes were applied for the stratification of drug subgroups in the other two datasets. Stratification with the suspected/interacting drugs by their active ingredient name only (without application of their ATC code) yielded similar results.
Ibuprofen is assigned to more than one ATC class. Thus, not all cases could be retrieved by ATC code N02 (analgesics), and ibuprofen was identified by its active ingredient name.
Chi‐squared test/Fischer exact test; P < 0.001. Further information for calculation of P values is included in Section 2.7.
Female gender was more frequently reported in the validated than in the basic dataset (51.6% vs 43.4%). Gender differences were also noted depending on the drug administered (eg, MRI contrast media [female gender] 73.7% vs 49.1%).
The drug classes most frequently suspected in the validated cases were less often reported in the basic dataset (antibiotics 30.2% vs 11%, analgesics/antipyretics 22.0% vs 5.6%; P values less than 0.001).
Intravenous administration was clearly more often reported in the validated compared with the basic dataset (38.4% versus 6.7%; P value less than 0.001, based on the number of suspected drugs) and differed depending on drug class.
For most parameters, larger (but similar) differences were observed between the validated and the basic dataset than between the all‐anaphylactic and the basic dataset. However, the number of cases that reported the seriousness criterion death was larger in the all‐anaphylactic (6.1%) than in the validated dataset (0.6%).
3.2. Analysis of validated cases
3.2.1. Demographic parameters
The mean age of validated cases was 8.9 years (SD = 5.4) (Table 2). Slightly more reports were found for preschoolers (≥3 to ≤6 years; 28.9%) and adolescents (≥16 to ≤17 years; 17.6%). Drug‐related age and gender differences were observed, eg, mean age: iron (14.7 years); gender: MRI contrast media (14 females vs five males). These gender differences were also observed in the stratified age groups (female 0‐5 years: 38.2%; female 13‐17 years: 62.7%).
Table 2.
Characterization of validated cases of anaphylactic reactionsa
| All Validated Cases (n = 159)b | Cases Attributed to Antibiotics n= 48 (30.2%) | Cases Attributed to Analgesics/Antipyretics n=35 (22.0%) | Cases Attributed to MRI Contrast media n= 19 (11.9%) | Cases Attributed to Alglucosidase (enzymes)c n = 12 (7.5%) | Cases Attributed to Iron n = 7 (4.4%) | Cases Attributed to Atracurium n = 5 (3.1%) | All Other Cases n = 36 (22.6%) | |
|---|---|---|---|---|---|---|---|---|
| Seriousd | 88.1% (140/159) | 75.0% (36/48) | 100.0% (35/35) | 84.2% (16/19) | 100.0% (12/12) | 85.7% (6/7) | 100.0% (5/5) | 91.7% (33/36) |
| Hospitalization | 45.3% (72/159) | 43.8% (21/48) | 62.9% (22/35) | 42.1% (8/19) | 25.0% (3/12) | 14.3% (1/7) | 40.0% (2/5) | 44.4% (16/36) |
| Life‐threatening | 23.3% (37/159) | 31.3% (15/48) | 22.9% (8/35) | 5.3% (1/19) | 8.3% (1/12) | 14.3% (1/7) | 60.0% (3/5) | 27.8% (10/36) |
| Mean age (years ± SD) | 8.9 (3.5‐14.3)e |
8.8 (3.4‐14.2) Cefaclor 5.8 (1.7‐9.9)* |
7.9 (3.2‐12.6) Ibuprofen 7.3 (2.8‐11.8)* |
11.5 (6.4‐16.6)* | 3.3 (0.4‐6.2)* | 14.7 (10.4‐19.0)* | 9.4 (3.0‐15.8) | 9.6 (4.3‐14.9) |
| Female | 51.6% (82/159) | 54.2% (26/48) | 34.3%* (12/35; 75.0% [9/12] ibuprofen) | 73.0% (14/19) | 33.3% (4/12) | 71.4% (5/7) | 20.0% (1/5) | 61.1% (22/36) |
| Male | 48.4% (77/159) | 45.8% (22/48) | 65.7% (23/35; 91.3% [21/23] ibuprofen) | 26.3% (5/19) | 66.7% (8/12) | 28.6% (2/7) | 80,0% (4/5) | 38.9% (14/36) |
| Intravenous administrationf | 39.6% (63/159) | 20.8%* (10/48) | 0% | 78.9%* (15/19) | 100.0% (12/12) | 85.7%* (6/7) | 80.0% (4/5) | 44.4% (16/36) |
| Drug administered before N = information contained(yes/no); (T = tolerated; NT = not tolerated; NA = unknown)g |
n = 78 No = 15.1% (24/159) Yes = 34.0% (54/159) T = 44.4% (24/54) NT = 40.1% (22/54) NA = 14.8% (8/54) |
n = 19 No = 12.5% (6/48) Yes = 27.1% (13/48) T = 61.5% (8/13) NT = 30.8% (4/13) NA = 7.7% (1/13) |
n = 21 No = 2.9% (1/35) Yes = 85.7% (20/35) T = 55.0% (11/20) NT = 40.0% (8/20) NA = 5.0% (1/20) |
n = 9 No = 42.1% (8/19) Yes = 5.3% (1/19) T = 100.0% (1/1) NT = 0% NA = 0% |
n = 12 No = 0% Yes = 100% (12/12) T = 8.3% (1/12) NT = 50.0% (6/12) NA = 41.7% (5/12) |
n = 3 No = 28.6% (2/7) Yes = 14.3% (1/7) T = 100.0% (1/1) NT = 0% NA = 0% |
n = 1 No = 0% Yes = 20.0% (1/5) T = 0% NT = 100.0% (1/1) NA = 0% |
n = 16 No = 19.4% (7/36) Yes = 25.0% (9/36) T = 22.2% (2/9) NT = 66.7% (6/9) NA = 11.1% (1/9) |
This table shows the validated cases (n = 159; validated dataset) stratified by drug class and seriousness criteria, age and gender, proportion of intravenous administration, and drug‐specific history. In 48 antibiotic cases, 49 antibiotics (one case with cefotaxime and cefixim) were reported. One case reporting cefaclor and ibuprofen as suspected drugs was also counted for the drugs class analgesics. In 35 analgesic cases, 36 analgesics (one case with ibuprofen and metamizole) were reported. One case reporting cefaclor and ibuprofen as suspected drugs was also counted for the drug class antibiotics. One report included metamizole and metoclopramide as suspected drugs and was therefore also counted in the group “all other cases.” One of the 5 atracurium reports included atracurium and propofol as suspected drugs and, thus, was also counted in the group “all other cases.” In 36 “all other cases,” atracurium and propofol were reported as suspected drugs and, hence, were also counted for atracurium. One report included metamizole and metoclopramide as suspected drugs and was therefore also counted in the group analgesics.
A total of 159 case reports contained 164 suspected drugs. Cases with more than one drug were counted in each drug class. However, they were not counted twice if they belonged to the same drug class. Therefore, the sum of cases of all drug subgroups exceeds 159 cases.
Twelve case reports for alglucosidase. Among these 12 cases, there was one patient accounting for five cases (each at a different date). In these cases, there was no evidence that the reactions occurred in context with a desensitization procedure.
The “seriousness” assessment may not reflect the clinical severity of the reaction since they refer to the legal definition of the Medicinal Products Act: An adverse drug reaction (ADR) is considered serious when the ADR results in death, is life‐threatening, requires in‐patient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or is a congenital anomaly/birth defect. One case may contain more than one of these criteria.
One case with age unknown.
Since this table refers to the number of cases (n = 159), the calculation of percentages is also based on the number of cases per drug subgroup. The respective figures relating to the number of incriminated drugs (n = 164) are: all validated cases: 38.4% (63/164), antibiotics: 20.4% (10/49), analgesics/antipyretics: 0%, MRI: 78.9% (15/19), alglucosidase 100% (12/12), iron: 85.7% (6/7), atracurium: 80.0% (4/5), and all other cases 44.4% (16/36).
The relative distributions if a “drug was tolerated” or “not tolerated” or “tolerated is unknown after previous administration” refer to the number reporting “drug administered before.” The 13.8% (22/159) cases, which reported previous hypersensitivity to the administered drug, included repeated readministration (four times) in one patient (assigned to the drug subgroup alglucosidase). A total of 11.3% (18/159) of cases remained if these four reports were excluded.
Chi‐squared test/Fischer exact test; P < 0.05. Further information on calculation of P values is included in Section 2.7.
3.2.2. Classification and description of anaphylactic reactions
A total of 10.1% of the validated cases were classified as grade I, 67.3% as grade II, 17.0% as grade III, and 0.6% as grade IV. Grade I/II (moderate; 77.4%) and grade III/IV (severe; 17.6%) cases were pooled for subanalysis. More severe than moderate reactions were only reported in atracurium cases (Table 3).
Table 3.
Classification of anaphylactic reactionsa
| All Cases of Anaphylactic Reactions (n = 159)b | Cases Attributed to Antibiotics n = 48 (30.2%) | Cases Attributed to Analgesics/Antipyretics n = 35 (22.0%) | Cases Attributed to MRI Contrast Media n = 19 (11.9%) | Cases Attributed to Alglucosidase n = 12 (7.5%)c | Cases Attributed to Iron n = 7 (4.4%) | Cases Attributed to Atracurium (Muscle Relaxants)n = 5 (3.1%) | All Other Cases n = 36 (22.6%) | |
|---|---|---|---|---|---|---|---|---|
| Anaphylactic reaction grades I‐II (n = 123) | 77.4% of cases (123/159) | 66.7% (32/48)* | 80.0% (28/35) | 89.5% (17/19) | 91.7% (11/12) | 85.7% (6/7) | 0% (0/5) | 83.3% (30/36) |
| Anaphylactic reaction grades III‐IV (n = 28) | 17.6% of cases (28/159) | 22.9% (11/48) | 17.1% (6/35) | 10.5% (2/19) | 8.3% (1/12) | 14.3% (1/7) | 80.0% (4/5)* | 13.9% (5/36) |
| Anaphylactic reaction NOS (n = 8) | 5.0% of cases (8/159) | 10.4% (5/48) | 2.9% (1/35) | 0% (0/19) | 0% (0/12) | 0% (0/7) | 20.0% (1/5) | 2.8% (1/36) |
In n = 8 cases, the anaphylactic reaction was classified as NOS (not otherwise specified). Only one out of 159 (0.6%) of cases (atracurium) had a fatal outcome. This table shows the stratification of the validated cases (n = 159; validated dataset) by drug class and assigned grade of anaphylactic reaction (moderate (grade I/II), severe (grade III/IV), classification not possible [NOS]).
A total of 159 case reports contained 164 suspected drugs. Cases with more than one drug were counted in each drug class. However, they were not counted twice if they belonged to the same drug class.
Twelve case reports for alglucosidase. Among these 12 cases there was one patient accounting for five cases (each at a different date). In these cases there was no evidence that the reactions occurred in context with a desensitization procedure.
Chi‐squared test/Fischer exact test; P < 0.05. Further information on the calculation of P values is included in Section 2.7.
The most frequently reported symptom was dyspnea (35.8%; 57/159 cases) followed by urticaria (33.3%; 53/159). Differences were noted for analgesics/antipyretics (urticaria: 40.0%) and for atracurium cases (anaphylactic shock: 60.0%) (Table 4). Urticaria (43.6%) was the leading symptom reported for the age class 0 to 5 years, whereas this was dyspnea for age classes 6 to 12 (32.7%) and 13‐17 years (33.3%) (data not shown).
Table 4.
Distribution of designated allergy/atopy and reported symptoms according to suspected underlying pathophysiologya
| Validated Dataset (n = 159)b | Cases Attributed to Antibioticsn = 48 (30.2%) | Cases Attributed to Ironn = 7 (4.4%) | Cases Attributed to Analgesics/Antipyreticsn = 35 (22.0%) | Cases Attributed to Atracurium n = 5 (3.1%) | Cases Attributed to MRI Contrast Median = 19 (11.9%) | Cases Attributed to Alglucosidase (enzymes)cn = 12 (7.5%) | |
|---|---|---|---|---|---|---|---|
| Suspected pathophysiology according to literature5, 35, 38, 39, 40, 41, 42, 43 | Immune mediatedd | Non–immune mediated | Non–immune mediatede | Immune or non–immune mediated | Immune or non–immune mediated | Immune (IgE) or non–immune mediated | |
| Allergy/atopyf | 25.2% (40/159) | 14.6%* (7/48) | 14.3% (1/7) | 42.9%*(15/35) | 20.0% (1/5) | 31.6% (6/19) | 0% |
| Reported symptomsg |
35.8% dyspnea (57/159) 33.3% urticaria (53/159) 22.0% rash (35/159) |
50.0%* dyspnea (24/48) 31.3% urticaria (15/48) 27.1% rash (13/48) |
42.9% dyspnea (3/7) 42.9% urticaria (3/7) |
40.0% urticaria (14/35) 31.4% anaphylactic reaction (11/35) 31.4%* angioedema (11/35) | 60.0%* anaphylactic shock (3/5) 40.0%* bronchospasm (2/5) |
42.1% dyspnea (8/19) 31.6%* erythema (6/19) 31.6%* cough (6/19) |
58.3%* rash (7/12) 50.0% urticaria (6/12) 50.0%* vomiting (6/12) |
Non–immune‐mediated reactions cover different pathomechanisms, like NSAID‐induced inhibition of COX enzymes,5, 35, 38 complement activation by intravenously administered iron,39 direct degranulation of mast cells in non‐IgE‐mediated hypersensitivity reactions induced by MRI contrast media,40, 41 or by neuromuscular blocking agents like atracurium.38, 43 In this table, the validated cases (n = 159; validated dataset) are stratified according to drug class, the reported underlying allergic/atopic conditions, the assumed underlying pathophysiological mechanisms, and the three most frequently reported symptoms.
A total of 159 case reports contained 164 suspected drugs. Cases with more than one drug were counted in each drug class. However, they were not counted twice if they belonged to the same drug class.
Twelve case reports for alglucosidase. Among these 12 cases, there was one patient accounting for five cases (each at a different date). In these cases, there was no evidence that the reactions occurred in context with a desensitization procedure.
This group also contained four fluoroquinolone cases. Both immune‐mediated and non–immune‐mediated reactions have been described for fluoroquinolones. The first is reported as being more common.38
Five subtypes of NSAID‐induced hypersensitivity reactions have been proposed,35 including non–immune‐mediated and immune‐mediated reactions. In one publication, it is assumed that non–immune‐mediated cases account for more than 75% of cases.38
Cases with patients designated as atopic (n = 22) or allergic (n = 29) were pooled for subgroup analysis (see section Results). Not mentioned does not exclude allergic/atopic condition.
Reported symptoms by analyzing the assigned preferred terms. The diagnosis “anaphylactic reaction” is based on specific symptoms reported. Some symptoms may be reported more often than others. In some cases only the diagnosis “anaphylactic reaction” is reported.
Chi‐squared test/Fischer exact test; P < 0.05. Further information on the calculation of P values is included in Section 2.7.
3.2.3. Atopy/allergy
Only 15.1% and 27.7% of the cases respectively yielded information on atopy (24/159) and allergy (44/159). A total of 13.8% (22/159) of the cases were designated as atopic, and allergy was determined in 18.2% (29/159) of the cases. In 23/29 of the allergy cases, specific information about the allergen was provided (pollen/house dust mites/animals [n = 13], food [nuts, milk, eggs, etc; n = 9], antibiotics [n = 2], and hymenoptera [n = 1]) (some patients reported more than one allergen). Histamine intolerance was reported in one case. For subgroup analysis, the atopy cases (n = 22) and allergy cases (n = 29) were pooled (altogether 40 cases, since 11 cases reported atopy and allergy). This was considered reasonable since the reported allergens are common in immediate‐type allergic reactions (eg, allergic rhinoconjunctivitis), which is also a characteristic of atopy.
Thirty‐two (26.0%) of the pooled atopy/allergy cases were classified as grade I/II (n = 123) and n = 6 (21.4%) as grade III/IV (n = 28) reactions (two cases NOS).
The largest number of reports designated as atopic/allergic was observed in the analgesics/antipyretics drug class (42.9%; 15/35; P < 0.05), followed by MRI contrast media (31.6%; 6/19) (Table 4), whereas only 14.6% (7/48; P < 0.05) of the antibiotic cases were designated as atopic/allergic.
3.2.4. Drug‐related findings
Table 5 shows the 10 drugs most frequently assessed as causal inducers.
Table 5.
The ten drugs most frequently assessed as causal inducers among the 159 casesa of the validated dataset
| Ranking | Drug Substance | Drug Class |
|---|---|---|
| 1. | Ibuprofen (n = 30) | Analgesics |
| 2. | Cefaclor (n = 13) | Antibiotics |
| 3. | Alglucosidase (n = 12) | Alglucosidase |
| 4. | Gadobutrol (n = 9) | MRI |
| 5. | Azithromycin (n = 5) | Antibiotics |
| 5. | Cefuroxime (n = 5) | Antibiotics |
| 5. | Etoposide (n = 5) | Other |
| 5. | Atracurium (n = 5) | Atracurium |
| 5. | Gadopentetate (n = 5) | MRI |
| 5. | Gadoteric acid (n = 5) | MRI |
A total of 159 cases with 164 incriminated drugs.
Ibuprofen ranked first with 18.9% (30/159; 85.7% [30/35] of analgesic/antipyretic cases) and was observed more frequently in males (21 vs 9; P < 0.05) and ages 0 to 12 years (86.7%). In 56.7% (17/30) of the reports, the drug had been administered orally. Of the oral formulations, 41.2% (7/17) contained flavors (eg, strawberry). Allergy/atopy was stated in 43.3% (13/30) of the reports.
Cefaclor ranked second and accounted for 52.0% (13/25) of the reports attributed to cephalosporins and for 27.1% (13/48) of the antibiotic cases. Of these cases, 46.2% (6/13) reported the seriousness criterion life‐threatening (compared with 23.3% of all cases). Age‐stratified analysis showed a larger number of reports for the ages 0 to 12 years (92.3%), and no gender differences were observed. None of the cefaclor cases reported allergy or atopy.
Three of five atracurium cases (rank 5) were classified as anaphylactic reactions grade III (1 grade IV (fatal outcome), 1 NOS); four out of five of these cases were in males.
Four of seven iron‐related cases referred to ferric carboxymaltose (intravenous; rank 6) and one case each to ferric gluconate (intravenous), ferric dextran (intravenous), and ferric sulfate (oral). In all cases, the reaction occurred within 30 minutes.
Four cases of anaphylactic reaction after intravenous corticosteroid therapy with asthma as comorbidity (rank 6) were identified.
Another four cases reported anaphylactic reactions (3/4 grade II, 1/4 NOS) after topical application of an ointment with the ingredients methyl nicotinate and Symphytum officinale (rank 6).
In 15.1% (24/159) of the reports, the drug had never been taken previously (Table 2). In 34.0% (54/159) of the cases, the drug had been given previously (not tolerated before: 40.7% [22/54] [33.3% if excluding repeated readministration in one person]; tolerated before: 44.4% [24/54]; unknown: 8/54). Cases reporting “not tolerated before” (13.8% of all cases [22/159] or 11.3% [18/159] if excluding repeated readministration in one person) were more often designated as severe (grade III/IV 22.7% vs 8.3%), life‐threatening (36.4% vs 20.8%), and serious (100% vs 83.3%) than cases reporting “drug never used before.”
4. DISCUSSION
The present study analyzed 159 validated cases of drug‐induced anaphylactic reactions in children and compared this dataset with a reference (basic dataset) containing all ADR reports excluding anaphylactic reactions.
4.1. Comparison of datasets
The drugs most frequently suspected in the validated dataset compared with the basic dataset were antibiotics (30.2% vs 11.0%), analgesics/antipyretics (22.0% vs 5.6%), and MRI contrast media (11.9% vs 0.5%). Hence, these may play a prominent role in drug‐induced anaphylactic reactions in children as also reported in literature.5, 13, 30, 31 Different drug exposure rates may also account for this finding. However, in Germany, analgesics and antiinfectives ranked only fourth and eighth in this respect.32
Intravenous administration was reported more frequently in the validated compared with the basic dataset (38.4% versus 6.7%). Hence, intravenous administration may entail a higher risk for anaphylactic reactions as also reported in other investigations14; alternatively, drugs with a higher risk may be more likely to be given intravenously.
In contrast to the basic dataset, the average number of cases reporting anaphylactic reactions did not increase in the past 16 years (validated dataset). Although this finding is reassuring, it cannot be concluded whether it also applies in real life because of the limitations of the spontaneous reporting system.
The reports of anaphylactic reactions appeared to be more severe based on the legally defined criteria of seriousness life‐threatening and hospitalization but were astonishingly less frequently reported as fatal (0.6% [validated] vs 3.5% [basic]). This particular finding may however result from the validation since fatal outcome was even higher (6.1%) in the all‐anaphylactic‐reaction dataset (not validated).
The differences between the validated and the basic dataset were mostly similar but larger than between the all‐anaphylactic and the basic datasets. Therefore, the discussed differences between the basic and the validated dataset are unlikely to have resulted from the validation process. On the other hand, validation improves data quality, as could be seen with regard to the outcome fatal.
4.2. Analysis of the validated dataset
Consistent with literature,32, 33, 34 we observed no obvious gender predominance over all validated cases (51.6% female vs 48.4% male). Likewise, gender‐related drug exposure in Germany from 2003 to 2006 for children reported similar figures (53.1% females; 48.7% males).32 However, we did observe a gender predominance for certain drugs (eg, female gender: iron). Since literature only reports a significant gender difference in drug exposure for drugs related to the urogenital system/sexual hormones (contraceptives),32 the observed differences could be due to chance or unknown factors.
Largely in accordance with a recent study,12 the majority of anaphylactic reactions was classified as moderate (77.4%; grade I/II). Likewise, only one of 159 cases reported a fatal outcome. Although others reported similar findings,11 fear of legal consequences might have discouraged reporting.
Dyspnea was the leading reported symptom (35.8%) over all validated cases, whereas urticaria (40.0%) ranked first in analgesics/antipyretics‐induced cases. Regarding the differentiation of NSAID‐induced hypersensitivity,35 this finding could reflect a higher proportion of the “NSAID‐induced urticaria/angioedema” type or the “NSAID‐exacerbated cutaneous disease” type in our cases. Children aged 0 to 5 years more often reported urticaria and vomiting than older age classes. In contrast, decreased blood pressure was more frequent in adolescents (13‐17; data not shown) as also reported by others.11
About one quarter of the cases was designated as atopic/allergic; similar results were reported in other studies.8, 36 Although preferential underreporting cannot be excluded, atopy was not confirmed as a risk factor for severe reactions in our study, which is also in accordance with literature.12, 15, 18, 37
Atopic patients are IgE antibody high responders.1 We found a lower percentage (14.6%) of patients reporting atopy/allergy in “antibiotics cases” with assumed preferential immune‐mediated pathophysiology (according to literature5). On the other hand, in the “analgesics/antipyretics cases” with assumed preferential non–immune‐mediated pathophysiology (according to literature5, 35, 38, 39, 40, 41, 42, 43), a higher percentage (42.9%) was observed. No significant association with atopy for beta‐lactam allergy in children44, 45 was found in other studies either. Instead, varying associations of atopy with different phenotypes of NSAID‐induced hypersensitivity have been described, suggesting that atopy may predispose to selected forms of NSAID hypersensitivity.46 However, in one study in patients of all ages, no differences were found.14 Therefore, our findings could also be due to chance or varying documentation.
Ibuprofen accounted for nearly every fifth of all incriminated drugs (18.9%; 30/164) and nearly every fourth in the age groups 0 to 5 and 6 to 12 years (data not shown). No matching exposure data are available. However, ibuprofen passed paracetamol in terms of exposure in 2007 and accounted for 76% of all analgesics prescribed to children within the statutory insurance system in Germany in 2013.47 Over‐the‐counter sales may further increase this exposure. Nevertheless, if the large number of reports is seen in context with the large exposure, we arrive at a more reassuring scenario.
Cefaclor accounted for 27.1% (13/48) of cases attributed to antibiotics, and nearly every second (46.2%; 6/13) was designated as life‐threatening. Cefaclor accounted for 10.4% of all antibiotics prescribed to children (0‐15 years) in Germany in 2004 and for 18.6% in 2013. In contrast, amoxicillin accounted for only four reports (none designated as life‐threatening), although it was the most frequently prescribed antibiotic for children in Germany in 2013 (28.7% of all antibiotics); this ratio has remained relatively stable since 2004.47 However, because of the limitations of the spontaneous reporting system, we cannot determine whether this finding reflects drug‐preferential reporting, different potentials of these drugs to induce anaphylactic reactions, or other reasons.
All five atracurium cases were designated as serious (one fatal). It remains unclear whether atracurium is associated with more severe anaphylactic reactions or whether severe anaphylactic reactions occurring under anesthesia are more likely to be noticed/reported. The latter would also apply to other drugs used in anesthesia, which were not seen in our analysis. Nevertheless, our finding could also reflect different exposure rates. An analysis in France48 also reported a higher ratio of grade III/IV hypersensitivity reactions for neuromuscular blocking agents.
In 13.8% of the cases (11.3% if excluding reported readministration in one person), previous hypersensitivity to the drug had been reported, and these reactions appeared to be more severe than cases designated as “drug never used before.” Hence, serious anaphylactic reactions might have been avoided in about every seventh case if taking the patient's history had included previous hypersensitivity reactions and if this factor had been considered prior to treatment. Concerning the 22/54 (40.7%) cases where previous administration had been tolerated, sensitization could have occurred in the immune‐mediated cases. Finally, we cannot rule out that there may have been cases for which no alternative medication was available.
The strengths of the spontaneous reporting system encompass the large number of potential cases, the inclusion of vulnerable patient populations (eg, children), and the possibility to detect very rare/long latency ADRs. Its limitations include underreporting, preferential and stimulated reporting, a varying degree of documentation in the reports, and the impossibility to calculate ADR frequencies due to lack of exposure data.49 Hence, epidemiological studies not based on spontaneous data are usually required to further investigate the signals observed.
In conclusion, a heterogeneous clinical phenotype with differences in associated factors was observed, suggesting different underlying mechanisms triggered by the different drug groups. Future studies may thus focus on defined drug groups. Exploration of larger databases like EudraVigilance could be helpful in order to gain access to further of such cases.
ETHICS STATEMENT
The study has been approved by the local ethics committee (009/17).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DISCLAIMER
The information and views set out in this manuscript are those of the authors and do not necessarily reflect the official opinion of the Federal Institute for Drugs and Medical Devices.
ACKNOWLEDGEMENTS
This project received funding from the Federal Institute for Drugs and Medical Devices (BfArM) own resources and the Institute for Medical Biometry, Informatics, and Epidemiology (IMBIE), University Hospital of Bonn (V‐16703/68502/2016‐2020).
The authors would like to thank the ADR database research team of BfArM's pharmacovigilance division for their excellent support.
Sachs B, Dubrall D, Fischer‐Barth W, Schmid M, Stingl J. Drug‐induced anaphylactic reactions in children: A retrospective analysis of 159 validated spontaneous reports. Pharmacoepidemiol Drug Saf. 2019;28:377–388. 10.1002/pds.4726
Diana Dubrall and Wilma Fischer‐Barth contributed equally to the study and the manuscript.
Statement about prior postings and presentations
An excerpt of some results has been presented as a poster at the 3rd Drug Hypersensitivity Meeting, which took place from April 19 to 21, 2018, in Amsterdam, the Netherlands.
REFERENCES
- 1. Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunology. 2004;113(5):832‐836. [DOI] [PubMed] [Google Scholar]
- 2. Simons FER, Ardusso LRF, Bilò MB, et al. World Allergy Organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4(2):13‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ring J, Beyer K, Biedermann T, et al. Leitlinie zu Akuttherapie und Management der Anaphylaxie. Allergo J Int. 2014;23(3):96‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montañez MI, Mayorga C, Bogas G, et al. Epidemiology, mechanisms, and diagnosis of drug‐induced anaphylaxis. Front Immunol. 2017;8:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thong BY, Tan TC. Epidemiology and risk factors for drug allergy. Br J Clin Pharmacol. 2011;71(5):684‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffer V, Scheuerman O, Marcus N, et al. Anaphylaxis in Israel: experience with 92 hospitalized children. Pediatr Allergy Immunol. 2011;22(2):172‐177. [DOI] [PubMed] [Google Scholar]
- 7. Moneret‐Vautrin DA, Morisset M, Flabbee J, Beaudouin E, Kanny G. Epidemiology of life‐threatening and lethal anaphylaxis: a review. Allergy. 2005;60(4):443‐451. [DOI] [PubMed] [Google Scholar]
- 8. Dinakar C. Anaphylaxis in children: current understanding and key issues in diagnosis and treatment. Curr Allergy Asthma Rep. 2012;12(6):641‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orhan F, Canitez Y, Bakirtas A, et al. Anaphylaxis in Turkish children: a multi‐centre, retrospective, case study. Clin Exp Allergy. 2011;41(12):1767‐1776. [DOI] [PubMed] [Google Scholar]
- 10. West SL, D'Aloisio AA, Ringel‐Kulka T, Waller AE, Clayton Bordley W. Population‐based drug‐related anaphylaxis in children and adolescents captured by South Carolina Emergency Room Hospital Discharge Database (SCERHDD) (2000–2002). Pharmacoepidemiol Drug Saf. 2007;16(12):1255‐1267. [DOI] [PubMed] [Google Scholar]
- 11. Xing Y, Zhang H, Sun S, et al. Clinical features and treatment of pediatric patients with drug‐induced anaphylaxis: a study based on pharmacovigilance data. Eur J Pediatr. 2018;177(1):145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cavkaytar O, Karaatmaca B, Cetinkaya PG, et al. Characteristics of drug‐induced anaphylaxis in children and adolescents. Allergy Asthma Proc. 2017;38:e56‐e63. [DOI] [PubMed] [Google Scholar]
- 13. Ribeiro‐Vaz I, Marques J, Demoly P, Polónia J, Gomes E. Drug‐induced anaphylaxis: a decade review of reporting to the Portuguese Pharmacovigilance Authority. Eur J Clin Pharmacol. 2013;69(3):673‐681. [DOI] [PubMed] [Google Scholar]
- 14. Faria E, Rodrigues‐Cernadas J, Gaspar A, et al. Drug‐induced anaphylaxis survey in Portuguese Allergy Departments. J Investig Allergol Clin Immunol. 2014;24(1):40‐48. [PubMed] [Google Scholar]
- 15. Jares EJ, Sanchez‐Borges M, Cardona‐Villa R, et al. Multinational experience with hypersensitivity drug reactions in Latin America. Ann Allergy Asthma Immunol. 2014;113(3):282‐289. [DOI] [PubMed] [Google Scholar]
- 16. Yocum MW, Butterfield JH, Klein JS, Volcheck GW, Schroeder DR, Silverstein MD. Epidemiology of anaphylaxis in Olmsted County: a population‐based study. J Allergy and Clin Immunol. 1999;104(2):452‐456. [DOI] [PubMed] [Google Scholar]
- 17. Webb LM, Lieberman P. Anaphylaxis: a review of 601 cases. Ann Allergy Asthma Immunol. 2006;97(1):39‐43. [DOI] [PubMed] [Google Scholar]
- 18. Banerji A, Rudders S, Clark S, Wei W, Long AA, Camargo CA Jr. Retrospective study of drug‐induced anaphylaxis treated in the emergency department or hospital: patient characteristics, management, and 1‐year follow‐up. J Allergy Clin Immunol Pract. 2014;2(1):46‐51. [DOI] [PubMed] [Google Scholar]
- 19. Simons FER, Ebisawa M, Sanchez‐Borges M, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sachs B, Fischer‐Barth W, Erdmann S, Merk HF, Seebeck J. Anaphylaxis and toxic epidermal necrolysis or Stevens‐Johnson syndrome after nonmucosal topical drug application: fact or fiction? Allergy. 2007;62(8):877‐883. [DOI] [PubMed] [Google Scholar]
- 21. Sachs B, Riegel S, Seebeck J, et al. Fluoroquinolone‐associated anaphylaxis in spontaneous adverse drug reaction reports in Germany: differences in reporting rates between individual fluoroquinolones and occurrence after first‐ever use. Drug Saf. 2006;29(11):1087‐1100. [DOI] [PubMed] [Google Scholar]
- 22. Federal Institute for Drugs and Medical Devices (BfArM) . http://www.bfarm.de/EN/Home/home_node.html
- 23. Paul‐Ehrlich‐Institut (PEI) . http://www.pei.de/EN/home/node.html
- 24. Gesetz über den Verkehr mit Arzneimitteln (Arzneimittelgesetz ‐ AMG) . Arzneimittelgesetz in der Fassung der Bekanntmachung vom 12. Dezember 2005 (BGBl. I S. 3394), das zuletzt durch Artikel 5 des Gesetzes vom 4. Mai 2017 (BGBl. I S. 1050) geändert worden ist.
- 25. German Institute of Medical Documentation and Information (DIMDI): Anatomical Therapeutic Chemical (ATC) classification system . http://www.dimdi.de/static/en/amg/atcddd/index.htm
- 26. Medical Dictionary for Regulatory Activities (MedDRA) . https://www.meddra.org/how‐to‐use/support‐documentation/english [DOI] [PubMed]
- 27. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255‐1259. [DOI] [PubMed] [Google Scholar]
- 28. Bergvall T, Norén GN, Lindquist M. vigiGrade: a tool to identify well‐documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37(1):65‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. European Medicines Agency . Guideline on good pharmacovigilance practices (GVP) Annex I ‐ Definitions (Rev 4). 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/05/WC500143294.pdf
- 30. Renaudin JM, Beaudouin E, Ponvert C, Demoly P, Moneret‐Vautrin DA. Severe drug‐induced anaphylaxis: analysis of 333 cases recorded by the Allergy Vigilance Network from 2002 to 2010. Allergy. 2013;68(7):929‐937. [DOI] [PubMed] [Google Scholar]
- 31. Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: an investigation into its epidemiology. Arch Intern Med. 2001;161(1):15‐21. [DOI] [PubMed] [Google Scholar]
- 32. Knopf H. Arzneimittelanwendung bei Kindern und Jugendlichen. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2007;50:863‐870. [DOI] [PubMed] [Google Scholar]
- 33. Ensina LF, de Lacerda AE, de Andrade DM, Machado L, Camelo‐Nunes I, Solé D. Drug‐induced anaphylaxis in children: nonsteroidal anti‐inflammatory drugs and drug provocation test. J Allergy Clin Immunol Pract. 2014;2(6):825. [DOI] [PubMed] [Google Scholar]
- 34. Liew WK, Williamson E, Tang MLK. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123(2):434‐442. [DOI] [PubMed] [Google Scholar]
- 35. Kowalski ML, Makowska JS, Blanca M, et al. Hypersensitivity to nonsteroidal anti‐inflammatory drugs (NSAIDs)—classification, diagnosis, and management: review of the EAACI/ENDA and GA2LEN/HANNA. Allergy. 2011;66(7):818‐829. [DOI] [PubMed] [Google Scholar]
- 36. Bohlke K, Davis RL, DeStefano F, Marcy SM, Braun MM, Thompson RS. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization. J Allergy Clin Immunol. 2004;113(3):536‐542. [DOI] [PubMed] [Google Scholar]
- 37. Aun MV, Blanca M, Garro LS, et al. Nonsteroidal anti‐inflammatory drugs are major causes of drug‐induced anaphylaxis. J Allergy Clin Immunol. 2014;2(4):414‐420. [DOI] [PubMed] [Google Scholar]
- 38. Dona I, Barrionuevo E, Blanca‐Lopez N, et al. Trends in hypersensitivity drug reactions: more drugs, more response patterns, more heterogeneity. J Investig Allergol Clin Immunol. 2014;24(3):143‐153. [PubMed] [Google Scholar]
- 39. Szebeni J, Fishbane S, Hedenus M, et al. Hypersensitivity to intravenous iron: classification, terminology, mechanisms and management. Br J Pharmacol. 2015;172(21):5025‐5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fok JS, Smith WB. Hypersensitivity reactions to gadolinium‐based contrast agents. Curr Opin Allergy Clin Immunol. 2017;17(4):241‐246. [DOI] [PubMed] [Google Scholar]
- 41. Carr TF. Pathophysiology of immediate reactions to injectable gadolinium‐based contrast agents. Top Magn Reson Imaging. 2016;25(6):265‐268. [DOI] [PubMed] [Google Scholar]
- 42. El‐Gharbawy AH, Mackey J, DeArmey S, et al. An individually, modified approach to desensitize infants and young children with Pompe disease, and significant reactions to alglucosidase alfa infusions. Mol Genet Metab. 2011;104(0):118‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McNeil B, Pundir P, Meeker S, et al. Identification of a mast‐cell‐specific receptor crucial for pseudo‐allergic drug reactions. Nature. 2015;519(7542):237‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ponvert C, Le Clainche L, de Blic J, Le Bourgeois M, Scheinmann P, Paupe J. Allergy to β‐lactam antibiotics in children. Pediatrics. 1999;104(4):e45. [DOI] [PubMed] [Google Scholar]
- 45. Ponvert C, Perrin Y, Bados‐Albiero A, et al. Allergy to betalactam antibiotics in children: results of a 20‐year study based on clinical history, skin and challenge tests. Pediatr Allergy Immunol. 2011;22(4):411‐418. [DOI] [PubMed] [Google Scholar]
- 46. Quiralte J, Blanco C, Delgado J, et al. Challenge‐based clinical patterns of 223 Spanish patients with nonsteroidal anti‐inflammatory‐drug‐induced‐reactions. J Investig Allergol Clin Immunol. 2007;17(3):182‐188. [PubMed] [Google Scholar]
- 47. Kapellen T, Telschow C, Zawinell A. Trends bei der Verordnung von Arzneimitteln bei Kindern und Jugendlichen In: Klauber J, Günster C, Gerste B, Robra B‐P, Schmacke N, eds. Versorgungsreport 2015/2016. Schwerpunkt: Kinder und Jugendliche. Stuttgart: Schattauer; 2015:71‐88 https://www.wido.de/themenbereiche/versorgungsanalysen/vsreport/versorgungs‐report‐2015‐160.html [Google Scholar]
- 48. Reitter M, Petitpain N, Latarche C, et al. Fatal anaphylaxis with neuromuscular blocking agents: a risk factor and management analysis. Allergy. 2014;69(7):954‐959. [DOI] [PubMed] [Google Scholar]
- 49. Hazell L, Shakir SAW. Under‐reporting of adverse drug reactions. Drug Saf. 2006;29(5):385‐396. [DOI] [PubMed] [Google Scholar]


