Abstract
The sodium glucose co‐transporter‐2 inhibitor dapagliflozin has been shown to decrease urinary albumin‐to‐creatinine ratio (UACR). This effect, however, varies among individual patients. In this study, we assessed the baseline characteristics and concurrent changes in other cardiovascular risk markers that might be associated with UACR response to dapagliflozin. A pooled analysis of 11 phase 3 randomized, controlled clinical trials was performed. UACR change from baseline after 24 weeks treatment with dapagliflozin 10 mg/d in 531 patients with type 2 diabetes and UACR ≥30 mg/g at baseline was determined. UACR response was defined as >30% reduction from baseline at 24 weeks, whereas UACR non‐response was defined as ≤30% reduction at 24 weeks. A total of 288 (54%) patients were classified as responders and 243 (46%) as non‐responders. At 24 weeks, the UACR‐adjusted mean change from baseline was −71.2% and 25.9% in responders and non‐responders, respectively. Baseline characteristics were similar between both groups. Changes in HbA1c and body weight were comparable across groups. Responders showed a numerically larger reduction in estimated glomerular filtration rate and systolic blood pressure versus non‐responders. UACR reduction to dapagliflozin is an individual characteristic that cannot be predicted by baseline clinical features or changes in metabolic variables. Whether UACR response would improve long‐term renal and cardiovascular outcomes remains to be determined.
Keywords: albuminuria, dapagliflozin, diabetes, hypertension, sodium glucose co‐transporter‐2
1. INTRODUCTION
Dapagliflozin, a selective sodium glucose co‐transporter‐2 (SGLT2) inhibitor, has been shown to improve glycemic control and to have beneficial effects on other cardiovascular risk markers, including body weight and blood pressure.1, 2 Studies of SGLT2 inhibitors in patients with type 2 diabetes with and without kidney impairment have consistently reported modest reversible reductions in estimated glomerular filtration rate (eGFR) and reductions of 30% to 40% in urinary albumin excretion.3, 4, 5, 6 Recent large clinical outcome trials in patients with type 2 diabetes at high cardiovascular risk have shown that SGLT2 inhibitors delay the progression of kidney disease and reduce the risk of end‐stage kidney disease.3, 7 Altogether, these data indicate that SGLT2 inhibitors hold great promise as renoprotective agents for patients with type 2 diabetes with prevalent kidney disease.
Although SGLT2 inhibitors have been shown to be effective in lowering albuminuria and stabilizing eGFR on a population level, individual patients may show large variations in their albuminuria response to these drugs, similar to what has been reported for ACE inhibitors (ACEi) and angiotensin receptor blockers (ARBs). It has been shown that the inter‐individual variation in albuminuria response to dapagliflozin is consistent in both the initial and subsequent exposure to the drug,4 suggesting that this variation is based on a specific pharmacodynamic response as opposed to merely random variation.
In order to precisely characterize the inter‐individual variations in albuminuria responses to dapagliflozin, we performed a patient‐level analysis of a large pooled phase 3 clinical trial database. To this end, we classified patients into responders and non‐responders based on their individual change in urinary albumin : creatinine ratio (UACR) with dapagliflozin. We then assessed baseline clinical characteristics and concurrent changes in other cardiovascular risk markers in these two groups.
2. METHODS
2.1. Study pool and patient population
This post hoc analysis was performed using pooled data from 11 phase 3, randomized, placebo‐controlled clinical trials (Table S1) assessing the effect of dapagliflozin 10 mg/d or placebo administered for 24 weeks in patients with type 2 diabetes. These studies compared dapagliflozin 10 mg with placebo as monotherapy or in combination with other glucose‐lowering therapies, including metformin, sulphonylureas, thiazolidinediones, insulin, and dipeptidyl peptidase 4 inhibitors. Two studies enrolled patients at high cardiovascular risk.8, 9 Detailed methods for individual trials have been described previously.1, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 All clinical study protocols were approved by the relevant institutional review board/ethics committee and written informed consent was provided by the enrolled patients.
2.2. UACR responder and non‐responder definition
Patients with UACR ≥30 mg/g at baseline and assigned to dapagliflozin 10 mg/d were identified and selected for the main analysis. The patient population was then divided into UACR responders and non‐responders. UACR response was defined as >30% reduction in UACR from baseline at week 24 of the treatment period. This threshold has been used in prior observational studies and prospective randomized clinical trials.18 UACR non‐response was defined as ≤30% reduction in UACR from baseline at week 24. For sensitivity analyses, UACR response/non‐response was defined as >30% reduction in UACR from baseline at least at one time point during the 24‐week treatment period or ≤30% reduction in UACR from baseline at all time points during the 24‐week treatment period, respectively. In a second sensitivity analysis, UACR response/non‐response was defined as >20% or ≤20% reduction in UACR from baseline, respectively, at week 24. In a third sensitivity analysis, UACR response/non‐response was defined as >20% reduction in UACR from baseline at least at one time point during the 24‐week treatment period or ≤20% reduction in UACR from baseline at all time points during the 24‐week treatment period, respectively.
2.3. Assessments and outcomes
This pooled analysis assessed changes in HbA1c, fasting plasma glucose (FPG), eGFR, body weight, systolic blood pressure (SBP), serum uric acid, haematocrit, and serum bicarbonate levels, as proxy for sodium‐hydrogen exchange transporter activity, from baseline to week 24 with dapagliflozin treatment in UACR responders and non‐responders. Urinary albumin and creatinine levels were measured in spot urine samples, with the ratio calculated as urine albumin (mg/L)/urine creatinine (g/L). eGFR was calculated using the Modification of Diet in Renal Disease equation. All biochemical assessments were conducted in central laboratories in each study. The frequencies of adverse events (AEs) and serious AEs (SAEs) were also assessed in both groups.
2.4. Statistical analyses
Descriptive statistics were used for presenting baseline characteristics and safety data. For efficacy variables, mean change from baseline values and 95% CI were derived using a longitudinal repeated measures mixed model with fixed terms for treatment, week, subgroup, study, week‐by‐treatment interaction, treatment‐by‐subgroup interaction, and treatment‐by‐week‐by‐subgroup interaction, as well as the fixed covariates of baseline, baseline‐by‐study, and baseline‐by‐week interactions. UACR values were log transformed (using the natural log). Change in UACR was calculated as log(UACRweek24 − UACRbaseline) and entered in the repeated measures mixed model. Results from the model were then exponentiated back to the original scale. The Kenward‐Roger method was used. When the model did not converge, the Satterthwaite approximation method was used. If this model also did not converge, the Kenward‐Roger method was used with the baseline‐by‐week terms removed. Finally, if this model still did not converge, ANCOVA was performed for each week separately. Correlation (Pearson) analyses of baseline and changes in UACR with changes in eGFR, serum uric acid, SBP, FPG and body weight (log‐transformed values) were also performed.
3. RESULTS
A total of 531 patients treated with dapagliflozin 10 mg/d and who had UACR ≥30 mg/g at baseline were available for analysis. In this cohort, UACR was reduced at 24 weeks by 19.1% (95% CI: −27.8 to −9.4) relative to placebo; however, there was a large variation among individual patients in the UACR response to dapagliflozin as reflected by the wide range in the 2.5th to 97.5th percentile values (−93.1% to +200.9%; Figure 1).
Figure 1.
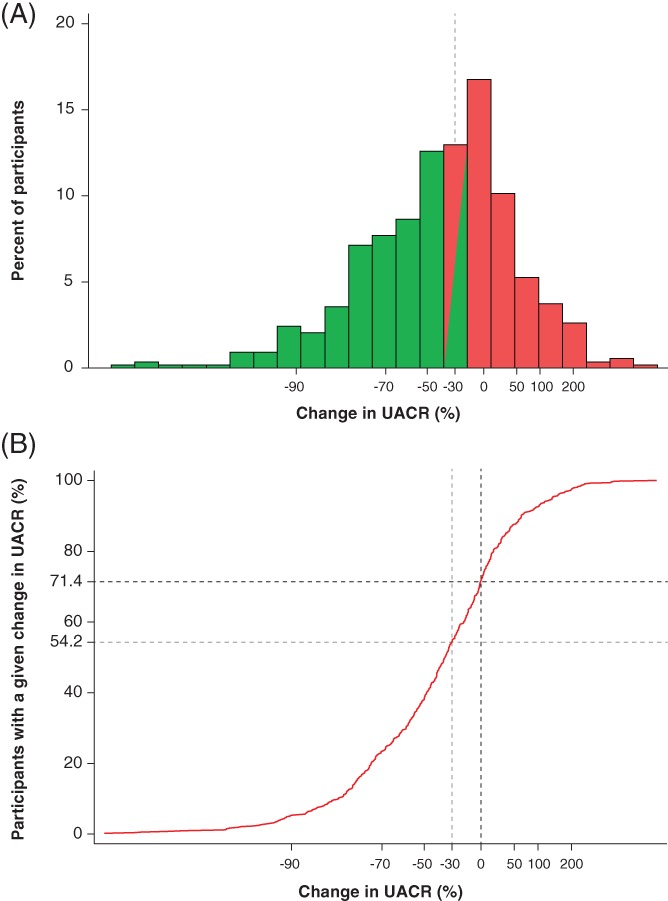
Distribution of albuminuria changes from baseline at week 24. A, Histogram and B, cumulative distribution of UACR changes at week 24; 54.2% of patients experienced >30% reduction in UACR and 71.4% of patients experienced >0% reduction in UACR. Abbreviation: UACR, urine albumin : creatinine ratio
A total of 288 patients (54.2%) showed >30% reduction in UACR from baseline (responders), whereas 243 (45.8%) showed ≤30% reduction (non‐responders). Among non‐responders, UACR levels remained stable over time (Figure 2A). After 24 weeks, the mean change in UACR from baseline in non‐responders was 25.9% (95% CI: 17.1 to 35.4). Among responders, UACR was substantially reduced after 4 weeks of treatment and this effect was stable and sustained throughout the 24‐week treatment period. At week 24, the average reduction in responders was −71.2% (95% CI: −73.7 to −68.3; Figure 2A). Among 437 patients with microalbuminuria, 136 (56.7%) UACR responders and 6 (3.0%) UACR non‐responders regressed to normoalbuminuria at week 24.
Figure 2.
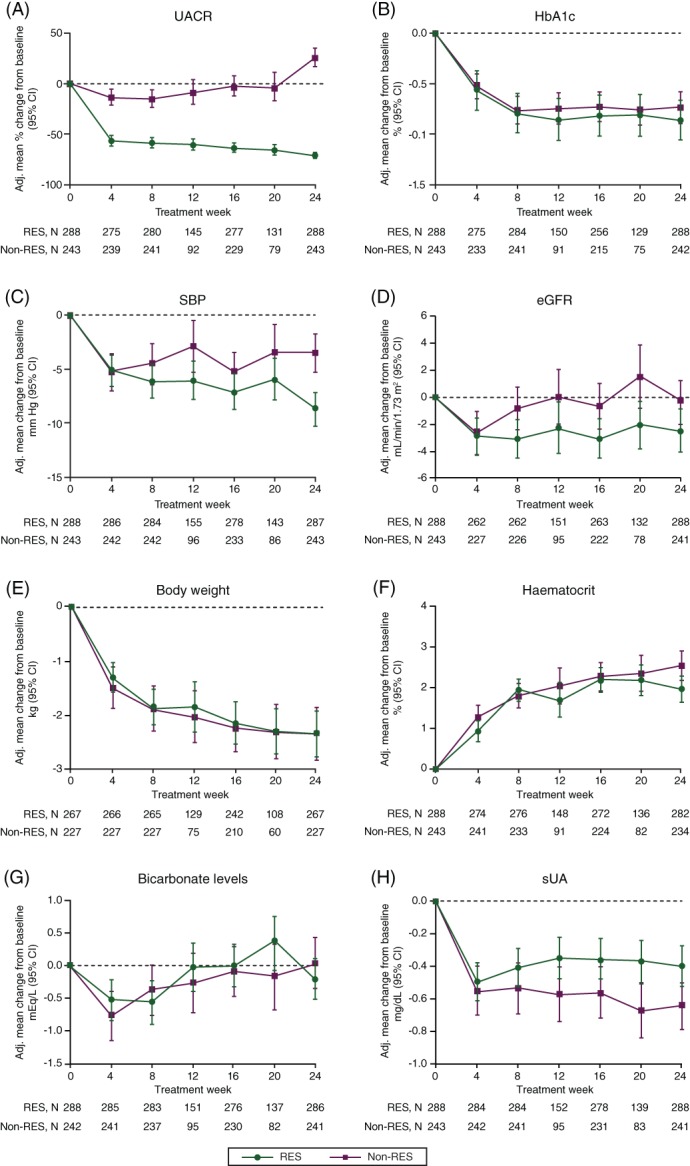
Changes in renal and cardiovascular risk markers over time in UACR responders and non‐responders. A, UACR; B, HbA1c; C, SBP; D, eGFR; E, body weight; F, haematocrit; G, bicarbonate levels; H, sUA. Abbreviations: Adj., adjusted; eGFR, estimated glomerular filtration rate; non‐RES, non‐responders; RES, responders; SBP, systolic blood pressure; sUA, serum uric acid; UACR, urine albumin : creatinine ratio
The baseline demographic, clinical, and biochemical characteristics of UACR responders and non‐responders were generally similar (Table 1). Specifically, baseline HbA1c, eGFR, SBP and body weight were similar between the responders and non‐responders. Cardiovascular disease history, as well as the use of renin‐angiotensin‐aldosterone system (RAAS) inhibitor, diuretics and insulin, was similar between both groups. A greater proportion of women were classified as responders versus 49% of the men. When analyzed on a continuous scale none of the baseline markers correlated with UACR change (Table S2), except HbA1c and FPG, which showed significant but weak correlations.
Table 1.
Baseline characteristics of patients who received dapagliflozin stratified by UACR response
| Baseline characteristics | Responders (n = 288) | Non‐responders (n = 243) |
|---|---|---|
| Age, y | 59.3 (10.3) | 60.0 (8.8) |
| Women | 121 (42.0) | 68 (28.0) |
| Men | 167 (58.0) | 175 (72.0) |
| Race | ||
| White | 244 (84.7) | 202 (83.1) |
| Black | 12 (4.2) | 9 (3.7) |
| Asian | 28 (9.7) | 22 (9.1) |
| Other | 4 (1.4) | 10 (4.1) |
| Duration of type 2 diabetes, y | 10.6 (8.9) | 10.6 (8.2) |
| UACR, mg/g | 86.0 (48.5–165.5) | 69.0 (42.0–162.0) |
| ≥30 to <300 | 240 | 197 |
| ≥300 | 44 | 46 |
| Body weight, kg | 91.5 (19.6) | 93.9 (22.7) |
| sUA, mg/dL | 5.60 (1.5) | 5.88 (1.7) |
| HbA1c, % | 8.38 (0.9) | 8.15 (0.9) |
| HbA1c, mmol/mol | 68 | 66 |
| Haematocrit, % | 42.1 (4.2) | 42.6 (4.0) |
| Serum bicarbonate, mEq/L | 25.3 (2.8) | 25.8 (3.0) |
| SBP, mm Hg | 136.8 (15.3) | 136.6 (14.8) |
| DBP, mm Hg | 79.6 (9.9) | 78.7 (8.8) |
| Pulse pressure, mm Hg | 57.2 (13.8) | 57.9 (13.1) |
| eGFR, mL/min/1.73 m2 | 80.3 (19.2) | 80.7 (21.1) |
| ≥30 to <45 | 7 (2.4) | 7 (2.9) |
| ≥45 to <60 | 26 (9.0) | 36 (14.8) |
| ≥60 to <90 | 176 (61.1) | 118 (48.6) |
| ≥90 | 79 (27.4) | 82 (33.7) |
| Baseline medications | ||
| Loop diuretics | 35 (12.2) | 42 (17.3) |
| Thiazide diuretics | 31 (10.8) | 18 (7.4) |
| ACEi/ARB | 226 (78.5) | 190 (78.2) |
| Insulin | 125 (43.4) | 103 (42.4) |
| SU | 82 (28.5) | 81 (33.3) |
| TZD | 72 (25.0) | 55 (22.6) |
| DPP4 inhibitors | 27 (9.4) | 18 (7.4) |
| Disease history | ||
| CVD and/or HF | 160 (55.6) | 158 (65.0) |
| Hypertension | 237 (82.3) | 208 (85.6) |
| Dyslipidaemia | 195 (67.7) | 190 (78.2) |
| PVD/PAD | 42 (14.6) | 47 (19.3) |
Abbreviations: ACEi, ACE inhibitor; ARB, angiotensin receptor blocker; CVD, cardiovascular disease; DBP, diastolic blood pressure; DPP4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; HF, heart failure; PAD, peripheral arterial disease; PVD, peripheral vascular disease; SBP, systolic blood pressure; SD, standard deviation; SU, sulphonylurea; sUA, serum uric acid; T2D, type 2 diabetes; UACR, urine albumin : creatinine ratio. Data are presented as mean (SD) or number (%). UACR values represent median (25th, 75th percentile) values in patients with UACR ≥30 mg/g at baseline.
3.1. Cardiovascular risk marker changes and UACR response
FPG was decreased to a slightly larger extent in UACR responders compared to non‐responders (24‐week FPG change: responders −34.6 mg/dL [95% CI: −39.6 to −29.6] versus non‐responders −24.2 mg/dL [95% CI: −29.9 to −18.6]). HbA1c reduction was similar between the groups (Figure 2B). Four weeks after treatment initiation, SBP decreased to a similar degree in responders (−5.1 mm Hg, 95% CI: −6.6 to −3.7) and non‐responders (−5.3 mm Hg, 95% CI: −7.0 to −3.6; Figure 2C). However, at the end of week 24, the mean reduction in SBP was numerically larger in the responders (−8.7 mm Hg, 95% CI: −10.2 to −7.2) than the non‐responders (−3.5 mm Hg, 95% CI: −5.3 to −1.8). Notably, eGFR change was −2.8 mL/min/1.73 m2 (95% CI: −4.0 to −1.5) in responders and −2.6 mL/min/1.73 m2 (95% CI: −4.1 to −1.0) in non‐responders after 4 weeks. While the eGFR returned to baseline values in non‐responders at week 24, the initial reduction in eGFR was sustained in responders over the 24‐week treatment period (Figure 2D). Changes in body weight, haematocrit and bicarbonate levels were similar between the two groups (Figure 2E–G). Finally, the mean reduction in serum uric acid was larger in non‐responders than in responders at week 24 (Figure 2H).
In a continuous analysis, the change in UACR at week 4 and week 24 in the overall population (responders and non‐responders together) was correlated with changes in other cardiovascular risk markers (Table S3). After 4 weeks, a weak positive correlation was observed between changes in UACR and eGFR, whereas a weak negative correlation was observed between changes in UACR and uric acid. At week 24, changes in UACR remained positively correlated with changes in eGFR, while the correlation of UACR with uric acid was lost. Weak correlations were observed with SBP and FPG.
In sensitivity analyses, similar results were obtained when the UACR response was defined as a >20% reduction from baseline at week 24 (Figure S1), as a >30% reduction from baseline at least at one time point during the 24‐week treatment period (Figure S2), and as a >20% reduction from baseline at least at one time point during the 24‐week treatment period (Figure S3).
3.2. Safety
Dapagliflozin was well tolerated in both responders and non‐responders. The number of AEs, hypoglycaemic events and SAEs was balanced between both groups. The numbers of AEs and SAEs leading to study drug discontinuation were low (Table S4).
4. DISCUSSION
SGLT2 inhibition with dapagliflozin has been shown to decrease albuminuria by approximately 35% on a population level, but with large inter‐individual variability. In this pooled analysis of multiple clinical trials, we showed that approximately half of all patients treated with dapagliflozin displayed a sustained reduction in UACR of >30% from baseline, and this group experienced a mean reduction of >70% at 24 weeks of treatment. In contrast, UACR essentially did not reduce on average in the other half of patients across the treatment period. The reduction in UACR for individuals could not be explained by any of the baseline characteristics analysed here, except that women tended to be more likely to be a responder. Despite the large separation in UACR reduction between the responders and non‐responders, no differences were observed in HbA1c and body weight. However, modest differences in eGFR and SBP were noted. This suggests that UACR change in individuals cannot be explained by metabolic variables and can only be partially explained by haemodynamic factors.
The baseline biochemical and physical characteristics in responders and non‐responders were similar, indicating that none of these variables could predict future responder status. Moreover, the similarity in baseline UACR levels between the two groups implies that the greater reduction in albuminuria in responders cannot be explained by a regression to the mean phenomenon. Further studies are required to determine if novel biomarkers measured before dapagliflozin exposure could aid in predicting reduction in albuminuria with dapagliflozin therapy.
It is improbable that the small differences observed in metabolic variables and blood pressure could explain the large separation in UACR response between the two groups. The similarity in changes in HbA1c, body weight and hematocrit between the two groups is in accordance with previous studies.5, 19, 20 The larger and sustained reduction in eGFR in the responder population is interesting and is most probably of haemodynamic origin because SGLT2 inhibitors acutely decrease renal blood flow and glomerular filtration pressure.21 The reduction in glomerular filtration pressure is clinically manifested by an acute fall in eGFR and may, at least in part, explain the reduction in UACR. The significant but weak correlation between changes in eGFR and UACR after 4 weeks of dapagliflozin treatment provides some support for this notion. However, it also indicates that a large part of the UACR‐lowering effect remained unexplained, which has been observed in prior studies as well.5, 20
The mean reduction in serum uric acid was numerically lower in responders compared with non‐responders. Urate levels depend on a balance between uric acid generation and excretion. Uric acid is freely filtered in the glomerulus and reabsorbed in the proximal tubule.22 The observed reduction in eGFR in the UACR responder group may have decreased uric acid filtration, resulting in less pronounced lowering of serum uric acid in the responders.
The studies used to generate the data described in this paper were not designed to characterize individual responses of albuminuria to dapagliflozin. Hence, the results from this post hoc analysis can only be interpreted as hypothesis‐generating. A large randomized cross‐over study where patients are exposed to placebo and dapagliflozin during various consecutive treatment periods would be the optimal design to assess individual treatment responses. Additionally, UACR was measured in single spot urine samples in most studies. The measurement of UACR in spot urine samples is subject to a large day‐to‐day variability causing random noise that may weaken the reported associations. Future studies with more frequent urine sampling using first morning void or 24‐hour urine samples, which have been shown to be less variable over time in an individual, should be used to gain further mechanistic understanding of the differential response in UACR. For example, a better understanding of the alterations in intra‐glomerular pressure and other determinants of glomerular albumin permeability, such as the glycocalyx, would be needed to better assess how these factors determine individual UACR responses to SGLT2 inhibition.
In conclusion, the albuminuria response to dapagliflozin is an individual characteristic that is only partly associated with changes in blood pressure and eGFR; however, it is not readily explained by routinely available clinical laboratory variables or changes in metabolic variables, but is partly associated with changes in eGFR and SBP. Further studies are required to determine whether UACR responders to SGLT2 inhibitors show improved renal and cardiovascular outcomes compared with non‐responders.
CONFLICTS OF INTEREST
H.J.L.H. has consultancy agreements with the following companies: Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Janssen, Fresenius, Gilead, and Merck, and has a policy of honoraria going to his employer; he has received grant support from Boehringer Ingelheim, AstraZeneca and Janssen (funding to his employer), and is a member of the steering committee for the DAPA‐CKD study. P.R. has received honoraria to his institution for advisory committee work or presentations for AstraZeneca, Astellas, Boehringer Ingelheim, Bayer, Novo Nordisk, Eli Lilly, MSD, has received unrestricted research grants from AstraZeneca and Novo Nordisk, is a member of the steering committee for the DAPA‐CKD study, and has shares in Novo Nordisk.
C.D.S., B.V.S. and P.S. are employed by AstraZeneca and may own stocks.
Author contributions
P.S., C.D.S., and B.V.S. designed the study. H.J.L.H. and P.S. wrote the first draft of this manuscript. V.C. performed all statistical analyses. All authors contributed to interpretation and critical revisions of the manuscript. All authors approved the submission for publication. H.J.L.H. and P.S. take full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Supporting information
Figure S1.
Figure S2.
Figure S3.
Table S1. Study characteristics of studies included in this analysis.
Table S2. Association between baseline cardiovascular risk markers and change in UACR at 4 and 24 weeks treatment with dapagliflozin.
Table S3. Association between change in UACR and change in cardiovascular risk markers at 4 and 24 weeks treatment with dapagliflozin.
Table S4. SAEs and AEs in UACR responders and non‐responders to dapagliflozin.
ACKNOWLEDGMENTS
The authors thank all the site investigators and patients who participated in the reported dapagliflozin trials.
Heerspink HJL, Sjöström CD, Inzucchi SE, et al. Reduction in albuminuria with dapagliflozin cannot be predicted by baseline clinical characteristics or changes in most other risk markers. Diabetes Obes Metab. 2019;21:720–725. 10.1111/dom.13579
Funding information This study was sponsored by AstraZeneca
REFERENCES
- 1. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2010;375:2223‐2233. [DOI] [PubMed] [Google Scholar]
- 2. Weber M, Mansfield T, Cain V, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211‐220. [DOI] [PubMed] [Google Scholar]
- 3. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 4. Petrykiv SI, Laverman GD, de Zeeuw D, Heerspink HJL. The albuminuria‐lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes Metab. 2017;19:1363‐1370. [DOI] [PubMed] [Google Scholar]
- 5. Heerspink HJ, Johnsson E, Gause‐Nilsson I, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin‐angiotensin blockers. Diabetes Obes Metab. 2016;18:590‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59:2036‐2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 8. Cefalu WT, Leiter LA, de Bruin TW, Gause‐Nilsson I, Sugg J, Parikh SJ. Dapagliflozin's effects on glycemia and cardiovascular risk factors in high‐risk patients with type 2 diabetes: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. Diabetes Care. 2015;38:1218‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leiter LA, Cefalu WT, de Bruin TW, Gause‐Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. J Am Geriatr Soc. 2014;62:1252‐1262. [DOI] [PubMed] [Google Scholar]
- 10. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care. 2010;33:2217‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020‐1031. [DOI] [PubMed] [Google Scholar]
- 12. Wilding JP, Woo V, Soler NG, et al. Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405‐415. [DOI] [PubMed] [Google Scholar]
- 13. Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24‐week, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2011;13:928‐938. [DOI] [PubMed] [Google Scholar]
- 14. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66:446‐456. [DOI] [PubMed] [Google Scholar]
- 16. Jabbour SA, Hardy E, Sugg J, Parikh S, Study G. Dapagliflozin is effective as add‐on therapy to sitagliptin with or without metformin: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study. Diabetes Care. 2014;37:740‐750. [DOI] [PubMed] [Google Scholar]
- 17. Kaku K, Kiyosue A, Inoue S, et al. Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab. 2014;16:1102‐1110. [DOI] [PubMed] [Google Scholar]
- 18. Heerspink HJL, Andress DL, Bakris G, et al. Rationale and protocol of the Study Of Diabetic Nephropathy with AtRasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab. 2018;20:1369‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28:368‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59:1860‐1870. [DOI] [PubMed] [Google Scholar]
- 21. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587‐597. [DOI] [PubMed] [Google Scholar]
- 22. Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19:358‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Figure S3.
Table S1. Study characteristics of studies included in this analysis.
Table S2. Association between baseline cardiovascular risk markers and change in UACR at 4 and 24 weeks treatment with dapagliflozin.
Table S3. Association between change in UACR and change in cardiovascular risk markers at 4 and 24 weeks treatment with dapagliflozin.
Table S4. SAEs and AEs in UACR responders and non‐responders to dapagliflozin.


