Abstract
Objectives
The objectives of this study were to compare short‐ and intermediate‐term clinical outcomes, procedural complications, TAVR prosthesis hemodynamics, and paravalvular leak (PVL) in stentless and stented groups.
Background
Valve‐in‐valve (ViV) transcatheter aortic valve replacement (TAVR) is an alternative to surgical redo for bioprosthetic valve failure. There have been limited data on ViV in stentless surgical valves.
Methods
We retrospectively analyzed 40 patients who underwent ViV TAVR in prior surgical bioprosthetic valves at Wake Forest Baptist Medical Center from October 2014 to September 2017. Eighty percent (32/40) ViV TAVRs were in stentless, while 20% (8/40) were in stented bioprosthetic valves.
Results
The primary mode of bioprosthetic valve failure for ViV implantation in the stentless group was aortic insufficiency (78%, 25/32), while in the stented group was aortic stenosis (75%, 6/8). The ViV procedure success was 96.9% (31/32) in stentless group and 100% in stented group (8/8). There were no significant differences in all‐cause mortality at 30 days between stentless and stented groups (6.9%, 2/31 versus 0%, 0/8, P = 0.33) and at 1 year (0%, 0/25 versus 0%, 0/5). In the stentless group, 34.4% (11/32) required a second valve compared to the stented group of 0% (0/8). There was a significant difference in the mean aortic gradient at 30‐day follow‐up (12.33 ± 6.33 mmHg and 22.63 ± 8.45 mmHg in stentless and stented groups, P < 0.05) and at 6‐month follow‐up (9.75 ± 5.07 mmHg and 24.00 ± 11.28 mmHg, P < 0.05), respectively.
Conclusions
ViV in the stentless bioprosthetic aortic valve has excellent procedural success and intermediate‐term results. Our study shows promising data that may support the application of TAVR in stentless surgical aortic valve. However, further and larger studies need to further validate our single center's experience.
Keywords: aortic valve replacement, homograft, stented, stentless, transcatheter aortic valve implantation
Abbreviations
- AVR
aortic valve replacement
- iEOA
indexed effective orifice area
- PVL
paravalvular leak
- TAVI
transcatheter aortic valve implantation
- TAVR
transcatheter aortic valve replacement
- THV
transcatheter heart valve
- VARC
Valve Academic Research Consortium
- ViV
valve‐in‐valve
1. INTRODUCTION
Bioprosthetic valve has been widely used in surgical aortic valve replacement (AVR). It can be categorized as stentless or stented. The stented valves are mounted on structure support such as a stent or frame.1 Stented valves provided easier implantation, however, sacrificed orifice area and increased stress at the stent attachment sites.2 The stentless bioprosthetic valve has been reported to provide better hemodynamic properties compared with stented bioprosthetic valves with less turbulent flow and larger effective orifice area, which reduces the risk of patient‐prosthesis mismatch. However, some of the disadvantages of stentless aortic bioprosthetic valves include association with significant calcification of the aortic root, therefore, making reoperation more difficult. In the past, the standard of care for bioprosthetic aortic valve failure has been redo AVR surgery. Redo surgery is associated with increased risk of morbidity and mortality of 3–7%, rising to 30% in high risk patients.3
With the introduction of transcatheter aortic valve replacement (TAVR), patients with increased surgical risks now have a promising alternative to surgical redo AVR, referred to as valve‐in‐valve (ViV) implantation.4, 5, 6 Stentless bioprosthetic valves make ViV implantation especially challenging given the lack of a frame or structural support to anchor the new transcatheter aortic valve, differences in modes of index valve failure, as well as lack of radiographic markers to help with proper positioning.1
2. METHODS
Our study retrospectively investigated 40 consecutive patients who underwent ViV implantation with previous surgical AVRs at Wake Forest Baptist Medical Center from October 2014 to September 2017. Patients were identified using electronic medical record system with CPT diagnosis codes in patients that underwent past‐SAVR and received ViV‐TAVR and cross confirmed with internal TAVR database. Baseline patient demographics, comorbidities, post‐ViV complications, and valve hemodynamics were followed longitudinally at 1‐, 6‐, and 12‐month follow‐ups with repeat echocardiograms, clinic visits, and phone calls. Follow‐ups were displayed as percentages per group at 1‐, 6‐, and 12‐month intervals. All patients provided written informed consent for the procedure and data collection according to the policy of the Institutional Review Board of Wake Forest Baptist Medical Center.
2.1. Pre‐procedural protocol
All patients were evaluated by the Structural Heart Team consisting of interventional cardiologists, cardiothoracic surgeons, cardiovascular imaging specialists, and coordinators. Patients were triaged based on their risk assessment by the Structural Heart Team, utilizing overall risk assessment with STS, Euroscores, as well as functional status. Consecutive patients with high or extreme risks were referred for ViV‐TAVR. All candidates for a ViV procedure underwent computed tomography to analyze the aortic annulus dimensions, aortic anatomy, and peripheral vascular access. In patients with renal insufficiency, a 3D transesophageal echocardiogram was obtained to evaluate the aortic annulus dimensions for TAVR size. Information from prior surgical AVR was reviewed, including the type and manufacturer of the bioprosthesis, size, and if stented or stentless. This is all standard work up protocol for all TAVR candidates in our institution.
2.2. Procedural description
2.2.1. Percutaneous trans‐femoral approach
The patients were prepped and draped in the usual sterile fashion. The most commonly accessed site was the common femoral artery (TAVR delivery site) using a micropuncture needle under fluoroscopic and ultrasound guidance. Two Perclose ProGlide® devices (Abbott Vascular, Santa Clara, CA) were used to pre‐close the common femoral artery over a J‐wire before a large bore sheath (14–18 F) was introduced. After crossing aortic valve, an Amplatz Super Stiff™ Guidewire (Boston Scientific, Marlborough, MA) was advanced to the apex of the left ventricle. Pre‐balloon dilation was not performed in ViV TAVR. TAVR was advanced and positioned at the surgical bioprosthetic valve. Aortography, positioning of a Pigtail at the non‐coronary cuspid, and echocardiography were utilized to assist with positioning. TAVR was then deployed according to manufacturer instructions. Hemodynamic and imaging evaluations with fluoroscopy and intraprocedural TEE were carried out to ensure proper deployment of TAVR. All patients underwent general anesthesia for ViV procedure.
After completion of TAVR deployment with satisfactory hemodynamics and imaging assessment with fluoroscopy and intraprocedural TEE, the TAVR sheath was removed, and sutures from the two previously placed Perclose ProGlide® devices were tightened. A subsequent angiogram was performed to ensure hemostasis.
2.3. Study endpoints
The primary endpoint of our study was all‐cause mortality at 1 year. Secondary endpoints included stroke, vascular complications, valve embolization and migration, device success, procedural success, valve hemodynamics, permanent pacemaker implantation, and hospitalization rates. The Valve Academic Research Consortium 2 (VARC‐2) criteria were used to major and minor vascular complications, myocardial infarction, arrhythmia, cerebrovascular events, and death.6 Device success was defined as successful vascular access, delivery and deployment of the device and successful retrieval of the delivery system, correct position of the device in the proper anatomical location, intended performance of the prosthetic heart valve (aortic valve area > 1.2 cm2 and mean aortic valve gradient <20 mmHg or peak velocity < 3 m/s, without moderate or severe prosthetic valve AR), and only one valve implanted in the proper anatomical location according to VARC criteria.7 Procedural success was defined as final device in proper anatomic position with satisfactory hemodynamics according to VARC‐2 criteria, patient survival within 72 hr post‐procedure, and no conversion to surgical operation.
2.4. Statistical analysis
Clinical endpoints were analyzed at 1, 6, and 12 months. Comparisons between groups were made using chi‐square tests for categorical variables and t‐tests for continuous variables. Due to our modest sample size and non‐normality, we conducted non‐parametric Wilcoxon tests to assess continuous variables. Other inferential statistical analyses were conducted when appropriate. A P value of less than 0.05 was considered statistically significant in this study. Of note, the P values were calculated with relatively small sample sizes in this study.
3. RESULTS
3.1. Baseline patient characteristics
Of those patients enrolled in the study, 80% (32/40) with prior stentless valves and 20% (8/40) with prior stented bioprosthetic valves underwent ViV implantation. Of the stentless group, 31% (10/32) were homograft aortic valves and the 69% (22/32) were commercial bioprosthetic valves, with the majority being Medtronic Freestyle® valve. Baseline demographics were similar in both groups (Table 1). The average age in the stentless and stented group was 63 ± 14 and 74 ± 13, respectively. Both groups consisted of the same proportion of males (75%) to females (25%). The majority of patients in both the stentless and stented groups had baseline NYHA class III scores (63% and 75%, respectively). The average STS score for the stentless and stented groups was 6.45 ± 7.02 and 6.98 ± 6.66, respectively. The average logistic Euroscore of the stentless and stented groups was 10.65 ± 9.06 and 14.1 ± 13.2, respectively.
Table 1.
Demographics
| Stented (N = 8) | Stentless (N = 32) | P‐value | |
|---|---|---|---|
| Age (years) | 73.75, 13.26 | 62.75, 14.37 | 0.0625 |
| Male | 6/8 (75%) | 24/32 (75%) | 1 |
| White | 8/8 (100.00%) | 31/32 (96.88%) | 0.6126 |
| Weight (kg) | 93.62, 15.75 | 84.53, 22.11 | 0.2013 |
| Height (m) | 1.75, 0.13 | 1.73, 0.11 | 0.6914 |
| BMI (kg/m2) | 30.6, 4.19 | 28.24, 7.28 | 0.2446 |
| BSA (m2) | 2.09, 0.24 | 1.98, 0.27 | 0.2798 |
| CAD | 4/8 (50%) | 18/32 (56.25%) | 1 |
| Hypertension | 5/8 (62.50%) | 22/32 (68.75%) | 0.7357 |
| Smoking | 0/8 (0%) | 13/32 (40.63%) | 0.0373 |
| Hyperlipidemia | 3/8 (37.50%) | 17/32 (53.13%) | 0.6984 |
| Diabetes mellitus | 2/8 (25%) | 11/32 (34.38%) | 0.6126 |
| Congestive heart failure | 3/8 (37.50%) | 14/32 (43.75%) | 0.7491 |
| NYHA baseline | I is 1/8 (12.50%) | I is 1/32 (3.33%) | 0.6016 (overall) |
| II is 0/8 (0%) | II is 4/32 (13.33%) | ||
| III is 6/8 (75%) | III is 20/32 (63.33%) | ||
| IV is 1/8 (12.50%) | IV is 26/32 (0.00%) | ||
| Atrial fibrillation | 2/8 (25%) | 10/32 (31.25%) | 0.7301 |
| Chronic atrial fibrillation | 2/8 (25%) | 9/32 (28.13%) | 0.8595 |
| Previous pacemaker | 0/8 (0%) | 2/32 (6.25%) | 0.4682 |
| Previous PCI | 0/8 (0%) | 3/32 (9.38%) | 0.3679 |
| Previous CABG | 4/8 (50%) | 12/32 (37.50%) | 0.5186 |
| Peripheral vascular disease | 1/8 (12.50%) | 2/32 (6.25%) | 0.5483 |
| COPD | 0/8 (0%) | 5/32 (15.63%) | 0.232 |
| Redo AVR | 0/8 (0%) | 12/32 (37.50%) | 0.079 |
| STS score | 6.98, 6.66 | 6.45, 7.02 | 0.8474 |
| Logistic Euroscore | 14.1, 13.20 | 10.65, 9.06 | 0.5353 |
| Pre‐creatinine | 1.14, 0.31 | 1.49, 1.50 | 0.2286 |
| Pre‐BNP | 295.71, 288.03 | 929.268, 966.732 | 0.0707 |
| AV diameter with CT (max) | 25.86, 2.67 | 26.85, 4.36 | 0.4506 |
| AV diameter with CT (min) | 23.14, 1.35 | 23.13, 3.57 | 0.9866 |
| AV area with Echo | 0.729 | 1.96 | 0.0008 |
3.2. Procedural outcomes
Complete procedural data are listed in Table 2. Aortic regurgitation as an indication for ViV TAVR was more prevalent in the stentless (78%) versus the stented (25%, P < 0.05) group. Aortic stenosis was more common in the stented (75%) than in the stentless (28%, P < 0.05) group. The most commonly used transcatheter heart valve (THV) was the Medtronic Evolut‐R (Medtronic, Minneapolis, MN), 56% (18/32) in the stentless group and 63% (5/8) in the stented group. Twenty‐eight percent (9/32) in the stentless and 38% (3/8) in the stented groups utilized the Medtronic CoreValve Classic (Medtronic Company, Minneapolis, MN), which were concentrated at the beginning of the study. Only one (3%) of the stentless patients utilized the Edwards SAPIEN (Edwards Lifesciences Corporation, Irvine, CA) valve, whereas none were utilized in the stented group. The average prior surgical aortic valve size in the stentless and stented group was 26.27 ± 2.07 mm and 23.75 ± 2.12 mm (P < 0.05), respectively, and the average THV size was 28.38 ± 3.30 mm and 24.50 ± 1.60 mm (P < 0.005), respectively. Prior surgical AVR valve sizing was determined from prior surgical operative reports and confirmed by cardiac CT images. The stentless group required more contrast amount (219.29 ± 119.94 mL) compared to the stented group (136.88.1 ± 94.65 mL). The ViV procedure success was 96.9% (31/32) in stentless group and 100% (8/8) in stented group. One patient in the stentless group expired within 72 hr after transcatheter aortic valve implantation (TAVI) due to bilateral anterior cerebral artery stroke with hemorrhagic transformation. The most commonly used approach was the femoral access, 94% in the stentless group and 88% in the stented group. The other less commonly utilized approaches included subclavian and transapical as noted in Table 2.
Table 2.
Procedural data
| Stented (N = 8) | Stentless (N = 32) | P‐value | |
|---|---|---|---|
| Indication | |||
| AI | 2/8 (25%) | 25/32 (78.13%) | 0.0085 |
| AS | 6/8 (75%) | 9/32 (28.13%) | 0.0358 |
| Mixed | 1/8 (12.50%) | 3/32 (9.38%) | 0.7921 |
| TAVR valve type | |||
| Medtronic | |||
| CoreValve (classic) | 3/8 (37.50%) | 9/32 (28.13%) | 0.6048 |
| EvolutR | 5/8 (62.50%) | 18/32 (56.25%) | 0.7491 |
| EvolutPRO | 0/8 (0%) | 2/32 (6.25%) | 0.4682 |
| Edwards | |||
| SAPIEN | 0/8 (0%) | 1/32 (3.13%) | 0.6126 |
| SAPIEN XT | 0/8 (0%) | 0/32 (0%) | |
| SAPIEN S3 | 0/8 (0%) | 0/32 (0%) | |
| Approach | |||
| Femoral | 7/8 (87.50%) | 30/32 (93.75%) | 0.5483 |
| Subclavian | 1/8 (12.50%) | 1/32 (3.13%) | 0.2765 |
| Transapical | 0/8 (0%) | 1/32 (3.13%) | 0.6126 |
| Previous surgical aortic valve size (mm) | 23.75, 2.12 | 26.27, 2.07 | 0.0092 |
| TAVR prosthesis size (mm) | 24.50, 1.60 | 28.38, 3.30 | 0.0028 |
| Contrast amount (mL) | 136.88, 94.65 | 219.29, 119.94 | 0.0606 |
| Device success (VARC) | 5/8 (62.5%) | 16/32 (50.0%) | 0.698 |
| • AVA > 1.2 cm2 | 5/8 (62.5%) | 25/32 (78.1%) | 0.3613 |
| • Mean aortic gradient <20 mmHg | 8/8 (100%) | 31/32 (96.9%) | 0.6126 |
| • Moderate–severe aortic paravalvular regurgitation | 0/8 (0%) | 3/32 (9.4%) | 0.3679 |
| • Requiring second valve | 0/8 (0%) | 11/32 (34.4%) | 0.0514 |
| Procedure success | 8/8 (100%) | 31/32 (96.9%) | 0.5064 |
|
Indexed effective orifice area (iEOA) pre‐TAVR
• Mean iEOA |
0.36, 0.11 | 0.87, 0.36 | 0.0016 |
|
Indexed effective orifice area (iEOA) post‐TAVR
• Mean iEOA |
0.69, 0.34 | 0.87, 0.31 | 0.1496 |
Complete procedural complications are listed in Table 3. Device success was 50.0% (16/32) for stentless and 62.5% (5/8) for stented group (P = 0.698). Intraprocedural valve embolization was more common in the stentless group that had 12.5% (4/32) compared to 0% (0/8) in the stented group. With regard to intraprocedural valve migration, the stentless group had 9.38% (3/32) of patients that had proximal migration toward the ascending aorta and 18.75% (6/32) of patients that had distal migration into the left ventricle. The stented group did not have any cases of valve migration. There were no incidences of post‐procedural valve migration or embolization. The stentless group required more second valves compared to the stented group (34% [11/32] versus 0% [0/8, P = 0.05]). In those that required second valves, 36% (4/11) utilized the Medtronic Classic, 45% (5/11) Evolut‐R, and 9% (1/11) Evolut‐Pro. No patients required conversion to open heart surgery.
Table 3.
Procedural complications
| Stented (N = 8) | Stentless (N = 32) | P value | |
|---|---|---|---|
| Valve embolization (Intraprocedural) | 0/8 (0%) | 4/32 (12.5%) | 0.2918 |
| Valve migration (intraprocedural) | |||
| Proximally | 0/8 (0%) | 3/32 (9.38%) | 0.3679 |
| Distally | 0/8 (0%) | 6/32 (18.75%) | 0.184 |
| Conversion to open heart surgery | 0/8 (0%) | 0/32 (0%) | |
| Need 2nd valve | 0/8 (0%) | 11/32 (34.38%) | 0.0514 |
| CAD occlusion | 0/8 (0%) | 0/32 (0%) | |
| Annulus rupture | 0/8 (0%) | 0/32 (0%) | |
| Tamponade | 0/8 (0%) | 0/32 (0%) | |
| Arrhythmia | 0/8 (0%) | 3/32 (9.38%) | 0.3679 |
| Hemodynamic support | 0/8 (0%) | 0/32 (0%) | |
| Hemodialysis post‐TAVI | 0/8 (0%) | 1/32 (3.13%) | 0.6126 |
| Pacemaker post‐TAVI | 0/8 (0%) | 2/32 (6.25%) | 0.4682 |
| CVA | 0/8 (0%) | 2/32 (6.25%) | 0.4682 |
| Length of stay (days) | 4.5, 1.93 | 7.13, 10.21 | 0.1874 |
3.3. Early and late outcomes
One‐month follow‐up outcomes are listed in Table 4. Follow‐up rates for stentless and stented groups for 1, 6, and 12 months were the following: 87.5% (28/32) and 100% (8/8), 50% (16/32) and 50% (4/8), and 50% (16/32) and 62.5% (5/8), respectively. There were patients that were lost to follow‐up and some patients had missing data, therefore were excluded. Stroke rates were similar in both groups, 3% (1/31) and 0% (0/8) in the stentless and stented groups, respectively. New permanent pacemaker implantation was more prevalent in the stentless group (6%) compared to the stented group (0%). The stentless group had 6% (2/31) of patients that had a major vascular complication compared to none in the stented group. At 6‐ and 12‐month follow‐up, there were no vascular complications according to VARC‐2 criteria.
Table 4.
Early outcomes
| 1 month | |||
|---|---|---|---|
| Stented (N = 8) | Stentless (N = 32) | P value | |
| CVA (TIA/stroke) | 0/8 (0%) | 2/32 (6.25%) | 0.445 |
| Sepsis | 0/8 (0%) | 0/32 (0%) | |
| New LBBB | 1/8 (12.50%) | 2/32 (6.25%) | 0.5483 |
| MI | 0/8 (0%) | 0/32 (0%) | |
| AKI | 0/8 (0%) | 0/32 (0%) | |
| New permanent pacemaker | 0/8 (0%) | 2/32 (6.25%) | 0.4682 |
| Major vascular complication | 0/8 (0%) | 0/32 (0%) | |
| Minor vascular complication | 1/8 (12.50%) | 0/32 (0%) | 0.2 |
| Major bleeding | 0/8 (0%) | 0/32 (0%) | |
| Threatening bleeding | 0/8 (0%) | 0/32 (0%) | |
| Valve embolization | 0/8 (0%) | 2/32 (6.25%) | 0.4682 |
The available 1‐year all‐cause mortality data showed that neither the stentless or stented group had deaths within this time frame (0%, 0/25 versus 0%, 0/5, respectively). All‐cause mortality for 30 days, 6 months, and 1 year are shown in Table 5. There was no significant difference in re‐hospitalization rates between the two groups. Re‐hospitalization data for 30 days, 6 months, and 1 year are shown in Table 5. Stroke rates were also similar between the two groups (Table 5).
Table 5.
Long‐term outcomes
| Death (%) | Stented | Stentless | |
|---|---|---|---|
| Death (inpatient) | 0/8 (0%) | 1/31 (3.23%) | 0.4949 |
| Death at 30 days | 0/8 (0%) | 2/31 (6.45%) | 0.3308 |
| Death at 6 months | 1/7 (14.29%) | 1/29 (3.45%) | 0.3156 |
| Death at 1 year | 0/5 (0%) | 0/25 (0%) | |
| CV death (inpatient) | 0/8 (0%) | 0/31 (0%) | |
| CV death (30 days) | 0/8 (0%) | 1/31 (3.23%) | 0.4949 |
| CV death (6 months) | 0/7 (0%) | 1/29 (3.45%) | 0.5365 |
| CV death (1 year) | 0/5 (0%) | 0/25 (0%) | |
| Stroke (%) | |||
| Stroke (30 days) | 0/8 (0%) | 1/31 (3.23%) | 0.4949 |
| Stroke (6 months) | 0/7 (0%) | 0/29 (0%) | |
| Stroke (12 months) | 0/5 (0%) | 0/25 (0%) | |
| Rehospitalization (%) | |||
| Rehospitalization (30 days) | 3/8 (37.50%) | 4/31 (12.90%) | 0.1309 |
| Rehospitalization (6 months) | 2/7 (28.57%) | 5/29 (17.24%) | 0.4965 |
| Rehospitalization (1 year) | 1/5 (20%) | 4/25 (16%) | 0.8259 |
| Vascular complications (%) | |||
| Major (30 days) | 0/8 (0%) | 2/31 (6.25%) | 0.5483 |
| Minor (30 days) | 1/8 (12.5%) | 0/31 (0%) | 0.2 |
| Major (6 months) | 0/7 (0%) | 0/29 (0%) | |
| Minor (6 months) | 0/7 (0%) | 0/29 (0%) | |
| Major (12 months) | 0/5 (0%) | 0/25 (0%) | |
| Minor (12 months) | 0/5 (0%) | 0/25 (0%) | |
3.4. Echocardiographic data and outcomes
Complete longitudinal echocardiographic data for baseline and 1‐, 6‐, and 12‐month follow‐ups are listed in Table 6. Baseline ejection fraction (EF) was similar in both the stentless and stented groups (52.2 ± 11.9% and 55.6 ± 9.4%, respectively). Etiologies for valve failure were different in stentless versus stented groups with 78% (25/32) of stentless and 25% (2/8) of stented patients having moderate to severe aortic insufficiency (P < 0.01). As such, baseline aortic valve area in the stentless and stented groups was 1.70 ± 0.78 mm and 0.73 ± 0.23 mm (P < 0.05), respectively. The mean indexed effective orifice area (iEOA) pre‐ViV for stentless and stented groups was 0.87 ± 0.36 cm2/m2 and 0.36 ± 0.11 cm2/m2 (P < 0.005), respectively. Post‐VIV, the iEOA was 0.87 ± 0.31 cm2/m2 and 0.69 ± 0.34 cm2/m2 (P = 0.15), respectively, as listed in Table 2. The baseline aortic valve peak gradient was significantly lower in the stentless versus the stented group (37.8 ± 29.5 mmHg versus 75.8 ± 29.6 mmHg, P < 0.01, respectively). The stentless group had a significantly lower aortic valve mean gradient relative to the stented group (23.3 ± 25.4 mmHg versus 43.0 ± 17.8 mmHg, P < 0.01, respectively).
Table 6.
Echocardiogram data
| Stented (N = 8) | Stentless (N = 32) | P value | |
|---|---|---|---|
| Baseline | |||
| EF (%) | 55.63, 9.43 | 52.16, 11.92 | 0.3947 |
| AVA (cm2) | 0.73, 0.23 | 1.70, 0.78 | 0.0104 |
| Aortic mean gradient (mmHg) | 43.03, 17.8 | 23.33, 25.37 | 0.0094 |
| Aortic peak gradient (mmHg) | 75.81, 29.59 | 37.78, 29.51 | 0.0058 |
| None | 4/8 (50%) | 5/32 (15.63%) | 0.0594 |
| Trace‐mild AI | 2/8 (25%) | 2/32 (6.25%) | 0.1138 |
| Moderate–severe AI | 2/8 (25%) | 25/32 (78.13%) | 0.0085 |
| Pre‐discharge (post‐TAVR) | Stented (N = 8) | Stentless (N = 32) | P value |
| EF (%) | 56.25, 10.26 | 47.19, 12.95 | 0.0669 |
| AVA (cm2) | 1.60, 0.669 | 1.69, 0.50 | 0.6132 |
| Aortic mean gradient (mmHg) | 7.35, 7.40 | 11.33, 5.69 | 0.1862 |
| Aortic peak gradient (mmHg) | 44.21, 21.79 | 25.43, 11.45 | 0.0129 |
| None | 4/8 (50%) | 9/32 (28.13%) | 0.3995 |
| Trace‐mild PVL | 4/8 (50%) | 20/32 (62.50%) | 0.6905 |
| Moderate–severe PVL | 0/8 (0%) | 3/32 (9.38%) | 1.000 |
| 30 day follow‐up | Stented (N = 8) | Stentless (N = 28) | P value |
| EF (%) | 56.88, 7.53 | 46.96, 14.80 | 0.0445 |
| AVA (cm2) | 1.11, 0.48 | 1.66, 0.68 | 0.0202 |
| Aortic mean gradient (mmHg) | 22.63, 8.45 | 12.33, 6.33 | 0.0046 |
| Aortic peak gradient (mmHg) | 43.1, 17.68 | 21.79, 10.57 | 0.002 |
| None | 4/8 (50%) | 6/28 (21.43%) | 0.0091 |
| Trace‐mild PVL | 2/8 (25%) | 19/28 (67.86%) | 0.0461 |
| Moderate–severe PVL | 2/8 (25%) | 3/28 (10.71%) | 0.3336 |
| 6 month follow‐up | Stented (N = 4) | Stentless (N = 16) | P value |
| EF (%) | 59, 11.43 | 55.93, 4.70 | 0.633 |
| AVA (cm2) | 1.61, 0.464 | 1.77, 0.510 | 0.5865 |
| Aortic mean gradient (mmHg) | 24, 11.28 | 9.75, 5.07 | 0.0143 |
| Aortic peak gradient (mmHg) | 33.08, 11.63 | 19.56, 10.74 | 0.0966 |
| None | 3/4 (66.67%) | 8/16 (50%) | 0.5957 |
| Trace‐mild PVL | 1/4 (33.33%) | 4/16 (25%) | 0.7636 |
| Moderate–severe PVL | 0/4 (0%) | 0/16 (0%) | 0.3297 |
| 12 month follow‐up | Stented (N = 5) | Stentless (N = 16) | P value |
| EF (%) | 55, 6.12 | 55.31, 6.45 | 0.9244 |
| AVA (cm2) | 1.53, 1.03 | 1.76, 0.57 | 0.685 |
| Aortic mean gradient (mmHg) | 22.5, 11.17 | 9.48, 4.37 | 0.0088 |
| Aortic peak gradient (mmHg) | 43.2, 28.05 | 18.49, 9.70 | 0.0761 |
| None | 3/5 (60%) | 7/16 (43.75%) | 0.5254 |
| Trace‐mild PVL | 2/5 (40%) | 5/16 (31.25%) | 0.7171 |
| Moderate–severe PVL | 0/5 (0%) | 4/16 (25%) | 0.214 |
At 30‐day follow‐up, the stentless group had a larger aortic valve area compared to the stented group (1.66 ± 0.68 mm versus 1.11 ± 0.48 mm [P < 0.05], respectively). The aortic valve mean gradient in the stentless and stented groups was 12.3 ± 6.3 mmHg versus 22.6 ± 8.5 mmHg (P < 0.005), respectively. The aortic peak gradient in the stentless and stented groups was 21.8 ± 10.6 mmHg versus 43.1 ± 17.7 mmHg (P < 0.01), respectively. At 30 days, 68% in the stentless and 25% in the stented groups had trace to mild paravalvular leak (PVL) (P < 0.05), however, no significant difference in moderate to severe PVL. The stentless group had a significantly lower EF compared to the stented group (47.0 ± 14.8% versus 56.9 ± 7.5%, P < 0.05). There was no significant increase in EF change from pre‐discharge in both groups (Figure 1).
Figure 1.
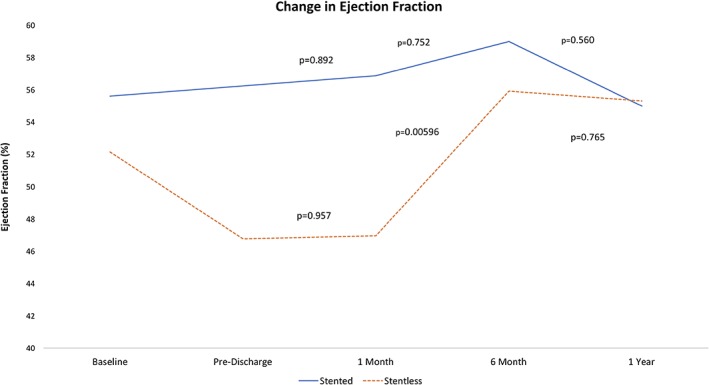
Longitudinal change in ejection fraction (%). This figure displays the average ejection fraction at each follow‐up and P‐values for the change in ejection fraction between follow‐up periods
At 6 months, the aortic valve area was similar in both groups (Table 4). The stentless group had a significant increase in EF from 1‐month follow‐up (P < 0.005), whereas the stented group remained similar (P = 0.75) (Figure 1). The aortic valve mean gradient remained lower in the stentless group compared to the stented group (9.8 ± 5.1 mmHg versus 24.0 ± 11.3 mmHg, respectively [P < 0.05]). At 6 months, 0% in both the stentless and stented groups had moderate to severe PVL.
At 12 months, the EF was similar in both groups and no significant change in EF from 6‐month follow‐up. The aortic valve area of the stentless and stented groups was found to be similar (1.76 ± 0.57 mm versus 1.53 ± 1.03 mm, respectively). The aortic valve mean gradient was found to be significantly lower in the stentless compared to the stented group (9.5 ± 4.4 mmHg versus 22.5 ± 11.2 mmHg, P < 0.01, respectively). There were no significant differences in PVL severity. Comparisons of mean gradient and aortic valve area between both groups over a 12‐month period are shown in Figure 2. Comparisons of aortic PVL severity between both groups over a 12‐month period are shown in Figure 3.
Figure 2.
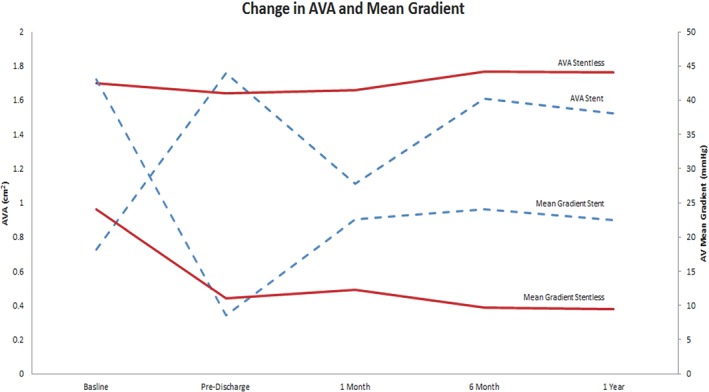
Mean aortic valve gradient comparison. This figure compares the mean aortic valve gradients (mmHg) and aortic valve area (AVA) from preoperative period to 12‐month follow‐up in both the stented (blue) and stentless (red) groups
Figure 3.
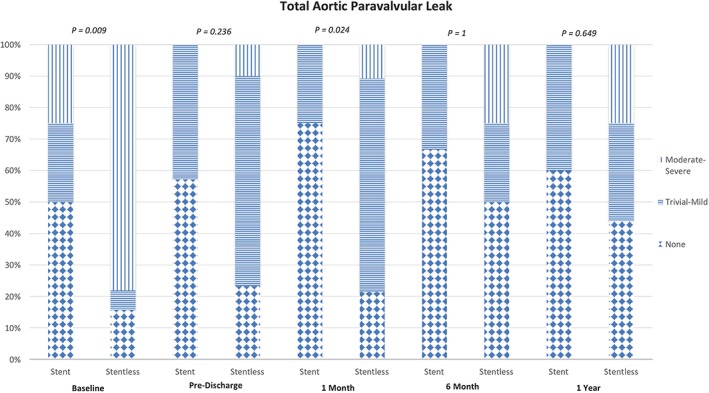
Comparison of paravalvular leak (PVL) severity. This figure compares the paravalvular leak from preoperative period to 12‐month follow‐up in both the stented and stentless group
4. DISCUSSION
The current study has demonstrated that ViV in stentless surgical bioprosthetic valve is feasible and safe with comparable clinical outcomes as in stented surgical bioprosthetic valve. Hemodynamics of ViV in the stentless group were superior with a larger effective orifice area and lower residual gradient as compared to the stented group. However, one should be cautious to generalize these findings as our stent group had a much smaller sample size compared to stentless group, such as the stentless group having a younger average age than stented group (62.75 ± 14.37 vs 73.75 ± 13.26), however not statistically significant. ViV in stentless group was technically more challenging than the stented group. More TAVR malposition/embolization was present in the stentless group. Using the first TAVR device as an anchor may facilitate appropriate positioning of the second TAVR device and favorable outcomes.
The first experiences of the ViV TAVI for failing bioprosthetic valves were performed by Walther et al. in an animal model.8 Wenaweser et al. performed the first published ViV procedure in the aortic valve in a human subject utilizing the Medtronic CoreValve.9 Seiffert et al. published the first ViV series in four patients with a ViV in aortic or mitral bioprosthetic failure.10 Further series included Webb et al. with 24 patients with aortic, mitral, pulmonary or tricuspid bioprosthetic valve failure.11 Piazza et al. published the German Heart Center series of 20 aortic valve patients with a 90% procedural success rate,12 and Ussia et al. in the Italian CoreValve Registry reported a 98% procedural success rate.13 Bapat et al. demonstrated a 100% procedural success rate in 23 consecutive patients utilizing the Edwards SAPIEN ViV for failing aortic bioprosthesis.14 PARTNER 2 Valve‐in‐Valve Registry by Webb et al. examined 30‐day and 1‐year outcomes in a large cohort of high‐risk patients undergoing ViV TAVR. At 30 days, all‐cause mortality was 2.7%, stroke was 2.7%, major vascular complication was 4.1%, and 1‐year all‐cause mortality was 12.4%. Majority of failed surgical bioprosthetic aortic valve in those studies were stented surgical bioprosthetic aortic valve.15 In a recent meta‐analysis by Tam et al., there was no difference in perioperative mortality (4.4% vs. 5.7%, P = 0.83) or late mortality, reported at median one‐year follow‐up (IRR 0.93, 95%CI: 0.74–1.16, P = 0.51) between ViV TAVR and redo SAVR groups.16
Data on ViV in stentless surgical bioprosthetic aortic valve is limited. Grubitzsch et al. compared redo SAVR (n = 25) versus ViV‐TAVR (n = 27) in patients with degenerated stentless aortic xenografts. There were similar 30‐day mortality (10% for both groups, P = 1.0) and one‐year survival (83.1 ± 7.7% versus 81.5 ± 7.5%, P = 0.76).17 Our study has demonstrated comparable clinical outcomes of ViV in stentless group as compared to stented group. In the Global Valve‐in‐Valve Registry, high post‐procedural gradients were common at 28%. Interestingly, we have observed superior hemodynamics of ViV in the stentless as compared to the stented group. The stentless group had a larger effective orifice area at 30 days and lower mean aortic gradient throughout 1‐year follow‐ups. It is easily understandable that the absence of a stent rendered a larger effective orifice area of prosthesis and reduction in the mean aortic gradient.18
The stentless had significantly more overall PVL (i.e., trivial‐severe) than the stented group at 1 month (P < 0.05), however, the PVL improved at 6‐month follow‐up and remained stable in the stentless and relatively stable in the stented groups. Lack of a rigid ring or calcified annulus to secure the device in the stentless ViV may have contributed to the outcomes. A standard approach to address immediate post TAVR PVL would apply to this group. If severe, we usually proceeded with post‐TAVR balloon expansion to reduce PVL. If it was not effective and the valve was low, we deployed a second valve at a higher level. PVL in the stentless group is usually diffuse and non‐focal. Plugging may not be practical. Interestingly, similar to native TAVR with the Medtronic CoreValve, our study demonstrated that although PVL was more prevalent in the stentless group, it improved as time progressed.
Stentless bioprosthetic valves make ViV implantation especially challenging given the lack of a frame or structural support to anchor the TAVR, as well as lack of radiographic markers to help with proper positioning. Our study has confirmed such concerns. In patients with stentless valve and primary mode of aortic insufficiency, identifying appropriate aortic annulus is challenging. We have used a multi‐modality imaging approach to assist in positioning the TAVR device. We also used multiple Pigtail catheters that were placed in the right and non‐coronary cuspids to assist in positioning. It proved to be less of a problem in patients with aortic stenosis as the primary mode of failure. Positioning was similar as in the native valve TAVR procedure.
More TAVR migration and embolization were present in the stentless group, which were either secondary to utilizing a smaller size THV or improper placement (placement is usually too low). To help reduce this complication, it is essential to select an appropriately sized THV by utilizing the true internal diameter of the prior surgical aortic valve as a guide and ideal placement.18 THV oversizing is commonly recommended to prevent complications of valve migration, embolization, and malpositioning.18, 19 However, one must exercise caution given the risk of coronary obstruction with oversized valve implantation. In particular, stentless bioprosthetic valves will have a smaller gap between the THV and the coronary ostia given the free‐style suturing nature of the prior surgically placed aortic valve.20
At the first deployment, if the device had a tendency to migrate distally, we adopted a strategy to deploy valve deep in the ventricle using the waist of the Medtronic CoreValve as an anchor to secure the second THV in a proper position. In our limited experience and follow up, we have not observed adverse effects with the first THV deployed relatively deep in the ventricle. In patients with aortic regurgitation, the hemodynamics tended to be stable as they tolerated the first THV regurgitation well as second THV was prepared. However, in patients with aortic stenosis, the hemodynamics may deteriorate quickly. Therefore, it is critical to have the second THV loaded and ready to deploy before releasing the first valve.
Finally, we observed a reduction in left ventricular systolic function immediately after ViV TAVR in the stentless group but not in the stented group. This phenomenon may reflect the differences in hemodynamic changes in these two groups. Most valve failure in the stentless group is aortic regurgitation. Therefore, placement of ViV TAVR does not change and may potentially increase left ventricular afterload resulting in a transient reduction of left ventricular systolic function. In contrast, most valve failure in the stented group is aortic stenosis. Placement of ViV TAVR reduces left ventricular afterload that is favorable for left ventricular hemodynamics. Fortunately, left ventricular function in the stentless group recovers as time progresses. There is no difference in left ventricular function between the two groups at 12 months.
4.1. Study limitations
This was a retrospective observational study of a single center's experience of comparing ViV TAVR in failed stentless versus stented bioprosthetic aortic valves. Our study was limited by the small sample size in both groups, particularly in the stented group, as well as being limited to a single center's experience. However, one of the study's strengths is the relatively larger number of stentless cases compared to what is currently available in the literature. Another limitation is that patients in those groups were not completely similar, more specifically in regards to primary mode of valve failure, therefore, must also consider these differences during the Heart Team evaluation and local expertise. Future and larger prospective multi‐center studies are needed to further validate our current findings.
5. CONCLUSION
ViV in the stentless bioprosthetic aortic valve has excellent procedural success and intermediate‐term results. Our study shows promising data that may support the application of TAVR in stentless surgical aortic valve.
CONFLICT OF INTEREST
All authors confirm that no conflicts of interest exist for this manuscript, the manuscript represents valid work and that neither this manuscript nor one with substantially similar content under their authorship has been or is being considered for publication elsewhere.
ACKNOWLEDGMENTS
All authors substantially contributed to the conception, design, drafting and final approval of this manuscript.
Choi CH, Cheng V, Malaver D, et al. A comparison of valve‐in‐valve transcatheter aortic valve replacement in failed stentless versus stented surgical bioprosthetic aortic valves. Catheter Cardiovasc Interv. 2019;93:1106–1115. 10.1002/ccd.28039
REFERENCES
- 1. Gurvitch R, Cheung A, Ye J, et al. Transcatheter valve‐in‐valve implantation for failed surgical bioprosthetic valves. J Am Coll Cardiol. 2011;58:2196‐2209. [DOI] [PubMed] [Google Scholar]
- 2. Kobayashi J. Stentless aortic valve replacement: An update. Vasc Health Risk Manage. 2011;7:345‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones JM, O'kane H, Gladstone DJ, et al. Repeat heart valve surgery: Risk factors for operative mortality. J Thorac Cardiovasc Surg. 2001;122:913‐918. [DOI] [PubMed] [Google Scholar]
- 4. Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Results from the global valve‐in‐valve registry. Circulation. 2012;126:2335‐2344. [DOI] [PubMed] [Google Scholar]
- 5. Camboni D, Holzamer A, Flörchinger B, et al. Single‐institution experience with transcatheter valve‐in‐valve implantation emphasizing strategies for coronary protection. Ann Thorac Surg. 2015;99:1532‐1538. [DOI] [PubMed] [Google Scholar]
- 6. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The valve academic research Consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438‐1454. [DOI] [PubMed] [Google Scholar]
- 7. Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the valve academic research consortium. Eur Heart J. 2011;32:205‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walther T, Falk V, Dewey T, et al. Valve‐in‐valve concept for transcatheter minimally invasive repeat xenograft implantation. J Am Coll Cardiol. 2007;50:56‐60. [DOI] [PubMed] [Google Scholar]
- 9. Wenaweser P, Buellesfeld L, Gerckens U, Grube E. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: The first valve in valve procedure using the CoreValve revalving system. Catheter Cardiovasc Interv. 2007;70:760‐764. [DOI] [PubMed] [Google Scholar]
- 10. Seiffert M, Franzen O, Conradi L, et al. Series of transcatheter valve‐in‐valve implantations in high‐risk patients with degenerated bioprosthesis in aortic and mitral position. Catheter Cardiovasc Interv. 2010;76:608‐615. [DOI] [PubMed] [Google Scholar]
- 11. Webb J, Wood D, Ye J, et al. Transcatheter valve‐in‐valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121:1848‐1857. [DOI] [PubMed] [Google Scholar]
- 12. Piazza N, Bleiziffer S, Brockmann G, et al. Transcatheter aortic valve implantation for failing surgical aortic bioprosthesis: From concept to clinical application and evaluation (part 2). J Am Coll Cardiol Interv. 2011;4:733‐742. [DOI] [PubMed] [Google Scholar]
- 13. Ussia GP, Barbanti M, Ramondo A, et al. The valve‐in‐valve technique for treatment of aortic bioprosthesis malposition an analysis of incidence and 1‐year clinical outcomes from the Italian CoreValve registry. J Am Coll Cardiol. 2011;57:1062‐1068. [DOI] [PubMed] [Google Scholar]
- 14. Bapat V, Attia R, Redwood S, et al. Use of transcatheter heart valves for a valve‐in‐valve implantation in patients with degenerated aortic bioprosthesis: Technical considerations and results. J Thorac Cardiovasc Surg. 2012;144:1372‐1380. [DOI] [PubMed] [Google Scholar]
- 15. Webb JG, Mack MJ, White JM, et al. Transcatheter aortic valve implantation within degenerated aortic surgical bioprosthesis. J Am Coll Cardiol. 2017;69:2253‐2262. [DOI] [PubMed] [Google Scholar]
- 16. Tam DY, Vo TX, Wijeysundera HC, Dvir D, Friedrich JO, Fremes SE. Transcatheter valve‐in‐valve versus redo surgical aortic valve replacement for the treatment of degenerated bioprosthetic aortic valve: A systematic review and meta‐analysis. Catheter Cardiovasc Interv. 2018;92:1404‐1411. 10.1002/ccd.27686 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17. Grubitzsch H, Zobel S, Christ T, et al. Redo procedures for degenerated stentless aortic xenografts and the role of valve‐in‐valve transcatheter techniques. Eur J Cardiothorac Surg. 2017;51:653‐659. [DOI] [PubMed] [Google Scholar]
- 18. Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162‐170. [DOI] [PubMed] [Google Scholar]
- 19. Borger MA, Prasongsukarn K, Armstrong S, Feindel CM, David TE. Stentless aortic valve reoperations: A surgical challenge. Ann Thorac Surg. 2007;84:737‐744. [DOI] [PubMed] [Google Scholar]
- 20. Noorani A, Radia R, Bapat V. Challenges in valve‐in‐valve therapy. J Thorac Dis. 2015;7:1501‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]


