Summary
To understand the role of micrometer‐scale oxygen (O2) gradients in facilitating dinitrogen (N2) fixation, we characterized O2 dynamics in the microenvironment around free‐floating trichomes and colonies of Trichodesmium erythraeum IMS101.
Diurnal and spatial variability in O2 concentrations in the bulk medium, within colonies, along trichomes and within single cells were determined using O2 optodes, microsensors and model calculations. Carbon (C) and N2 fixation as well as O2 evolution and uptake under different O2 concentrations were analyzed by stable isotope incubations and membrane inlet mass spectrometry.
We observed a pronounced diel rhythm in O2 fluxes, with net O2 evolution restricted to short periods in the morning and evening, and net O2 uptake driven by dark respiration and light‐dependent O2 uptake during the major part of the light period. Remarkably, colonies showed lower N2 fixation and C fixation rates than free‐floating trichomes despite the long period of O2 undersaturation in the colony microenvironment.
Model calculations demonstrate that low permeability of the cell wall in combination with metabolic heterogeneity between single cells allows for anoxic intracellular conditions in colonies but also free‐floating trichomes of Trichodesmium. Therefore, whereas colony formation must have benefits for Trichodesmium, it does not favor N2 fixation.
Keywords: colony, microenvironment, N2 fixation, oxygen, Trichodesmium
Introduction
Fixation of dinitrogen (N2) by marine diazotrophic bacteria and cyanobacteria provides a significant source of nitrogen to phytoplankton in oligotrophic systems. The N2‐fixing enzyme nitrogenase is inhibited by oxygen (O2) via oxidative damage to the iron sulfur clusters (Burgess & Lowe, 1996), proteolysis (Durner et al., 1996) as well as suppression of nitrogenase synthesis and posttranslational modification (Gallon, 1992). To protect nitrogenase from O2, many single‐celled cyanobacteria separate photosynthesis from N2 fixation in time, conducting N2 fixation during the night, whereas many filamentous cyanobacteria restrict N2 fixation to specialized cells termed heterocysts. The genus Trichodesmium spp., a globally important, colony‐forming diazotroph, is an exception in that it fixes N2 during the day although it lacks heterocysts. A range of O2‐protective mechanisms has been suggested for Trichodesmium: the formation of anoxic microzones within colonies (Paerl & Bebout, 1988); a downregulation of photosynthesis during the peak of N2 fixation at midday (Berman‐Frank et al., 2001); a restriction of N2 fixation to specialized cells termed diazocytes (Bergman & Carpenter, 1991; Berman‐Frank et al., 2001); and dynamic switches between different photosynthetic activity states by reversible uncoupling of phycobilisomes from the photosystems on the time scale of minutes (Küpper et al., 2004, 2009). The prevalence and coordination of these different mechanisms is still debated, partly due to conflicting results in previous studies.
Regarding cell specialization, several studies have reported nitrogenase to be present only in 10–15% of cells within a trichome (diazocytes), which are not terminally differentiated cells, do not have thicker cell walls than vegetative cells, and contain photosystem II, by contrast to heterocysts (Carpenter et al., 1990; Siddiqui et al., 1992a; Fredriksson & Bergman, 1995; Berman‐Frank et al., 2001). Other studies observed no differences in nitrogenase expression and/or N2 fixation between single cells and therefore questioned the prevalence of diazocytes (Paerl & Bebout, 1988; Finzi‐Hart et al., 2009; Ohki & Taniuchi, 2009). Also the formation of anoxic microzones in colonies has been challenged by field measurements showing strong O2 supersaturation in Trichodesmium colonies (Eichner et al., 2017), questioning the assumed benefit of colony formation for N2 fixation. Direct comparisons of colonies and single trichomes are scarce. Field studies have traditionally shown a bias towards studying colonies rather than single trichomes (Letelier & Karl, 1998), whereas most laboratory studies on Trichodesmium physiology, including those demonstrating the inhibitory effects of O2 on N2 fixation in vivo (Küpper et al., 2004; Berman‐Frank et al., 2005, 2008; Staal et al., 2007), have been conducted on cultures of Trichodesmium erythraeum IMS101 grown as single trichomes.
To re‐evaluate the hypothesis that anoxic microenvironments allow for higher N2 fixation in colonies compared with single trichomes, we conducted an in‐depth analysis of O2 dynamics and feedbacks on N2 fixation in colonies and free‐floating trichomes of Trichodesmium erythraeum IMS101. We directly compared carbon (C) and N2 fixation rates in colonies vs single trichomes and characterized cellular gross and net O2 fluxes, diurnal variations in O2 concentrations in the bulk medium, and O2 concentrations within colonies, on the surface of single trichomes and within single cells.
Materials and Methods
Culture conditions
Cultures of Trichodesmium erythraeum IMS101 were grown on YBCII culture medium (Chen et al., 1996) with decreased phosphate concentrations (4.61 ± 0.79 μmol l−1, determined with QuAAtro39, Seal Analytics), at 27–29°C and 240–280 μmol photons m−2 s−1 under a 12 h: 12 h, light : dark cycle. Stock cultures were grown on a shaking table (80 rounds min−1, IKA KS 130 basic), and transferred to roller tanks once colonies had started to form in order to maintain colonies physically intact for longer. Additional colonies formed in roller tanks, including mostly puffs, but also tuft‐ and needle‐shaped colonies. Depending on the culture volume required for the specific measurements, different roller tank set‐ups were used, including bottles with a volume between 60 ml and 2.5 l. pH levels (National Bureau of Standards (NBS) scale) were determined with a two‐point calibrated glass electrode (Aquatrode plus Pt1000; Metrohm, Herisau, Switzerland).
Elemental composition
For determination of elemental ratios, samples for particulate organic carbon, nitrogen and phosphorus (POC, PON and POP) and chlorophyll a (Chla) were taken c. 7 h after beginning of the light phase. Technical duplicate samples of culture including both free trichomes and colonies were filtered onto precombusted GF/F filters and stored at −20°C. Samples for analysis of POC and PON were acidified with 200 μl HCl (0.2 mol l−1) and subsequently measured on an elemental analyzer (EuroEA, Eurovector). POP was determined spectrophotometrically (UV‐1202; Schimadzu, Kyoto, Japan) according to Hansen & Koroleff (1999). Chla was extracted in 90% acetone at 4°C for > 12 h with ultrasonication (10 s) and measured fluorometrically (10‐AU, Turner Designs; Holm‐Hansen & Riemann, 1978).
Optode measurements
The diurnal cycle of O2 concentrations in cultures was monitored with contactless optical O2 sensors glued into culture vessels (silicon glue, sensor spots of 5 mm diameter and a FireStingO2/FireStingGO2 oxygen meter, Pyroscience). Measurements were performed in 120 ml serum bottles closed without headspace and incubated in roller tanks. Additionally, O2 concentrations were monitored in the set‐up used for stock cultures, that is, culture vials closed with a gas permeable frit (VWR) that were incubated on a shaking table. O2 concentrations were recorded at 2 Hz for up to 7 d. Optodes were two‐point calibrated using medium that was bubbled with either N2 gas or air to achieve 0% and 100% air saturation, respectively.
Microsensor measurements
Microsensor measurements on colonies were performed in a custom‐made flow system (Ploug & Jørgensen, 1999) in YBCII medium at 26°C. Single colonies were suspended in the flow chamber (flow < 0.1 mm s−1), fixed with a thin glass needle and observed through a stereomicroscope (Stemi SV6; Zeiss). For recording depth profiles through the colony center, microsensors were moved towards and into the colony with a motor‐driven micromanipulator (VT‐80, Micos/Faulhaber Minimotor SA).
In a total of 13 colonies, O2 concentrations in and around colonies were measured with Clark‐type microelectrodes (tip diameter c. 10–15 μm, response time c. 1 s). Rates of respiration and net photosynthesis were calculated from the steady‐state O2 gradient at the colony surface according to Fick's first law of diffusion:
where J represents the interfacial O2 flux, D the diffusion coefficient for O2 (2.26 × 10−9 m2 s−1 at 25°C and salinity 34; Broecker & Peng, 1974), and ΔC the concentration difference measured over the respective distance, Δr, at the colony surface. Surface area and volume were determined for each colony assuming ellipsoid geometry and used to convert interfacial flux to volume‐normalized rates. The theoretical maximum O2 uptake supported by diffusive O2 supply (that is, the O2 uptake rate yielding anoxia at the center of the colony) was calculated as a function of colony volume as described by Ploug et al. (1997). An apparent diffusivity of O2 within the colony of 0.95× that of seawater, a Sherwood number of 1 (that is, no difference between the motion of the colony and that of the surrounding water, resulting in mass transfer merely by molecular diffusion but not advection), a bulk O2 concentration of 212 μmol l−1 (that is, air saturation), and a uniform respiration rate throughout the colony were assumed.
Additionally, measurements of chlorophyll fluorescence were performed on nine of the colonies using a MicrofiberPAM (Walz) with a microfiber tapered to 10–20 μm width at the tip. A red LED (650 nm) was used for excitation. Measurements were performed in the dark, between 1 and 9 h after beginning of the light phase. Photochemical quantum yield (F v/F m) is reported only for those positions in and around colonies for which signal strength was high enough for a clear fluorescence induction curve to be observed.
For microsensor measurements on single trichomes with higher spatial resolution, the trichomes were embedded in 0.5% agar, in which the effective diffusion coefficient of O2 is similar as in seawater (Ploug & Passow, 2007). Ultra‐pure low melting point agar (Invitrogen) was dissolved, allowed to cool down to < 30°C, and then mixed in a ratio of c. 1 : 1 with culture in a Petri dish. Measurements were performed on a total of 29 trichomes with Clark‐type microelectrodes (tip diameter 5–10 μm, response time c. 1 s) under an inverted microscope (Axiovert 25; Zeiss) at ×20 magnification, room temperature and c. 150 μmol photons m−2 s−1 (unless specified otherwise), at various time points between the start and 1 h after the end of the light period. Differences in O2 concentrations along trichomes were probed by moving the sensor along the trichome within c. 3 μm distance from the cell surface as observed in the microscope, and recording O2 concentrations in consecutive steps of three to four cells. Additionally, O2 concentrations were recorded continuously at the surface of individual cells while switching the light on and off.
Membrane inlet mass spectrometry
Gross and net O2 fluxes were measured with a custom‐built membrane inlet mass spectrometer (MIMS; mass spectrometer Isoprime, custom‐built 8 ml cuvette) using the 18O2‐based approach described by Fock & Sültemeyer (1989). Cultures containing colonies and single trichomes were grown in 2.5 l Schott bottles at 25°C and 300 μmol photons m−2 s−1 (Biolux; Osram, Garching, Germany). For measurements, 18O2 gas (Chemotrade, Düsseldorf, Germany) was dissolved in previously N2‐bubbled YBCII medium buffered with HEPES (50 mmol l−1, pH 8.29 ± 0.06), reaching a final concentration of c. 150% air saturation. Cultures concentrated by gentle filtration over a polycarbonate filter, as well as additional colonies picked with a Pasteur pipette, were suspended in the 18O2‐enriched medium, reaching a final Chla concentration of 0.41 ± 0.26 μg ml−1. The production of 16O2 and the uptake of 16O2 and 18O2 were then monitored in light and dark under three different O2 concentrations (346 ± 39 μmol l−1, 191 ± 51 μmol l−1 and 101 ± 35 μmol l−1) obtained by bubbling with N2 gas. Samples were stirred during measurements, resulting in disassembly of the colonies. Two replicate light and dark phases lasting c. 4 min each were conducted at each O2 level. O2 signals were corrected for abiotic consumption and influx of O2 into the cuvette by subtracting O2 slopes recorded in abiotic controls at the respective O2 concentrations. In total, 11 samples containing free‐floating trichomes and colonies in various proportions were analyzed. Measurements were performed between 1.5 and 4 h after the beginning of the light phase (except one measurement at 8–9 h after the beginning of the light phase that yielded results that were not different from the others).
Stable isotope incubations
C and N2 fixation rates of free‐floating trichomes and colonies were determined by stable isotope incubations with NaH13CO3 (Sigma Aldrich) and 15N2 gas (Cambridge Isotope Laboratories, Tewksbury, MA, USA). To ensure dissolution of 15N2 gas, 15N2 and NaH13CO3 were predissolved in YBCII medium for > 12 h before incubations. Final enrichment was 3.44 ± 0.24 atom percent excess (at% excess) for 13C and 2.2 ± 1.06 at% excess for 15N, as determined at the end of incubations for each incubation bottle by cavity ring‐down spectroscopy (G2201‐i; Picarro, Santa Clara, CA, USA) and MIMS (GAM2000; InProcess, Bremen, Germany). O2 concentrations in the incubation medium were adjusted to low (24 ± 11 μmol l−1), ambient (238 ± 13 μmol l−1) or high (475 ± 32 μmol l−1) levels by bubbling with a mixture of helium and N2 (20% : 80%, low O2 treatment), room air (ambient O2 treatment), or a mixture of O2 and N2 (40% : 60%, high O2 treatment). Subsequently, Trichodesmium biomass was transferred to the respective medium and amended with 15N‐ and 13C‐enriched stock solutions. For incubations of colonies, approx. 50 colonies per incubation vial were picked from culture bottles with a Pasteur pipette. For incubations of free‐floating trichomes, the remaining culture volume was concentrated by filtration over a polycarbonate filter before transfer to the incubation medium. Samples where free‐floating trichomes formed colonies during the incubation period, resulting in colony‐dominated biomass, were classified as colonies. Incubations were performed in triplicate, in 60 ml serum vials at 25°C and 150 μmol photons m−2 s−1 (100–210 μmol photons m−2 s−1 in roller tanks), for 11 h starting within 1 h after beginning of the light period. The O2 concentration in each incubation vial was measured with O2 microsensors before and after incubations. Vials with single trichomes were gently agitated by hand c. 4 times over the incubation period. Vials with colonies were incubated in roller tanks. At the end of the incubation time, cultures were filtered on precombusted GF/F filters and stored at −20°C. Before analysis, filters were acidified in an HCl fume (overnight) to remove all inorganic carbon. Amounts of POC and PON and their isotopic composition were measured by elemental analyser isotope ratio mass spectrometry (EA‐IRMS, Delta Plus XP and Flash EA 112; Thermo Fisher Scientific, Waltham, MA, USA). 13C and 15N enrichment of the POC and PON relative to the bulk solution was then converted to C and N2 fixation rates normalized to cellular C and N biomass, respectively, yielding C‐ and N‐specific fixation rates. The dissolved inorganic carbon concentration in incubation vials at the end of the incubation period was never below 1820 μmol l−1.
Results
Culture characteristics
Single trichomes and colonies co‐occurred in the cultures in varying proportions. Cultures generally started forming colonies 1 wk or longer after dilution with fresh medium and stayed in a colony‐dominated state for more than a week. Colonies were often formed in high numbers within a short time period, starting with needle‐ or string‐shaped colonies that formed around 200 mostly puff‐shaped colonies l−1 within a day, which were morphologically similar to field‐collected puffs. Continuous monitoring of bulk O2 concentrations within culture vials over several days showed that the cultures were net phototrophic most of the time (Fig. 1). This trend was independent of the relative abundance of colonies vs single trichomes, that is, no changes were observed once cultures went from a single‐trichome‐ to a colony‐dominated state (Fig. 1b). Key characteristics of the cultures are summarized in Table 1. pH levels were higher in cultures than in abiotic reference medium (t‐test, P < 0.005, Table 1), confirming net phototrophic growth. Monitoring of phosphate concentrations in two representative bottles over 8 d showed phosphate consumption. Elemental ratios in biomass were variable, with average values close to the Redfield ratio. POC : PON ratios in samples taken from stable isotope incubations at the end of the light period were higher than those taken c. 7 h after the beginning of the light period, and showed no significant difference between colonies and trichomes (t‐test, P > 0.05, Table 1).
Figure 1.
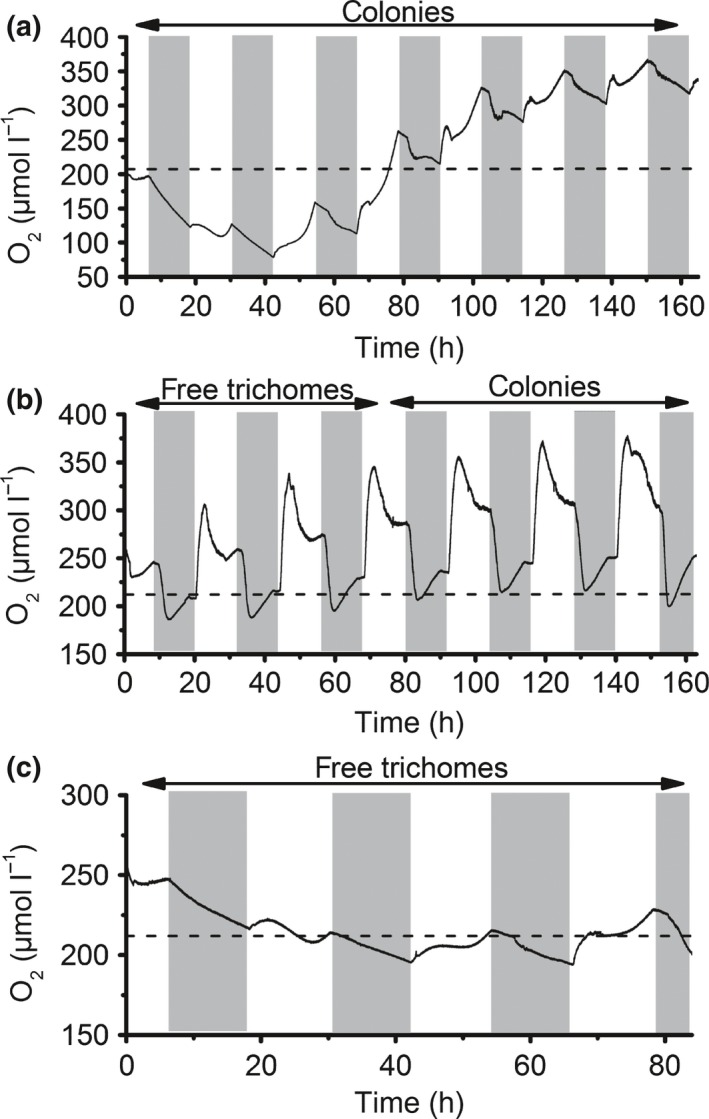
O2 concentrations in the bulk medium of Trichodesmium cultures incubated in roller tanks, monitored in three replicate vials (a–c). Dominant morphology (free‐floating trichomes or colonies) is indicated above each panel. Note that total biomass differed between vials and changed over time. Dashed lines indicate O2 concentration at air saturation, and grey shaded areas indicate dark phases (that is, night‐time).
Table 1.
Key characteristics of Trichodesmium cultures used in the experiment (mean ± SD)
| pH in culture medium | 8.52 ± 0.06 | n = 7 | |
| pH in abiotic reference medium | 8.38 ± 0.06 | n = 9 | |
| Phosphate uptake (replicate 1) | μmol l−1 d−1 | 0.08 | |
| Phosphate uptake (replicate 2) | μmol l−1 d−1 | 0.12 | |
| Fv/Fm a | 0.46 ± 0.07 | n = 9 | |
| POC : PON (7 h)b | mol mol−1 | 5.5 ± 1.2 | n = 4 |
| POC : PON (12 h)c | mol mol−1 | 8.3 ± 2.6 | n = 28 |
| POC : POPb | mol mol−1 | 100 ± 75 | n = 4 |
| PON : POPb | mol mol−1 | 20 ± 16 | n = 4 |
| POC : chlorophyll a b | μg μg−1 | 256 ± 116 | n = 3 |
Fv/Fm, photochemical quantum yield; POC, particulate organic carbon; PON, particulate organic nitrogen; POP, particulate organic phosphorus.
Measured along transects across individual colonies.
Bulk culture samples taken 7 h after beginning of the photoperiod.
Samples taken at the end of the photoperiod, average value including colonies and single trichomes incubated under different O2 concentrations.
Diel variation in bulk O2 concentrations
Continuous measurements of O2 concentrations in the culture medium revealed strong variations over the diel cycle, both in closed vials (Fig. 1), and in culture flasks with a gas permeable lid (up to 300 μmol l−1; data not shown). Often, a fast rise in O2 concentrations was observed within a short period of 1–3 h in the morning, followed by a slow decrease in O2 concentrations over several hours (Fig. 1). A second phase of net O2 evolution followed in most cases 2–4 h before the beginning of the dark phase. Net O2 evolution over the 12 h light period ranged between −3 μmol l−1 (12 h)−1 (that is, net O2 consumption) and 146 μmol l−1 (12 h)−1. During the night, a net decrease of O2 concentrations was observed, sometimes including a short and steep decrease during the first hours of the night. Remarkably, this initial decrease was followed by an increase to air saturation level or above, while the incubation was still in the dark (Fig. 1b). This pattern was confirmed by tracking O2 concentrations close to the center of single colonies with microsensors over several hours (Supporting Information Fig. S1b,d). In the morning, maximum O2 concentrations of 570 and 326 μmol l−1 were observed within colonies (0.5 and 1.3 h after beginning of the light period, respectively; Fig. S1a,c). In the evening, three out of six colonies examined showed elevated O2 concentrations (e.g. 450 μmol l−1 measured 2 h before the beginning of the dark period, Figs 2d, S1). During most of the day, however, colonies were undersaturated with O2 (Fig. 2a–c) and net photosynthesis calculated from O2 profiles was negative (Fig. 2e). To shed light on the regulation of O2 concentrations specifically during this phase of O2 undersaturation, O2 microenvironments and cellular gross and net O2 fluxes were analyzed in more detail during the middle of the day.
Figure 2.
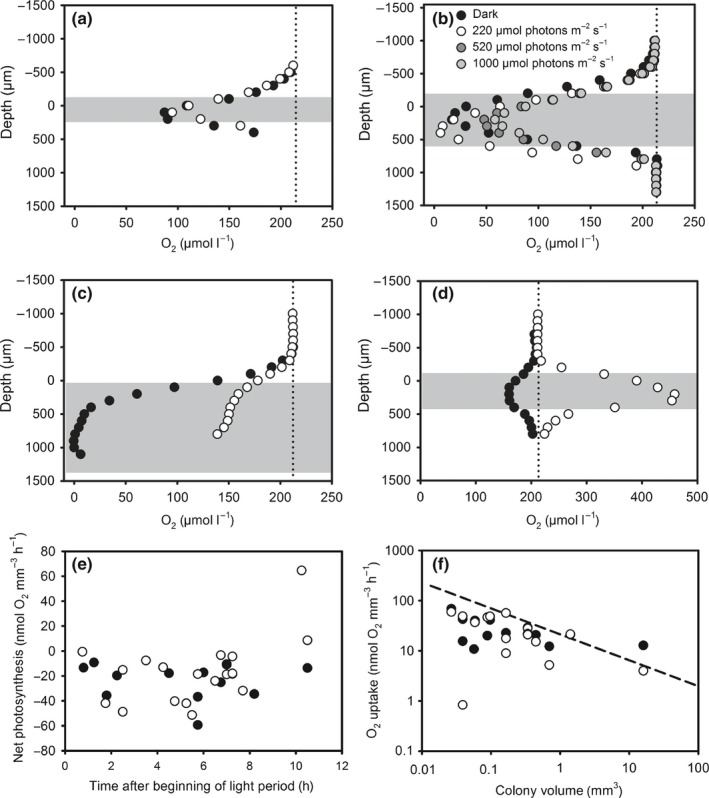
(a–d) Examples of O2 profiles measured in Trichodesmium colonies of different sizes (a, 0.02 mm3; b, 0.38 mm3; c, 2.4 mm3; d, 0.16 mm3) at different light intensities and times of the day (a, 6 h; b, 6 h; c, 7 h; d, 10 h, after the beginning of the light phase). Note the different scale of x‐axis. Grey shading indicates approximate position of the colony, dotted lines indicate O2 concentration at air saturation. (e) Net photosynthesis rates calculated from depth profiles through colonies measured at different times of the day. (f) Net O2 uptake by colonies in dependence of colony volume. Dashed line indicates diffusion limitation (calculated according to Ploug et al., 1997).
O2 microenvironments in colonies during the middle of the day
O2 profiles were measured on a total of 13 colonies of different size (250–1500 μm diameter), shape (tufts and puffs), buoyancy and trichome density between 0.45 and 8 h after beginning of the light period, that is, covering the time period of decreasing O2 evolution rates in the bulk medium (Fig. 1). O2 concentrations in the center of the colony ranged from anoxic conditions to close to air saturation (Fig. 2a–c). Net O2 uptake rates ranged from 0.02 to 26.5 nmol colony−1 h−1. O2 uptake in light was correlated to dark respiration with an R 2 of 0.699 (linear regression). Volume‐normalized O2 uptake was not significantly affected by light intensity (t‐test, P > 0.05; Table 2) and colonies undersaturated with O2 were observed under light intensities up to 1000 μmol photons m−2 s−1 (Fig. 2b). A comparison of the measured O2 uptake rates with calculated O2 diffusion rates into the colony revealed that O2 uptake in both light and dark ranged up to the predicted maximum rate allowed by diffusive O2 supply (Fig. 2f). Measurements of chlorophyll fluorescence did not show any spatial patterns of photosynthetic activity along colony transects (data not shown).
Table 2.
Net O2 uptake rates (mean ± SD) by Trichodesmium colonies based on O2 profiles measured at different light intensities at 45 min to 8 h after beginning of the light period
| Light intensity | Net O2 uptake | ||
|---|---|---|---|
| μmol photons m−2 s−1 | nmol O2 colony−1 h−1 | nmol mm−3 h−1 | |
| Dark | 4.0 ± 6.8 | 23 ± 14 | n = 14 |
| 200 | 2.4 ± 2.3 | 24 ± 17 | n = 16 |
| 500 | 1.4 ± 1.8 | 13 ± 8 | n = 8 |
| 1000 | 3.1 ± 3.4 | 15 ± 1 | n = 2 |
Cellular O2 fluxes during the middle of the day
Measurements of O2 fluxes in colonies and single trichomes during late morning/midday using an 18O2‐based MIMS approach yielded net O2 production rates that were often close to zero or negative (Fig. 3), in line with microsensor measurements performed during this part of the day (Fig. 2). The low net photosynthesis was a result of 4–5 times higher gross O2 uptake balancing gross O2 evolution. Dark respiration and light‐dependent O2 uptake (e.g. classical Mehler reaction and/or flavoprotein‐mediated O2 uptake) amounted to 56 ± 31% and 42 ± 63% of gross O2 evolution, respectively. Measurements under different external O2 concentrations mimicking the range observed in the center of colonies (e.g. Fig. 2) showed that gross O2 evolution, dark respiration, and light‐dependent O2 uptake increased with increasing external O2 concentration, whereas net photosynthesis decreased (analysis of variance (ANOVA), P < 0.05; Fig. 3) and was always negative when O2 concentrations above air saturation were applied. Test measurements revealed that these effects of elevated O2 levels were reversible; for instance, gross O2 evolution measured under ambient O2 was not significantly affected by O2 concentration in the preceding light phase (t‐test, P > 0.05, n ≥ 4; data not shown). The relative amount of colonies and free‐floating trichomes did not have consistent effects on O2 fluxes, with higher dark respiration in colonies than free trichomes observed only under ambient O2 (t‐test, P < 0.05, n ≥ 4) and higher total O2 uptake in colonies observed only under low O2 (t‐test, P < 0.05, n ≥ 4).
Figure 3.
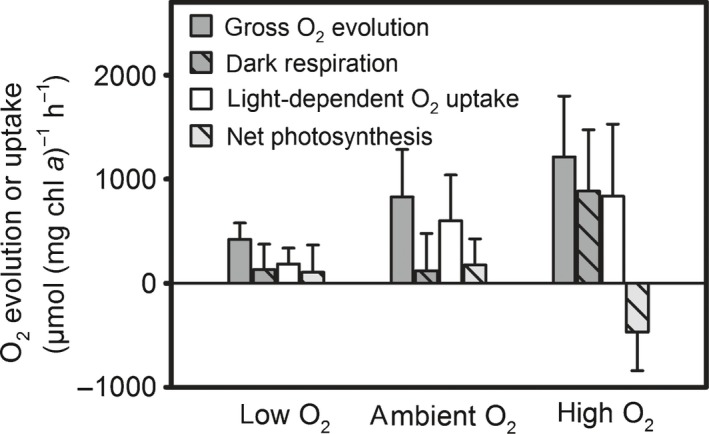
O2 production and uptake rates in Trichodesmium cultures measured during late morning/midday under different external O2 concentrations. Cultures contained both colonies and free trichomes. Note that positive values on the y‐axis indicate rates of both O2 evolving and O2 consuming processes. Light‐dependent O2 uptake is the difference between total O2 uptake measured in the light and total O2 uptake measured in the dark. Low O2, 101 ± 35 μmol l−1; ambient O2, 191 ± 51 μmol l−1; high O2, 346 ± 39 μmol l−1. n = 11 for low and ambient O2, n = 7 for high O2. Error bars ± SD.
O2 on the surface of single trichomes
To analyze variability at the level of single cells, O2 concentrations on the surface of single trichomes were measured with microsensors at a spatial resolution equivalent to c. 2–4 cells (Fig. 4). O2 concentrations on the surface of single trichomes as well as in the surrounding agar never deviated more than c. 30 μmol l−1 from air saturation level. Changes in O2 concentrations on the cell surface when switching between light and dark ranged between 1 and 8 μmol l−1 (2.8 ± 1.8 μmol l−1, recorded in 27 measurements on a total of nine filaments; examples given in Fig. 4a–c). To test comparability of results from this set‐up with those obtained on colonies in the flow system, O2 concentrations were measured on the surface of a trichome located in the periphery of a tuft‐shaped colony, yielding a deviation in light vs dark of 70 μmol l−1 (data not shown). In the light, O2 concentrations on the trichome surface differed by up to 6 μmol l−1 between cells/regions within a single trichome (Fig. 4d–f). Distinct areas with lower than average O2 concentrations were observed in 11 out of the 26 trichomes analyzed. These areas were between 10 and 50 cells long and often located in the middle of trichomes.
Figure 4.
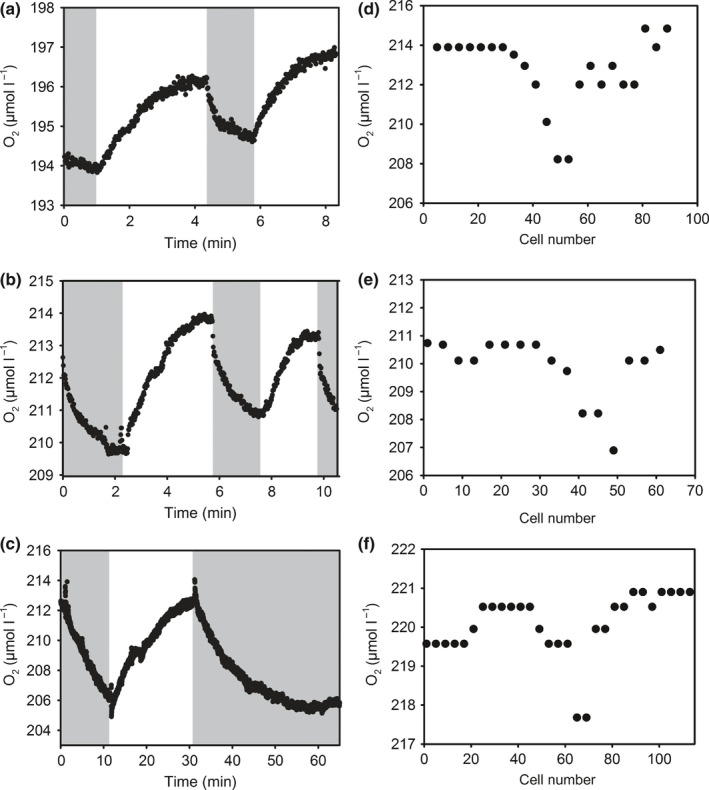
Examples of O2 concentrations measured within c. 3 μm of the surface of single Trichodesmium filaments during consecutive light and dark phases (a–c) and while sliding along the trichome with a microsensor (d–f; total duration of the measurement c. 20 min). Grey shaded areas indicate dark phases.
Effects of O2 concentrations on C and N2 fixation
N2 fixation was strongly O2‐dependent, with a clear inhibition in treatments with air‐saturated medium and medium containing elevated O2 concentrations compared to medium undersaturated in O2 (two‐way ANOVA and Holm‐Sidak test, P < 0.05; Fig. 5a). At low O2 concentrations, representative for minimum O2 levels observed in the colony center (e.g. Fig. 2), N2 fixation was increased by a factor of up to 4 compared to ambient O2 concentrations (Fig. 5a). At elevated O2 concentrations, representative for maximum O2 levels observed in the colony center (e.g. Fig. 2), N2 fixation rates were close to zero (Fig. 5a). Colonies showed lower N2 fixation rates than free‐floating trichomes (two‐way ANOVA, P < 0.05; Fig. 5a). Pair‐wise comparison under each O2 level showed that this effect was only significant under low O2 (Holm−Sidak test, P < 0.05). Similarly, colonies also showed lower C fixation rates than free‐floating trichomes (two‐way ANOVA, P < 0.05; Fig. 5b), with pair‐wise comparison showing a significant difference only under low O2 (Holm‐Sidak test, P < 0.05). C fixation was not significantly affected by the O2 treatment (two‐way ANOVA, P > 0.05). C‐specific O2 evolution was not significantly affected by the O2 treatment and was not different between colonies and free‐floating trichomes (two‐way ANOVA, P > 0.05; Fig. 5c).
Figure 5.
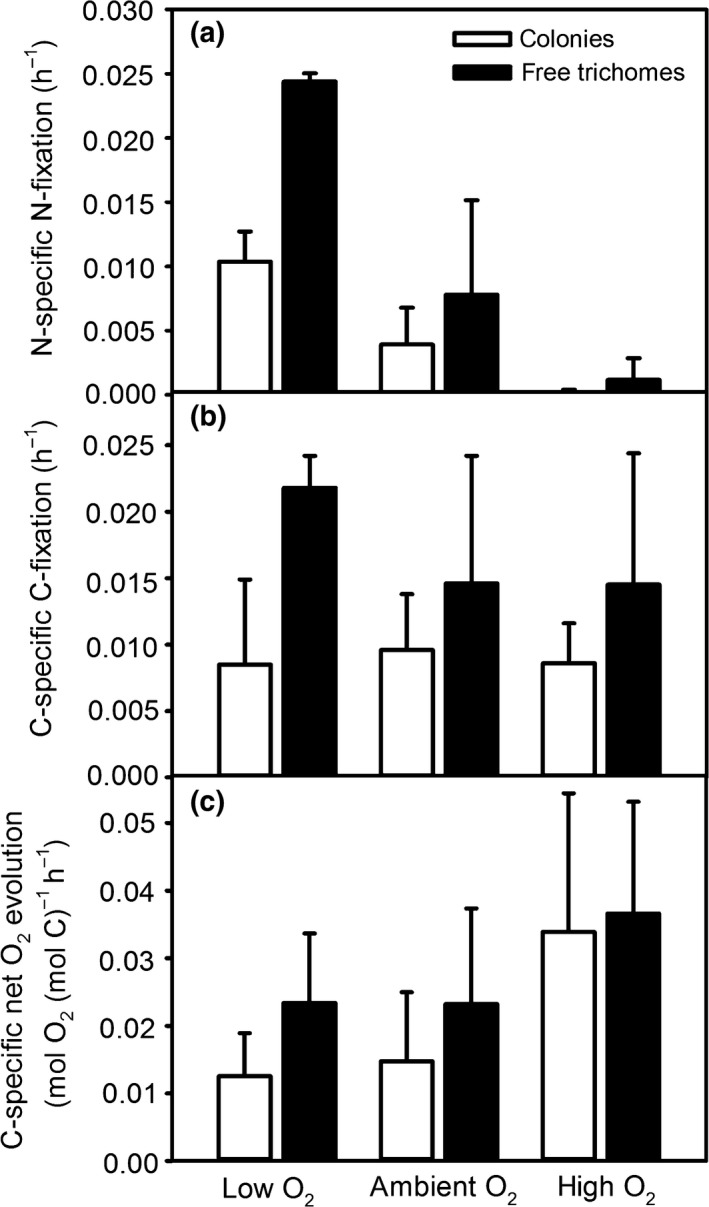
(a) N2 fixation, (b) C fixation and (c) O2 evolution in free‐floating trichomes and colonies of Trichodesmium measured under different O2 concentrations in the bulk medium over the duration of the light phase (12 h). Low O2, 24 ± 11 μmol l−1; ambient O2, 238 ± 13 μmol l−1; high O2, 475 ± 32 μmol l−1. n ≥ 3, except for trichomes at low O2 with n = 2 in (a, b), and colonies at high O2 with n = 2 in (c). Error bars indicate SD.
Discussion
Physiological state of the cultures
Several observations indicate net biomass production in the colony‐forming cultures in this study. Firstly, the increases in pH and phosphate consumption in the medium demonstrate that the cultures were growing (Table 1). Although brief net heterotrophic phases were observed (e.g. first day in Fig. 1a,c), which could potentially be sustained by uptake of dissolved organic matter accumulated in the medium (Benavides et al., 2017), O2 concentrations generally increased over the duration of several days, confirming net autotrophy (Fig. 1). Both free‐floating trichomes and colonies were able to significantly increase N2 fixation rates within a day when exposed to reduced O2 concentrations (Fig. 5a). Colonies formed during the 12 h incubation period had similar N2 fixation rates as older colonies (data not shown), indicating that ‘old’ colonies were not in a senescent state. These findings are in contrast with a previous report attributing low N2 fixation rates in colonies of Trichodesmium NIBB 1067 to colony formation only during stationary growth phase (Ohki & Fujita, 1988).
C : N and C : Chla ratios in particulate organic matter as well as C and N2 fixation rates were in a similar order of magnitude as previous data on field‐collected Trichodesmium colonies (Letelier & Karl, 1998; Eichner et al., 2017). POC : POP ratios were lower than those previously measured in the field (Letelier & Karl, 1998; Orcutt et al., 2013), and similar to P‐replete cultures of Trichodesmium IMS101 (Spungin et al., 2014), indicating that our cultures were not P‐limited. F v/F m, a general indicator for the health status of photosynthetic organisms that is often used to detect iron (Fe) limitation, was higher in our study than previous measurements on free‐floating, exponentially growing trichomes of the same strain (0.44 ± 0.11 (our study) vs 0.18–0.35 (Eichner et al., 2014; measured with a blue LED)), suggesting that colonies were not stressed or severely Fe‐depleted. By contrast with a recent laboratory study by Tzubari et al. (2018), we therefore assumed that, in our experiment, colony formation was not induced by P‐ or Fe‐depletion. As we did not observe any differences in abiotic conditions or cellular composition between free‐floating trichomes and colonies, the triggers for colony formation could not be identified. As cultures were grown under the same (macroscale) conditions, we relate the observed physiological differences to microenvironments within colonies.
Variation over the diel cycle
The downregulation of net photosynthesis during the middle of the day (Figs 1, 2e) is in line with previous observations on Trichodesmium in the field and laboratory (Berman‐Frank et al., 2001; Eichner et al., 2014) and coincides with the peak in N2 fixation observed 4–5 h after the beginning of the light period in Trichodesmium IMS101 (e.g. Kranz et al., 2010; Eichner et al., 2014). The switch from N2 fixation back to photosynthesis in the afternoon was also reflected in higher POC : PON ratios measured at the end of the light period compared with the afternoon (Table 1), in line with previous observations (Kranz et al., 2009). The magnitude of diel variations in O2 was exceptionally large, reaching net O2 uptake during the middle of the day (Figs 1, 2e), whereas previous studies merely showed a reduction of net O2 evolution by 40–70% (Berman‐Frank et al., 2001; Eichner et al., 2014). To understand the physiological mechanisms leading to this net O2 uptake during the N2 fixation phase, we investigated gross O2 fluxes in this period by MIMS.
These measurements revealed a high ratio of gross to net O2 evolution comparable with previous field observations on Trichodesmium (e.g. Kana, 1993), and exceeding previous estimates for free‐floating trichomes of Trichodesmium IMS101 under comparable conditions (Kranz et al., 2010; Eichner et al., 2014). Such high O2 uptake suggests that Trichodesmium decreases O2 concentrations during the day by an upregulation of O2‐consuming processes such as dark respiration, classical Mehler reaction and/or flavoprotein‐mediated O2 uptake, rather than a downregulation of O2 evolution. This way, electron transport through the photosynthetic and respiratory transport chain, and therefore proton translocation, is maintained even through the phase of low net O2 production. This may serve as a means to maintain the production of ATP to support N2 fixation, and is also in line with the relatively high Fv/Fm observed at the same time (Table 1). While O2 uptake by Mehler reaction does not involve carbon fluxes, the high dark respiration rates observed here require the breakdown of a significant amount of carbohydrates produced in the preceding morning hours. For instance, maintaining the dark respiration rates observed at high O2 concentrations (Fig. 3) for 7 h would consume c. 30% of the POC at a typical cell density observed in our cultures (200 μmol C l−1). In Crocosphaera, a similar diel cycle including the build‐up of carbohydrates during the day and their breakdown at night has been proposed to support the high energy demand related to protecting nitrogenase under ambient O2 levels (Großkopf & LaRoche, 2012). In Trichodesmium, energy and carbon budgets may differ substantially on a single‐cell level due to cell specialization, depending on how diazocytes acquire carbohydrates and/or energy equivalents to support respiration and N2 fixation. As the downregulation of net photosynthesis under elevated O2 observed during MIMS measurements in the late morning (Fig. 3) was not observed in 12 h incubations (Fig. 5c), it seems to be a transient effect that is outbalanced on a diurnal time scale. It might represent a regulatory response that induces a decrease in O2 concentrations once a certain threshold is reached during the accumulation of O2 in the phase of high O2 evolution in the morning. Such a switch in metabolism towards higher O2 uptake would ultimately lead to transient O2 undersaturation (Fig. 2a–c) within colonies, allowing for N2 fixation to start during midday.
Impacts of colony formation
Colony formation resulted in pronounced microenvironments with O2 undersaturation during a large part of the day, reaching nearly anoxic conditions in the center of some of the colonies (Fig. 2a–c). However, as colonies in this study were mostly net phototrophic over a period of several days (Fig. 1), the long phase of O2 undersaturation must have been outbalanced by a c. 10 times shorter phase of strong O2 supersaturation. Indeed, microsensor measurements revealed O2 concentrations within colonies of up to 570 μmol l−1 (Figs S1, 2d). Even under air‐equilibrated bulk conditions, O2 concentrations within colonies therefore temporarily exceeded those supplied in the high O2 treatment, where N2 fixation was completely inhibited (Fig. 5a). However, this inhibitory effect of O2 on N2 fixation was reversible, as N2 fixation rates could be increased by exposure to low O2 concentrations (Fig. 5a), in line with previous studies showing recovery of nitrogenase after exposure to high O2 (Zehr et al., 1993). Hence, the lowered N2 fixation in colonies compared with single trichomes is most likely not caused by damage to nitrogenase during the phase of high O2 accumulation in the morning.
However, elevated O2 concentrations may lead not only to inactivation or degradation of O2‐sensitive proteins such as nitrogenase, but also induce damage to other cellular components relevant for both N2 fixation and C fixation, including lipids, proteins and DNA, through a concurrent increase in reactive oxygen species such as superoxide (e.g. Lesser, 2006). In line with this, high superoxide production by field‐collected Trichodesmium colonies has been observed (Hansel et al., 2016). Accumulation of O2 and reactive oxygen species in the colony microenvironment may therefore increase the risk of oxidative stress in colonies compared with single trichomes and might be part of the reason why colonies showed lower N2 and C fixation rates. The high rates of both gross O2 evolution and O2 uptake observed under elevated O2 concentrations (Fig. 3) might place additional strain on the turnover of the photosynthetic machinery during the periods of elevated O2 concentrations in colonies.
Moreover, the high rates of photosynthesis during the morning and evening (Fig. 1) may lead to a temporary depletion of carbon dioxide (CO2) within the colony microenvironment. In line with this, a correlation of 13C composition with colony size has been observed in field‐collected Trichodesmium colonies, which may reflect cellular responses to CO2 limitation in larger colonies (Tchernov & Lipschultz, 2008). In combination with the concurrently elevated O2 concentrations, CO2 depletion is likely to induce photorespiration and therefore lower C fixation rates. While CO2 depletion can be partly compensated by carbon concentrating mechanisms (CCMs) such as the active uptake of bicarbonate (HCO3 −), the elevated energy expenditure for CCMs poses an additional disadvantage for colonies compared with free‐floating trichomes. Diffusion limitation in the colony microenvironment therefore involves several negative effects that may counteract the benefits of temporary O2 depletion for N2 fixation.
Our combined measurements of N2 fixation rates and small‐scale O2 gradients contradict the hypothesis of colony formation as a mechanism to foster N2 fixation by protecting nitrogenase from O2 (Paerl & Bebout, 1988). Instead, we were able to demonstrate that O2 undersaturation in the microenvironment (Fig. 2a–c) during the phase of typically highest nitrogenase activity (e.g. Berman‐Frank et al., 2001; Eichner et al., 2014) did not result in elevated N2 fixation rates in colonies (Fig. 5a). Consequently, we suggest that previous observations of higher or similar N2 fixation rates in colonies compared with free‐floating trichomes in the field (Saino & Hattori, 1982; Letelier & Karl, 1998) were not due to O2 microenvironments, but due to other factors not included in our laboratory setting. These might include positive effects of colony formation on the nutritional status of Trichodesmium that are manifested only under nutrient‐limited conditions in the field, such as enhanced dust dissolution (Rubin et al., 2011) and more efficient interaction with bacterial associates producing siderophores and/or alkaline phosphatase (Chappell & Webb, 2010; Orcutt et al., 2013; Lee et al., 2017). By excluding these additional factors in our laboratory study under nutrient‐replete conditions, we revealed that O2 microenvironments per se did not lead to higher N2 fixation rates in colonies compared with free‐floating trichomes. In addition to microbial interactions and higher nutrient (P and Fe) availability, reduced grazing and efficient vertical movement along light and nutrient gradients (e.g. reviewed by Beardall et al., 2009; Stal, 2017) may contribute to making colony formation a selective advantage in natural ecosystems.
Intracellular O2 concentrations
Ultimately, nitrogenase activity is controlled by intracellular O2 concentrations in the vicinity of the protein rather than extracellular O2 conditions. On the cell surface of single trichomes, O2 concentrations were never below 180 μmol l−1. Hence, assuming that anoxic conditions are required in the vicinity of nitrogenase for it to function, cells must be able to establish a large gradient in O2 concentrations across the cell wall. If cells are relatively impermeable, O2 concentrations may differ strongly between neighboring cells in a trichome, depending on their specific metabolic activity. Our analysis of cell surface O2 concentrations on a single‐cell level revealed regions with reduced O2 concentrations (Fig. 4b). While we cannot exclude that these are senescing or dividing cells, it is interesting to note that their occurrence and preferential localization in the center of single trichomes is also in line with the proposed concept of transiently or permanently nonphotosynthetic diazocytes (e.g. Lin et al., 1998; Berman‐Frank et al., 2001).
To examine variation in intracellular O2 in more detail, we modeled the relation between membrane permeability, intracellular O2 concentrations and respiratory O2 fluxes based on our data, using a model described in Damm et al. (2015; Notes S1). Previous studies examining the interplay of O2 diffusion, consumption and N2 fixation in Trichodesmium assumed either no or very low (1–7 × 10−1 m s−1) diffusion resistance to O2 for Trichodesmium cells (Staal et al., 2003; Milligan et al., 2007). Here, we chose a substantially different approach in that permeability of the cell wall was the ultimate model output. Assuming that nitrogenase can only function under locally anoxic conditions, we calculated the permeability that would be necessary to yield an O2 concentration close to zero in the center of a diazocyte, based on cell dimensions and respiration rates measured by MIMS (Table 3). Since, strictly speaking, respiration cannot be sustained under anoxic conditions, the optimum intracellular O2 concentration is most likely to be slightly above zero, and our model output represents a conservative estimate of the required diffusion resistance. As histological studies on Trichodesmium indicated that thylakoids are distributed relatively homogenously in the cytosol (Siddiqui et al., 1992a) we assumed that there was uniform O2 consumption throughout the cell.
Table 3.
Calculated permeability of the cell wall necessary to support anoxia in the interior of a Trichodesmium cell, based on measured rates of O2 uptake (that is, dark respiration), extracellular O2 concentrations in colonies and cell dimensions (average 5 μm length, 7 μm width)
| Respiration distribution | External O2 (μmol l−1) | Average O2 uptake (fmol cell−1 h−1) | Intensity of O2 consumption (mol s−1 m−3) | Permeability for O2 (m s−1) | Permeability for CO2 (m s−1) |
|---|---|---|---|---|---|
| Homogeneous | 329 | 608 | 1.49 | 4.6 × 10−6 | 3.7 × 10−6 |
| Heterogeneous | 329 | 3648 | 8.96 | 2.9 × 10−5 | 2.3 × 10−5 |
| Homogeneous | 187 | 194 | 0.48 | 2.6 × 10−6 | 2.1 × 10−6 |
| Heterogeneous | 187 | 1164 | 2.86 | 1.6 × 10−5 | 1.3 × 10−5 |
| Homogeneous | 101 | 198 | 0.49 | 4.9 × 10−6 | 3.9 × 10−6 |
| Heterogeneous | 101 | 1188 | 2.92 | 3.1 × 10−5 | 2.4 × 10−5 |
Scenarios with heterogeneous distribution of respiration assume a six‐fold higher respiration rate in diazocytes compared to vegetative cells. Permeability for CO2 was calculated from O2 permeability using a conversion factor of 0.7961 according to Ramsing & Gundersen (1994). For further details on the model, please refer to Supporting Information Notes S1.
Our model calculations revealed that single cells can achieve anoxic conditions in the center with a cell wall permeability for O2 between 2.6 × 10−6 and 4.9 × 10−6 m s−1 if they perform dark respiration at the average rate observed in this study (homogenous respiration, Table 3). Based on a previous study demonstrating single‐cell variation in cytochrome oxidase content that was correlated to nitrogenase content in Trichodesmium thiebautii (Bergman et al., 1993), we also considered the effects of a six‐fold higher respiration rate in diazocytes compared with vegetative cells, yielding somewhat higher permeability estimates between 1.6 × 10−5 m s−1 and 3.1 × 10−5 m s−1 (heterogeneous respiration, Table 3). These estimates exceed a previous one for heterocysts (4 × 10−7 m s−1; Walsby, 1985), in line with the fact that Trichodesmium cell walls lack the thick glycolipid layer that acts as a diffusion barrier in heterocysts. In contrast with eukaryotic cells, plasma membranes of Trichodesmium are surrounded by a peptidoglycan layer, which was also reported to be more pronounced than in other Gram‐negative bacteria (Siddiqui et al., 1992b), and can explain why our estimates for CO2 permeability (calculated from O2 permeability according to Ramsing & Gundersen, 1994; Table 3) are lower than estimates for diatoms (10−3 m s−1; Hopkinson et al., 2011). Carboxysomes, in turn, with their protein shell acting as a diffusion barrier to CO2 (e.g. Rae et al., 2013), are expected to have a lower permeability to CO2 than the plasma membrane and cell wall. Accordingly, our estimates for CO2 permeability exceeded previous estimates for the cyanobacterium Synechococcus that were based on measurements of cellular CO2 efflux and therefore represent an integrated estimate for the cell wall and carboxysome (10−6–10−7 m s−1, Badger et al., 1985; 10−8 m s−1, Salon et al., 1996).
As there are no indications for differences in membrane permeability between individual Trichodesmium cells, anoxic conditions in diazocytes must be accompanied by O2 accumulation in photosynthesizing cells. Hence, while low permeability favors N2 fixation in diazotrophic (nonphotosynthetic) cells, it also increases the risk for photorespiration and oxidative stress in vegetative (photosynthetic) cells. Applying membrane permeabilities between 2.6 × 10−6 and 4.9 × 10−6 m s−1 (calculated for diazocytes as described above; Table 3) and a photosynthesis rate of 1013 fmol cell−1 h−1 (based on an O2 profile measured 10 h into the light period; Fig. 2d), we calculated intracellular O2 concentrations representative for the net O2 evolution phase in the morning/evening, yielding concentrations between 623 and 1166 μmol l−1 (Table 4). Intracellular O2 concentrations can therefore differ strongly between single cells, depending on their instantaneous rates of photosynthesis and respiration. These rates may change significantly not only over the diel cycle but potentially also as cells switch between N2‐fixing and non‐N2‐fixing states. The unexpected, transient nighttime increase in O2 concentrations observed with microsensors within colonies (Fig. S1) and with optodes in the bulk medium in closed vials (Fig. 1b) furthermore suggests that intracellular O2 dynamics may additionally be influenced by intracellular O2 reservoirs and/or chemical binding of O2, which should be targeted in future research.
Table 4.
Calculated intracellular O2 concentrations in Trichodesmium under net O2 producing conditions (representative of early morning or evening), based on measured rates of O2 production, extracellular O2 concentrations in colonies, cell dimensions (5 μm length, 7 μm width), and membrane permeability calculated for diazocytes with an anoxic cell interior (Table 3)
| Net O2 production (fmol cell−1 h−1) | External O2 concentration (μmol l−1) | Intensity of O2 production (mol s−1 m−3) | Permeability for O2 (m s−1) | Intracellular O2 concentration (μmol l−1) |
|---|---|---|---|---|
| 1031 | 329 | 2.53 | 4.6 × 10−6 | 884 |
| 1031 | 187 | 2.53 | 2.6 × 10−6 | 1166 |
| 1031 | 101 | 2.53 | 4.9 × 10−6 | 623 |
Cell‐specific O2 production was calculated from a colony‐specific measurement assuming 10 000 cells per colony. For further details on the model, please refer to Supporting Information Notes S1.
Notably, our model calculations demonstrate that O2 undersaturation on the cell surface is not a prerequisite for anoxic conditions in diazocytes. In fact, anoxic conditions could be achieved within nonphotosynthetic cells even under elevated ambient O2 concentrations (Table 3). Colony formation as a means to form anoxic microenvironments is therefore not required, explaining why also single trichomes can fix N2 at high rates (Fig. 5a). More generally, our model results highlight that due to the low permeability of the cell wall, intracellular O2 concentrations in Trichodesmium are largely determined by cellular O2 fluxes rather than extracellular microenvironments. Importantly, extracellular O2 microenvironments can, however, indirectly affect intracellular conditions by inducing physiological responses of cellular O2 fluxes to external O2 concentrations (Fig. 3).
Conclusions
In summary, our measurements and model calculations demonstrate strong variations in O2 concentrations in colony microenvironments and also indicate large variations within single cells (Fig. 6). These were induced by changes in the metabolic activity of Trichodesmium over the diel cycle, including high O2 uptake during midday. Although colonies were undersaturated with O2 during most of the day, this did not result in higher N2 fixation rates by colonies compared with single trichomes, potentially as the O2 undersaturation over part of the day was accompanied by high extra‐ and intracellular O2 concentrations during a short time window in the morning and evening. Based on our model calculations and high resolution microsensor measurements, we hypothesize that the diffusion barrier provided by the cell wall in combination with physiological heterogeneity between single cells allows for anoxic conditions within diazocytes, that is, respiring, nonphotosynthetic cells, under air‐saturated bulk conditions (Fig. 6). Hence, O2 undersaturation in colony microenvironments is not required to support N2 fixation. Future research should therefore focus on the benefits of colony formation in Trichodesmium that outweigh the negative effects on C and N2 fixation.
Figure 6.
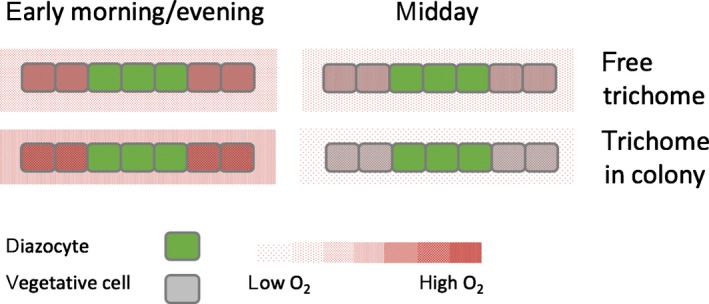
Conceptual model of intra‐ and extracellular O2 concentrations in Trichodesmium at different times of the day. Note that (nonphotosynthetic) diazocytes are always anoxic, while O2 concentrations in vegetative (photosynthetic) cells vary with time of the day and morphology (colony vs free trichomes). Estimates are based on O2 measurements (by microsensors and membrane inlet mass spectrometry (MIMS)) and model calculations, assuming the same photosynthesis rate and permeability for all cells. Red pattern in and around cells indicates O2 concentration, grey and green backgrounds indicate vegetative cells and diazocytes, respectively.
Author contributions
ME, BR, WM and DdB designed the study, ME and SA performed the experiments and analyzed the data, ST performed the model calculations, ME, ST, BR, WM, SA, HP, MMMK and DdB discussed data interpretation and wrote the manuscript.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 O2 concentrations recorded within Trichodesmium colonies over several hours.
Notes S1 Calculation of the diffusion resistance to oxygen.
Acknowledgements
We thank the technical assistants in the microsensor group for construction of microsensors and Gabriele Klockgether, Philip Hach and Clarissa Karthäuser for help with EA‐IRMS, 13C‐DIC and MIMS measurements. We also thank Laura Wischnewsky for phosphate measurements, as well as Tong Li and Dirk Koopmanns for helpful discussions. Funding was provided by the Alexander von Humboldt Foundation and the Max Planck Society.
References
- Badger MR, Bassett M, Comins HN. 1985. A model for HCO3 − accumulation and photosynthesis in the cyanobacterium Synechococcus sp. Theoretical predictions and experimental observations. Plant Physiology 77: 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardall J, Allen D, Bragg J, Finkel Z, Flynn K, Quigg A, Rees TAV, Richardson A, Raven JA. 2009. Allometry and stoichiometry of unicellular, colonial and multicellular phytoplankton. New Phytologist 181: 295–309. [DOI] [PubMed] [Google Scholar]
- Benavides M, Berthelot H, Duhamel S, Reimbault P, Bonnet S. 2017. Dissolved organic matter uptake by Trichodesmium in the Southwest Pacific. Scientific Reports 7: 41315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman B, Carpenter EJ. 1991. Nitrogenase confined to randomly distributed trichomes in the marine cyanobacterium Trichodesmium thiebautii . Journal of Phycology 27: 158–165. [Google Scholar]
- Bergman B, Siddiqui PJA, Carpenter EJ, Peschek GA. 1993. Cytochrome oxidase: subcellular distribution and relationship to nitrogenase expression in the nonheterocystous marine cyanobacterium Trichodesmium thiebautii . Applied and Environmental Microbiology 59: 3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman‐Frank I, Chen Y‐B, Gao Y, Fennel K, Follows MJ, Milligan AJ, Falkowski PG. 2008. Feedbacks between the nitrogen, carbon and oxygen cycles In: Capone DG, Bronk DA, Mulholland MR, Carpenter EJ, eds. Nitrogen in the marine environment, 2nd edn Amsterdam, the Netherlands: Elsevier Inc., 1537–1563. [Google Scholar]
- Berman‐Frank I, Chen YB, Gerchman Y, Dismukes GC, Falkowski PG. 2005. Inhibition of nitrogenase by oxygen in marine cyanobacteria controls the global nitrogen and oxygen cycles. Biogeosciences Discussion 2: 261–273. [Google Scholar]
- Berman‐Frank I, Lundgren P, Chen YB, Küpper H, Kolber Z, Bergman B, Falkowski P. 2001. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium . Science 294: 1534–1537. [DOI] [PubMed] [Google Scholar]
- Broecker WS, Peng TH. 1974. Gas exchange rates between air and sea. Tellus 26: 21–35. [Google Scholar]
- Burgess BK, Lowe DJ. 1996. Mechanism of molybdenum nitrogenase. Chemical Reviews 96: 2983–3012. [DOI] [PubMed] [Google Scholar]
- Carpenter EJ, Chang J, Cottrell M, Schubauer J, Paerl HW, Bebout BM, Capone DG. 1990. Reevaluation of nitrogenase oxygen‐protective mechanisms in the planktonic marine cyanobacterium Trichodesmium . Marine Ecology Progress Series 65: 151–158. [Google Scholar]
- Chappell PD, Webb EA. 2010. A molecular assessment of the iron stress response in the two phylogenetic clades of Trichodesmium . Environmental Microbiology 12: 13–27. [DOI] [PubMed] [Google Scholar]
- Chen Y‐B, Zehr JP, Mellon M. 1996. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101 in defined media: evidence for a circadian rhythm. Journal of Phycology 32: 916–923. [Google Scholar]
- Damm E, Thoms S, Beszczynska‐Möller A, Nöthig EM, Kattner G. 2015. Methane excess production in oxygen‐rich polar water and a model of cellular conditions for this paradox. Polar Science 9: 327–334. [Google Scholar]
- Durner J, Böhm I, Knörzer OC, Böger P. 1996. Proteolytic degradation of dinitrogenase reductase from Anabaena variabilis (ATCC 29413) as a consequence of ATP depletion and impact of oxygen. Journal of Bacteriology 178: 606–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner MJ, Klawonn I, Wilson ST, Littmann S, Whitehouse MJ, Church MJ, Kuypers MMM, Karl DA, Ploug H. 2017. Chemical microenvironments and single‐cell carbon and nitrogen uptake in field‐collected colonies of Trichodesmium under different pCO2 . ISME Journal 11: 1305–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M, Kranz SA, Rost B. 2014. Combined effects of different CO2 levels and N sources on the diazotrophic cyanobacterium Trichodesmium . Physiologia Plantarum 152: 316–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi‐Hart JA, Pett‐Ridge J, Weber PK, Popa R, Fallon SJ, Gunderson T, Capone DG. 2009. Fixation and fate of C and N in the cyanobacterium Trichodesmium using nanometer‐scale secondary ion mass spectrometry. Proceedings of the National Academy of Sciences, USA 106: 6345–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fock HP, Sültemeyer DF. 1989. O2 evolution and uptake measurements in plant cells by mass spectrometer In: Liskens HF, Jackson JF, eds. Modern methods of plant analysis, vol. 9. Heidelberg, Germany: Springer‐Verlag, 3–18. [Google Scholar]
- Fredriksson C, Bergman B. 1995. Nitrogenase quantity varies diurnally in a subset of cells within colonies of the non‐heterocystous cyanobacteria Trichodesmium spp. Microbiology 141: 2471–2478. [Google Scholar]
- Gallon JR. 1992. Reconciling the incompatible: N2 fixation and O2 . New Phytologist 122: 571–609. [Google Scholar]
- Großkopf T, LaRoche J. 2012. Direct and indirect costs of dinitrogen fixation in Crocosphaera watsonii WH8501 and possible implications for the nitrogen cycle. Frontiers in Microbiology 3: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel CM, Buchwald C, Diaz JM, Ossolinski JE, Dyhrman ST, Van Mooy BAS, Polyviou D. 2016. Dynamics of extracellular superoxide production by Trichodesmium colonies from the Sargasso Sea. Limnology and Oceanography 61: 1188–1200. [Google Scholar]
- Hansen HP, Koroleff F. 1999. Determination of nutrients In: Grasshoff K, Kremling K, Ehrhardt M, eds. Methods of seawater analysis. Weinheim, Germany: Wiley‐VCH, 159–228. [Google Scholar]
- Holm‐Hansen O, Riemann B. 1978. Chlorophyll a determination: improvements in methodology. Oikos 30: 438–447. [Google Scholar]
- Hopkinson BM, Dupont CL, Allen AE, Morel FMM. 2011. Efficiency of the CO2‐concentrating mechanism of diatoms. Proceedings of the National Academy of Sciences, USA 108: 3830–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana TM. 1993. Rapid oxygen cycling in Trichodesmium thiebautii . Limnology and Oceanography 38: 18–24. [Google Scholar]
- Kranz SA, Levitan O, Richter KU, Prášil O, Berman‐Frank I, Rost B. 2010. Combined effects of CO2 and light on the N2‐fixing cyanobacterium Trichodesmium IMS101: physiological responses. Plant Physiology 154: 334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz SA, Sültemeyer D, Richter K‐U, Rost B. 2009. Carbon acquisition in Trichodesmium: the effect of pCO2 and diurnal changes. Limnology and Oceanography 54: 548–559. [Google Scholar]
- Küpper H, Andresen E, Wiegert S, Šimek M, Leitenmaier B, Šetlík I. 2009. Reversible coupling of individual phycobiliprotein isoforms during state transitions in the cyanobacterium Trichodesmium analysed by single‐cell fluorescence kinetic measurements. Biochimica et Biophysica Acta (BBA)‐Bioenergetics 1787: 155–167. [DOI] [PubMed] [Google Scholar]
- Küpper H, Ferimazov N, Šetlík I, Berman‐Frank I. 2004. Traffic lights in Trichodesmium: regulation of photosynthesis for nitrogen fixation studied by chlorophyll fluorescence kinetic microscopy. Plant Physiology 135: 2120–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MD, Walworth NG, McParland EL, Fu F‐X, Mincer TJ, Levine NM, Hutchins DA, Webb EA. 2017. The Trichodesmium consortium: conserved heterotrophic co‐occurrence and genomic signatures of potential interactions. ISME Journal 11: 1813–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser MP. 2006. Oxidative stress in marine environments: biochemistry and physiological ecology. Annual Reviews in Physiology 68: 253–278. [DOI] [PubMed] [Google Scholar]
- Letelier RM, Karl DM. 1998. Trichodesmium spp. physiology and nutrient fluxes in the North Pacific subtropical gyre. Aquatic Microbial Ecology 15: 265–276. [Google Scholar]
- Lin S, Henze S, Lundgren P, Bergman B, Carpenter EJ. 1998. Whole‐cell immunolocalization of nitrogenase in marine diazotrophic cyanobacteria, Trichodesmium spp. Applied and Environmental Microbiology 64: 3052–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan AJ, Berman‐Frank I, Gerchman Y, Dismukes GC, Falkowski PG. 2007. Light‐dependent oxygen consumption in nitrogen‐fixing cyanobacteria plays a key role in nitrogenase protection. Journal of Phycology 43: 845–852. [Google Scholar]
- Ohki K, Fujita Y. 1988. Aerobic nitrogenase activity measured as acetylene reduction in the marine non‐heterocystous cyanobacterium Trichodesmium spp. grown under artificial conditions. Marine Biology 98: 111–114. [Google Scholar]
- Ohki K, Taniuchi Y. 2009. Detection of nitrogenase in individual cells of a natural population of Trichodesmium using immunocytochemical methods for fluorescent cells. Journal of Oceanography 65: 427–432. [Google Scholar]
- Orcutt KM, Gundersen K, Ammerman JW. 2013. Intense ectoenzyme activities associated with Trichodesmium colonies in the Sargasso Sea. Marine Ecology Progress Series 478: 101–113. [Google Scholar]
- Paerl HW, Bebout BM. 1988. Direct measurement of O2‐depleted microzones in marine Oscillatoria: relation to N2 fixation. Science 241: 442–445. [DOI] [PubMed] [Google Scholar]
- Ploug H, Jørgensen BB. 1999. A net‐jet flow system for mass transfer and microsensor studies of sinking aggregates. Marine Ecology Progress Series 176: 279–290. [Google Scholar]
- Ploug H, Kühl M, Buchholz‐Cleven B, Jørgensen BB. 1997. Anoxic aggregates – an ephemeral phenomenon in the pelagic environment? Aquatic Microbial Ecology 13: 285–294. [Google Scholar]
- Ploug H, Passow U. 2007. Direct measurement of diffusivity within diatom aggregates containing transparent exopolymer particles. Limnology and Oceanography 52: 1–6. [Google Scholar]
- Rae BD, Long BM, Badger MR, Price GD. 2013. Functions, compositions, and evolution of the two types of carboxysomes: polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiology and Molecular Biology Reviews 77: 357–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsing N, Gundersen J. 1994. Seawater and Gases: Tabulated physical parameters of interest to people working with microsensors in marine systems, Techn Rep MPI Mar Microbiology Bremen. [WWW document] URL http://www.unisense.com/files/PDF/Diverse/Seawater%20&%20Gases%20table.pdf [accessed 2 October 2018].
- Rubin M, Berman‐Frank I, Shaked Y. 2011. Dust‐and mineral‐iron utilization by the marine dinitrogen‐fixer Trichodesmium . Nature Geoscience 4: 529–534. [Google Scholar]
- Saino T, Hattori A. 1982. Aerobic nitrogen fixation by the marine non‐heterocystous cyanobacterium Trichodesmium (Oscillatoria) spp.: its protective mechanism against oxygen. Marine Biology 70: 251–254. [Google Scholar]
- Salon C, Mir NA, Canvin DT. 1996. Influx and efflux of inorganic carbon in Synechococcus UTEX625. Plant, Cell & Environment 19: 247–259. [Google Scholar]
- Siddiqui PJA, Carpenter EJ, Bergman B. 1992a. Ultrastructure and immunolocalization of phycobiliproteins and Ribulose‐1,5‐bisphosphate carboxylase/oxygenase in the marine cyanobacterium Trichodesmium thiebautii . Journal of Phycology 28: 320–327. [Google Scholar]
- Siddiqui PJ, Carpenter EJ, Bergman B. 1992b. Trichodesmium: ultrastructure and protein localization In: Carpenter EJ, Capone DG, Rueter JG, eds. Marine pelagic cyanobacteria: Trichodesmium and other diazotrophs. Dordrecht, the Netherlands: Springer Netherlands, 9–28. [Google Scholar]
- Spungin D, Berman‐Frank I, Levitan O. 2014. Trichodesmium's strategies to alleviate phosphorus limitation in the future acidified oceans. Environmental Microbiology 16: 1935–1947. [DOI] [PubMed] [Google Scholar]
- Staal M, Meysman FJ, Stal LJ. 2003. Temperature excludes N2‐fixing heterocystous cyanobacteria in the tropical oceans. Nature 425: 504–507. [DOI] [PubMed] [Google Scholar]
- Staal M, Rabouille S, Stal LJ. 2007. On the role of oxygen for nitrogen fixation in the marine cyanobacterium Trichodesmium sp. Environmental Microbiology 9: 727–736. [DOI] [PubMed] [Google Scholar]
- Stal LJ. 2017. Gregarious cyanobacteria. Environmental Microbiology 19: 2105–2109. [DOI] [PubMed] [Google Scholar]
- Tchernov D, Lipschultz F. 2008. Carbon isotopic composition of Trichodesmium spp. colonies off Bermuda: effects of colony mass and season. Journal of Plankton Research 30: 21–31. [Google Scholar]
- Tzubari Y, Magnezi L, Be'er A, Berman‐Frank I. 2018. Iron and phosphorus deprivation induce sociality in the marine bloom‐forming cyanobacterium Trichodesmium . ISME Journal 12: 1682–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby AE. 1985. The permeability of heterocysts to the gases nitrogen and oxygen. Proceedings of the Royal Society of London. Series B, Biological Sciences 226: 345–366. [Google Scholar]
- Zehr JP, Wyman M, Miller V, Duguay L, Capone DG. 1993. Modification of the Fe protein of nitrogenase in natural populations of Trichodesmium thiebautii . Applied and Environmental Microbiology 59: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 O2 concentrations recorded within Trichodesmium colonies over several hours.
Notes S1 Calculation of the diffusion resistance to oxygen.


