Abstract
Aims
Enrollment criteria vary substantially among cardiovascular outcome trials (CVOTs) of sodium‐glucose cotransporter‐2 inhibitors (SGLT‐2is), which impacts the relationship between a trial population and the general type 2 diabetes (T2D) population. The aim of this study was to evaluate the representativeness of four SGLT‐2i CVOTs of a general T2D population.
Methods
T2D patients from Germany, The Netherlands, Norway and Sweden were included in the study. Given the available data per country, key inclusion and exclusion criteria were defined by diagnoses, procedures and drug treatments to facilitate comparability among countries. Representativeness was determined by dividing the number of patients fulfilling the key enrolment criteria of each CVOT (CANVAS, DECLARE‐TIMI 58, EMPA‐REG OUTCOME, VERTIS‐CV) by the total T2D population.
Results
In 2015, a total T2D population of 803 836 patients was identified in Germany (n = 239 485), in The Netherlands (n = 36 213), in Norway (n = 149 782) and in Sweden (n = 378 356). These populations showed a 25% to 44% cardiovascular (CV) disease baseline prevalence and high CV‐preventive drug use (>80%). The general T2D population had less prevalent CV disease and patients were slightly older than those included in the CVOTs. The DECLARE‐TIMI 58 trial had the highest representativeness, 59% compared to the general T2D population, and this representativeness was almost 2‐, 3‐ and 4‐fold higher compared to the CANVAS (34%), EMPA‐REG OUTCOME (21%) and VERTIS‐CV (17%) trials, respectively.
Conclusions
In large T2D populations within Europe, consistent patterns of representativeness of CVOTs were found when applying the main enrolment criteria. The DECLARE‐TMI 58 trial had the highest representativeness, indicating that it included and examined patients who are most representative of the general T2D patients in the studied countries.
Keywords: cardiovascular disease, cardiovascular outcome trial, observational study, representativeness, SGLT2 inhibitors
1. INTRODUCTION
Patients with type 2 diabetes are at increased risk of mortality and cardiovascular (CV) disease.1 Recent cardiovascular outcome trials (CVOTs) have shown clinically important results in reducing CV risk when using sodium‐glucose cotransporter‐2 inhibitors (SGLT‐2is), and further studies are upcoming.2, 3, 4, 5, 6 The generalizability of the results of clinical trials to common clinical practice is recognized as a major issue. This is often evaluated as external validity of the study as it is important in clinical decision making and in implementation of new clinical guidelines. As enrolment criteria vary among SGLT2‐i CVOTs, it is expected that they will have an impact on the external validity of the results. An understanding of external validity is particularly important as the recently updated ADA/EASD position statement concerning glucose‐lowering therapy in patients with T2D has strengthened the position of SGLT‐2is.7 Many CVOTs include patients with a high risk of CV and with an expected high rate of CV events, to ensure that differences in CV outcomes may be reported with sufficient statistical power. As a consequence, various degrees of strict CVOT patient enrolment criteria may impact the representativeness of the trial population of a general T2D population and, thus, may also impact the external validity of study results.8, 9
Four CVOTs that examined treatment with SGLT‐2is are within the scope of this study.3, 4, 5, 6 Major differences exist among these CVOTs concerning inclusion criteria. Two of the trials included only patients with established CV disease (EMPA‐REG OUTCOME5 and VERTIS‐CV10), while the other two trials also included patients with additional CV risk factors (DECLARE‐TIMI 58,6 ≥ 2 CV risk factors, and CANVAS,4 ≥ 3 CV risk factors).
The aim of this analysis was to evaluate the representativeness of four SGLT‐2i CVOTs (CANVAS, EMPA‐REG OUTCOME, VERTIS‐CV, DECLARE‐TIMI 58) by applying the respective main inclusion and exclusion criteria to a general T2D population within four European countries.
2. MATERIALS AND METHODS
2.1. Data sources
The present study is part of a large‐scale diabetes investigation initiative to acquire an understanding of T2D and its treatment with drugs.11 The unique features of available health care registries and the corresponding secondary data from health care systems (claims data from Germany, electronic health care records data from The Netherlands, full population registry data from Norway and Sweden) were utilized, in order to include all T2D patients who filled prescriptions for glucose‐lowering drugs.11 For a detailed description of data sources, see Online Supplemental Appendix, Section 1.
2.1.1. Germany
Data from Germany were obtained from the Betriebskrankenkassen (BKK), a sickness‐fund database consisting of up to 4.9 million insured individuals who are covered by statutory health insurance (Online Supplemental Appendix 1.1). The BKK includes routine billing data from 2007 to 2015 and includes core data concerning the insured individual and full billing information concerning the hospital health services utilized, the ambulatory sector and pharmaceuticals. Information concerning filled drug prescriptions and hospital visits is updated on a daily basis and primary care visits are updated on a quarterly basis. Thus, patients were described on a quarterly basis. Patient data were fully anonymized before analyses were performed by TeamGesundheit Gesellschaft für Gesundheitsmanagement mbH, Essen, Germany. All required study approvals were obtained.
2.1.2. The Netherlands
Data for the study were obtained from the PHARMO Database Network in The Netherlands (Online Supplemental Appendix 1.2). This population‐based network of electronic healthcare databases combines and links, through validated algorithms, patient‐level data from different primary and secondary healthcare settings, including data from general practices, in‐ and out‐patient pharmacies, clinical laboratories, hospitals and the cancer, pathology and perinatal registries. Detailed information on the methodology and the validation of the record linkage method has been described previously.12, 13
2.1.3. Norway and Sweden
Both Norway and Sweden have comprehensive, nationwide public health care systems (Online Supplemental Appendix 1.3–S14).14, 15 All citizens have a unique personal identification number (PIN), which is mandatory for all administrative purposes, including any contact with the health‐care system, as well as drug purchases, thus providing a comprehensive medical history of the population. Individual patient‐level data from the Prescribed Drug Registers, the Cause of Death Registers and the National Patient Registers covering all hospitalizations with discharge diagnoses and all out‐patient hospital visits, were linked using the PIN. The linked databases were separately managed by Statisticon AB (Uppsala, Sweden).
In Norway (DAPHNE study database), the study protocol was approved by the Regional Ethics Committee, Helse Sør‐Øst (reference number 2015/1337/REK sør‐øst A) and was authorized by the Norwegian Data Inspectorate (Datatilsynet). In Sweden (DAISY study database), the study protocol was approved by the Stockholm Regional Ethics Committee (reference number 2013/2206‐31), with data linkage performed by the Swedish National Board of Health and Welfare.
2.2. Study population
All patients who were using glucose‐lowering drugs within the year prior to 31 December 2015 or during the last quarter of 2015 in Germany, were included (Online Supplemental Appendix 1.2).
2.2.1. Baseline data
Patient baseline data included characteristics (eg, age and sex), and comorbidities retrieved from all available data prior to and including the index date, with the exception of cancer (within 5 years prior to the index date). Prior medications were defined as any dispensed 12 months (3 months in the Netherlands) prior to and including the index date.
2.2.2. Inclusion and exclusion criteria
Adaptation of the inclusion and exclusion criteria of the CVOTs to the respective countries' health care data was performed by using detailed diagnostic codes, procedure codes and drug codes (Table S1a–S1d, Online Supplemental Appendix). Criteria were adapted to the codes similarly in all countries. The main difference between the included CVOTs concerns inclusion criteria; the EMPA‐REG OUTCOME5 and VERTIS‐CV3 trials included only patients with established CV disease, while the DECLARE‐TIMI 5816 and CANVAS4 trials also included patients with CV risk factors only (DECLARE‐TIMI 58, ≥2 and CANVAS, ≥3).
2.2.3. Outcome
Representativeness was determined by dividing the number of patients fulfilling the four CVOT key inclusion and exclusion criteria by the total enrolled T2D population.
2.2.4. Statistical analysis
Demographic data are presented as mean (SD) or n (%). No statistical comparisons were performed among country results. All analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina) or R statistical software (R version 3.1.1 or 3.2.3).17
3. RESULTS
3.1. Baseline
In total, a T2D population of 803 836 patients was identified in Germany (n = 239 485), in The Netherlands (n = 36 213), in Norway, (n = 149 782) and in Sweden (n = 378 356) (Table 1). The use of metformin was high in all countries (67%–85%). The use of newer oral glucose‐lowering drugs (DPP‐4i, SGLT‐2i and GLP‐1RA) was the highest in Germany and Norway, whereas use of sulphonylurea was the highest in The Netherlands and use of insulin was the highest in Sweden. All four populations had similar CV disease profiles at baseline, with a prevalence ranging from 25% in Norway to 44% in Germany. The use of CV preventive drug treatment was similarly high, more than 80%, in all countries.
Table 1.
Baseline description of general type 2 diabetes populations in 2015
| Germany | The Netherlands | Norway | Sweden | |
|---|---|---|---|---|
| N = 239 485 | N = 36 213 | N = 149 782 | N = 378 356 | |
| Age, years (SD) | 67.7 (13.0) | 68.6 (11.5) | 64.1 (13.4) | 67.5 (12.3) |
| Sex (female) | 105 406 (44.0) | 16 571 (45.8) | 64 207 (42.9) | 158 030 (41.8) |
| First GLD, years (SD) | 6.1 (2.8) | 5.3 (3.6) | 6.7 (3.8) | 6.8 (4.0) |
| CV disease | 104 547 (43.7) | 12 594 (34.8) | 37 547 (25.1) | 118 852 (31.4) |
| Myocardial infarction | 28 671 (12.0) | 3752 (10.4) | 10 647 (7.1) | 41 444 (11.0) |
| Unstable angina | 13 312 (5.6) | 1428 (3.9) | 5391 (3.6) | 20 843 (5.5) |
| Heart failure | 64 956 (27.1) | 2274 (6.3) | 10 063 (6.7) | 35 980 (9.5) |
| Stroke | 23 566 (9.8) | 3491 (9.6) | 7499 (5.0) | 33 560 (8.9) |
| Peripheral artery disease | 35 831 (15.0) | 3715 (10.3) | 11 769 (7.9) | 24 338 (6.4) |
| Chronic kidney disease | 30 746 (12.8) | 6078 (20.4)a | 7915 (5.3) | 14 856 (3.9) |
| Cancerb | 42 386 (17.7) | 5051 (13.9) | 18 903 (12.6) | 71 158 (18.8) |
| Metformin use | 160 094 (66.8) | 30 831 (85.1) | 110 600 (73.8) | 294 704 (77.9) |
| Sulphonylurea use | 32 941 (13.8) | 15 516 (42.8) | 32 832 (21.9) | 55 030 (14.5) |
| DPP‐4i use | 59 644 (24.9) | 2373 (6.6) | 20 157 (13.5) | 48 365 (12.8) |
| SGLT‐2i use | 8708 (3.6) | 219 (0.6) | 11 572 (7.7%) | 14 048 (3.7) |
| GLP‐1RA use | 4267 (1.8) | 507 (1.4) | 10 290 (6.9%) | 22 949 (6.1) |
| Metiglinides use | 6783 (2.8) | 0 (0) | 183 (0.1) | 13 836 (3.7) |
| Thiazolidinediones use | 212 (0.1) | 273 (0.8) | 1513 (1.0) | 2944 (0.8) |
| Insulin use | 68 055 (28.4) | 9532 (26.3) | 34 182 (22.8) | 135 027 (35.7) |
| CV preventive drug use | 202 022 (84.4) | 31 868 (88.0) | 123 222 (82.3) | 335 910 (88.8) |
| Low‐dose aspirin use | 34 869 (14.6) | 8183 (22.6) | 56 726 (37.9) | 120 218 (31.8) |
| Statins use | 102 527 (42.8) | 24 912 (68.8) | 87 784 (58.6) | 243 862 (64.5) |
| Antihypertensives use | 192 832 (80.5) | 26 750 (73.9) | 105 220 (70.2) | 298 238 (78.8) |
| Receptor P2Y12 antagonists use | 11 861 (5.0) | 2,356 (6.5) | 6265 (4.2) | 21 190 (5.6) |
Abbreviations: CV, cardiovascular; DPP‐4i, dipeptidyl‐peptidase‐4 inhibitors; GLP‐1RA, glucagon‐like peptide‐1 receptor agonists; SGLT‐2i, sodium‐glucose‐cotransporter‐2 inhibitors.
In The Netherlands, chronic kidney disease was defined by both diagnosis and laboratory kidney function.
Cancer diagnosis within five years prior to index.
When comparing the four European T2D populations with the respective CVOT patients (Table 2), the general T2D patients were slightly older, were more often women, and had less prevalent CV disease.
Table 2.
Characteristics of patients in the cardiovascular outcome trials and a general T2D population from four European countries
| DECLARE‐TIMI 58 (dapagliflozin) References 6, 16 | CANVAS (canagliflozin) Reference 3 | EMPA‐REG OUTCOME (empagliflozin) Reference 5 | VERTIS‐CV (ertugliflozin) References 3 | General T2D population | |
|---|---|---|---|---|---|
| Number of patients | 17 160 | 10 142 | 7020 | 8237 | 803 836 |
| Age, years | 63.8 | 63.3 | 63.1 | 64.4 | 67.0 |
| Sex, female | 37% | 36% | 29% | 30% | 43% |
| Cardiovascular disease | 41% | 66% | 99% | 99% | 34% |
| Heart failure | 10% | 14% | 10% | 22% | 14% |
3.2. Representativeness results
The DECLARE‐TIMI 58 trial showed the highest representativeness (59%), followed by the CANVAS (34%), EMPA‐REG OUTCOME (21%) and VERTIS‐CV (17%) trials (Figure 1). Compared to the other CVOTs, representativeness in the DECLARE‐TIMI 58 trial was 2‐, 3‐ and 4‐fold, respectively. Representativeness results were consistent across all four countries (Figure 2 and Table S2, Online Supplemental Appendix).
Figure 1.
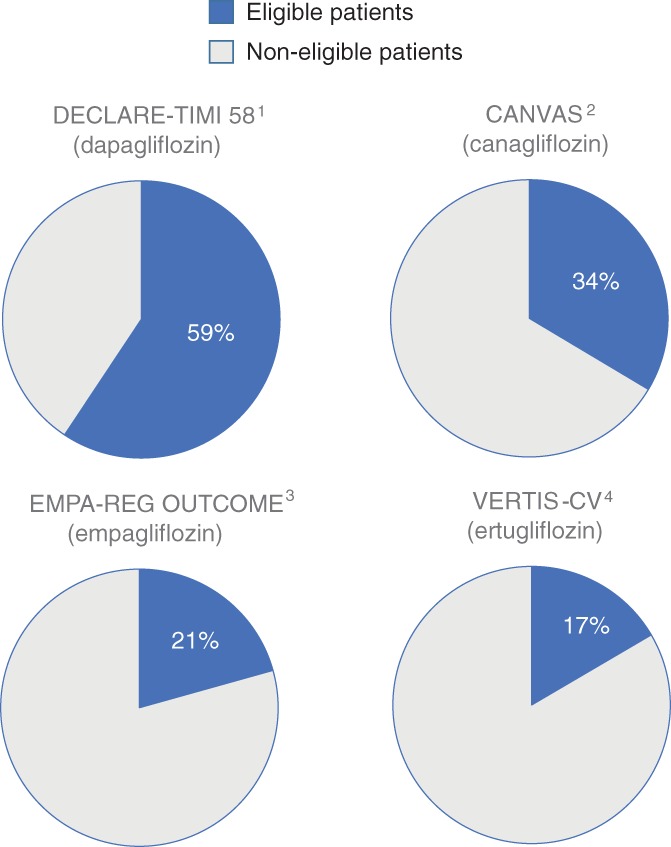
Representativeness of patients in cardiovascular outcome trials when compared to the general type 2 diabetes population across four European countries. 1. Wiviott S, et al. NEJM. 2018: DOI: 10.1056/NEJMoa1812389. 2. Neal B, et al. NEJM. 2017;377(7):644‐57. 3. Zinman B, et al. NEJM. 2015;373(22):2117‐28. 4. Cannon CP, et al. Am Heart J. 2018;206:11‐23
Figure 2.
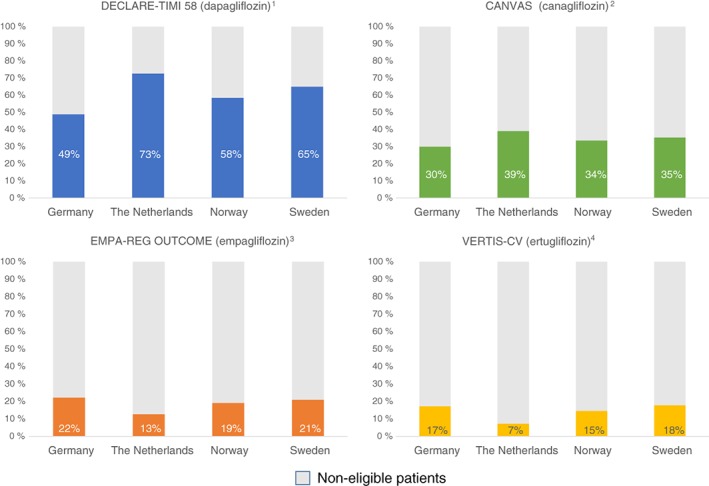
Representativeness of patients in cardiovascular outcome trials when compared to the general type 2 diabetes population in four European countries 1. Wiviott S, et al. NEJM. 2018: DOI: 10.1056/NEJMoa1812389. 2. Neal B, et al. NEJM. 2017;377(7):644‐57. 3. Zinman B, et al. NEJM. 2015;373(22):2117‐28. 4. Cannon CP, et al. Am Heart J. 2018;206:11‐23
3.3. Sensitivity analysis
When laboratory data available in the database from The Netherlands were used additionally as inclusion and exclusion criteria, the representativeness compared to the general T2D populations was, in general, lower for all four CVOTs (Figure S1, Online Supplemental Appendix). The most important reason for the lower representativeness was the HbA1c inclusion criteria, explained by missing HbA1c data or by HbA1c being outside the defined range. The DECLARE‐TIMI 58 trial consistently showed the highest representativeness, but with higher relative differences compared to the primary analysis described above.
4. DISCUSSION
In this observational study, we have investigated how four different SGLT‐2i CVOT populations, defined by applying main inclusion and exclusion criteria from the respective trials, are representative of general T2D populations in four European countries, Germany, The Netherlands, Norway and Sweden. We found that the DECLARE‐TIMI 58 trial had the highest representativeness, covering approximately 60% of patients in a general T2D population, and this was almost 2‐, 3‐ and 4‐fold higher, respectively, as compared to the CANVAS (34%), EMPA‐REG OUTCOME (21%) and VERTIS‐CV (17%) trials.3, 4, 5, 6 With only small variations, findings were consistent across all four countries. Similar patterns, but with more pronounced differences among the CVOTs compared to the main findings, were seen when also including laboratory data in the inclusion and exclusion criteria (available in The Netherlands only).
The most important differences among designs of the respective CVOTs are the inclusion criteria determining CV risk at baseline, relevant when using CV outcomes as measures. Here, two of the studies, DECLARE‐TIMI 58 and CANVAS, allowed for inclusion of patients without established CV disease, but with multiple CV risk factors, whereas the EMPA‐REG OUTCOME and VERTIS‐CV studies limited participants to T2D patients with established CV disease.3, 4, 5, 6 Our results clearly show that the use of less restrictive CV inclusion criteria enhances representativeness, as in the DECLARE‐TIMI 586 and CANVAS4 trials as compared to the EMPA‐REG OUTCOME and VERTIS‐CV trials.3, 5
Wittbrodt et al. recently reported a comparison of representativeness of the CVOTs as compared to a US population when also using laboratory data to define enrolment criteria.18 Interestingly, despite large differences in data sources as well as demographics and methods, striking similarities were found in the comparison of US and Dutch results when laboratory data were used in a sensitivity analysis (Figure S1, Online Supplemental Appendix). Compared to the results of our primary analysis without laboratory data, representativeness was reduced for all CVOTs and the most important explanation for this was the HbA1c inclusion limits. Also, the relative difference in representativeness between the DECLARE‐TIMI 58 trial and the other CVOTs increased substantially when including laboratory data, mainly HbA1c driven, as found in the study by Wittbrodt et al.,18 with less restrictive HbA1c inclusion criteria than that of the DECLARE‐TIMI 58 trial compared to the other trials (Figure S1, Online Supplemental Appendix).
In principle, CVOT representativeness can be assessed with or without access to laboratory data, which are not always sufficiently present in many health care registries. The level of CV risk at baseline mainly determines the level of CV outcome in CVOTs and must be comparable to that in the T2D population to enhance the representativeness of a CVOT. HbA1c is used for trial‐specific reasons, has less impact on CV outcomes, and affects the representativeness of a CVOT. Using laboratory data to assess representativeness requires an HbA1c reading within a reasonable period before index; many patients will therefore be lost because they do not fulfill HbA1c inclusion criteria or values for them are missing in the registries. Also, in the eligibility assessment prior to CVOT inclusion, trial‐specific HbA1c measurements are performed to secure enrolment. Hence, the HbA1c measurement used to determine representativeness in registries is requested for reasons other than those for HbA1c measurements prior to trial enrolment. One might discuss which method, with or without laboratory data, is the most accurate to determine the representativeness of a CVOT of a general T2D population, and the truth is probably somewhere between the two. However, we have shown that comparison of representativeness among CVOTs remains robust and can be accessed via health care registries without laboratory data.
With consistent evidence of the beneficial effects of SGLT‐2is on CV risk and their strengthened position in guidelines, it is of high importance to understand the actual patient profiles in these CVOTs and, thereby, how representative of a general T2D population the included patient populations actually are.2, 4, 5, 6, 7, 14, 15, 19, 20, 21 From the present analysis, it is clear that the main inclusion criteria, determining the baseline CV risk in the respective CVOT, are closely correlated to how representative of a general T2D population the trials are in comparison with each other. Trials that include patients with less CV risk and, consequently, with higher representativeness, require a larger number of patients to fulfill sufficient power criteria concerning CV outcomes. Outcomes of such trials may support a broader implementation of the study results in a real‐world setting with a broad spectrum of CV risk levels.
The strength of the present study involves the populations, which are either representative samples (Germany and The Netherlands) or full populations (Norway and Sweden). The real‐world design provides high external validity and large populations. In addition, the utilized registers have full coverage for hospitalizations and filled drug prescriptions with established public or private healthcare systems and few patients are lost to follow‐up. CV diagnoses in the registries from Norway and Sweden have been reported to have high validity.22, 23, 24, 25, 26
This analysis is based on registries and therefore carries some limitations relating to the completeness and quality of the registries. Also, there may be differences among the registries from the four countries, and it is not possible to analyse the actual cause of the differences in representativeness as many different criteria seem to interact. For example, differences among countries in age and CV disease at baseline seem to play an important role in explaining the differences in representativeness.
From our analysis, we can determine only which prescriptions were filled, which does not guarantee actual ingestion of the drug. As such, we have no information on medication adherence once it is picked up from the pharmacy. The present work has limited information on laboratory measurements, which were available only in The Netherlands as discussed above. Nevertheless, in light of our findings, a high representativeness of CVOTs concerning level of CV risk, may help in applying the study results to a real‐world setting.
In conclusion, consistent patterns of representativeness for four cardiovascular outcome trials involving large T2D populations from European countries were found when applying main inclusion and exclusion criteria. In this study, the DECLARE‐TIMI 58 trial represented two out of three general T2D patients, and the estimated representativeness was 2‐, 3‐ and 4‐fold higher as compared to the CANVAS, EMPA‐REG OUTCOME and VERTIS‐CV trials, respectively. This indicates that the DECLARE‐TIMI 58 trial included and examined patients who are most representative of the general T2D patient in the studied countries.
CONFLICTS OF INTEREST
K. I. B. has received grants to his institution from AstraZeneca for this study and has received honoraria for lectures and consulting from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck Sharp & Dohme. J. B. holds a full‐time position at AstraZeneca as an epidemiologist. A. N. has received honoraria from MSD, Astra Zeneca, Eli Lilly, Boehringer Ingelheim and Novo Nordisk. J. G. K. is an employee of the PHARMO Institute for Drug Outcomes Research which is an independent research institute that performs financially supported studies for government and related healthcare authorities and for several pharmaceutical companies. E. G. is an employee of Team Gesundheit GmbH and conducted work on behalf of Kantar Health. W. B. H. is an employee of AstraZeneca BV in The Netherlands, working as Medical Evidence Delivery Manager. M. T. is employed by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, of which AstraZeneca Nordic‐Baltic is a client. M. P. is an employee of Kantar Health GmbH, Munich, of which AstraZeneca Germany is a client. R. M. C. H. is an employee of the PHARMO Institute for Drug Outcomes Research, which is an independent research institute that performs financially supported studies for government and related healthcare authorities and for several pharmaceutical companies. A. K. is a researcher and head of the Bethesda Diabetes Research Center, University Medical Center Groningen, The Netherlands, without relevant disclosures concerning this manuscript.
Author contributions
All authors participated in the research design. M. T., J. G. K. and E. G. performed the data management and statistical analyses after discussion with all authors. All authors participated in data interpretation and in writing the manuscript. All authors took final responsibility for the decision to submit for publication. All authors are guarantors of the manuscript.
Supporting information
Online Supplemental Appendix
ACKNOWLEDGMENTS
We are grateful to Susanna Jerström and Helena Goike at AstraZeneca for logistic support and valuable comments on the manuscript. Urban Olsson, Statisticon AB, is acknowledged for database management. Fernie Penning and Edith Heintjes, PHARMO Institute, provided important support with analyses and review of the manuscript. Data from the Norwegian Patient Register have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian patient register is intended nor should be inferred.
Birkeland KI, Bodegard J, Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2019;21:968–974. 10.1111/dom.13612
Funding information This study was sponsored by AstraZeneca AB.
REFERENCES
- 1. Norhammar A, Bodegård J, Nyström T, Thuresson M, Eriksson JW, Nathanson D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose‐lowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006–2013. Diabetologia. 2016;59:1692‐1701. [DOI] [PubMed] [Google Scholar]
- 2. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2018. 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 3. Cannon CP, McGuire D, Pratley R, et al. Design and baseline characteristics of the evaluation of ertugliflozin efficacy and safety cardiovascular outcomes trial (VERTIS‐CV). Am Heart J. 2018;206:11‐23. [DOI] [PubMed] [Google Scholar]
- 4. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018. 10.1056/NEJMoa1812389 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461‐2498. [DOI] [PubMed] [Google Scholar]
- 8. Saunders C, Byrne CD, Guthrie B, et al. External validity of randomized controlled trials of glycaemic control and vascular disease: how representative are participants? Diabet Med. 2013;30:300‐308. [DOI] [PubMed] [Google Scholar]
- 9. Sen A, Goldstein A, Chakrabarti S, et al. The representativeness of eligible patients in type 2 diabetes trials: a case study using GIST 2.0. J Am Med Inform Assoc. 2017. 10.1093/jamia/ocx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. VERTIS‐CV‐Study . Cardiovascular outcomes following ertugliflozin treatment in type 2 diabetes mellitus participants with vascular disease, The VERTIS CV study (MK‐8835‐004): ClinicalTrials.gov; https://clinicaltrials.gov/ct2/show/NCT01986881. Updated May 17, 2013.
- 11. Lindh A, Persson F, Sobocki P, Bodegard J, Lindarck N. Nordic longitudinal data from electronic medical records and full population national registers: unique opportunities for new insights in benefit of diabetes patients. Value Health. 2015;18:A726. [Google Scholar]
- 12. van Herk‐Sukel MP, van de Poll‐Franse LV, Lemmens VE, et al. New opportunities for drug outcomes research in cancer patients: the linkage of the Eindhoven Cancer Registry and the PHARMO Record Linkage System. Eur J Cancer. 2010;46:395‐404. [DOI] [PubMed] [Google Scholar]
- 13. Herings RM, Pedersen L. Pharmacy‐based medical record linkage systems In: Kimmel S, Hennessey S, eds. Pharmacoepidemiology. 5th ed. Chichester, West Sussex, UK; John Wiley & Sons, Ltd; 2012:270‐286. [Google Scholar]
- 14. Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709‐717. [DOI] [PubMed] [Google Scholar]
- 15. Persson F, Nystrom T, Jorgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all‐cause mortality in people with type 2 diabetes (CVD‐REAL Nordic) when compared with dipeptidyl peptidase‐4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20:344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wiviott SD, Raz I, Bonaca MP, et al. The design and rationale for the Dapagliflozin Effect on Cardiovascular Events (DECLARE)‐TIMI 58 Trial. Am Heart J. 2018;200:83‐89. [DOI] [PubMed] [Google Scholar]
- 17. R Core Team . A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 18. Wittbrodt ET, Eudicone JM, Bell KF, Enhoffer DM, Latham K, Green JB. Eligibility varies among the 4 sodium‐glucose cotransporter‐2 inhibitor cardiovascular outcomes trials: implications for the general type 2 diabetes US population. Am J Manag Care. 2018;24(suppl 8):S138‐S145. [PubMed] [Google Scholar]
- 19. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose cotransporter‐2 inhibitors). Circulation. 2017;136:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL 2 study. J Am Coll Cardiol. 2018;71:2628‐2639. [DOI] [PubMed] [Google Scholar]
- 21. Petrykiv S, Sjostrom CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12:751‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brynildsen J, Hoiseth AD, Nygard S, et al. [Diagnostic accuracy for heart failure ‐ data from the Akershus Cardiac Examination 2 study]. Tidsskr Nor Laegeforen. 2015;135:1738‐1744. [DOI] [PubMed] [Google Scholar]
- 23. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787‐791. [DOI] [PubMed] [Google Scholar]
- 24. Kumler T, Gislason GH, Kirk V, et al. Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail. 2008;10:658‐660. [DOI] [PubMed] [Google Scholar]
- 25. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sundboll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplemental Appendix


