Abstract
Introduction
Human leukocyte antigen (HLA)‐B*5701 screening identifies patients at increased risk for abacavir (ABC) hypersensitivity reaction (HSR). Screening was adopted in GlaxoSmithKline and ViiV Healthcare clinical trials in 2007 and human immunodeficiency virus treatment guidelines in 2008. Company meta‐analyses of trials pre–HLA‐B*5701 screening reported HSR rates of 4–8%. We analyzed the effectiveness of HLA‐B*5701 screening on reducing HSR rates using clinical trial, Observational Pharmaco‐Epidemiology Research & Analysis (OPERA) cohort, and spontaneous reporting data.
Methods
A meta‐analysis examined 12 trials in 3063 HLA‐B*5701–negative patients receiving an ABC‐containing regimen from April 9, 2007, to September 22, 2015. Potential cases were identified using prespecified Medical Dictionary for Regulatory Activities (MedDRA) preferred terms (drug hypersensitivity, hypersensitivity, anaphylactic reaction, anaphylaxis) and adjudicated against a Company ABC HSR case definition. Investigator‐diagnosed cases were identified and rates were calculated. In the OPERA cohort, 9619 patients initiating their first ABC‐containing regimen from January 1, 1999, to January 1, 2016, were identified. Patients were observed from regimen start until the earliest‐following censoring event: ABC discontinuation, loss to follow‐up, death, or study end (July 31, 2016). OPERA physicians evaluated events against OPERA definitions for definite/probable cases of ABC HSR; rates were calculated pre‐ and post‐2008. The Company case definition was used to identify spontaneously reported cases for four marketed ABC‐containing products; reporting rates were calculated using estimated exposure from sales data, through December 31, 2016.
Results
Suspected ABC HSR rates were 1.3% or less in the meta‐analysis. In the OPERA cohort, the rate was 0.4% among patients initiating ABC post‐2008 versus 1.3% pre‐2008 (p<0.0001). Spontaneous reporting rates were low post‐2008 (54 to 22 cases per 100,000 patient‐years exposure [PYE]) versus pre‐2008 (618 to 55 cases per 100,000 PYE).
Conclusions
Clinically suspected ABC HSR rates were 1.3% or less in HLA‐B*5701–negative patients. Recognizing their limitations, data from the OPERA cohort and spontaneous reporting indicate that HLA‐B*5701 screening has reduced reporting rates of suspected HSR in clinical practice. Where screening for HLA‐B*5701 is standard care, patients should be confirmed negative for this allele before starting ABC treatment.
Keywords: abacavir, hypersensitivity reaction, HLA‐B*5701 screening, pharmacogenetics, HIV
Abacavir (ABC) is a nucleoside reverse transcriptase inhibitor (NRTI) approved since 1998 to treat human immunodeficiency virus (HIV) infection as part of combination antiretroviral therapy (ART).1 Originally marketed as ZIAGEN (ViiV Healthcare, Research Triangle Park, NC), ABC was subsequently co‐formulated with other NRTIs, zidovudine (ZDV) and lamivudine (3TC) (i.e., ABC/3TC/ZDV; TRIZIVIR and ABC/3TC; EPZICOM and KIVEXA; ViiV Healthcare) and more recently with 3TC and dolutegravir (DTG) (i.e., ABC/DTG/3TC; TRIUMEQ; ViiV Healthcare).
Hypersensitivity reaction (HSR) to ABC is a well‐characterized systemic syndrome, usually presenting with multiple symptoms involving several organ systems (Box 1, part B, outlines common symptoms).2, 3, 4, 5, 6 Abacavir HSR can occur at any time during treatment, but it usually occurs within the first 6 weeks of therapy (median time to onset [TTO] 9–11 days).4, 5 The reaction generally evolves over several days, can be detected early with clinical monitoring, and is reversible on ABC discontinuation. More severe life‐threatening or fatal reactions can rarely occur as a result of prolonged ABC treatment in the face of evolving HSR symptoms or ABC rechallenge.2, 3, 4 Reporting rates for ABC HSR from meta‐analyses of up to 34 GlaxoSmithKline–sponsored clinical trials conducted through 2002 ranged from 4–8%.2, 4, 7, 8
Box 1.
The Company ABC HSR case definition is consistent with the description of HSR in the global, regulatory authority approved product labeling for the ABC‐containing products including the EU Summary of Product Characteristics6 and the U.S. Prescribing Information.5 This definition was developed based on minimum criteria required to make a diagnosis of HSR. Cumulative analyses of adverse event reports from clinical trials and postmarketing experience with ABC have shown that the case definition is a conservative way to identify HSR cases for all Company analyses and regulatory reporting activities for clinically suspected ABC, and no single symptom or combination of symptoms is consistently present.2
Company ABC HSR Case Definition
A case of ABC HSR is one in which conditions in parts A or B are fulfilled and where the exclusion criteria do not apply.
-
Part A:
Hypersensitivity, anaphylactic reaction, allergic reaction or drug allergy to ABC is reported.
OR
-
Part B:
Two or more events are reported from two or more of the following groups of signs/symptoms:
rash
fever
gastrointestinal symptoms (nausea, vomiting, diarrhea, abdominal pain)
constitutional symptoms (lethargy, fatigue, malaise, myalgia, arthralgia, general ill feeling)
respiratory symptoms (dyspnea, sore throat, cough, chest x‐ray changes, predominantly infiltrates, which can be localized)
Exclusion Criteria
Other causes of the HSR‐like events appear significantly more likely
Cases where there is a negative rechallenge with ABC
Cases where symptoms resolved (or did not worsen/result in withdrawal from the study)† with continued ABC treatment
Cases of possible hypersensitivity to ABC [in part A]† that do not fulfill the criteria in part B
†Note: Applied to clinical trial meta‐analysis only because cases were captured in more detail in clinical trials than in most spontaneous reports of clinically suspected ABC HSR.
Carriage of the human leukocyte antigen (HLA)‐B*5701 allele increases the risk of HSR.9, 10, 11, 12, 13, 14, 15 The clinical utility of screening for HLA‐B*5701 before beginning ABC treatment was established by the PREDICT‐1 trial that eliminated immunologically confirmed HSR with a negative predictive value of 100% and a positive predictive value of 47.9%.16 It became standard practice in 2007 for GlaxoSmithKline and then ViiV Healthcare (“Company”)–sponsored clinical trials to require study participants to have tested HLA‐B*5701 negative before initiating an ABC‐containing medication. Treatment guidelines for HIV were revised in 2008 to require (depending on region) or recommend HLA‐B*5701 screening for all patients before ABC treatment.
The objective of the present analysis was to assess the effectiveness of HLA‐B*5701 screening as a risk‐mitigation measure in the 10 years since adoption in clinical practice, by calculating reporting rates for suspected ABC HSR from aggregate data from Company‐sponsored clinical trials in HLA‐B*5701–negative patients, the Observational Pharmaco‐Epidemiology Research & Analysis (OPERA) cohort,17 and cases spontaneously reported to the Company.
Methods
Clinical Trials
This analysis used data from 12 phase IIb/IV clinical trials in HIV‐infected adults for ABC/3TC, DTG, ABC/DTG/3TC, or cabotegravir conducted since January 2007 (Table 1).18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 As of April 2017, studies had between 20 and 144 weeks of data. Study designs for each trial were previously described. Briefly, patients received either ABC/3TC plus an anchor drug or ABC/DTG/3TC as study regimens per protocol design. All patients had to be negative for the HLA‐B*5701 allele before receiving ABC. Ethics committee approval was obtained for all studies in accordance with the principles of the 2008 Declaration of Helsinki. Written informed consent was obtained from all patients before enrollment.
Table 1.
Overview of the 12 GSK/VH–Sponsored Clinical Trials Included in the Meta‐analysisa
| Study details | ABC study regimen: (non‐ABC comparator arm) | ABC‐exposed, n | Investigator‐diagnosed cases, n | Company‐adjudicated cases, n | Company adjudication details |
|---|---|---|---|---|---|
|
ING112276 (SPRING‐1)18
ART naive, open‐label, week 96 |
ABC/3TC+DTGb
,
c
ABC/3TC+EFVb , d |
68 | 0 | 0 | NA |
|
ING113086 (SPRING‐2)19
ART naive open‐label NRTI backbone, week 96 |
ABC/3TC+DTGb
,
c
ABC/3TC+RALb , d |
333 | 2 | 5 | Three additional cases. Two met parts A and B of the case definition: 1/2 with positive dechallenge to ABC+DTG, investigator‐implicated DTG only; however, Company could not rule out ABC causality; 1/2 with positive dechallenge to ABC+RAL and was confounded by confirmed influenza infection and amoxicillin use. Remaining case was a nonserious AE, met part A of the definition only and reported a positive dechallenge to ABC and a negative rechallenge to RAL. |
|
ING114467 (SINGLE)20
ART naive, double‐blind,f week 144 |
ABC/3TC+DTGc
,
e
(EFV/TDF/FTC) |
414 | 1 | 2 | One additional case. Met parts A and B of the case definition, with a positive dechallenge to ABC + DTG. Investigator‐implicated DTG only; however, Company could not rule out ABC causality. |
|
ING114915 (FLAMINGO)21
ART naive, open‐label, week 96 |
ABC/3TC+DTGb
,
c
ABC/3TC+DRV+RTVb , d |
159 | 2 | 0 | Two unconvincing cases. One met part A of the case definition; the other was reported as rash only (i.e., met neither part A nor part B), but an ABC HSR CRF Module was completed. Symptoms for both indicated single body system involvement only (i.e., skin and subcutaneous disorders MedDRA SOC); thus were excluded from the Company‐adjudicated analysis. Positive dechallenge to ABC for both and DRV+RTV for 1/2. |
|
ING11607022
ART naive, open‐label, week 96 |
ABC/3TC+DTGc
(None) |
13 | 0 | 0 | NA |
|
ING117172 (ARIA)23
ART naive, open‐label, week 48 |
ABC/DTG/3TCc
,
e
(ATV+RTV+TDF/FTC) |
248 | 1 | 0 | One unconvincing case. Met part A but not part B of the case definition, with symptoms indicating single body system involvement only (i.e., skin and subcutaneous disorders MedDRA SOC); thus was excluded from the Company‐adjudicated analysis. Positive dechallenge to ABC/DTG/3TC. |
|
201147 (STRIIVING)24
ART experienced, open‐label, week 48 |
ABC/DTG/3TCc
,
e
(CAR)g |
519h | 0 | 0 | NA |
|
CNA109586 (ASSERT)25
ART naive, open‐label, week 96 |
ABC/3TC+EFVd
,
e
(TDF/FTC+EFV) |
192 | 6 | 10 |
Six additional cases. Two met parts A and B of the case definition. Investigator‐implicated EFV or NVP only. Four were nonserious and met part A only; verbatim terms implicated EFV only. Positive dechallenge to ABC+EFV (or NVP) in all six; thus Company could not rule out a possible ABC HSR for all cases. Two unconvincing cases. Both met part A but not part B, with symptoms indicating single body system involvement only (i.e., skin and subcutaneous disorders MedDRA SOC). 1/2 with rash only; thus both were excluded from the Company‐adjudicated analysis. Positive dechallenge to ABC for both and EFV for 1/2. |
|
EPZ108859 (ARIES)26
ART naive, open‐label, week 144 |
ABC/3TC+ATV+RTVd
ABC/3TC+ATVd |
515h | 4 | 3 | One unconvincing case. Met part A but not part B with symptoms indicating single body system involvement only (i.e., skin and subcutaneous disorders MedDRA SOC); thus was excluded from the Company‐adjudicated analysis. Positive dechallenge to ABC and negative rechallenge to ATV+RTV. |
|
EPZ113734 (ASSURE)27
ART experienced, open‐label, week 48 |
ABC/3TC+ATVd
,
e
(TDF/FTC+ATV+RTV) |
199 | 1 | 1 | Complete concordance with investigator‐diagnosed cases. |
|
LAI116482 (LATTE)28
ART naive, open‐label, week 96 |
ABC/3TC+CABb
,
d
(CAB+RTV) |
94h | 0 | 0 | NA |
|
200056 (LATTE‐2)29
ART naive, open‐label, week 32i |
ABC/3TC+CABd
,
e
(CAB+RTV) |
309h | 0 | 0 | NA |
ABC = abacavir; AE = adverse event; ART = antiretroviral therapy; ATV = atazanavir; CAB = cabotegravir; CAR = current antiretroviral regimen; CRF = case report form; DRV = darunavir; DTG = dolutegravir; EFV = efavirenz; FTC = emtricitabine; GSK = GlaxoSmithKline; HSR = hypersensitivity reaction; MedDRA = Medical Dictionary for Regulatory Activities; NA = not applicable; NRTI = nucleoside reverse transcriptase inhibitor; NVP = nevirapine; RAL = raltegravir; RTV = ritonavir; SOC = system organ class; 3TC = lamivudine; TDF = tenofovir disoproxil fumarate; VH = ViiV Healthcare.
aWith a breakdown of investigator‐diagnosed and company‐adjudicated cases of suspected ABC HSR.
bInvestigator's choice background therapy was either ABC/3TC or TDF/FTC.
cIncluded in the subanalysis of patients who received ABC/DTG/3TC (or the equivalent components of DTG in combination with the ABC/3TC): the “ABC/DTG/3TC subpopulation.”
dIncluded in the subanalysis of patients who received ABC/3TC in combination with a non‐DTG anchor drug: the “ABC/3TC subpopulation.”
eRandomized with respect to ABC therapy (i.e., an experimental control for ABC).
fDouble‐blind phase occurred from initiation to week 96 followed by an open‐label phase from week 96 to week 144.
gLate switch to ABC/DTG/3TC at 24 weeks.
hExposure calculation for each phase of the study (e.g., early switch and late switch; induction and maintenance with and without extension).
iAlthough the analysis was 32 weeks, all subjects took CAB+ABC/3TC for a 20‐week induction phase and were then randomized 4:1 to either switch to CAB+RTV or continue CAB+ABC/3TC, during the maintenance phase post–20 weeks.
A definition for clinically suspected ABC HSR, similar to the Company case definition (Box 1), was provided in the study protocols. Investigators were instructed to record any case postbaseline that met this definition as a suspected ABC HSR in the case report form (CRF) as either an adverse event (AE) or a serious AE. Verbatim AE terms, including “suspected abacavir hypersensitivity reaction” (or similar), were coded to the Medical Dictionary for Regulatory Activities (MedDRA) AE preferred term of “drug hypersensitivity.” Completion of a separate standardized follow‐up form (ABC HSR CRF) was also required.
To identify cases for this meta‐analysis, relevant AE listings for each trial were reviewed by a Company pharmacovigilance specialist for MedDRA AE preferred terms considered indicative of HSR: hypersensitivity, drug hypersensitivity, anaphylactic reaction, and anaphylaxis only, and no derivatives. Data listings for the ABC HSR CRF were also reviewed. All such identified cases formed the “initial HSR data set” for three separate analyses calculating reporting rates for suspected ABC HSR, as follows.
Investigator‐diagnosed Cases
All cases specifically reported by investigators as clinically suspected ABC HSR, as identified by AE terms, serious AE case narratives, or ABC HSR CRF completion.
Company‐adjudicated Cases
All cases from the initial HSR data set were adjudicated against the Company ABC HSR case definition (Box 1) by the same pharmacovigilance specialist. For this particular analysis, because clinical trial cases were captured in more detail than most spontaneously reported cases of suspected HSR, cases were excluded if they met part A of the ABC HSR case definition (Box 1) but reported symptoms did not meet part B. Reactions in this data set were characterized.
All Possible Cases of ABC HSR
Combined investigator‐diagnosed cases with any additional Company‐adjudicated cases considered to fulfill the ABC HSR case definition stated previously.
These three analyses were performed for the “all ABC‐exposed patients” population (N=3063) and in subpopulations of patients exposed to either ABC/DTG/3TC (or DTG plus ABC/3TC) in 7 of 12 clinical trials (the “ABC/DTG/3TC subpopulation”; N=1494) or ABC/3TC plus a non‐DTG anchor drug (the “ABC/3TC subpopulation”; N=1569) in 10 of 12 clinical trials.
Exact binomial two‐sided confidence intervals (CIs) were used to calculate 95% CIs. Mean exposure in days was calculated from patient‐level data available for each study. Total exposure time was obtained by taking the sum of each participant's exposure time from start to end of ABC treatment. Baseline demographics, clinical characteristics, and event frequencies were summarized using descriptive statistics.
OPERA Cohort
OPERA is a multisite observational database and research network built from complete patient health records managed in electronic health record systems from more than 400 participating caregivers in over 80 locations throughout the United States.17 Real‐world data from OPERA were used to describe the annual incidence of HSR associated with ABC use. Patients who initiated an ABC‐containing regimen for the first time between January 1, 1999, and January 1, 2016, while in the care of an OPERA caregiver were identified (N=15,648). To be eligible for inclusion, patients were required to have at least one clinic contact in the 12 months before ABC initiation to ensure the start date was accurate, and another clinic contact in the first 12 months following ABC initiation to increase the likelihood of observing outcomes (N=9619).
Patients included in the analysis population were observed from their index date (first start date of ABC) until the earliest of the following censoring events: discontinuation of ABC, loss to follow‐up, death, or study end (July 31, 2016). Loss to follow‐up was defined as patients not seen in the clinic for 12 months. Patients were compared by the HLA‐B*5701 testing time period in which they initiated ABC (pre–HLA‐B*5701 screening period: January 1, 1999, to June 14, 2008; or post–HLA‐B*5701 screening period: June 15, 2008, to January 1, 2016).
A panel of three OPERA physicians evaluated all events against OPERA definitions for either definite or probable cases of ABC HSR. A definite case of HSR was defined as one of the following within 6 weeks of ABC initiation: diagnosis of “hypersensitivity reaction” or “allergic drug reaction” with specific reference to ABC followed by drug discontinuation within 2 weeks from the TTO; two or more symptoms indicative of HSR including abdominal pain, allergic reaction, cough, diarrhea, drug reaction, dyspnea, fatigue, fever, flushing, headache, hypersensitivity, malaise, nausea, pharyngitis, rash, or vomiting with complete remission within 14 days from ABC discontinuation; or death within 14 days of a diagnosis or a symptom of HSR (cause of death data were not available). The first two parts of this OPERA “definite” ABC HSR definition were similar to the Company ABC HSR case definition. Probable cases were defined as a single “hypersensitivity” symptom or other atypical complaints within 6 weeks of initiating ABC that did not progressively worsen but did not remit until ABC was discontinued. Patients who subsequently tolerated ABC rechallenge were deemed not to be a case. Case determination was based on agreement between at least two physicians.
Descriptive statistics were used to compare baseline clinical and demographic characteristics and event frequencies. Statistical comparisons between the pre– and post–HLA‐B*5701 screening periods were made using Pearson's χ2/Fisher exact tests for categorical variables and the Wilcoxon rank sum test for continuous variables.
Spontaneously Reported Cases
All spontaneously reported postmarketing cases (i.e., nonsolicited) for ABC, ABC/3TC, ABC/3TC/ZDV, and ABC/DTG/3TC that were received before a data cutoff date of December 31, 2016, and prospectively assessed as clinically suspected ABC HSR against the Company case definition (Box 1) by Company pharmacovigilance specialists for regulatory reporting purposes, were identified from the Company Global Safety Database (OASIS, based on Oracle Argus Safety 2013; Oracle Corp., Redwood Shores, CA) (N=2291).
Because it is not possible to estimate the true incidence of an event from spontaneous data, reporting rates were calculated using estimated exposure from sales data for the four marketed ABC‐containing products, which provide an indication of reporting frequencies. These were calculated as the number of spontaneously reported cases during an estimated 2,972,612 patient‐years of exposure (PYE) to all four ABC‐containing products pooled, and also for each product individually, and were expressed as the number of cases per 100,000 PYE. Annual reporting rates and 95% CIs (calculated using Wald's method) were plotted. Data for December 1998 were included in 1999.
Results
ABC HSR Reporting Rate in Clinical Trials
The median exposure to ABC for all ABC‐exposed patient population was 342 days (range 1–1126 days) and was similar for both subpopulations (Table 2). Figure 1 details the case identification and classification results. Reporting rates of possible ABC HSR in HLA‐B*5701–negative patients were less than 1% for the all ABC‐exposed patient population (0.6–0.9%) and the ABC/DTG/3TC subpopulation (0.3–0.4%), in each of the three case analyses (Table 2). Reporting rates were lower for the ABC/DTG/3TC subpopulation than the ABC/3TC subpopulation (0.8–1.3%) (Table 2). None of the suspected ABC HSR cases resulted in a fatal outcome. The baseline characteristics of the treatment subpopulations were generally similar, although the ABC/DTG/3TC subpopulation had a greater proportion of study participants who were female, ART experienced, and acquired HIV through heterosexual transmission; a lower median viral load; and a higher median CD4+ cell count compared with the ABC/3TC subpopulation (Table 3).
Table 2.
Clinical Trial Reporting Rates of Possible ABC HSR Among HLA‐B*5701–Negative Patients Treated with ABC/3TC or ABC/DTG/3TC
| Parameter | All ABC‐exposed patientsa N=3063 | ABC/DTG/3TC subpopulationb N=1494 | ABC/3TC subpopulationc N=1569 |
|---|---|---|---|
| Exposure in days, median (min, max) | 342.00 (1.00, 1126.00) | 340.00 (1.00, 1124.00) | 347.00 (1.00, 1126.00) |
| Investigator‐diagnosed cases | 0.6% (n=17; 95% CI 0.32–0.89) | 0.3% (n=4; 95% CI 0.07–0.68) | 0.8% (n=13; 95% CI 0.44–1.41) |
| Company‐adjudicated cases | 0.7% (n=21; 95% CI 0.42–1.05) | 0.3% (n=5; 95% CI 0.11–0.78) | 1.0% (n=16; 95% CI 0.58–1.65) |
| All possible cases of ABC HSR | 0.9% (n=27; 95% CI 0.58–1.28) | 0.4% (n=6; 95% CI 0.15–0.87) | 1.3% (n=21; 95% CI 0.83–2.04) |
ABC = abacavir; CI = confidence interval; DTG = dolutegravir; HSR = hypersensitivity reaction; 3TC = lamivudine.
aPatients exposed to ABC/3TC or ABC/DTG/3TC.
bPatients exposed to ABC/DTG/3TC or DTG+ABC/3TC.
cPatients exposed to ABC/3TC in combination with a non‐DTG anchor drug that was atazanavir + ritonavir (RTV), cabotegravir, darunavir + RTV, efavirenz, or raltegravir.
Figure 1.
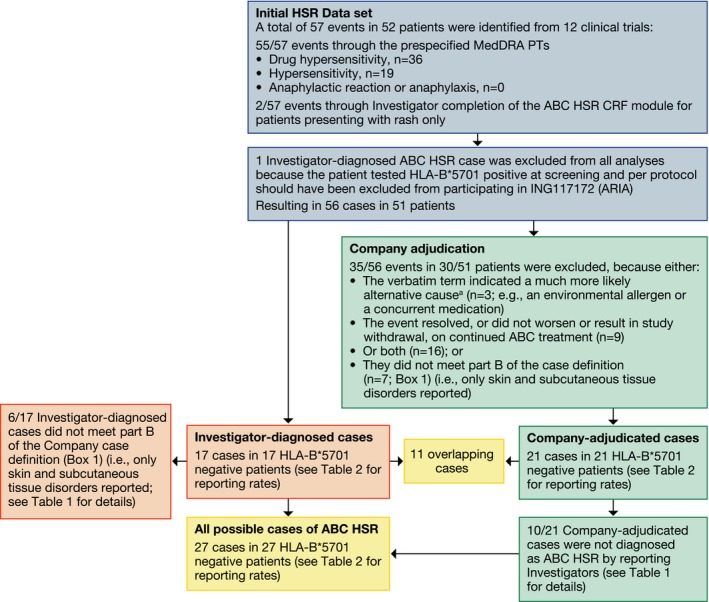
Clinical trial case identification and classification results. ABC = abacavir; CRF = case report form; HSR = hypersensitivity reaction; MedDRA = Medical Dictionary for Regulatory Activities; PT = preferred term. aCoded to MedDRA PTs “Hypersensitivity” or “Drug hypersensitivity.”
Table 3.
Clinical Trial Baseline Demographics and Clinical Characteristics
| Parameter | All ABC‐exposed patientsa (N=3063) | ABC/DTG/3TC subpopulationb (N=1494) | ABC/3TC subpopulationc (N=1569) |
|---|---|---|---|
| Age, yrs, median (min, max) | 38.0 (18.0, 80.0) | 39.0 (18.0, 80.0) | 37.0 (18.0, 75.0) |
| Sex, n (%) | |||
| Female | 699 (22.8) | 454 (30.4) | 245 (15.6) |
| Male | 2364 (77.2) | 1040 (69.6) | 1324 (84.4) |
| Race, n (%) | |||
| White | 2105 (68.7) | 987 (66.1) | 1118 (71.3) |
| Nonwhite | 956 (31.2) | 505 (33.8) | 451 (28.7) |
| Missing | 2 (0.1) | 2 (0.1) | 0 |
| Geographic region, n (%) | |||
| Europed | 911 (29.7) | 416 (27.8) | 495 (31.5) |
| North Americae | 1881 (61.4) | 881 (59.0) | 1000 (63.7) |
| South Americaf | 38 (1.2) | 34 (2.3) | 4 (0.3) |
| Rest of worldg | 233 (7.6) | 163 (10.9) | 70 (4.5) |
| ART status, n (%) | |||
| Naive | 2345 (76.6) | 975 (65.3) | 1370 (87) |
| Experienced | 718 (23.4) | 519 (34.7) | 199 (13) |
| Baseline values, median (range) | |||
| HIV‐1 RNA PCR, log10 copies/ml | 4.36 (1.59–6.93) | 4.02 (1.59–6.66) | 4.64 (1.59–6.93) |
| CD4+ cell count, cells/mm3 | 363.0 (10.0–1831.0) | 411.0 (19.0–1831.0) | 313.0 (10.0–1196.0) |
| CDC classification of HIV, n (%) | |||
| A: Asymptomatic, lymphadenopathy, or acute HIV | 2408 (78.6) | 1132 (79.2) | 1225 (78.1) |
| B: Symptomatic, not AIDS | 415 (13.6) | 190 (12.7) | 225 (14.3) |
| C: AIDS | 240 (7.8) | 121 (8.1) | 119 (7.6) |
| HIV risk factor, n (%) | |||
| Hemophilia‐associated injections | 1 (0) | 1 (0.1) | 0 |
| Heterosexual contact | 960 (31.4) | 600 (40.2) | 360 (22.9) |
| Homosexual contact | 1828 (59.7) | 788 (52.7) | 1040 (66.3) |
| Injectable drug use | 71 (2.3) | 40 (2.7) | 31 (2.0) |
| Occupational exposure | 7 (0.2) | 2 (0.1) | 5 (0.3) |
| Transfusion | 6 (0.2) | 3 (0.2) | 3 (0.2) |
| Other | 89 (2.9) | 6 (0.4) | 83 (5.3) |
| Missing | 101 (3.3) | 54 (3.6) | 47 (3.0) |
ABC = abacavir; AIDS = acquired immunodeficiency syndrome; ART = antiretroviral therapy; CAB = cabotegravir; CDC = Centers for Disease Control and Prevention; DRV = darunavir; DTG = dolutegravir; EFV = efavirenz; HIV = human immunodeficiency virus; PCR = polymerase chain reaction; RAL = raltegravir; RTV = ritonavir; 3TC = lamivudine.
aPatients exposed to ABC/3TC or ABC/DTG/3TC.
bPatients exposed to ABC/DTG/3TC or DTG+ABC/3TC.
cPatients exposed to ABC/3TC in combination with a non‐DTG anchor drug that was ATV+RTV, CAB, DRV+RTV, EFV, or RAL.
dEurope designated countries: Austria, Belgium, Denmark, France, Germany, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Spain, Switzerland, United Kingdom.
eNorth America designated countries: Canada, United States of America.
fSouth America designated countries: Argentina, Mexico, Puerto Rico.
gRest of world designated countries: Australia, Russia, South Africa, Thailand.
Reporting rates for investigator‐diagnosed cases were similar to those for Company‐adjudicated cases in each of the three ABC‐exposed populations analyzed (0.3–0.8% and 0.3–1.0%, respectively) (Table 2). Eleven of the 17 investigator‐diagnosed cases were included in the 21 Company‐adjudicated cases; however, 6 of 17 cases did not meet part B of the Company ABC HSR case definition (Box 1) because reported symptomatology indicated single body system involvement only. Ten additional Company‐adjudicated cases were identified that were not reported as investigator‐diagnosed cases. All 10 cases resolved following the permanent discontinuation of ABC/3TC (and the anchor drug in 9/10 cases) (Figure 1; Table 1).
Characterization of the 21 Company‐adjudicated cases was no different from historical Company‐sponsored clinical trial data pre–HLA‐B*5701 screening2, 4, 7, 8 in terms of TTO, symptomatology, and intensity/toxicity grade or duration, although no cases were fatal, life threatening, or disabling (Table 4).
Table 4.
Characterization of 21 Company‐Adjudicated Clinical Trial Cases
| Parameter | Company‐adjudicated cases (N=21) |
|---|---|
| Time to onset, days, median (min, max) | 10 (2.0, 89.0) |
| Intensity/DAIDS toxicity grading, n (%) | |
| Grade 1 | 0 |
| Grade 2 | 16 (76) |
| Grade 3 | 3 (14) |
| Grade 4 | 2 (10)a |
| Seriousness criteria, n (%) | |
| Fatal | 0 |
| Life threatening | 0 |
| Hospitalization | 3 (14.3)b |
| Disabling or incapacitating | 0 |
| Clinically significant | 11 (52.4) |
| Nonserious AE | 7 (33.3) |
| Duration, days, median (min, max) | 20 (4.0, 172.0)c |
| Details of individual signs and symptoms reported, n (%) | |
| Yes | 16 (76) |
| No | 5 (24) |
| Symptomatology, n (%) | (N=16) |
| Rash | 14 (87.5) |
| Fever | 12 (75) |
| Rash and fever | 10 (62.5) |
| Lethargy/malaise | 11 (69) |
| Gastrointestinal complaints | 10 (62.5) |
| Respiratory symptoms | 6 (37.5) |
| Musculoskeletal symptoms | 4 (25) |
ABC = abacavir; AE = adverse event; DAIDS = Division of AIDS; DTG = dolutegravir; EFV = efavirenz; 3TC = lamivudine.
aOne grade 4 case involved liver dysfunction with ABC/3TC+DTG from SPRING‐2, resulting in hospitalization; the other grade 4 case involved hypotension with EFV+ABC/3TC from ASSERT and was considered clinically significant.
bHospitalization in 2 of 3 cases was due to liver dysfunction with DTG+ABC/3TC from SPRING‐2 (and was previously described);19, 30 the reason for hospitalization was not clear for the third case involving EFV+ABC/3TC from ASSERT.
cUpper limit due to normalization of liver chemistries for the grade 4 case from SPRING‐2, as noted above.
ABC HSR Reporting Rate in the OPERA Cohort
Of the 9619 patients in the OPERA database who were eligible for this analysis, one‐third (n=3215) initiated ABC in the pre–HLA‐B*5701 period (January 1, 1999, to June 14, 2008); two‐thirds (n=6404) initiated ABC in the post–HLA‐B*5701 period (June 15, 2008, to January 1, 2016).
In this post hoc analysis, HSR events were significantly less common in patients who initiated ABC in the post–HLA‐B*5701 period compared with the pre–HLA‐B*5701 period (0.4% vs 1.3%; p<0.0001), respectively. Neither TTO for an HSR event nor frequency of deaths differed significantly between groups (Table 5). Annual event rates of HSR before 2008 ranged from 1.8 to 0.8% and decreased to a range of 0.8 to 0.2% between 2008 and 2015 (Table 6).
Table 5.
OPERA Cohort: Physician‐Adjudicated Definite or Probable Hypersensitivity Reaction Events and Deathsa
| Parameter | All ABC initiators (N=9619) | Started ABC in pre–HLA‐B*5701 screening period (N=3215) | Started ABC in post–HLA‐B*5701 screening period (N=6404) | p value |
|---|---|---|---|---|
| Definite or probable HSR events, n (%) | 69 (0.7) | 42 (1.3) | 27 (0.4) | < 0.0001 |
| Time to onset for HSR event, days | 17.0 | 17.0 | 16.0 | 0.7028 |
| Median (IQR) | (10.0–27.0) | (11.0–27.0) | (9.0–27.0) | |
| Deathsb within 14 days of HSR diagnosis, n (%) | 7 (0.1) | 1 (0.0) | 6 (0.1) | 0.4370 |
ABC = abacavir; HSR = hypersensitivity reaction; IQR = interquartile range.
aWithin 6 weeks (42 days) of abacavir initiation.
bCause of death data were not available.
Table 6.
OPERA Cohort: Physician‐Adjudicated Definite or Probable Hypersensitivity Reaction Eventsa
| ABC initiation by year | All ABC initiators (N=9619) | Definite or probable HSR events only (%) (N=69) |
|---|---|---|
| 2000 | 141 | 2 (1.4) |
| 2001 | 599 | 7 (1.2) |
| 2002 | 544 | 10 (1.8) |
| 2003 | 264 | 2 (0.8) |
| 2004 | 294 | 4 (1.4) |
| 2005 | 388 | 4 (1.0) |
| 2006 | 319 | 5 (1.6) |
| 2007 | 408 | 7 (1.7) |
| 2008 | 505 | 4 (0.8) |
| 2009 | 648 | 5 (0.8) |
| 2010 | 411 | 2 (0.5) |
| 2011 | 458 | 3 (0.7) |
| 2012 | 470 | 2 (0.4) |
| 2013 | 763 | 2 (0.3) |
| 2014 | 1299 | 6 (0.5) |
| 2015 | 2099 | 4 (0.2) |
ABC = abacavir; HSR = hypersensitivity reaction.
aWithin 6 weeks (42 days) of abacavir initiation by year of abacavir initiation.
Patient characteristics differed between the two observation periods (Table 7). The pre–HLA‐B*5701 screening period population was younger and more likely to be white, non‐Hispanic, and men who have sex with men from the West Coast of the United States, with higher viral loads and lower CD4 counts. They were also more likely to be ART experienced with acquired immunodeficiency syndrome at ABC initiation than during the post–HLA‐B*5701 screening period.
Table 7.
OPERA Cohort: Baseline Demographic and Clinical Characteristics of Patients Initiating Treatment with Abacavira
| Parameter | All ABC initiators N=9619 | Started ABC in pre–HLA‐B*5701 screening period (N=3215) | Started ABC in post–HLA‐B*5701 screening period (N=6404) | p value |
|---|---|---|---|---|
| Age, yrs, n (%) | ||||
| Median (IQR) | 42.8 (34.7–50.5) | 40.4 (34.9–46.4) | 44.6 (34.6–52.0) | < 0.0001 |
| 13–25 | 670 (7.0) | 127 (4.0) | 543 (8.5) | < 0.0001 |
| 26–49 | 6424 (66.8) | 2577 (80.2) | 3847 (60.1) | |
| 50+ | 2525 (26.3) | 511 (15.9) | 2014 (31.4) | |
| Sex, n (%) | ||||
| Male | 8089 (84.1) | 2759 (85.8) | 5330 (83.2) | < 0.0001 |
| Female | 1500 (15.6) | 429 (13.3) | 1071 (16.7) | |
| Unknown | 30 (0.3) | 27 (0.8) | 3 (0.05) | |
| Race, n (%) | ||||
| African American | 3240 (33.7) | 865 (26.9) | 2375 (37.1) | < 0.0001 |
| Not African American | 6379 (66.3) | 2350 (73.1) | 4029 (62.9) | |
| Ethnicity, n (%) | ||||
| Hispanic | 2161 (22.5) | 615 (19.1) | 1546 (24.1) | < 0.0001 |
| Not Hispanic | 7458 (77.5) | 2600 (80.9) | 4858 (75.9) | |
| Risk of infection, n (%) | ||||
| MSM | 5014 (52.1) | 1969 (61.2) | 3045 (47.5) | < 0.0001 |
| Not MSM | 4605 (47.9) | 1246 (38.8) | 3359 (52.5) | |
| Region, n (%) | ||||
| Northeast | 397 (4.1) | 40 (1.2) | 357 (5.6) | < 0.0001 |
| South | 4330 (45.0) | 917 (28.5) | 3413 (53.3) | |
| Midwest | 52 (0.5) | 0 | 52 0.8) | |
| West | 4840 (50.3) | 2258 (70.2) | 2582 (40.3) | |
| Treatment naive at index, n (%) | 3940 (41.0) | 1188 (37.0) | 2752 (43.0) | < 0.0001 |
| Baseline HIV RNA viral load | 685 | 8051 | 110 | < 0.0001 |
| Median copies/ml (IQR) | (47–43,755) | (151–71,079) | (19–29,462) | |
| Baseline CD4 count | 389 | 274 | 452 | < 0.0001 |
| Median cells/μL (IQR) | (212–606) | (142–452) | (270–660) | |
| AIDS‐defining illness at or before index | 1924 (20.0) | 965 (30.0) | 959 (15.0) | < 0.0001 |
ABC = abacavir; AIDS = acquired immunodeficiency syndrome; IQR = interquartile range; MSM = men who have sex with men.
aBy screening period.
ABC HSR Reporting Rate from Spontaneously Reported Cases
Most of the 2291 spontaneous cases received through December 31, 2016, and assessed by the Company as clinically suspected ABCHSR were reported from North America (48%) and Europe (39%) by health care providers (HCPs) (66%).
The cumulative global reporting rate of suspected ABC HSR for all four ABC‐containing products was 77 cases per 100,000 PYE. This reporting rate generally decreased annually between 1999 and 2007 (618 to 55 cases per 100,000 PYE), with further decreases from 2008 (37 to 22 cases per 100,000 PYE; Figure 2). A numerical increase was observed for 2014 (54 cases per 100,000 PYE); however, this pooled reporting rate subsequently decreased toward previous levels during 2015 and 2016 (42 cases per 100,000 PYE during 2016) (Figure 2). Similar reporting patterns were observed for ABC, ABC/3TC/ZDV, and ABC/3TC individually (Figure 3). Estimated reporting rates also decreased annually for ABC/DTG/3TC, from 232 cases per 100,000 PYE in 2014 when first introduced to 54 cases per 100,000 PYE during 2016 (Figure 3), resulting in a cumulative estimated reporting rate of 66 cases per 100,000 PYE to December 31, 2016, for this product.
Figure 2.
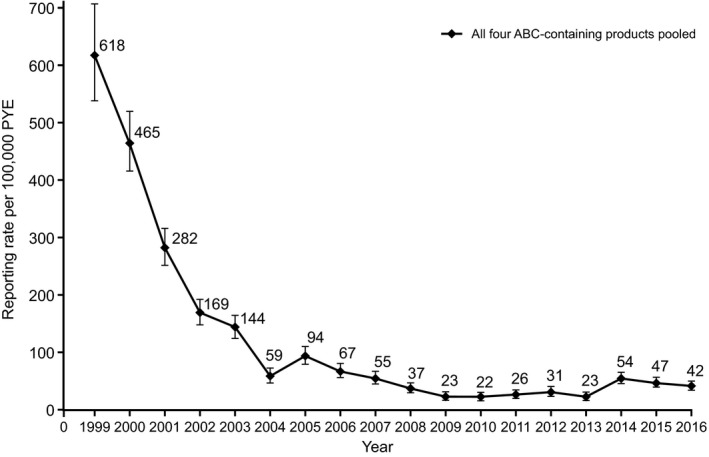
Annual spontaneous reporting rates for all cases fulfilling the Company ABC HSR case definition to December 31, 2016, for all four ABC‐containing products pooled. ABC = abacavir; HSR = hypersensitivity reaction; PYE = patient‐years of exposure.
Figure 3.
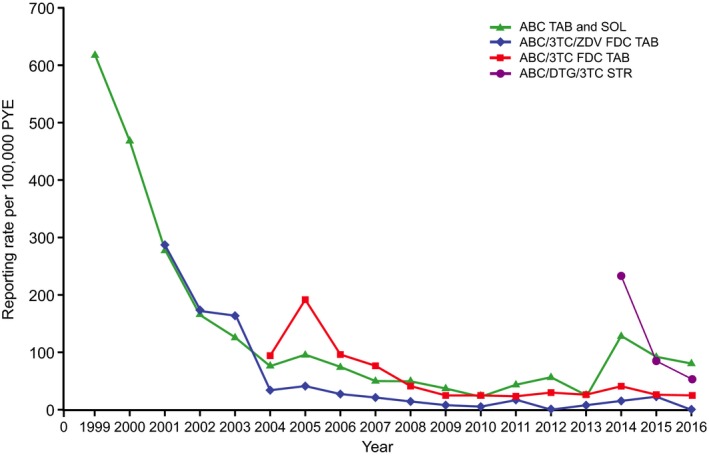
Annual spontaneous reporting rates for all cases fulfilling the Company ABC HSR case definition to December 31, 2016, for each individual ABC‐containing product. ABC = abacavir; DTG = dolutegravir; FDC = fixed‐dose combination; HSR = hypersensitivity reaction; PYE = patient‐years of exposure; SOL = solution; STR = single‐tablet regimen; TAB = tablet; 3TC = lamivudine; ZDV = zidovudine. Cumulative PYE to December 31, 2016, for ABC = 837,429, ABC/3TC/ZDV = 510,913, ABC/3TC = 1,419,426, and ABC/DTG/3TC = 204,844.
A total of 118 of the 2291 spontaneous cases involved positive rechallenge, and 34 of 2291 cases resulted in a fatal outcome (cumulative reporting rates: 4 cases per 100,000 PYE for a positive rechallenge; 1 case per 100,000 PYE for fatal outcome), with 11 of 34 fatal cases involving a positive rechallenge. Most of the positive rechallenge and fatal cases were reported in the first 4 years of ABC availability (Figure 4).
Figure 4.
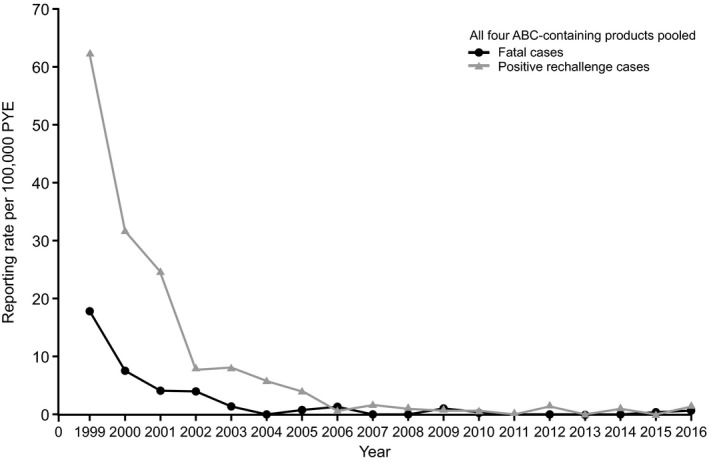
Annual spontaneous reporting rates for positive rechallenge and fatal cases fulfilling the Company ABC HSR case definition to December 31, 2016, for all four ABC‐containing products pooled. ABC = abacavir; HSR = hypersensitivity reaction; PYE = patient‐years of exposure.
Discussion
This analysis evaluated the effectiveness of HLA‐B*5701 screening as a risk‐mitigation measure for ABC HSR by assessing reporting rates for suspected ABC HSR in the 10 years since adoption of this test in clinical practice. As expected from previously published research,9, 10, 11, 12, 13, 14, 15, 16 suspected ABC HSR occurred at a lower frequency in HLA‐B*5701–negative patients treated with ABC in clinical trials. In the observational cohort (OPERA), events were significantly less common in patients initiating ABC after the HLA‐B*5701 test became available in June 2008, and spontaneous reporting rates were also low during the same period. Analyses of these diverse data sources use the strength of each source for a comprehensive assessment.
Clinical trial data are valuable because investigator training and controlled protocol conditions allow for more consistent clinical care and thorough classification, reporting, and safety assessment of adverse drug reactions (ADRs). All calculations from the clinical trial meta‐analysis resulted in reporting rates of 1.3% or less for suspected ABC HSR and were lower for the ABC/DTG/3TC subpopulation than those observed for the ABC/3TC subpopulation. Differences in baseline characteristics between the two ABC‐exposed subpopulations were due to two specific ABC/DTG/3TC studies, ARIA23 (women only) and STRIIVING24 (stable switch study), and they are not believed to have influenced incidence rates.
A total of 16 cases were discordant between investigator‐diagnosed and Company‐adjudicated cases. Six investigator‐diagnosed cases from 4 of 12 trials reported symptoms indicating only single body system involvement (i.e., rash with or without other skin disorders); they were not considered to meet the Company ABC HSR case definition. In 10 additional Company‐adjudicated cases, although causality with the anchor drug may have appeared more likely by the reporting investigators (as indicated by the verbatim event term of “allergy to EFV” [or equivalent] in six cases from ASSERT), all these cases involved a positive dechallenge to ABC (and the anchor drug in 9/10); therefore, a diagnosis of clinically suspected ABC HSR could not be ruled out by the Company.
The Company ABC HSR case definition is a pragmatic and conservative definition used for the purposes of Company analyses and regulatory reporting activities; it is not meant to replace medical diagnoses. The investigator diagnosis of suspected ABC HSR in the six cases of “rash” only, and withdrawal of ABC treatment in all 16 discordant cases, may have been clinically appropriate at the time of patient presentation. None of the ABC HSR cases identified in this meta‐analysis involved either an ABC rechallenge or a fatal outcome, which is an indication of effective protocol guidance on management of this risk, as per the product labeling.5, 6
Although the PREDICT‐1 study represented pioneering research in the field of pharmacogenetics and ABC HSR risk mitigation, investigators were blinded to the patients’ HLA‐B*5701 status, which may have resulted in overreporting of ABC HSR in this study (27/803 [3.4%])16 due to a more cautious and conservative approach to diagnosing clinically suspected ABC HSR in symptomatic patients (e.g., not willing to monitor patients presenting with single key symptoms, or consider or manage possible confounders). We consider that the present meta‐analysis more accurately reflects experience and reporting rates in clinical practice because patient HLA‐B*5701 status was known during the included trials.
The OPERA cohort data enable the calculation of incidence rates based on known denominators. Additionally, 97% of patients eligible for this analysis were seen more than once following initiation of ABC, thus allowing for more complete data capture. Therefore, OPERA represents the real‐world setting more accurately than data from spontaneous reporting. The OPERA data support conclusions from the clinical trial meta‐analysis that clinically suspected ABC HSRs were less common (less than 1%) in patients who initiated ABC from 2008 onward after the uptake of HLA‐B*5701 screening in clinical practice. Reporting rates after 2008 in OPERA were slightly lower than those observed in the clinical trial meta‐analysis, which would be expected due to more controlled protocols for clinical trials (previously described) and the recognized limitations of observational data in the following discussion. Patient characteristics were significantly different between the two observation periods in OPERA. These differences were consistent with the changing HIV epidemic in the United States since 1999 (notably as earlier initiation of effective ART results in better control of the infection31 and longer life expectancy),32 and they are not believed to have influenced ABC HSR reporting rates.
Spontaneous data reporting for the four ABC‐containing products spanned 17 years, allowing for longitudinal analysis and trend identification. Following adoption by HIV treatment guidelines in 2008, HLA‐B*5701 screening appears to have reduced estimated spontaneous reporting rates of suspected ABC HSR. A numerical increase was observed for 2014 that coincided with the introduction of a new ABC‐containing product (ABC/DTG/3TC). However, a similar increase was also observed in the same year for the ABC single‐active product (Figure 3), which may have been due to an influx of uninterpretable cases from an interactive digital media program. Although reporting rates for ABC/DTG/3TC appeared high in 2014, this is in the context of very few reported cases (eight) and estimated PYE (3444) during the 4 months following first product launch. Additionally, similar peaks in reporting rates were also observed for the other three ABC‐containing products following their initial licensures (Figure 3). By 2016, reporting rates for ABC/DTG/3TC decreased to levels similar to those previously calculated for the more established ABC‐containing products. This is consistent with the recognized pattern for waning spontaneous reporting after the first 2 years of product marketing.33
A minority of spontaneous reports still indicate positive rechallenge ABC HSR and/or involve fatal outcome. The Company case adjudication results from the present clinical trial meta‐analysis may illustrate some of the challenges faced by HCPs in diagnosing suspected ABC HSR, especially in the presence of coadministered drugs with overlapping ADR profiles. Thirteen of the 16 total discordant cases involved ABC/3TC in combination with anchor drugs with ADR profiles significant for rash and/or HSR (i.e., atazanavir plus ritonavir [RTV], darunavir plus RTV, efavirenz, or raltegravir). Ultimately, when a distinction cannot be made, ABC should always be discontinued, regardless of a patient's HLA‐B*5701 status.
Although our analysis used robust data sources, each has limitations. Clinical trials use specific patient selection criteria and therefore do not accurately represent real‐world populations. The OPERA cohort is a U.S.‐based population, so conclusions may not extrapolate to other countries. However, a recent analysis of a large European cohort also demonstrated a low discontinuation rate due to ABC HSR (0.3%) post‐uptake of HLA‐B*5701 screening.34 There may be underreporting of HSRs in observational cohorts because mild events may be recorded as symptoms instead of diagnoses. A detailed report of the OPERA data is under review for publication.
Spontaneous reporting has several limitations including underreporting (because reporting is voluntary); variable‐quality data for complete ABC HSR assessment (e.g., missing concomitant medications, medical history, temporal associations, drug action taken, event outcome, HLA‐B*5701 status, and reasons for patient mismanagement); and lack of denominator data biases (e.g., no control group, preferential reporting of unusual or more serious reactions, and adverse outcomes).35, 36 The Company ABC HSR case definition may also be open to subjective bias due to the potential for different interpretations between staff making the case assessments. However, because this definition is pragmatic and conservative, case assessments are likely overinclusive. Additionally, exposure estimates to marketed products require various assumptions and are less accurate than clinical trial exposure data.
Furthermore, all three present analyses are potentially limited because the patient populations were predominantly from North America and Europe. This is likely because these regions are the major markets for sales of the ABC‐containing products; have higher prevalence for the HLA‐B*5701 allele;37 testing is readily available; and treatment guidelines recommend screening as standard of care,38, 39 as compared with other regions.40, 41, 42
Despite these limitations, the analysis of three different data sources shows that HLA‐B*5701 screening is an effective risk‐mitigation measure in reducing reporting rates for clinically suspected ABC HSR. The data from the OPERA cohort and spontaneous reporting indicate the successful adoption of this risk‐mitigation measure in clinical practice that was facilitated by the Company through product labeling, global HCP education programs (ongoing since 1998), and active research and publications on this topic. However, just as in PREDICT‐1, while excluding HLA‐B*5701–positive patients from receiving ABC reduced the risk of ABC HSR, clinically suspected ABC HSR cases were still reported from HLA‐B*5701–negative patients in the present analyses.
All data sets emphasize the importance of adhering to the product labeling when managing patients with suspected ABC HSR: where HLA‐B*5701 testing is standard of care, patients must be confirmed negative before starting ABC treatment; for any patient receiving ABC, the clinical diagnosis of suspected HSR must remain the basis of clinical decision making; and regardless of HLA‐B*5701 status, it is important to discontinue ABC permanently and not rechallenge if an HSR cannot be ruled out clinically because of the potential for a severe or even fatal reaction.5, 6
In conclusion, clinically suspected ABC HSR occurred at a rate of less than 1% in HLA‐B*5701–negative patients and rarely leads to fatality when patients are managed according to the product labeling.
Data Sharing and Data Accessibility
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Acknowledgments
This study was funded by ViiV Healthcare. All listed authors meet the criteria for authorship set forth by the International Committee of Medical Journal Editors. Editorial assistance to the authors’ original creation was provided under the direction of the authors by Sherri Damlo, MedThink SciCom, and was funded by ViiV Healthcare. We thank L. Curtis, H. Vangerow, B. Shepherd, B. Win, J. Double, S. Thiagarajah, and A. Fettiplace from the GSK Global Clinical Safety and Pharmacovigilance Department for their contributions to Safety Evaluation and Risk Management activities for the abacavir (ABC)‐containing products, including ongoing evaluation of adverse drug reaction (ADR) cases received from all sources globally against the Company ABC hypersensitivity reaction (HSR) case definition for identification in the Company safety database, regulatory reporting and product labeling activities, and the Company ABC HSR education programs. We thank J. Hopking from the GSK Statistics, Programming and Data Strategy Department for contributions to the design of the clinical trial meta‐analysis. We also thank C. McCoig from ViiV Healthcare, Global Medical Sciences, for assistance with responding to reviewer comments. IQVIA (Durham, NC) has shared sales data with ViiV Healthcare but does not actively support or endorse findings within this publication. IQVIA is satisfied for ViiV Healthcare to issue this publication, but IQVIA does not take any responsibility for ViiV Healthcare's findings or conclusions.
Chris Stainsby is currently employed in Global Medical Sciences by ViiV Healthcare, London, UK.
Lindsay Carter is currently affiliated with Vidant Medical Center, Greenville, North Carolina.
Conflicts of interest: Chris Stainsby, James Oyee, Rimgaile Urbaityte, and Charlotte Lane are current or former employees of GlaxoSmithKline. Chris Stainsby, Teodora Perger, Vani Vannappagari, Cassidy Henegar, Lindsay Carter, Gary Pakes, and Mark Shaefer are current or former employees of ViiV Healthcare and may own stock in GlaxoSmithKline. Karam Mounzer reports research grants from Gilead Sciences, Janssen, Merck, and ViiV Healthcare and personal fees from Epividian, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. Ricky Hsu reports research grants from Gilead Sciences and personal fees from speaker honoraria and advisory boards from Bristol‐Myers Squibb, Gilead Sciences, Merck, and ViiV Healthcare.
Poster Presentations: Analyses of the clinical trial and spontaneous reporting data were presented as a poster at the 16th European AIDS Conference, October 25–27, 2017, Milan, Italy.
Analyses of the OPERA cohort/pharmaco‐epidemiological data were presented as a poster at the 9th International AIDS Society Conference on HIV Science, July 23–26, 2017, Paris, France.
References
- 1. Cruciani M, Mengoli C, Malena M, et al. Virological efficacy of abacavir: systematic review and meta‐analysis. J Antimicrob Chemother 2014;69:3169–80. [DOI] [PubMed] [Google Scholar]
- 2. Hetherington S, McGuirk S, Powell G, et al. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther 2001;23:1603–14. [DOI] [PubMed] [Google Scholar]
- 3. Hewitt RG. Abacavir hypersensitivity reaction. Clin Infect Dis 2002;34:1137–42. [DOI] [PubMed] [Google Scholar]
- 4. Symonds W, Cutrell A, Edwards M, et al. Risk factor analysis of hypersensitivity reactions to abacavir. Clin Ther 2002;24:565–73. [DOI] [PubMed] [Google Scholar]
- 5. ViiV Healthcare . Ziagen (abacavir) package insert. Research Triangle Park, NC; 2018.
- 6. ViiV Healthcare . Ziagen (abacavir) summary of product characteristics. Brentford, Middlesex, United Kingdom; 2018.
- 7. Hernandez JE, Cutrell A, Edwards M, et al. Clinical risk factors for hypersensitivity reactions to abacavir: retrospective analysis of over 8,000 subjects receiving abacavir in 34 clinical trials [abstract H‐2013]. Presented at 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14–17, 2003; Chicago, IL.
- 8. Cutrell AG, Hernandez JE, Fleming JW, et al. Updated clinical risk factor analysis of suspected hypersensitivity reactions to abacavir. Ann Pharmacother 2004;38:2171–2. [DOI] [PubMed] [Google Scholar]
- 9. Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA‐B region and hypersensitivity reactions to abacavir. Lancet 2002;359:1121–2. [DOI] [PubMed] [Google Scholar]
- 10. Easterbrook PJ, Waters A, Murad S, et al. Epidemiological risk factors for hypersensitivity reactions to abacavir. HIV Med 2003;4:321–4. [DOI] [PubMed] [Google Scholar]
- 11. Saag M, Balu R, Phillips E, et al. High sensitivity of human leukocyte antigen‐B*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis 2008;46:1111–8. [DOI] [PubMed] [Google Scholar]
- 12. Martin AM, Nolan D, Gaudieri S, et al. Predisposition to abacavir hypersensitivity conferred by HLA‐B*5701 and a haplotypic Hsp70‐Hom variant. Proc Natl Acad Sci USA 2004;101:4180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rauch A, Nolan D, Martin A, et al. Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV cohort study. Clin Infect Dis 2006;43:99–102. [DOI] [PubMed] [Google Scholar]
- 14. Reeves I, Churchill D, Fisher M. Screening for HLA‐B*5701 reduces the frequency of abacavir hypersensitivity reactions [abstract 14]. Antivir Ther 2006;11:L11. [Google Scholar]
- 15. Zucman D, De Truchis P, Majerholc C, et al. Screening for HLA B*5701 in a population of HIV patients of diverse ethnic origin exposed to abacavir in France [abstract P‐116]. Presented at 8th International Congress of Drug Therapy in HIV Infection; November 12–16, 2006; Glasgow, Scotland.
- 16. Mallal S, Phillips E, Carosi G, et al. HLA‐B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008;358:568–79. [DOI] [PubMed] [Google Scholar]
- 17. Epividian . Available from http://www.epividian.com/products/opera/. Accessed January 14, 2018.
- 18. Stellbrink H‐J, Reynes J, Lazzarin A, et al. Dolutegravir in antiretroviral‐naive adults with HIV‐1: 96‐week results from a randomized dose‐ranging study. AIDS 2013;27:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raffi F, Rachlis A, Stellbrink H‐J, et al. Once‐daily dolutegravir versus raltegravir in antiretroviral‐naive adults with HIV‐1 infection: 48 week results from the randomised, double‐blind, non‐inferiority SPRING‐2 study. Lancet 2013;381:735–43. [DOI] [PubMed] [Google Scholar]
- 20. Walmsley S, Baumgarten A, Berenguer J, et al. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV‐1 infection in antiretroviral therapy‐naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015;70:515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molina JM, Clotet B, van Lunzen J, et al. Once‐daily dolutegravir versus darunavir plus ritonavir for treatment‐naïve adults with HIV‐1 infection (FLAMINGO): 96 week results from a randomised, open‐label, phase 3b study. Lancet HIV 2015;2:e127–36. [DOI] [PubMed] [Google Scholar]
- 22. Letendre SL, Mills AM, Tashima KT, et al. ING116070: a study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV‐1–infected, ART‐naive subjects. Clin Infect Dis 2014;59:1032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orrell CHD, Belonosova E, Porteiro N, et al. Superior efficacy of dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) fixed dose combination (FDC) compared with ritonavir (RTV) boosted atazanavir (ATV) plus tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) in treatment‐naive women with HIV‐1 infection (ARIA Study). Presented at 21st International AIDS Conference; July 18–22, 2016; Durban, South Africa.
- 24. Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48‐week, randomized, non‐inferiority, open‐label, phase IIIb study. Antivir Ther 2017;22:295–305. [DOI] [PubMed] [Google Scholar]
- 25. Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once‐daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral‐naive, HIV‐1‐infected adults: 48‐week results from the ASSERT study. J Acquir Immune Defic Syndr 2010;55:49–57. [DOI] [PubMed] [Google Scholar]
- 26. Squires KE, Young B, DeJesus E, et al. Safety and efficacy of a 36‐week induction regimen of abacavir/lamivudine and ritonavir‐boosted atazanavir in HIV‐infected patients. HIV Clin Trials 2010;11:69–79. [DOI] [PubMed] [Google Scholar]
- 27. Wohl DA, Bhatti L, Small CB, et al. Simplification to abacavir/lamivudine + atazanavir maintains viral suppression and improves bone and renal biomarkers in ASSURE, a randomized, open label, non‐inferiority trial. PLoS ONE 2014;9:e96187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Margolis DA, Brinson CC, Smith GHR, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral‐naive adults with HIV‐1 infection (LATTE): a randomised, phase 2b, dose‐ranging trial. Lancet Infect Dis 2015;15:1145–55. [DOI] [PubMed] [Google Scholar]
- 29. Margolis DA, Gonzalez‐Garcia J, Stellbrink HJ, et al. Long‐acting intramuscular cabotegravir and rilpivirine in adults with HIV‐1 infection (LATTE‐2): 96‐week results of a randomised, open‐label, phase 2b, non‐inferiority trial. Lancet 2017;390:1499–510. [DOI] [PubMed] [Google Scholar]
- 30. Curtis L, Nichols G, Stainsby C, et al. Dolutegravir: clinical and laboratory safety in integrase inhibitor–naive patients. HIV Clin Trials 2014;15:199–208. [DOI] [PubMed] [Google Scholar]
- 31. Hanna DB, Buchacz K, Gebo KA, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment‐eligible HIV‐infected individuals in North America, 2001–2009. Clin Infect Dis 2013;56:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV‐positive individuals in the United States and Canada. PLoS ONE 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weber JCP. Epidemiology of adverse reactions to nonsteroidal anti‐inflammatory drugs In: Rainsford KD, Velo GD, eds. Side‐effects of anti‐inflammatory drugs, advances in inflammation research. New York, NY: Raven Press, 1984:1–7. [Google Scholar]
- 34. Roen A, Laut K, Pelchen‐Matthews A, et al. Abacavir usage patterns and hypersensitivity reactions in the EuroSIDA cohort. HIV Med 2018;19:252–60. [DOI] [PubMed] [Google Scholar]
- 35. Arora D. Pharmacovigilance: an industry perspective. Navi Mumbai, India: Pharma Publisher, 2012. [Google Scholar]
- 36. Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther 1998;20:C40–4. [DOI] [PubMed] [Google Scholar]
- 37. Small CB, Margolis DA, Shaefer MS, et al. HLA‐B*57:01 allele prevalence in HIV‐infected North American subjects and the impact of allele testing on the incidence of abacavir‐associated hypersensitivity reaction in HLA‐B*57:01‐negative subjects. BMC Infect Dis 2017;17:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. U.S. Department of Health and Human Services ; Panel on Antiretroviral Guidelines for Adults and Adolescents . Laboratory testing. In: Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Available from https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed September 4, 2018.
- 39. European AIDS Clinical Society . Guidelines, version 9.0. 2017: 6–11. Available from http://www.eacsociety.org/files/guidelines_9.0-english.pdf. Accessed September 4, 2018.
- 40. Shah R, Nabiswa H, Okinda N, et al. Prevalence of HLA‐B*5701 in a Kenyan population with HIV infection. J Infect 2018;76:212–4. [DOI] [PubMed] [Google Scholar]
- 41. Zhang H, Zhang T, Zhao H, et al. Low prevalence of human leukocyte antigen‐B*5701 in HIV‐1‐infected Chinese subjects: a prospective epidemiological investigation. AIDS Res Ther 2015;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. World Health Organization . Monitoring of and substitutions for ARV drug toxicities In: Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach, 2nd ed Geneva, Switzerland: WHO Press, 2016:136–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.


