Abstract
Background and purpose
Myasthenia gravis activities of daily living (MG‐ADL) is a commonly used questionnaire in MG trials. To investigate whether MG‐ADL is equally sensitive to oculobulbar and generalized weakness, its correlation with the oculobulbar and generalized domain of the quantitative myasthenia gravis (QMG) score was analyzed (QMGob and QMGgen, respectively). To test whether the sensitivity of MG‐ADL for generalized weakness could be improved, the additional value of ACTIVLIM on top of MG‐ADL in the prediction QMGgen in was investigated.
Methods
MG‐ADL, QMG and ACTIVLIM, an ADL questionnaire focusing on generalized weakness, were analyzed in a prospective cohort of 112 MG patients. A generalized linear model was used to calculate the correlation of MG‐ADL with QMGob and QMGgen and to assess the additional value of ACTIVLIM on top of MG‐ADL for its correlation with QMGgen.
Results
MG‐ADL had a higher correlation with QMGob than with QMGgen (B = 0.68, P < 0.001, and B = 0.38, P < 0.001, respectively). A similar trend was found for changes in the scores (B = 0.68, P = 0.132, and B = 0.39, P = 0.492, respectively). ACTIVLIM had a significant additional value on top of MG‐ADL in the prediction of QMGgen, both cross‐sectionally (B = −0.61, P < 0.001) and for changes within individual patients (B = −0.93, P = 0.041).
Conclusion
MG‐ADL has a lower sensitivity for generalized weakness than for oculobulbar weakness. Adding questions on generalized weakness would improve the sensitivity of the MG‐ADL for generalized weakness.
Keywords: myasthenia gravis, myasthenia gravis activities of daily living, neuromuscular disease, outcome measure, quantitative myasthenia gravis
Introduction
Myasthenia gravis (MG) is an autoimmune disease which can affect extra‐ocular, bulbar, limb and respiratory muscles 1. The degree to which the different muscles are affected varies greatly between MG patients 2. MG‐ADL (myasthenia gravis activities of daily living) is a commonly used questionnaire in MG trials 3, 4, 5. Of the eight questions, three query generalized weakness. These questions concern respiratory function, the ability to brush teeth or comb hair and the ability to rise from a chair. Alongside this ADL scale, the quantitative myasthenia gravis (QMG) score is often used in clinical trials as an objective physician‐reported scale. The QMG, however, includes more items on generalized weakness (eight of 13). In general, the MG‐ADL score correlates well with the QMG score 6, but in a recently published trial on the effect of eculizumab in generalized MG patients there was a non‐significant change in MG‐ADL whereas QMG improved significantly 4, 7. This raises the question whether MG‐ADL is equally sensitive to changes in generalized weakness and oculobulbar weakness and whether adding questions on generalized weakness would improve sensitivity. There are no other MG‐specific ADL measures with more questions on generalized weakness. In a prior study, a general neuromuscular ADL measure focusing on generalized weakness, the ACTIVLIM (acronym of activity limitations), was validated in a cohort of 118 MG patients 8, 9. To investigate whether the sensitivity of MG‐ADL for generalized weakness could be improved, the additional value of ACTIVLIM on top of MG‐ADL in the prediction of the generalized domain of the QMG score was investigated.
Methods
Patients
A prospective cohort of MG patients under treatment at Leiden University Medical Center (LUMC) between 2016 and 2017 was included. The QMG, MG‐ADL and ACTIVLIM scores were recorded in all MG patients who visited the outpatient clinic as part of routine clinical care. The diagnosis of MG was based on a combination of clinically confirmed fluctuating muscle weakness and the presence of serum autoantibodies to the acetylcholine receptor or muscle‐specific kinase. Seronegative myasthenia gravis was defined as fatigable muscle weakness in combination with an abnormal decrement (at least 10%) during low‐frequency repetitive nerve stimulation, increased jitter in single‐fiber electromyography testing or a positive neostigmine test 1. This study was approved by the medical ethics boards of the LUMC.
Outcome variables
Two ADL measures and one physician‐reported measure of muscle fatigability, MG‐ADL, ACTIVLIM and QMG, were compared. The QMG was subdivided into an oculobulbar (first five items of the QMG; QMGob) and a generalized domain (remaining eight items; QMGgen).
Statistical analysis
A generalized linear model was used to calculate the correlation of MG‐ADL with QMGob and QMGgen and to assess the additional value of ACTIVLIM on top of MG‐ADL in the prediction of QMGgen on the first visit. Furthermore, this additional value was analyzed for changes in the measures (ΔACTIVLIM, ΔMG‐ADL and ΔQMGgen) within the same patient. First, a linear model was fitted for QMGgen with MG‐ADL as the single predictor. Next, the residuals from that model were regressed on ACTIVLIM. The residual is the difference between observed and predicted QMGgen based on the MG‐ADL score of that patient. Results are expressed as B coefficients with 95% confidence intervals, which reflect the additional value of ACTIVLIM in the prediction of QMGgen compared to MG‐ADL alone. Regression lines show the degree to which ACTIVLIM can compensate for the mismatch between observed and predicted QMGgen. P values < 0.05 were considered significant. Statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, USA).
Results
One hundred and twelve consecutive patients with MG were included. Of this group, 14 patients had a second visit with all assessments. The baseline characteristics of all patients are shown in Table 1. MG‐ADL had a higher correlation with QMGob than with QMGgen (B = 0.68, P < 0.001, and B = 0.38, P < 0.001, respectively). A similar trend was found for changes in the scores (B = 0.68, P = 0.132, and B = 0.39, P = 0.492, respectively).
Table 1.
Baseline characteristics of 112 patients with MG included in this study
| MG patients N = 112 | |
|---|---|
| Age, years | 57.1 ± 18.0 |
| Age at onset, years | 46.7 ± 20.0 |
| Gender | |
| Male | 42 (38) |
| Female | 70 (62) |
| Antibodies | |
| AChR+ | 84 (75) |
| MuSK+ | 8 (7) |
| Seronegative | 20 (18) |
| Thymectomy | |
| Yes, with thymoma | 8 (7) |
| Yes, without thymoma | 27 (24) |
| No | 77 (69) |
| ACTIVLIM | 3.5 ± 2.2 |
| MG‐ADL | 4.5 ± 3.5 |
| QMG | 6.4 ± 4.5 |
| QMGgen | 4.3 ± 3.6 |
AChR, acetylcholine receptor; MG‐ADL, myasthenia gravis activities of daily living; MuSK, muscle‐specific kinase; QMG, quantitative myasthenia gravis. Data are presented as number of patients (%) for categorical variables and as mean ± SD for continuous variables.
The mean deviation of MG‐ADL from the predicted values of QMGgen was 2.6 ± 1.9. ACTIVLIM had an additional value on top of MG‐ADL in the prediction of QMGgen (B = −0.61, P < 0.0001; Fig. 1a). In addition, the mean deviation of ΔMG‐ADL from the predicted values of ΔQMGgen was 1.7 ± 1.3. ΔACTIVLIM had an additional value on top of ΔMG‐ADL in the prediction of ΔQMGgen (B = −0.93, P = 0.041; Fig. 1b).
Figure 1.
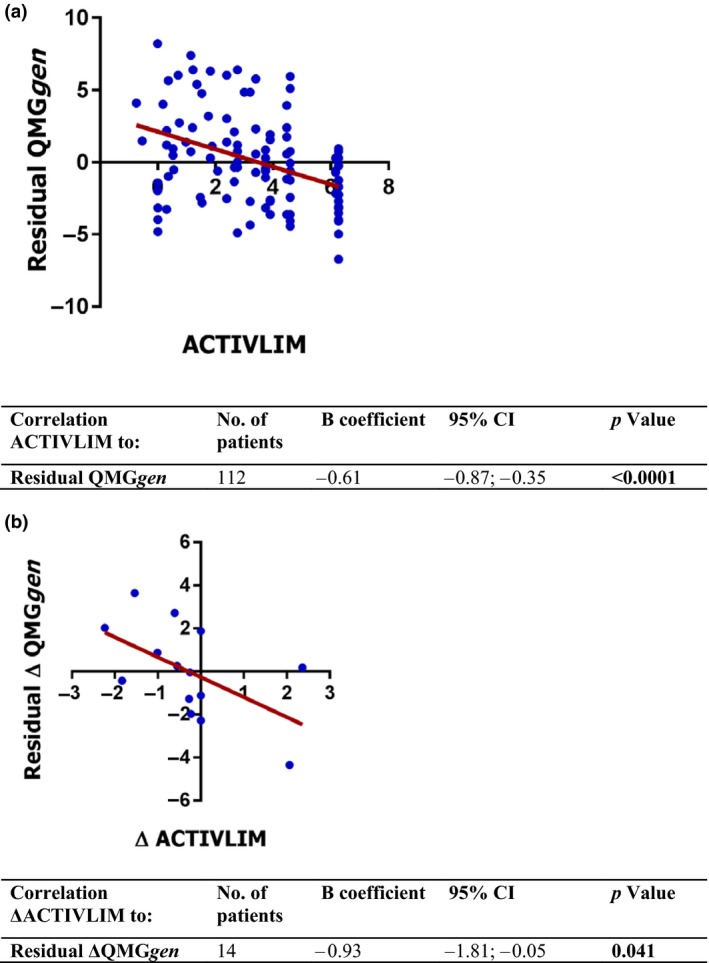
Analysis of the (Δ)ACTIVLIM, (Δ)MG‐ADL and (Δ)QMGgen scores in 112 individual patients and in 14 patients with a follow‐up visit. The dots show the (Δ)QMGgen residual for each individual patient. The (Δ)QMGgen residual is the difference between observed and predicted (Δ)QMGgen based on the (Δ)MG‐ADL score of that patient. The B coefficient (slope) shows the degree to which (Δ)ACTIVLIM correlates with the residual of (Δ)QMGgen. The significant correlation implies that (Δ)ACTIVLIM can (partly) compensate for the mismatch between observed and predicted (Δ)QMGgen and therefore has an additional value on top of the (Δ)MG‐ADL. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
In a cohort of 112 MG patients, it was found that MG‐ADL correlates better with QMGob than with QMGgen. ACTIVLIM had a significant additional value on top of MG‐ADL in the prediction of QMGgen, suggesting that adding questions on generalized weakness could improve the sensitivity of the MG‐ADL for generalized weakness. This additional value was even higher for changes in generalized weakness and was found to be significant, even in the small group of patients with a follow‐up visit (n = 14) in which this analysis could be performed.
Further research could focus on formulating and testing one or two additional questions on generalized weakness that could improve the MG‐ADL score by balancing oculobulbar and generalized items without losing a major positive trait of the MG‐ADL, time efficiency, as this minor addition would cost less than a minute. The importance of having a high sensitivity for changes in both oculobulbar and generalized weakness was shown by Barnett et al. 10 who found that ocular weakness appeared to respond better to treatment with prednisone whilst generalized weakness was more amenable to intravenous immunoglobulin or plasma exchange. Their newly developed outcome measure, the MG impairment index, takes changes in generalized weakness into account and may be a good measure for future studies. As QMG and MG‐ADL will probably remain the leading outcome measures in the coming years, it is suggested that the oculobulbar and generalized subscores of these outcome measures are reported separately. This might help to distinguish potential preferential effects on oculobulbar or generalized weakness.
The limitations of this study include the single center of inclusion and our study population within a tertiary referral center that may not fully reflect the total MG population due to a referral bias. Moreover, it may be argued that ACTIVLIM is not a proper reference for generalized weakness in MG, as little is known about this measure in MG besides our earlier study 8. ACTIVLIM is a validated, Rasch‐built ADL measure for neuromuscular diseases in general and has been shown to be highly responsive to decreases in functional status 11. Our previous study showed that ACTIVLIM is highly responsive to changes in generalized weakness in MG patients as well 8. It was therefore found appropriate to use ACTIVLIM instrumentally to investigate whether adding ADL questions on generalized weakness could improve the sensitivity of the MG‐ADL for generalized weakness. Importantly, it is not proposed to use ACTIVLIM as a primary outcome measure in MG as it lacks oculobulbar items. However, ACTIVLIM might also be useful in the future in assessing the generalized component of new MG outcome measures.
In conclusion, the current study shows that MG‐ADL has a lower sensitivity for generalized weakness than for oculobulbar weakness. Adding questions on generalized weakness would improve the sensitivity of the MG‐ADL for generalized weakness, thereby increasing the overall performance of the MG‐ADL in future clinical trials.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.
References
- 1. Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 2009; 8: 475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodolico C, Parisi D, Portaro S, et al Myasthenia gravis: unusual presentations and diagnostic pitfalls. J Neuromuscul Dis 2016; 3: 413–418. [DOI] [PubMed] [Google Scholar]
- 3. Pasnoor M, He J, Herbelin L, et al A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis. Neurology 2016; 87: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howard JF Jr, Utsugisawa K, Benatar M, et al Safety and efficacy of eculizumab in anti‐acetylcholine receptor antibody‐positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double‐blind, placebo‐controlled, multicentre study. Lancet Neurol 2017; 16: 976–986. [DOI] [PubMed] [Google Scholar]
- 5. Beecher G, Anderson D, Siddiqi ZA. Subcutaneous immunoglobulin in myasthenia gravis exacerbation: a prospective, open‐label trial. Neurology 2017; 89: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 6. Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology 1999; 52: 1487–1489. [DOI] [PubMed] [Google Scholar]
- 7. de Meel RHP, Verschuuren J, Tannemaat MR. Distinct representation of muscle weakness in QMG and MG‐ADL. Lancet Neurol 2018; 17: 204–205. [DOI] [PubMed] [Google Scholar]
- 8. De Meel RHP, Lipka AF, Van Der Lende M, Van Zwet EW, Tannemaat MR, Verschuuren J. Activity limitations in myasthenia gravis and relation to clinical variables. Muscle Nerve 2017; 56: 64–70. [DOI] [PubMed] [Google Scholar]
- 9. Vandervelde L, Van den Bergh PY, Goemans N, Thonnard JL. ACTIVLIM: a Rasch‐built measure of activity limitations in children and adults with neuromuscular disorders. Neuromuscul Disord 2007; 17: 459–469. [DOI] [PubMed] [Google Scholar]
- 10. Barnett C, Bril V, Kapral M, Kulkarni AV, Davis AM. Myasthenia gravis impairment index: responsiveness, meaningful change, and relative efficiency. Neurology 2017; 89: 2357–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vandervelde L, Van den Bergh PY, Goemans N, Thonnard JL. Activity limitations in patients with neuromuscular disorders: a responsiveness study of the ACTIVLIM questionnaire. Neuromuscul Disord 2009; 19: 99–103. [DOI] [PubMed] [Google Scholar]


