Abstract
Abnormal retinal neovascularization associated with various retinopathies can result in irreversible vision loss. Although the mechanisms involved in this occurrence is unclear, increasing evidence suggests that aberrant Wnt signaling participates in the pathogenesis of abnormal neovascularization. Because Wnt signaling upregulation can be induced by oxidative stress through the activation of disheveled (DVL), a key molecule in the Wnt signaling pathway, we investigated whether oxidative stress can activate Wnt signaling and induce angiogenic phenotypes in human retinal microvascular endothelial cells (HRMECs). We found that increased Wnt signaling activity, as well as enhanced angiogenic phenotypes, such as tube formation and cell migration, were detected in the hydrogen peroxide‐treated HRMECs. Moreover, these effects were effectively suppressed by a small‐molecule Wnt inhibitor targeting the PDZ domain of DVL. Therefore, we propose that targeting abnormal Wnt signaling at the DVL level with a small‐molecule inhibitor may represent a novel approach in retinal neovascularization treatment and prevention.
Keywords: angiogenesis, oxidative stress, pathological neovascularization, Wnt signaling
1 |. INTRODUCTION
Pathological retinal neovascularization is a hallmark of several retinal diseases including retinopathy of prematurity (ROP) and diabetic retinopathy (DR).1–4 The inner segment of the retina is vascularized by retinal vessels,5 where endothelial cells connect and communicate via tight junctions resulting in the formation of the inner blood-retinal barrier.5,6 This barrier provides a selective mechanism which protects the retina from circulating agents and small molecules, allowing the retina to function in a highly specialized environment.5 In healthy tissue, retinal blood vessels are maintained in a quiescent state with consideration to growth.7,8 However, in the event of pathological retinal neovascularization, abnormal angiogenic growth of weakened blood vessels is present at the juncture of the vascularized and avascular retina going into the vitreous. In turn, these weakened blood vessels may lead to blindness as a result of vascular leakage, rupture, scarring, and retinal detachment.2,9–12
Although the underlying mechanism of retinal neovascularization is unclear, increasing evidence finds that abnormal Wnt/β-catenin signaling participates in retinal neovascularization.1,10,12–16 Wnt signaling is essential for the development and differentiation of the retinal vasculature. Moreover, experimental mouse models of oxygen-induced retinopathy (OIR) which depict events that occur during ROP, have shown that abnormal Wnt/β-catenin activity promotes pathological retinal neovascularization in retinopathy.12,17,18 Vascular endothelial growth factor (VEGF), a potential downstream target gene of the Wnt signaling pathway,19,20 which controls endothelial cell processes including cell migration and proliferation, is linked to retinal neovascularization.21,22 Dysregulation of the Wnt signaling pathway has been linked to upregulation of VEGF in endothelial cells.23–25 Nevertheless, the cause of abnormal Wnt signaling activity is unknown.
Oxidative stress as a result of high concentrations of reactive oxygen species (ROS), also has been linked to pathological retinal neovascularization.26,27 Oxidative stress is reported to induce key properties of angiogenesis, including cell migration and proliferation, through the stimulation of VEGF in endothelial cells.28 The main pathogenesis of ROP and DR at the proliferative retinopathy stages is oxidative stress within blood vessel cells.29–32 We speculate that oxidative stress may be responsible for abnormal Wnt signaling activity in human retinal microvascular endothelial cells, which may be a major contributor to retinal neovascularization. Contrarily, an interrelationship between redox and Wnt signaling has been reported, where redox regulates Wnt signaling at the disheveled (DVL) level.33–35
In the current working model of canonical Wnt signaling, cytoplasmic DVL protein is activated via the binding of a secreted Wnt ligand to its transmembrane receptor, Frizzled (FZD).36–38 Upon activation, FZD interacts directly with the PDZ domain of DVL39 initiating downstream Wnt signaling.40–47 However, this interaction between DVL and FZD is also mediated by redox through nucleoredoxin (NXN), a thioredoxin-related redox-regulating protein.33,34 NXN also binds to the PDZ domain of DVL, blocking Wnt signaling activity by competing with Wnt receptor, FZD. However, oxidative stress can dissociate the interaction between NXN and DVL, enabling DVL to interact with FZD and initiate Wnt signaling.33,35 Therefore, it is likely that oxidative stress in the retinal activates abnormal Wnt signaling at the DVL level. Indeed, while Wnt activity is significantly increased in pathologic neovascularization in the OIR model,12,48 mutant mice lacking Dvl2, presented significantly reduced levels of neovascularization, suggesting that the DVL proteins play an essential role in the development of retinal neovascularization.12 Therefore, we reasoned that a Wnt inhibitor targeting the PDZ domain of DVL, may be helpful in developing a novel therapeutic approach for the treatment of retinal neovascularization. However, despite the extensive efforts, the current state-of-the-art DVL-PDZ domain inhibitors are not apposite to be used in the in vivo animal model studies.49 Thus, we decided to use different in vitro assays of angiogenesis50 to assess the idea of targeting DVL with a small-molecule inhibitor of the DVL-PDZ domain.
In this study, using compound 3289–8625, a known inhibitor of the DVL-PDZ domain,51 together with models of retinal angiogenesis in vitro, we first confirmed that the DVL protein is involved in Wnt-mediated angiogenesis through our analysis of human retinal microvascular endothelial cells (HRMECs) tube formation and cell migration. Then, using hydrogen peroxide (H2O2) to mimic oxidative stress in the retinal, we showed that H2O2 activated canonical Wnt signaling in HRMECs and further promoted tube formation and enhanced cell migration. Moreover, we found that Wnt inhibitor 3289-8625, effectively suppressed the H2O2- induced angiogenesis phenotypes in HRMECs, suggesting that selectively targeting the DVL-PDZ domain with small molecules may be a strategy against pathological retinal neovascularization.
2 |. MATERIALS AND METHODS
2.1 |. HRMECs culture and Wnt conditional medium preparation
HRMECs were purchased from Angio-Proteomie (Boston, MA). These cells were maintained in Endothelial Cell Medium MV (PromoCell, Heidelberg, Germany) supplemented with Endothelial Cell Supplement Mix (PromoCell, Heidelberg, Germany). Cells from passages 3 to 7 were used for all assays. For production of Wnt and control conditional media, mouse L Wnt-3a cell line stably expressing Wnt-3a (CRL2647; American Type Culture Collection [ATCC], Manassas, VA), and L-cell line (CRL2648; ATCC), were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum. Wnt-3a conditional medium (WCM) and L-cell control conditional medium (LCM) were collected from L Wnt-3a cells and L cells, respectively, after 7 days in culture. Upon assessment, the concentration of LCM and WCM used for all assays was maintained at 10%.
2.2 |. Compound preparation and HRMECs treatment
Wnt signaling inhibitor 3289–8625 (ENZO Life Sciences, Farmingdale, NY) was dissolved in 100% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St Louis, MO). The working concentration of compound 3289–8625 was 50 μM in 0.05% DMSO, therefore the concentration of the vehicle control and LCM control used contained 0.05% DMSO, unless stated otherwise. HRMECs were treated with conditional medium or 1 μM H2O2 in presence or absence of compound 3289–8625, unless specified differently. The medium was replaced with a growth medium or medium containing compound 3289–8625, following a 30-minute incubation for HRMECs treated with H2O2, or H2O2 in presence of compound 3289–8625.
2.3 |. Immunofluorescence assay
HRMECs were treated with either LCM, WCM, WCM in presence of compound 3289-8625, or vehicle, H2O2, and H2O2 in presence of compound 3289–8625 for 24 hours. These cells were washed once with PBS (Thermo Fisher Scientific, Waltham, MA) and fixed in 4% paraformaldehyde (Sigma-Aldrich) for 30 minutes at room temperature. Samples were then washed three times for 5 minutes each wash, with PBS. Samples were blocked with PBS containing 5% goat serum and 0.25% Triton-X-100. Primary rabbit anti-human β-catenin (ab32572; Abcam, Cambridge, MA) and phalloidin-TRITC (P5282; Sigma-Aldrich) were added overnight at 4°C in PBS containing 1% goat serum. Samples were washed once with PBS. Secondary goat anti-rabbit Alexafluor 594 (A-11037; Thermo Fisher Scientific, Waltham, MA) was added to the membranes and incubated for 1 hour at room temperature. These samples were washed three times with PBS for 5 minutes each wash, and then counterstained and mounted with 4′,6-diamidino-2-phenylindole (Vector Labs, Burlingame, CA). Samples were visualized using Keyence BZ-X700 fluorescence microscope (Keyence, El Segundo, CA) and five random fields of views were photographed.
2.4 |. Quantitative polymerase chain reaction
HRMECs were treated with LCM, WCM, and WCM in presence of compound 3289–8625, or vehicle, H2O2, and H2O2 in presence of compound 3289–8625 for 24 hours. Total cellular RNA was extracted from HRMECs using the Qiagen RNeasy mini kit (Qiagen, Valencia, CA) as per manufacturer’s protocol and the concentration of RNA was analyzed using a NanoDrop (Thermo Fisher Scientific, Waltham, MA). Quantitative polymerase chain reaction (qPCR) was carried out using qScript-XLT 1-Step RT-qPCR kit from Quanta Biosciences (Beverly, MA), with TaqMan primers for human glyceraldehyde 3-phosphate dehydrogenase (Hs02758991_g1) and human AXIN2 (Hs00610344_m1) purchased from Thermo Fisher Scientific (Canoga Park, CA). Manufacturer’s protocol was followed for the qPCR and the delta-delta method was used for analysis. Each experimental condition was repeated at least three times.
2.5 |. HRMEC tube formation assay
Tube formation assay was performed by using growth factor-reduced Matrigel (Corning Inc, Corning, NY). Matrigel was evenly coated into a six-well plate and HRMECs were seeded at 1.2 × 105 cells per well on these Matrigel-coated wells, and subsequently treated with serum-free medium containing either LCM, WCM, and WCM in presence of inhibitor 3289-8625, or vehicle, H2O2, and H2O2 in presence of inhibitor 3289–8625. Image acquisition was performed following incubation at 37°C for 6 hours. Acquired images were taken under a phase contrast microscope and were processed and evaluated for the total tube length, branching points, total loops, and area coverage by ImageJ (National Institutes of Health [NIH], Bethesda, MD).
2.6 |. HRMEC migration assay
The cell migration assay was performed using the migration and wound-healing system from ibidi (Madison, WI). HRMECs were seeded in a μ-35 culture-insert dish at 3 × 104 cells per well and incubated overnight. A flat-head forecep was used to carefully remove the culture inserts from culture dish after incubation to create a cell-free gap. Then, HRMECs were treated with serum-free medium containing LCM, WCM, WCM in presence of Wnt inhibitor 3289–8625, or H2O2, H2O2 with inhibitor 3289–8625. Images were acquired using a phase contrast microscope after 16 hours. HRMECs migration rates were quantified by analyzing the size of nonmigrated area using the ImageJ (NIH) program.52 Each experiment was repeated at least three times and there were five random fields of views acquired for each treatment group.
2.7 |. Luciferase assay
Stable transfected NIH3T3 cell line expressing luciferase under a TCF/LEF promoter was purchased from ENZO Life Sciences Inc (Farmingdale, NY). These 3T3 cells were seeded at 2 × 105 cells per well in a 96-well plate (Corning Inc) and incubated overnight. Cells were then treated with either LCM, WCM, and WCM in presence of compound 3289–8625; or vehicle, H2O2, H2O2 in presence of 3289-8625, and were cultured for 14 hours. Cell viability and firefly luciferase activity were measured using the ONE-Glo + Tox Luciferase Reporter and Cell Viability Assay kit (Promega, Madison, WI) following manufacturer’s protocol. A microplate reader, FilterMax F5 (Molecular Devices, Sunnyvale, CA) was used to measure the cell viability and firefly luciferase activity. All assays were performed in triplicates.
3 |. RESULTS
3.1 |. Compound 3289-8625 inhibited Wnt signaling and Wnt-induced angiogenic phenotypes in HRMECs
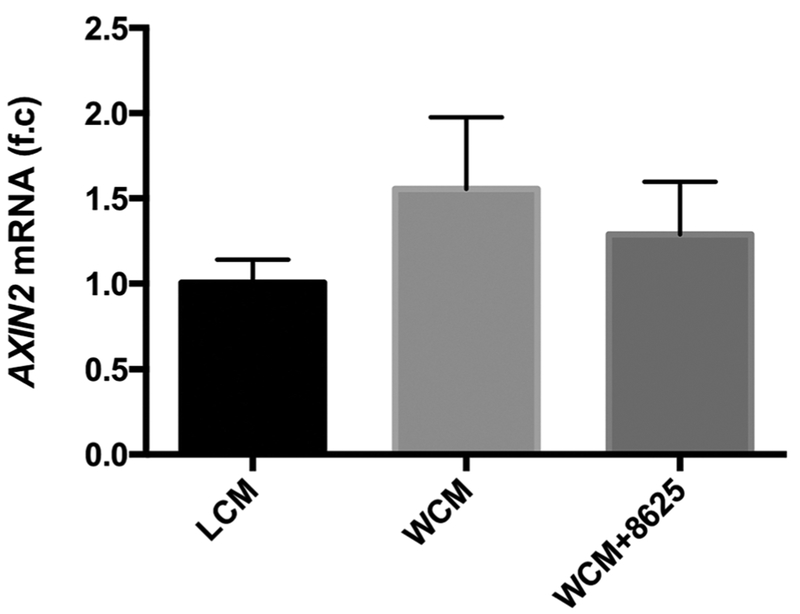
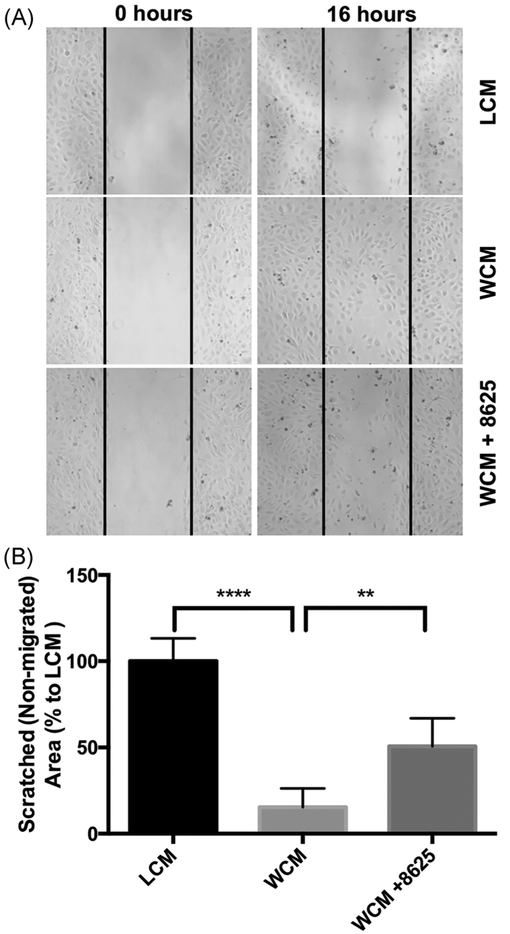
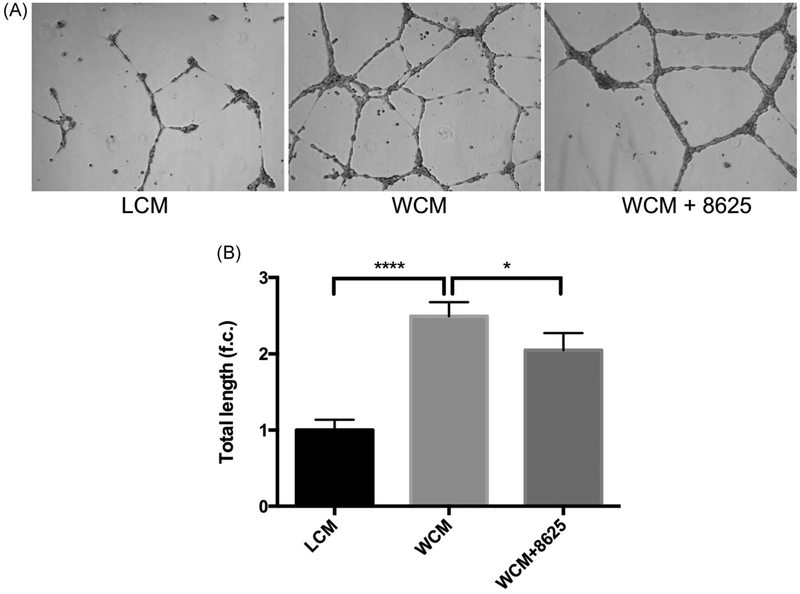
Compound 3289–8625 binds to the DVL-PDZ domain51 and blocks Wnt signaling through the inhibition of DVL in different biological systems.36,53–57 To further validate whether 3289–8625 can also block Wnt signaling in HRMECs, Wnt signaling was activated in HRMECs with Wnt-3a conditional media. AXIN2 is a known target of Wnt signaling.58,59 Whereas most Wnt signaling target genes are tissue-specific or developmental stage-specific, the AXIN2 gene is considered a global transcription target.60 As expected, we found that AXIN2 expression was elevated in HRMECs treated with Wnt-3a conditional media and that elevated AXIN2 expression was reduced when 3289–8625 was added to the Wnt-3a conditional media-treated cells (Figure 1). In addition, immunofluorescence analysis showed that HRMECs treated with WCM had enhanced β-catenin nuclear translocation compared to HRMECs treated with Wnt- 3a conditional media in presence of 3289–8625 (Figure 2). Our results indicate that 3289–8625 can effectively block Wnt signaling activity in HRMECs. Wnt signaling regulates retinal neovascularization.12,61–63 Consistent with a previous report,62 we found that treating HRMECs with Wnt-3a conditional media enhanced endothelial cell migration and stimulated tube formation (Figures 3,4). However, HRMECs treated with Wnt-3a conditional media in the presence of Wnt inhibitor 3289–8625, had a significantly slower rate of cell migration and regulated tube formation as opposed to cells treated with only Wnt-3a conditional media (Figures 3,4). These data suggest that Wnt signaling is a regulator of HRMECs migration and tube formation. Additionally, inhibiting Wnt signaling can reduce and control cell migration and tube formation in vitro, potentially affecting neovascularization.
FIGURE1.
Compound 3289–8625 regulates Wnt signaling activity in HRMECs. HRMECs were treated with either LCM, WCM, or WCM in the presence of compound 3289–8625 (50 μM) for 24 hours. AXIN2 mRNA expression levels were measured by qPCR. HRMECs treated with compound 3289-8625 presented reduced Wnt signaling levels, indicating that Wnt signaling activity can be regulated by compound 3289–8625 in HRMECs. HRMEC, human retinal microvascular endothelial cell; LCM, L-cell control conditional medium; mRNA, messenger RNA; qPCR, quantitative polymerase chain reaction; WCM, Wnt-3a conditional medium
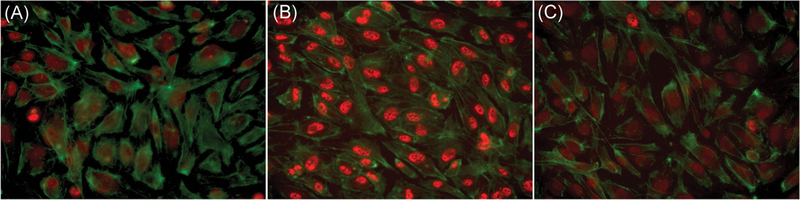
FIGURE 2.
Compound 3289–8625 suppressed β-catenin protein level, thus regulating Wnt activity in HRMECs. HRMECs were treated with LCM (A), WCM (B), or WCM in presence of compound 3289-8625 (50 μM) (C), for 24 hours. Five random fields were examined on the slide. The green channel of phalloidin staining represents the HRMECs cytoskeleton. Immunofluorescence analysis of total β-catenin protein levels, as indicated by the red channel in HRMECs, shows that WCM enhanced the translocation of β-catenin protein levels into the nucleus. Moreover, compound 3289-8625 can inhibit Wnt signaling activity in HRMECs. HRMEC, human retinal microvascular endothelial cell; LCM, L-cell control conditional medium; WCM, Wnt-3a conditional medium
FIGURE 3.
Wnt signaling inhibition reduces Wnt-3a- dependent migration in HRMECs. HRMECs were seeded into the μ-35 dishes with culture insert. Culture inserts were removed after 24 hours creating a uniformed cell-free surface. Cells were then treated with LCM, WCM, or WCM in the presence of compound 3289–8625 (50 μM). Cell images of five random views were taken after 16 hours (A). Image analysis was performed using ImageJ (B). These data show that Wnt-mediated HRMECs migration can be inhibited by compound 3289-8625 (**P 0.01, ****P 0.001 via one-way ANOVA with Dunnett’'s correction). ANOVA, analysis of variance; HRMEC, human retinal microvascular endothelial cell; LCM, L-cell control conditional medium; WCM, Wnt-3a conditional medium
FIGURE 4.
Wnt signaling inhibition reduces Wnt-3a-dependent tube formation in HRMECs. HRMECs were seeded into Matrigel-coated six-well plate. These cells were then treated with LCM, WCM, orWCM in presence of compound 3289-8625 (50 μM). Cell images of five random views were taken after 6 hours (A) then analyzed by ImageJ (B). Wnt-induced HRMECs tube formation was inhibited by compound 3289–8625 (*P < 0.05 and ****P < 0.001 via one-way ANOVA with the Dunnett correction). ANOVA, analysis of variance; HRMEC, human retinal microvascular endothelial cell; LCM, L-cell control conditional medium; WCM, Wnt-3a conditional medium
3.2 |. H2O2-induced Wnt signaling activity in HRMECs
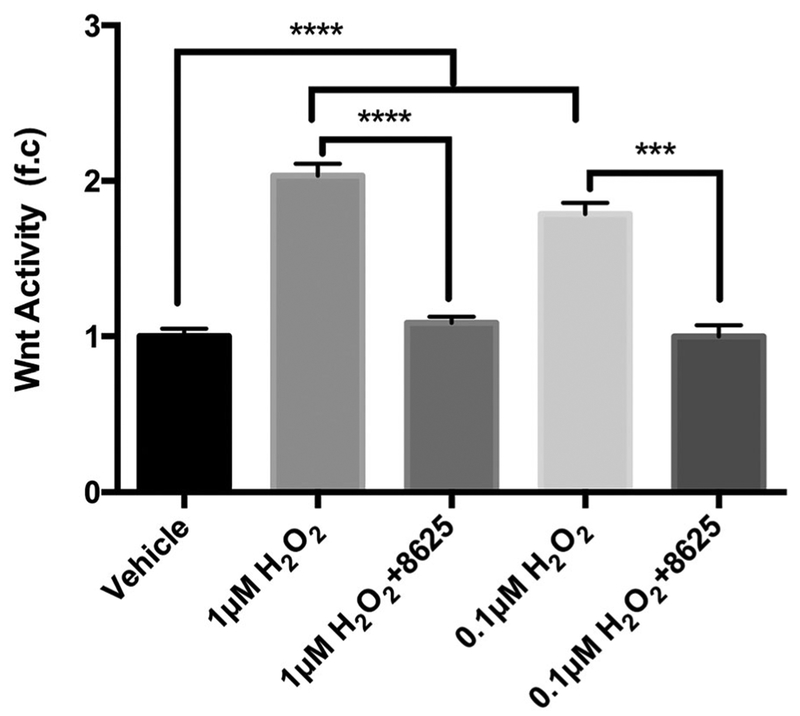
Oxidative stress stimulates Wnt signaling by activating DVL.33–35 Indeed, using low concentrations of H2O2 to mimic oxidative stress,33 enhanced levels of canonical Wnt signaling activity were detected in H2O2-treated NIH3T3 cells, and this increase of Wnt activity occurred in an H2O2 concentration-dependent manner (Figure 5). Moreover, consistent with the notion that oxidative stress activates canonical Wnt signaling at the DVL level,35 we found that compound 3289–8625, targeting the PDZ domain of DVL, significantly suppressed canonical Wnt signaling in cells treated with H2O2 (Figure 5).
FIGURE 5.
Hydrogen peroxide (H2O2) induces Wnt activation in the NIH3T3 cell, and compound 3289-8625 suppresses the H2O2-induced Wnt activation. Stable, transfected 3T3 cells expressing luciferase under control of the Wnt promoter were treated with vehicle, H2O2 (1 or 0.1 μM), or H2O2 (1 or 0.1 μM) presenting with 3289–8625 (50 μM). After 16 hours, Wnt-induced luciferase activities in 3T3 cells were detected (***P < 0.005 and ****P < 0.001 via one-way ANOVA with the Dunnett correction). ANOVA, analysis of variance; HRMEC, human retinal microvascular endothelial cell
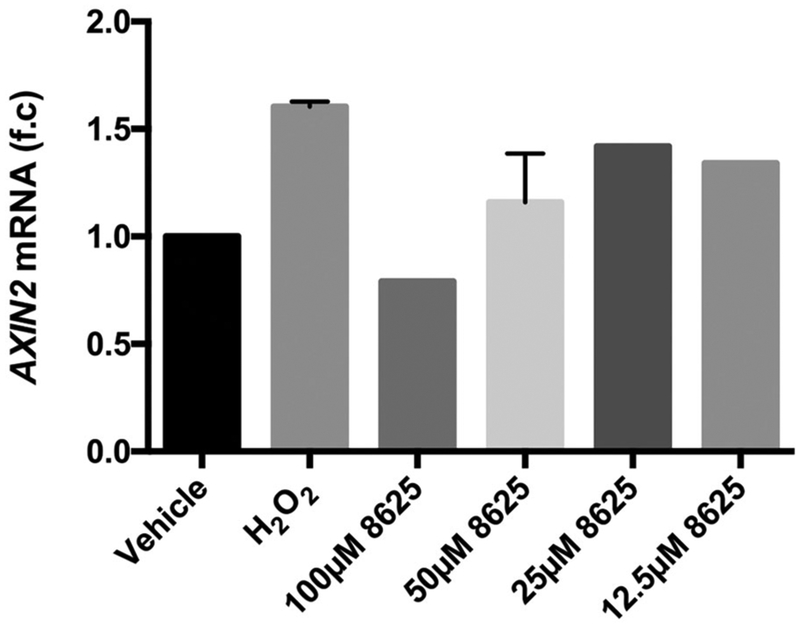
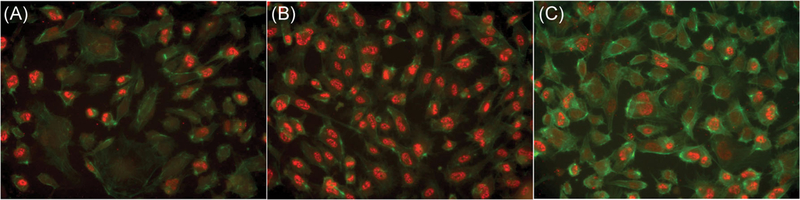
Oxidative stress has been implicated in the proliferative retinopathy stages of ROP and DR.26,27 Mouse models of OIR shows that upon removing mice from a high oxygen- concentrated environment and exposing them to normal oxygen levels, H2O2 accumulation induced by the change in environment, is observed in their retina.30,64,65 There fore, we examined whether H2O2 can also induce Wnt signaling in HRMECs. We found that following 30’minutes of H2O2 treatment, AXIN2 expression was elevated, and that AXIN2 elevation could be suppressed by compound 3289–8625 in a dose-dependent manner (Figure 6). Furthermore, immunofluorescence analysis shows that β-catenin levels were upregulated in the nucleus following 30 minutes of H2O2 treatment, and this effect could also be suppressed by adding the compound 3289–8625 to H2O2- treated HRMECs (Figure 7). These results suggest that H2O2 treatment upregulated the Wnt signaling pathway in HRMECs at the DVL level.
FIGURE 6.
Hydrogen peroxide induces Wnt activation in the HRMECs, which can be suppressed by compound 3289–8625. HRMECs were treated with vehicle, H2O2 (1 μM), or H2O2 (1 μM) presenting with compound 3289–8625 at a concentration of 100, 50, 25, or 12.5 μM. The AXIN2 mRNA expression levels in these cells were measured by qPCR. Compound 3289-8625 can reduce H2O2-induced Wnt signaling, in a concentration-dependent manner. HRMEC, human retinal microvascular endothelial cell; mRNA, messenger RNA; qPCR, quantitative polymerase chain reaction
FIGURE 7.
Hydrogen peroxide (H2O2) increases β-catenin protein level activation in HRMECs and compound 3289–8625 attenuates these effects. HRMECs were treated with (A) vehicle, (B) H2O2 (1 μM), and (C) H2O2 (1 μM) presenting with compound 3289–8625 (50 μM), for 24 hours. Total β-catenin protein levels were detected by immunofluorescence, as shown by the red channel. The green channel of phalloidin staining represents the HRMECs cytoskeleton. Data suggests H2O2 upregulated Wnt activity in the HRMECs. Compound 3289–8625 can inhibit H2O2-induced Wnt activation. HRMEC, human retinal microvascular endothelial cell
3.3 |. H2O2-induced Wnt signaling enhances cell migration and tube formation of HRMECs
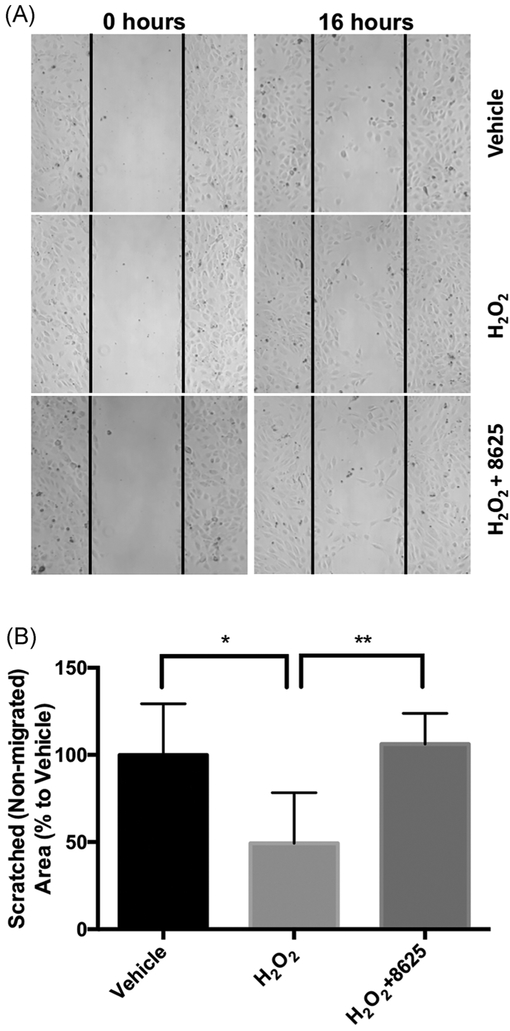
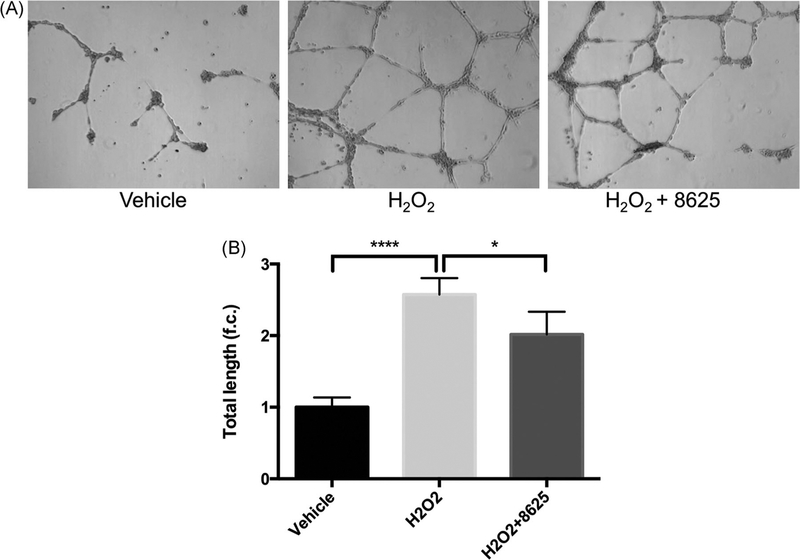
Since Wnt ligands, such as Wnt-3a, induce different angiogenic phenotypes in HRMECs,62 we next evaluated whether H2O2-activated Wnt signaling can also stimulate these similar angiogenic phenotypes in HRMECs. We found that after 30 minutes of H2O2 treatment, the cell migration rate of HRMECs was enhanced. Moreover, following the addition of 3289-8625 to the cell-culture media, this H2O2-induced cell migration was reduced (Figure 8). Similarly, we found that H2O2 treatment induced HRMECs tubular network formation by nearly threefold, yet, adding compound 3289-8625 abridged this effect (Figure 9).
FIGURE 8.
Inhibiting Wnt suppresses H2O2-induced HRMECs migration. HRMECs were seeded into the μ-35 dishes with culture-insert. Culture inserts were removed after 24 hours, creating a uniformed cell-free surface. Cells were then treated with vehicle, H2O2 (1 μM), or H2O2 (1 μM) presenting with compound 3289–8625 (50 μM). Images of five random views were taken after a 16-hour incubation (A) and then analyzed using ImageJ (B). Our analysis shows that H2O2-induced Wnt activation mediates HRMECs migration and this phenomenon is reduced with treatment of Wnt modulator, 3289-8625 (*P < 0.05 and **P < 0.01 via one-way ANOVA with the Dunnett correction). ANOVA, analysis of variance; HRMEC, human retinal microvascular endothelial cell
FIGURE 9.
Wnt inhibition decreases H2O2-induced tube formation in HRMECs. HRMECs were seeded into a Matrigel-coated six-well plate. These cells were then treated with vehicle, H2O2 (1 μM), or H2O2 (1 μM) presenting with compound 3289–8625 (50 μM). Cell images of five random views were taken after 6 hours (A) then analyzed by ImageJ (B). Tube formation stimulated by H2O2 induced Wnt signaling can be inhibited by compound 3289-8625 (*P < 0.05 and ****P < 0.001 via one-way ANOVA with the Dunnett correction). ANOVA, analysis of variance; HRMEC, human retinal microvascular endothelial cell
4 |. DISCUSSION
Wnt signaling plays a critical role in the development and differentiation of the retinal vasculature,13 including its restoration following OIR.66 Indeed, norrin, a secreted signaling molecule, which is also essential in the retinal vasculature during development, induces Wnt signaling in microvascular endothelial cells, by binding to Wnt receptor, FZD.66 Furthermore, norrin-induced Wnt signaling increases insulin-like growth factor (IGF-1) expression,66 a critical factor in the development in the retinal vasculature. Preterm babies with mutations in the norrin/FZD4/LRP5 signaling pathway have decreased levels of IGF-1 that have been associated with the impediment of retinal vascular growth and abnormal angiogenesis.23,67 Under disease conditions, dysregula tion of Wnt signaling has been linked to dysregulation of IGF-1 and has been postulated to be responsible for the ROP pathology.68
Mutations in the Wnt signaling pathway have also been linked to several pathologies including defective vascularization of the retina.48,63,69 Consistent with this notion, aberrant Wnt signaling activity has been shown in a mouse model depicting OIR.12,48 Furthermore, mutant mice lacking key components of the Wnt signaling pathway have significantly decreased levels of neovascularization,12 and this effect can be normalized using lithium, a known Wnt activator.70 In another study, a DR model showed that oxidative stress leads to Wnt signaling activation and is linked to retinal inflammation and neovascularization.71 Therefore, targeting specific proteins involved in Wnt signaling may provide insight on understanding and treating retinal neovascularization. Nevertheless, to effectively target abnormal Wnt signaling, a clear view of the mechanism which promotes aberrant Wnt signaling in the retina needs to be further elucidated.
Oxidative stress, as a result of high concentrations of ROS, has been implicated in the pathogenic role of various processes including abnormal retinal neovascularization in ROP and DR.29,30,72–74 It is known that ROS acts as an intracellular signaling mediator, triggering different signaling pathways including the Wnt signaling pathway. Specifically, it has been reported that oxidative stress can regulate Wnt/β-catenin signaling in a redox-dependent manner.33,35 In physiological conditions, a thioredoxin- related protein, NXN, is directly bound to the PDZ domain of DVL, through the thiol moiety (-SH) of cysteine, inhibiting the function of DVL in the Wnt/β-catenin signaling cascade. Inhibition of DVL allows β-catenin to form a “destruction complex” consisting of Axin, APC, and GSK-3β resulting in the phosphorylation of β-catenin by a proteasome.33–35 Treating cells with a low concentration of H2O2 can cause a thiol-disulfide bond to form, leading to the conformational change of NXN. As a result, the inhibitory function of NRX is effected, causing its dissociation from DVL and allowing for DVL to interact with FZD, thus allowing for the upregulation of Wnt signaling and triggering neovascularization.33 In this present study, we showed that H2O2 enhances vascular- related activities of HRMECs such as tube formation and cell migration. The phenotype induced by H2O2 is similar to that induced by Wnt-3a. However, in the presence of oxidative stress, a small-molecule Wnt inhibitor, targeting the PDZ domain of DVL, can take the place of NXN and modulate Wnt signaling as well as neovascularization. This prevents the accumulation of β-catenin and thus its translocation into the nucleus. As a result, transcription of downstream Wnt signaling genes, including VEGF, does not occur. Furthermore, taking advantage of this small- molecule, we demonstrate that oxidative stress activates Wnt signaling and stimulates the angiogenic phenotypes at the DVL level. Therefore, our findings suggest that ROS-mediated Wnt signaling can be regulated by a small- molecule Wnt inhibitor targeting the PDZ domain of DVL, which in turn can reduce the proangiogenic phenotype of HRMECs. This notion is consistent with the in vivo study showing that the mice lacking Dvl2 have significantly reduced levels of pathological neovascularization.12
Although oxidative stress may potentially cause pathological neovascularization, ROS still serves as a line of defense against bacteria and other agents.31,75 However, under diseased conditions, ROS, such as H2O2, can trigger angiogenesis by functioning as signaling molecules to regulate cellular signaling,27 with Wnt signaling being one of the pathways induced by ROS. However, Wnt signaling is important for normal retinal angiogenesis and retinal development, especially in premature infants.76 Because ROS activates abnormal Wnt signaling at the DVL level, therefore, dynamically easing aberrant ROS-mediated Wnt signaling by selectively targeting the DVL protein with a small-molecule reagent may lead to the development of a novel therapeutic approach for ROP treatment.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Sarah D Ahadome for her discussions regarding experimental setup. The authors would also like to thank the lab of Dr. Sophie X Deng for the use of the fluorescence microscope and qPCR machine. We also acknowledge Alexandro Guerrero, Alseena Thomas, Edmond Ma, and Freddi Tran for all their help within the lab. This study was supported in part by NIH grants R01GM100909 and R01EY028557, and by Research to Prevent Blindness.
Funding information
National Institute of General Medical Sciences; Research to Prevent Blindness, Grant/Award Numbers: R01GM100909, R01EY028557
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Moran EP, Wang Z, Chen J, Sapieha P, Smith LE, Ma JX. Neurovascular cross talk in diabetic retinopathy: pathophysio-logical roles and therapeutic implications. Am J Physiol Heart Circ Physiol. 2016;311:H738–H749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neely KA, Gardner TW. Ocular neovascularization: clarifying complex interactions. Am J Pathol. 1998;153:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohlmann A, Seitz R, Braunger B, Seitz D, Bosl MR, Tamm ER. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J Neurosci. 2010;30:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Che XIN, Fan X-Q, Wang Z-L. Mechanism of blood-retinal barrier breakdown induced by HIV-1 (Review). Exp Ther Med. 2014;7:768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engerman RL, Pfaffenbach D, Davis MD. Cell turnover of capillaries. Lab Invest. 1967;17:738–743. [PubMed] [Google Scholar]
- 8.Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto K, Khosrof S, Bursell SE, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA. 1999;96:10836–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robitaille J, MacDonald ML, Kaykas A, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32:326–330. [DOI] [PubMed] [Google Scholar]
- 11.Saint-Geniez M, Jiang A, Abend S, Liu L, Sweigard H, Connor KM, Arany Z, et al. PGC-1α regulates normal and pathological angiogenesis in the retina. Am J Pathol. 2013;182:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Stahl A, Krah NM, et al. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation. 2011;124:1871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimura N WNT/β-catenin signaling in vertebrate eye development. Front Cell Dev Biol. 2016;4:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi Y, Chen Q, Rajala RVS, Ma JX. MicroRNA-184 modulates canonical Wnt signaling through the regulation of frizzled-7 expression in the retina with ischemia-induced neovascularization. FEBS Lett. 2015;589:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Zhou KK, Ma J-X. Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the WNT/β-catenin pathway. Diabetes. 2010;59:1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou T, Hu Y, Chen Y, et al. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:4371–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura S, Imai S, Ogishima H, Tsuruma K, Shimazawa M, Hara H. Morphological and functional changes in the retina after chronic oxygen-induced retinopathy. PLOS One. 2012;7:e32167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeilbeck LF, Muller BB, Leopold SA, et al. Norrin mediates angiogenic properties via the induction of insulin-like growth factor-1. Exp Eye Res. 2016;145:317–326. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Ma J-X. Wnt pathway antagonists and angiogenesis. Protein Cell. 2010;1:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharadwaj AS, Appukuttan B, Wilmarth PA, et al. Role of the retinal vascular endothelial cell in ocular disease. Prog Retin Eye Res. 2013;32:102–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masckauchan TN, Kitajewski J. Wnt/Frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology (Bethesda). 2006;21:181–188. [DOI] [PubMed] [Google Scholar]
- 24.Cabral T, Mello LGM, Lima LH, et al. Retinal and choroidal angiogenesis: a review of new targets. Int J Retina Vitreous. 2017;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen JJ, Pohl SÖ-G, Deshmukh A, Visweswaran M, Ward NC, Arfuso F, Agostino M, Dharmarajan A, et al. The role of Wnt signalling in angiogenesis. Clin Biochem Rev. 2017;38:131–142. [PMC free article] [PubMed] [Google Scholar]
- 26.Katz ML, Robison WG Jr. Autoxidative damage to the retina: potential role in retinopathy of prematurity. Birth Defects Orig Artic Ser. 1988;24:237–248. [PubMed] [Google Scholar]
- 27.Wang H, Zhang SX, Hartnett ME. Signaling pathways triggered by oxidative stress that mediate features of severe retinopathy of prematurity. JAMA Ophthalmol. 2013;131:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y-W, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartnett ME. The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model. Doc Ophthalmol. 2010;120:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jivabhai Patel S, Bany-Mohammed F, McNally L, et al. Exogenous superoxide dismutase mimetic without scavenging H2O2 causes photoreceptor damage in a rat model for oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2015;56:1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calderon GD, Juarez OH, Hernandez GE, Punzo SM, De, la Cruz ZD. Oxidative stress and diabetic retinopathy: development and treatment. Eye (Lond). 2017;31:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tayyari F, Khuu LA, Flanagan JG, Singer S, Brent MH, Hudson C. Retinal blood flow and retinal blood oxygen saturation in mild to moderate diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56:6796–6800. [DOI] [PubMed] [Google Scholar]
- 33.Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin- related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–508. [DOI] [PubMed] [Google Scholar]
- 34.Funato Y, Miki H. Nucleoredoxin, a novel thioredoxin family member involved in cell growth and differentiation. Antioxid Redox Signal. 2007;9:1035–1057. [DOI] [PubMed] [Google Scholar]
- 35.Funato Y, Miki H. Redox regulation of Wnt signalling via nucleoredoxin. Free Radic Res. 2010;44:379–388. [DOI] [PubMed] [Google Scholar]
- 36.Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22:717–727. [DOI] [PubMed] [Google Scholar]
- 37.Habas R, Dawid IB. Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol. 2005;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wynshaw-Boris A Dishevelled: in vivo roles of a multi-functional gene family during development. Curr Top Dev Biol. 2012;101:213–35. [DOI] [PubMed] [Google Scholar]
- 39.Wong HC, Bourdelas A, Krauss A, et al. Direct binding of the PDZ domain of dishevelled to a conserved internal sequence in the C-terminal region of frizzled. Mol Cell. 2003;12:1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etheridge SL, Ray S, Li S, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLOS Genet. 2008;4:e1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamblet NS, Lijam N, Ruiz-Lozano P, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Yuan H, Xie W, et al. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J Biol Chem. 1999;274:129–134. [DOI] [PubMed] [Google Scholar]
- 43.Lijam N, Paylor R, McDonald MP, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. [DOI] [PubMed] [Google Scholar]
- 44.Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:1637–1647. [DOI] [PubMed] [Google Scholar]
- 45.Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. [DOI] [PubMed] [Google Scholar]
- 46.Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. DIshevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. [DOI] [PubMed] [Google Scholar]
- 47.Umbhauer M, Djiane A, Goisset C, et al. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J. 2000;19:4944–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Stahl A, Krah NM, et al. Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLOS One. 2012;7:e30203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran FH, Zheng JJ. Modulating the Wnt signaling pathway with small molecules. Protein Sci. 2017;26:650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res. 2007;74:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, Shi D-L, Zheng JJ, et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem. 2009;284:16256– 16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jati S, Kundu S, Chakraborty A, Mahata SK, Nizet V, Sen M. Wnt5A signaling promotes defense against bacterial pathogens by activating a host autophagy circuit. Front Immunol. 2018;9:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HJ, Shi DL, Zheng JJ. Conformational change of dishevelled plays a key regulatory role in the Wnt signaling pathways. Elife. 2015;4:e08142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lina TT, Luo T, Velayutham T-S, Das S, McBride JW. Ehrlichia activation of Wnt-PI3K-mTOR signaling inhibits autolysosome generation and autophagic destruction by the mononuclear phagocyte. Infect Immun. 2017;85:e00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo K, Gu X, Liu J, et al. Inhibition of disheveled-2 resensitizes cisplatin-resistant lung cancer cells through down-regulating Wnt/beta-catenin signaling. Exp Cell Res. 2016;347:105–13. [DOI] [PubMed] [Google Scholar]
- 57.Xu W, He L, Li Y, Tan Y, Zhang F, Xu H. Silencing of lncRNA ZFAS1 inhibits malignancies by blocking Wnt/β-catenin signaling in gastric cancer cells. Biosci Biotechnol Biochem. 2018;82:456–465. [DOI] [PubMed] [Google Scholar]
- 58.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of AXIN2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan D, Wiesmann M, Rohan M, et al. Elevated expression of AXIN2 and hnkd mRNA provides evidence that Wnt/beta-catenin signaling is activated in human colon tumors. Proc Natl Acad Sci USA. 2001;98:14973–14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. [DOI] [PubMed] [Google Scholar]
- 61.Busik JV, Grant MB. Wnting out ocular neovascularization: using nanoparticle delivery of VLDL receptor extracellular domain as Wnt pathway inhibitor in the retina. Arterioscler Thromb Vasc Biol. 2015;35:1046–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Cheng R, Lee K, et al. Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization. Arterioscler Thromb Vasc Biol. 2015;35:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high- affinity ligand-receptor pair. Cell. 2004;116:883–895. [DOI] [PubMed] [Google Scholar]
- 64.Beharry KD, Cai CL, Sharma P, et al. Hydrogen peroxide accumulation in the choroid during intermittent hypoxia increases risk of severe oxygen-induced retinopathy in neonatal rats. Invest Ophthalmol Vis Sci. 2013;54:7644–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stahl A, Connor KM, Sapieha P, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeilbeck LF, Müller BB, Leopold SA, et al. Norrin mediates angiogenic properties via the induction of insulin-like growth factor-1. Exp Eye Res. 2016;145:317–326. [DOI] [PubMed] [Google Scholar]
- 67.Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015;122:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shastry BS. Genetic susceptibility to advanced retinopathy of prematurity (ROP). J Biomed Sci. 2010;17:69–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smallwood PM, Williams J, Xu Q, Leahy DJ, Nathans J. Mutational analysis of Norrin-Frizzled4 recognition. J Biol Chem. 2007;282:4057–4068. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Liu CH, Sun Y, et al. Pharmacologic activation of Wnt signaling by lithium normalizes retinal vasculature in a murine model of familial exudative vitreoretinopathy. Am J Pathol. 2016;186:2588–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, Boulton M, Lyons TJ, Gao G, Ma J-X, et al. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol. 2009;175:2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong A, Xie B, Shen J, et al. Oxidative stress promotes ocular neovascularization. J Cell Physiol. 2009;219:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008;49:1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang H, Han X, Wittchen ES, Hartnett ME. TNF-alpha mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent beta-catenin activation. Mol Vis. 2016;22:116–128. [PMC free article] [PubMed] [Google Scholar]
- 75.Ozsurekci Y, Aykac K. Oxidative stress-related diseases in newborns. Oxid Med Cell Longev. 2016;2016:2768365–2768369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drenser KA. Wnt signaling pathway in retinal vascularization. Eye Brain. 2016;8:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]