Abstract
Background
Magnetic Resonance Imaging (MRI) techniques to image the larynx have evolved rapidly into a promising and safe imaging modality, without need for sedation or ionizing radiation. MRI is therefore of great interest to image pediatric laryngeal diseases. Our aim was to review MRI developments relevant for the pediatric larynx and to discuss future imaging options.
Methods
A systematic search was conducted to identify all morphological and diagnostic studies in which MRI was used to image the pediatric larynx, laryngeal disease, or vocal cords.
Results
Fourteen articles were included: three studies on anatomical imaging of the larynx, two studies on Diffusion Weighted Imaging, four studies on vocal cord imaging and five studies on the effect of anaesthesiology on the pediatric larynx. MRI has been used for pediatric laryngeal imaging since 1991. MRI provides excellent soft tissue contrast and good visualization of vascular diseases such as haemangiomas. However, visualization of cartilaginous structures, with varying ossification during childhood, and tissue differentiation remain challenging. The latter has been partly overcome with diffusion weighted imaging (DWI), differentiating between benign and malignant masses with excellent sensitivity (94‐94.4%) and specificity (91.2‐100%). Vocal cord imaging evolved from static images focused on vocal tract growth to dynamic images able to detect abnormal vocal cord movement.
Conclusion
MRI is promising to evaluate the pediatric larynx, but studies using MRI as diagnostic imaging modality are scarce. New static and dynamic MR imaging techniques could be implemented in the pediatric population. Further research on imaging of pediatric laryngeal diseases should be conducted.
Keywords: imaging, larynx, magnetic resonance imaging, pediatric
1. INTRODUCTION
Pathologies of the larynx in children are rare but severe conditions, and can have life‐long consequences. The development of the larynx, normally completed around the 10th week of gestation, can fail at various time‐points resulting in a variety of congenital laryngeal diseases.1 After birth, the pediatric larynx is sensitive to acquired laryngeal insults, such as infectious diseases, inflammatory processes, and traumatic injuries (Table 1).1 Severe cases might be in need for surgical intervention, such as micro‐ laryngeal surgery, laryngotracheal reconstruction, or tracheostomy.1 In the adult population, the most common diseases in the laryngeal region are of neoplastic origin.2
Table 1.
Pediatric laryngeal pathologies for which MR imaging of the laryngeal region could be of use, freely adapted from Cummings Otolaryngology46
| Congenital | Acquired | Infectious/inflammatory | Neoplasms | Vocal cord disorders |
|---|---|---|---|---|
| Laryngomalacia | Trauma | Laryngotracheobronchitis | Benign | Vocal cord nodule |
| Laryngeal atresia | Acquired glottic stenosis | Epiglottitis | Haemangioma | Vocal cord polyp |
| Laryngeal web | Mucosal trauma | Retropharyngeal abscess | Papillomatosis | Vocal cord granuloma |
| Laryngeal cleft | Blunt trauma | Diphtheria | Laryngeal/saccular cysts | Vocal cord paralysis |
| Congenital glottic stenosis | Tuberculosis | Laryngocele | Acquired | |
| Penetrating trauma | Granulomatosis Sarcoidosis | Subglottic cyst | Congenital Paradoxic vocal cord motion | |
| Gastro‐pharyngeal reflux | Rheumatic disease | Neurofibroma | ||
| Malignant | ||||
| Recurrent respiratory papillomatosis | Sarcoma | |||
| Relapsing polychondritis | Squamous cell carcinoma | |||
| Lymphoma | ||||
| Mucoepidermoid carcinoma | ||||
| Neuroectodermal tumour | ||||
| Metastatic carcinoma |
To understand the nature of laryngeal pathologies, multiple imaging modalities can be used.1, 3, 4 In the pediatric population, the choice of the right modality should be carefully considered according to the wide variety of pathologies and the patient's age. Direct laryngoscopy is the gold standard for early diagnostics of the pediatric larynx and vocal cords, but its disadvantages are the operator‐dependant diagnostic accuracy, the absence of objective measurements, limited detailed imaging, and the need for anesthetics to get a clear view of the subglottis.5, 6, 7 Computed Tomography (CT) is used for extensive imaging of the larynx after surgical intervention. A limitation of CT is the exposure to radiation, which is especially important in laryngeal imaging because it exposes the thyroid, one of the most sensitive organs, to radiation.8 This explains the limited use of CT for dynamic imaging of the larynx.
Over the past decades, Magnetic Resonance Imaging (MRI) techniques have rapidly evolved into a promising and safe modality to image the larynx, with the availability of faster sequences and specialized surface coils.3, 9, 10, 11 However, most studies have been conducted in the adult population and are focused on laryngeal malignancies. There is a dearth of pediatric MR laryngeal imaging. Though, MRI is of great interest for imaging pediatric laryngeal diseases, as sedation is not needed in older children and the use of MRI is not accompanied by exposure to radiation. This makes MRI ideal for dynamic evaluation of the larynx and vocal cords.
The aim of this study is to review the existing literature for recent developments in MR imaging of the pediatric larynx and discuss future innovative MR imaging.
2. METHODS
2.1. Search strategy
A systematic search was developed to identify all morphological and diagnostic studies in which MRI was used to image the pediatric larynx. The search protocol was set up according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement.12 A search was conducted on “pediatric” and “larynx” or “laryngeal disease” or “vocal cords” and “magnetic resonance imaging,” the detailed search strategy is attached in online supplement S1. Data bases used were: EMBASE, Medline Ovid, Web of Science, Cochran and Google Scholar. In addition, the reference lists of the included articles were searched for additional articles of interest. Only original papers were included in this review. Reviews and case reports on less than ten patients were excluded due to risk of bias. The search was conducted in October 2017 and updated in December 2018.
2.2. Article selection
The article selection was done by two independent reviewers (BE, SH). Each reviewer evaluated the abstracts on the type of article, the imaged anatomic region and cohort size (n ≥ 10) and age (≤18 years). Selected full‐text articles were again read on the type of article, imaged anatomic region, and the cohort size and age, and sufficient MR imaging data. “Sufficient” was defined as the following elements; MR scanner type and field strength, imaging parameters used, contrast, and/or sedation requirements. Disagreement on inclusion/exclusion based on abstract or full text of the articles was resolved by consensus.
3. RESULTS
3.1. Study selection
Eight hundred sixty‐three articles were identified. Duplicate studies were eliminated and 860 articles were screened for title and abstract based on article type, anatomical area imaged, and number of patients included. The full text of 76 selected articles was reviewed for sufficient methodological information. Five articles were excluded based on incorrect article type, 18 on incorrect anatomic region, 12 on cohort size, 9 on cohort age, and 18 on insufficient MRI data, resulting in a final inclusion of 14 articles. The flowchart of the article selection is shown in Figure 1. Table 2 shows the final 14 articles selected for this review, online supplement S2 shows technical MRI information on the articles selected.
Figure 1.
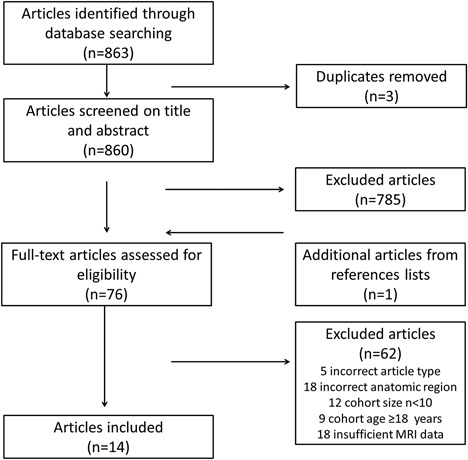
Flowchart of the article selection
Table 2.
Key features for all selected articles that resulted from our systematic review
| Study | Study population (n) | Age range (years) | Scanner | Sedation or anesthetics | Contrast (yes/no) | Sequences | Study aim | Main study result |
|---|---|---|---|---|---|---|---|---|
| Yuh13 | 53 | 0‐18 | Picker International, 0.5T GE, 1.5T | No | Both | T1, T2 | Characterization of pediatric head/neck masses | MRI can accurately characterize pediatric head/neck masses |
| Hudgins14 | 15 | 0‐16 | Not defined, 1.5T | No | No | T1, FSE T2 | Visualization of the normal pediatric larynx | The pediatric and adult larynx differ in size, position, consistency, and shape as seen on MRI |
| Fitch20 | 129 | 2.8‐25 | GE, 1.5T | No | No | T1 | Quantification of vocal tract morphology during development | The post pubertal vocal tract is larger in males compared to females |
| Faust21 | 10 | 0‐16 | Siemens, 1.5T | No | No | SE T1, SE T2, cine‐ MRI | Dynamic visualization of the pediatric airway | Cine MRI can be used to visualize vocal cord movement in children (feasibility study) |
| Mahboubi15 | 45 | 0‐2 | Siemens, 1.5T | No | Both | SE T1 | Visualization of pediatric upper airway obstruction | MRI can characterize pediatric airway abnormalities with high image quality |
| Litman25 | 99 | 0‐14 | GE, 1.5T | Sedation | No | SE T1 | Determination of the effect of age on pediatric laryngeal diameter | In sedated children of all ages the narrowest part of the airway is the glottic opening |
| Litman26 | 17 | 2‐11 | Siemens, 1.5T | Anesthesia | No | T1 | Evaluation of the effect of lateral positioning on the pediatric laryngeal diameter | Lateral positioning increases the airway dimensions in children |
| Vorperian22 | 63 | 0‐6.6 | GE, 1.5T Resonex, T not specified | Sedation | No | T1, T2 | Evaluation of the growth pattern of the vocal tract | The vocal tract continues to grow from birth until 6.6 years of age without gender differences |
| Vialet27 | 30 | 0‐8.8 | Siemens, 1.5T | Anesthesia | No | SE T1 | Evaluation of the effect of head extension on pediatric laryngeal diameter | Head extension increases the laryngeal visualization in pediatric patients |
| Abdel Razek18 | 78 | 0‐15 | Siemens, 1.5T | Sedation | Both | T1, FSE T2, DWI | Characterization of pediatric laryngeal masses with DWI | DWI can differentiate benign from malignant laryngeal masses with sensitivity 94.4% and specificity 91.2% |
| Vorperian24 | 307 | 0‐19 | GE, 1.5T Resonex, T not specified | Sedation | No | SE T1, FSE T2 | Evaluation of developmental sex differences in vocal tract length | Sex differences in vocal tract length exist before puberty |
| Taha19 | 49 | 5‐82 | Philips, 1.5T | No | Yes | T1, T2, DWI | Characterization of laryngeal masses with DWI | DWI can differentiate benign from malignant laryngeal masses with sensitivity 94% and specificity 100% |
| Bécret28 | 155 | 0‐18.5 | Siemens, 1.5T | Anesthesia | No | SE T1 | Quantification of the effect of age on airway modifications due to head extension | In children of all ages head extension increases the visualization of the larynx |
| Aqil29 | 60 | 0‐12 | Siemens, 3T | Anesthesia | No | RGE T1 | Visualization of anatomical changes caused by different pediatric airway devices | Supraglottic airway devices alter pediatric airway dimensions |
DWI, diffusion weighted imaging; FSE, fast spin‐ echo; RGE, rapid gradient echo; SE, spin‐echo; T, Tesla; T1, T1 weighted image; T2, T2 weighted imaging.
3.2. Anatomical imaging
The first description of the pediatric larynx on MRI dates from 1991.13 On 53 MRIs of patients aged between 1 day and 18 years, various laryngeal lesions, such as haemangioma, were evaluated and compared to CT in 25 cases. The images were evaluated by looking at four lesion characteristics: tissue contrast, detectability, extent, and origin. All MRIs were made on a 0.5 or 1.5T scanner, and at least one T1 weighted image and one T2 weighted image were obtained. Despite the use of these early scanning techniques, good anatomical visualization of mainly soft tissue was achieved. MRI was found to be excellent for all four lesion characteristics. T1 weighted images showed the best anatomical detail and T2 weighted images showed the best image contrast. Disadvantages of MRI were the difficulty to distinguish between inflammatory and malignant lesions, and difficulty to identify bony involvement due to changing ossification of cartilaginous structures in the developing pediatric larynx. Despite these disadvantages, MRI was proposed as an imaging tool to be superior to CT for the visualization of complex laryngeal structures, because of its excellent anatomical visualization.
In 1997 a 1.5T scanner was used to compare differences in anatomy and pathologies of the larynx between children and adults.14 Despite the increased field strength of the MRI scanner, difficulties in the visualization of bony involvement, motion artefacts, poor spatial resolution compared to CT, and the inability to generate thin slices were seen as disadvantages for the clinical use of MRI.
However, in 2001 Mahboubi et al15 using a 1.5T scanner, managed to produce remarkably good images of pediatric laryngeal lesions, such as haemangiomas and lymphangiomas, and their surrounding structures. Although visualization of bony involvement remained superior on CT, this study confirmed the great potential of MRI as a possible diagnostic modality for pediatric laryngeal lesions.
3.3. Diffusion weighted imaging
For use of MRI for the diagnostics of pediatric laryngeal lesions, the most important disadvantages had to be overcome, especially the visualization of bony structures and tissue characterization. Inflammatory and malignant masses may have similar MRI signal intensity and the extent of mass can be hard to visualize, but is important to determine treatment options and prognosis. To overcome these difficulties Diffusion Weighted Imaging (DWI) of the laryngeal region was introduced in 2001 to characterize masses by their cellular density based on free motion of water protons.16, 17 Structures with cellular swelling or a high cellular density, such as malignancies, have less free water proton movement. In the first study that used DWI to image the pediatric larynx, multiple benign, and malignant masses; such as haemangioma, neurofibroma, and non‐Hodgkin lymphoma, were visualized.18 Benign masses could easily be discriminated from malignant masses with a sensitivity of 94.4% and a specificity of 91.2%.18 Only two benign masses were falsely characterized as malignant due to the presence of high cellular compact bodies and fibrosis. In 2015 Taha et al19 confirmed the feasibility of DWI to differentiate between benign and malignant laryngeal masses. They were able to detect the nature of laryngeal masses with a sensitivity of 94% and a specificity of 100% in an adult and pediatric cohort.
3.4. Vocal cord imaging
The development of MRI sequences to visualize soft tissue allowed imaging of the vocal cords. The vocal cords were first imaged using MRI to define the differences in growth and maturation of the vocal tract between genders. In 129 children aged between 2 and 25 years the vocal tract was measured on MRI.20 The laryngeal length increased over the years and its length correlated to body composition. Only small differences in vocal tract length were observed between boys and girls before puberty. However, in post pubertal boys a highly significant lengthening of the vocal tract was observed compared to girls. These differences were seen as a possible explanation for voice differences between the post pubertal genders.
In 2001 the first dynamic study of the vocal tract was conducted.21 Faust et al21 used cine‐ MRI to image the vocal tract in pediatric patients with impaired vocal function and healthy volunteers. The images, obtained during respiration and phonation, were viewed in a cine loop format. These cine‐MRI images could easily identify impaired vocal cord movement and even showed patient‐reported symptoms that could not be seen on endoscopy or static MRI. Figure 2 shows an axial MRI image of a healthy larynx during respiration (A) and phonation (B) from this study.21
Figure 2.
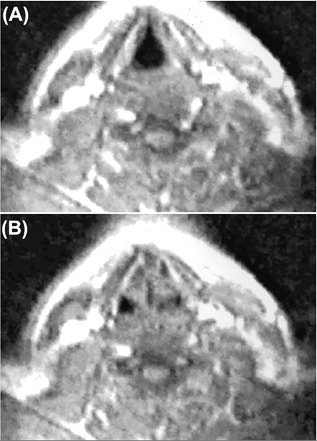
Axial TurboFLASH cine‐MRI image (TR 2.5 ms, TE 1.2 ms, acquisition time 10 s per slice) with the use of a 1.5T MR imaging system (Siemens) of a healthy larynx during respiration (A) and phonation (B), showing bilateral symmetric vocal cord adduction during phonation.21 (With permission)
Vorperian et al22 imaged the vocal tract in 63 children aged between 2 weeks and 6.6 years old. The MRIs were used to assess the effect of the development of soft and hard laryngeal structures on the vocal tract. During childhood the larynx descends from the spinal level C3‐C4 to C6‐C7.23 This laryngeal descent was found to account for 45‐65% of the vocal tract lengthening, depending on specific age ranges. These findings emphasized the contribution of the larynx on voice development and showed that MRI was able to visualize the laryngeal region in different age groups. Vorperian et al24 also evaluated vocal tract differences between genders, by measuring the vocal tract length on 307 MRIs of children aged between 0 and 19 years. The vocal tract length, measured on sagittal images as the length from the lips until the larynx, was significantly increased in boys compared to girls starting from an age of 12 years. This confirmed the gender differences shown in their earlier study.22
3.5. Anesthetics in imaging
An important advantage of MRI for imaging of the pediatric larynx is the limited need for anesthetics. However, many of the earlier studies on MR imaging of the pediatric larynx had the primary aim to visualize the effects of anesthesia and deep sedation on the airway.25, 26, 27, 28, 29 A reported side effect of anesthetics is respiratory distress, which in children is mainly caused by apnoea and upper airway collapse.30 Despite multiple studies reporting the anatomy of the sedated pediatric larynx, none of the studies made a direct comparison between sedated and non‐sedated patients.
The effect of sedation on the pediatric larynx was first described by Litman et al,25 using MRI to study the effect of deep sedation on the airway in children between 0 and 14 years. The most narrow part of the airway was at the level of the glottic opening in all age groups. In contrast to previous cadaver studies describing the cricoid ring as the most narrow part of the airway. This discrepancy might have been caused by the lack of volume standardization during the MRIs, such as the use of a spirometer controlled MRI acquisition.31 This might have resulted in images acquired during airway expiration, showing minor collapse of the laryngeal region. Another possible explanation is the use of propofol which can induce vocal cord tension, although this is not expected when sedation is maintained at a constant level.
Litman et al, Vialet et al, and Bécret et al further investigated the sedated laryngeal dimensions, concluding that the collapse of the pediatric airway during sedation can be partly overcome by positioning the child in either lateral or neutral position with a slight head extension.26, 27, 28
In the above mentioned studies, anesthetics was used as part of the research protocol, aiming to image the sedated airway. In other studies, anesthetics was used according to clinical protocols for scanning of non‐cooperative young children.14, 15, 18, 22, 24 In the study by Fitch et al,20 where children as young as 2 years were instructed to lie still instead of using sedation, some images had to be excluded because of motion artefacts, which made it impossible to visualize the glottis.
4. DISCUSSION
Current MRI protocols are well capable of visualizing soft tissue and vascular structures of the larynx (Figure 3 and online supplement S3). Fourteen publications on MR imaging of the larynx in the pediatric population were identified, mainly focusing on anatomy and anesthetics, however these findings show that MRI can be a valuable imaging tool for the visualization of several pediatric laryngeal diseases (Table 1).
Figure 3.
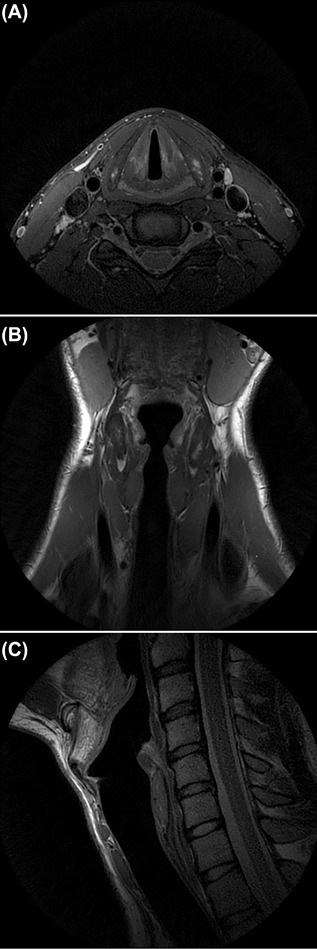
High‐resolution T2 FSE weighted (PROPELLER) axial (A), coronal (B), and sagittal (C) images of the larynx of a healthy volunteer. Pediatric laryngeal MRI protocol developed at the Erasmus MC—Sophia Children's Hospital, with the use of a 3T MRI (GE Healthcare) using a 6 Chanel Carotid coil (spatial resolution 0.5x.0.5 (in plane) x 2 mm).
4.1. Anatomical imaging
The studies found show that MRI is the best imaging modality for detailed anatomy of the healthy pediatric larynx,14 and laryngeal lesions such as haemangioma and cysts.13, 15
MR imaging of the pediatric larynx remains challenging due to the developing anatomy and the variety of laryngeal pathologies. Limitations of MRI include limited spatial resolution and longer scanning times when compared to CT. Images can be degraded by artefacts due to general patient movement or by swallowing or coughing.32, 33, 34 Interestingly in this series of publications MR was described as an imaging technique that is relatively insensitive to motion and since the larynx stays in the same horizontal plane during respiration, the presence of respiratory artefacts is unlikely.14, 20 Technical developments in recent years have resulted in improved spatial resolution and dedicated hardware, such as the availability of 3T scanners and neck surface coils, are expected to further decrease scanning times and increase image quality.35
The findings described in pediatric studies are in line with studies conducted in the adult population, describing T1 and T2 sequences to provide excellent soft tissue characterization of the larynx.11, 32, 36 This is particularly important for laryngeal diseases with complex anatomy, such as laryngotracheal stenosis, laryngoceles, and haemangioma which are easily identified on MRI.3, 32 Most selected studies compared the anatomy as shown on MRI to CT. No pediatric studies comparing MRI to laryngoscopy were found, but a comparative study in the adult population shows promising results in favor of MRI.6
4.2. Tissue characterization
The visualization of cartilaginous structures of the pediatric larynx, with varying ossification stages during childhood, and the differentiation between inflammatory and malignant masses remain challenging on MRI,11 but these are considered important factors in disease treatment and prognosis.9, 11, 32 In the pediatric population, malignant masses are extremely rare, but benign lesions such as cysts and haemangioma are more common.37, 38, 39 Two studies on the imaging of pediatric laryngeal lesions with T1 and T2 sequences were identified. These studies both described the differentiation between benign and malignant lesions and bony involvement to be inferior on MRI compared to CT. However, studies by Abdel Razek et al and Taha et al showed that the latter can be partly overcome with DWI,18, 19 which is in line with adult studies.9, 11, 40 Tissue characterization over time has been shown in adult cohorts to be challenging, because inflammation and fibrosis after surgery or radiation is hard to distinguish from the primary lesion.40 These challenges were not reviewed in this series, hence there is need for longitudinal studies in the pediatric population.
Another option to improve tissue characterization is the use of intravenous contrast. In three of the studies identified, contrast enhanced MRI was used.15, 18, 19 In the studies by Taha et al and Abdel Razek et al gadolinium contrast was used in a diagnostic setting, but the advantage of contrast enhancement is not described.18, 19 Only the study by Mahboubi et al15 described contrast administration to aid in the diagnosis of pediatric laryngeal lesions by enhancing vascular and malignant lesions. It should be taken into account that intravenous contrast has important disadvantages related to costs, renal impairment, possible allergic reactions, and the need for an intravenous cannula. Moreover, recent publications related to deposition of MRI contrast in human tissue have compelled the European Medicines Agency (EMA) to ban the use of specific gadolinium contrast media limiting their use to specific oncological purposes.41, 42, 43, 44
4.3. Vocal cord imaging
The imaging of the vocal cords has evolved from static images evaluating the growth of the vocal tract to dynamic cine‐MRI for the visualization of (impaired) vocal cord movement.
The studies identified show promising results of using MRI for dynamic imaging of vocal cord function. Studies in the adult population show that quantification of vocal cord function is possible using dynamic MRI.10, 45
4.4. Anesthetics in imaging
The narrow and noisy environment of an MRI scanner, together with the need for a subject to lie still during the MRI examination often requires the use of anesthetics for laryngeal MRI investigations in children younger than 5 years.18, 24 Many of the studies identified in relation to anesthetics were conducted primarily for the development of safer methods for pediatric airway management during sedation or anesthesia.25, 26, 27, 28, 29 These studies do not clearly answer the question if anesthetics is a prerequisite for laryngeal MRI in the pediatric population. However, these studies do show that the dimensions of the larynx on MRI change due to the administration of anesthetics and thus these images cannot be fully compared to the un‐sedated airway. With the development of faster scanning protocols the need for anesthetics is likely to be reduced. The studies identified report scanning times up to 10 min per sequence.21, 25, 29 However, a pediatric laryngeal MRI protocol developed at the Erasmus MC—Sophia Children's Hospital shows the possibility of an extensive anatomical and dynamic evaluation within 30 min. In addition, in the neonatal population where laryngeal MRI would be of interest to image congenital lesions, increasing experience with the use of the “feed and sleep”—method will make it possible to omit sedation. Likewise, dynamic laryngeal MRI techniques require full cooperation without the influence of anesthetics.
5. CONCLUSION
MRI is a promising modality for the evaluation of pediatric laryngeal diseases (Table 3). Most of the MRI studies on the anatomy of the pediatric larynx were conducted before 2001, so with the recent technological advancements of MRI, image quality has definitely improved. The most recent studies focused on tissue characterization and on the effect of anesthesia in the larynx. Studies using MRI as a diagnostic imaging modality and longitudinal studies are scarce. In addition, although dynamic imaging of the vocal cords has been proven feasible in the adult population, implementation in the pediatric population is still lacking. Clinical studies on the technical use of MRI in the spectrum of pediatric laryngeal diseases, such as laryngeal stenosis and vocal cord dysfunction, are lacking. Further research should be conducted to explore these options.
Table 3.
Advantages and disadvantages of pediatric laryngeal MRI
| Advantages | Disadvantages |
|---|---|
| No sedation needed | Inferior spatial resolution to CT |
| Free of ionizing radiation | Long scanning time compared to CT |
| Excellent soft tissue contrast | Differentiation between malignant and inflammatory lesions can be challenging |
| Good visualization of vascular structures | Bony involvement can be challenging |
| Dynamic imaging possible |
CONFLICTS OF INTEREST
Dr B. Elders, Dr S. Hermelijn, Dr B. Pullens, and P. Wielopolski report no disclosures. Prof Dr H. Tiddens reports to be involved in an industry symposium on cystic fibrosis with Roche, to be involved in lectures and the advisory board of Novartis. He has obtained grants from Vertex, Gilead, and Chiesi; outside this submitted work. He also has a patent licensed for Vectura and PRAGMA‐CF and he is head of the Erasmus MC—Sophia Children's Hospital core laboratory Lung Analysis. Dr P. Ciet reports to have obtained personal fees from Vertex, outside this submitted work.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Supporting Data S1.
Supporting Data S2.
Supporting Video S1.
ACKNOWLEDGEMENTS
We would like to acknowledge W.M. Bramer, from the Erasmus MC—medical library, for his assistance in the literature search. There is no funding to report for this study.
Elders BBLJ, Hermelijn SM, Tiddens HAWM, Pullens B, Wielopolski PA, Ciet P. Magnetic resonance imaging of the larynx in the pediatric population: A systematic review. Pediatric Pulmonology. 2019;54:478–486. 10.1002/ppul.24250
REFERENCES
- 1. Stephenson KA, Wyatt. Glottic stenosis. Semin Pediatr Surg. 2016;25:132–137. [DOI] [PubMed] [Google Scholar]
- 2. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. [DOI] [PubMed] [Google Scholar]
- 3. Becker M, Burkhardt K, Dulguerov P, Allal A. Imaging of the larynx and hypopharynx. Eur J Radiol. 2008;66:460–479. [DOI] [PubMed] [Google Scholar]
- 4. Huang BY, Solle M, Weissler MC. Larynx: anatomic imaging for diagnosis and management. Otolaryngol Clin North Am. 2012;45:1325–1361. [DOI] [PubMed] [Google Scholar]
- 5. Scott AD, Wylezinska M, Birch MJ, Miquel ME. Speech MRI: morphology and function. Phys Med. 2014;30:604–618. [DOI] [PubMed] [Google Scholar]
- 6. Henes FO, Laudien M, Linsenhoff L, et al. Accuracy of magnetic resonance imaging for grading of subglottic stenosis in patients with granulomatosis with polyangiitis: correlation with pulmonary function tests and laryngoscopy. Arthritis Care Res. 2017;70:777–784. [DOI] [PubMed] [Google Scholar]
- 7. Williamson JP, James AL, Phillips MJ, Sampson DD, Hillman DR, Eastwood PR. Quantifying tracheobronchial tree dimensions: methods, limitations and emerging techniques. Eur Respir J. 2009;34:42–55. [DOI] [PubMed] [Google Scholar]
- 8. Mazonakis M, Tzedakis A, Damilakis J, Gourtsoyiannis N. Thyroid dose from common head and neck CT examinations in children: is there an excess risk for thyroid cancer induction? Eur Radiol. 2007;17:1352–1357. [DOI] [PubMed] [Google Scholar]
- 9. Preda L, Conte G, Bonello L, et al. Diagnostic accuracy of surface coil MRI in assessing cartilaginous invasion in laryngeal tumours: do we need contrast‐agent administration? Eur Radiol. 2017;27:4690–4698. [DOI] [PubMed] [Google Scholar]
- 10. Baki MM, Menys A, Atkinson D, et al. Feasibility of vocal fold abduction and adduction assessment using cine‐MRI. Eur Radiol. 2017;27: 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agnello F, Cupido F, Sparacia G, et al. Computerised tomography and magnetic resonance imaging of laryngeal squamous cell carcinoma: a practical approach. Neuroradiol J. 2017;30:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yuh WTC, Sato Y, Loes DJ, et al. Magnetic‐resonance‐imaging and computed‐tomography in pediatric head and neck masses. Ann Otol Rhinol Laryngol. 1991;100:54–62. [DOI] [PubMed] [Google Scholar]
- 14. Hudgins PA, Siegel J, Jacobs I, Abramowsky CR. The normal pediatric larynx on CT and MR. Am J Neuroradiol. 1997;18:239–245. [PMC free article] [PubMed] [Google Scholar]
- 15. Mahboubi S, Gheyi V. MR imaging of airway obstruction in infants and children. Int J Pediatr Otorhinolaryngol. 2001;57:219–227. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Takashima S, Takayama F, et al. Head and neck lesions: characterization with diffusion‐weighted echo‐planar MR imaging. Radiology. 2001;220:621–630. [DOI] [PubMed] [Google Scholar]
- 17. Chavhan GB, Alsabban Z, Babyn PS. Diffusion‐weighted imaging in pediatric body MR imaging: principles, technique, and emerging applications. Radiographics. 2014;34:E73–E88. [DOI] [PubMed] [Google Scholar]
- 18. Abdel Razek AA, Gaballa G, Elhawarey G, Megahed AS, Hafez M, Nada N. Characterization of pediatric head and neck masses with diffusion‐weighted MR imaging. Eur Radiol. 2009;19:201–208. [DOI] [PubMed] [Google Scholar]
- 19. Taha MS, Amir M, Hassan O, Sabra R, Taha T, Riad MA. Pre‐treatment apparent diffusion coefficient mapping: differentiation of benign from malignant laryngeal lesions. J Laryngol Otol. 2015;129:57–62. [DOI] [PubMed] [Google Scholar]
- 20. Fitch WT, Giedd J. Morphology and development of the human vocal tract: a study using magnetic resonance imaging. J Acoust Soc Am. 1999;106:1511–1522. [DOI] [PubMed] [Google Scholar]
- 21. Faust RA, Remley KB, Rimell FL. Real‐time, cine magnetic resonance imaging for evaluation of the pediatric airway. Laryngoscope. 2001;111:2187–2190. [DOI] [PubMed] [Google Scholar]
- 22. Vorperian HK, Kent RD, Lindstrom MJ, Kalina CM, Gentry LR, Yandell BS. Development of vocal tract length during early childhood: a magnetic resonance imaging study. J Acoust Soc Am. 2005;117:338–350. [DOI] [PubMed] [Google Scholar]
- 23. Adewale L. Anatomy and assessment of the pediatric airway. Paediatr Anaesth. 2009;19:1–8. [DOI] [PubMed] [Google Scholar]
- 24. Vorperian HK, Wang S, Schimek EM, et al. Developmental sexual dimorphism of the oral and pharyngeal portions of the vocal tract: an imaging study. J Speech Lang Hear Res. 2011;54:995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Litman RS, Weissend EE, Shibata D, Westesson PL. Developmental changes of laryngeal dimensions in unparalyzed, sedated children. Anesthesiology. 2003;98:41–45. [DOI] [PubMed] [Google Scholar]
- 26. Litman RS, Wake N, Chan LML, et al. Effect of lateral positioning on upper airway size and morphology in sedated children. Anesthesiology. 2005;103:484–488. [DOI] [PubMed] [Google Scholar]
- 27. Vialet R, Nau A, Chaumoître K, Martin C. Effects of head posture on the oral, pharyngeal and laryngeal axis alignment in infants and young children by magnetic resonance imaging. Paediatr Anaesth. 2008;18:525–531. [DOI] [PubMed] [Google Scholar]
- 28. Becret A, Vialet R, Chaumoitre K, Loundou A, Lesavre N, Michel F. Upper airway modifications in head extension during development. Anaesth Crit Care Pain Med. 2017;36:285–290. [DOI] [PubMed] [Google Scholar]
- 29. Aqil M, Delvi B, Abujamea A, et al. Spatial relationship of i‐gel® and Ambu® AuraOnceTM on pediatric airway: a randomized comparison based on three dimensional magnetic resonance imaging. Minerva Anestesiol. 2017;83:23–32. [DOI] [PubMed] [Google Scholar]
- 30. Cote CJ, Notterman DA, Karl HW, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105:805–814. [DOI] [PubMed] [Google Scholar]
- 31. Salamon E, Lever S, Kuo W, Ciet P, Tiddens HA. Spirometer guided chest imaging in children: it is worth the effort!. Pediatr Pulmonol. 2017;52:48–56. [DOI] [PubMed] [Google Scholar]
- 32. Yousem DM, Tufano RP. Laryngeal imaging. Magn Reson Imaging Clin N Am. 2002;10:451–465. [DOI] [PubMed] [Google Scholar]
- 33. Mannelli G, Cecconi L, Gallo O. Laryngeal preneoplastic lesions and cancer: challenging diagnosis. Qualitative literature review and meta‐analysis. Crit Rev Oncol Hematol. 2016;106:64–90. [DOI] [PubMed] [Google Scholar]
- 34. Kuno H, Onaya H, Fujii S, Ojiri H, Otani K, Satake M. Primary staging of laryngeal and hypopharyngeal cancer: CT, MR imaging and dual‐energy CT. Eur J Radiol. 2014;83:e23–e35. [DOI] [PubMed] [Google Scholar]
- 35. Casselman JW. High‐resolution imaging of the skull base and larynx In: Schoenberg SO, Dietrich O, Reiser MF, eds. Parallel Imaging in Clinical MR Applications. Medical Radiology (Diagnostic Imaging). Berlin, Heidelberg: Springer; 2007. [Google Scholar]
- 36. Banko B, Dukic V, Milovanovic J, Kovac JD, Artiko V, Maksimovic R. Diagnostic significance of magnetic resonance imaging in preoperative evaluation of patients with laryngeal tumors. Eur Arch Otorhinolaryngol. 2011;268:1617–1623. [DOI] [PubMed] [Google Scholar]
- 37. Gindhart TD, Johnston WH, Chism SE, Dedo HH. Carcinoma of the larynx in childhood. Cancer. 1980;46:1683–1687. [DOI] [PubMed] [Google Scholar]
- 38. Parkes WJ, Propst EJ. Advances in the diagnosis, management, and treatment of neonates with laryngeal disorders. Semin Fetal Neonatal Med. 2016;21:270–276. [DOI] [PubMed] [Google Scholar]
- 39. Ahmad SM, Soliman AM. Congenital anomalies of the larynx. Otolaryngol Clin North Am. 2007;40:177–191. [DOI] [PubMed] [Google Scholar]
- 40. Maroldi R, Ravanelli M, Farina D. Magnetic resonance for laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22:131–139. [DOI] [PubMed] [Google Scholar]
- 41. Pullicino R, Radon M, Biswas S, Bhojak M, Das K. A review of the current evidence on gadolinium deposition in the brain. Clin Neuroradiol. 2018;28:159–169. [DOI] [PubMed] [Google Scholar]
- 42. McDonald JS, McDonald RJ, Jentoft ME, et al. Intracranial gadolinium deposition following gadodiamide‐enhanced magnetic resonance imaging in pediatric patients: a case‐control study. JAMA Pediatr. 2017;171:705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Young JR, Orosz I, Franke MA, et al. Gadolinium deposition in the paediatric brain: T1‐weighted hyperintensity within the dentate nucleus following repeated gadolinium‐based contrast agent administration. Clin Radiol. 2018;73:290–295. [DOI] [PubMed] [Google Scholar]
- 44. Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. International Society for Magnetic Resonance in M. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16:564–570. [DOI] [PubMed] [Google Scholar]
- 45. Ahmad M, Dargaud J, Morin A, Cotton F. Dynamic MRI of larynx and vocal fold vibrations in normal phonation. J Voice. 2009;23:235–239. [DOI] [PubMed] [Google Scholar]
- 46. Flint PW, Haughey BH, Lund VJ, et al. Cummings Otolaryngology‐head and neck surgery. Sixth edition. Philadelphia: Elsevier Health Sciences; 2014. 3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Supporting Data S1.
Supporting Data S2.
Supporting Video S1.


