Abstract
Data on the impact of human papillomavirus (HPV) vaccination on the population HPV prevalence are largely obtained from women. We assessed the impact of the girls‐only HPV16/18 vaccination program in the Netherlands that started in 2009, on trends in HPV prevalence among women and heterosexual men, using data from the PASSYON study. In this cross‐sectional study, the HPV prevalence among 16‐ to 24‐year‐old visitors to sexually transmitted infection clinics was assessed in 2009, 2011, 2013, and 2015. We compared the genital postvaccination HPV prevalence with the prevaccination prevalence (2009) using Poisson GEE models. In total, we included 4,996 women and 1,901 heterosexual men. The percentage of women who reported to be vaccinated increased from 2.3% in 2009 to 37% in 2015. Among all women, the HPV16/18 prevalence decreased from 23% prevaccination to 15% in 2015 (adjusted prevalence ratio [aPR] 0.62, p trend < 0.01). Among heterosexual men, the HPV16/18 prevalence decreased from 17% prevaccination to 11% in 2015 (aPR 0.52, p trend < 0.01). Of the heterosexual men with a steady partner, HPV16/18 prevalence was lower among those whose steady partner had been vaccine‐eligible in the national immunization program (aPR 0.13). Among unvaccinated women, the HPV16/18 prevalence in 2015 was not different from prevaccination. The decreasing HPV16/18 prevalence among heterosexual men and the reduced HPV16/18 prevalence among heterosexual men with a vaccine‐eligible steady partner strongly suggests herd protection from girls‐only vaccination. Absence of notable herd effects among unvaccinated women 6 years postvaccination may be due to the moderate vaccine uptake among girls in the Netherlands.
Keywords: human papillomavirus, vaccination, herd protection, population effects, public health
Short abstract
What's new?
Human papillomavirus (HPV) is a sexually transmitted virus that plays a causal role in the development of anogenital and oropharyngeal cancers in both men and women. The population‐level impact of HPV vaccination programs on the HPV prevalence has however mainly been studied in women. This study shows decreasing trends in the HPV16 and HPV18 prevalence among both women and heterosexual men after the introduction of a girls‐only HPV16/18 vaccination program in the Netherlands. The findings provide compelling evidence for herd protection in men. Because HPV16/18 are the most oncogenic types, HPV‐related cancers are expected to decline in both sexes after girls‐only HPV vaccination.
Abbreviations
- 95% CI
95% confidence interval
- aPR
adjusted prevalence ratio
- GEE
generalized estimating equation
- HPV
human papillomavirus
- hrHPV
high‐risk human papillomavirus
- NIP
National Immunization Program
- PR
prevalence ratio
- STI
sexually transmitted infection
Introduction
Human papillomavirus (HPV) is a sexually transmitted virus that plays a causal role in the development of anogenital and oropharyngeal cancers in both men and women.1 To prevent HPV‐related cancers, many countries have included HPV vaccination in their national immunization program (NIP), using one of the available vaccines that provide direct protection against two, four, or nine HPV types, all including HPV16 and HPV18.2
In the Netherlands, the bivalent vaccine (Cervarix®, GSK) is used in the NIP; to date, this has been a girls‐only program.3 In 2009, there was a catch‐up campaign for girls born from 1993 to 1996 with 52% that completed the 3‐dose schedule.4 Routine HPV vaccination was introduced in 2010 for girls in the year they turn 13, with an initial 3‐dose uptake of 56% (birth cohort 1997). The uptake increased to 61% for birth cohort 2001, but decreased again to 53% for birth cohort 2002. In 2014, routine HPV vaccination changed to a 2‐dose schedule.5
We previously reported on direct bivalent HPV vaccine effectiveness using cross‐sectional data from female sexually transmitted infection (STI) clinic visitors. We showed high effectiveness against the vaccine types HPV16 and HPV18 and cross‐protection against other oncogenic HPV types.6 These findings were reiterated in a longitudinal cohort study among vaccine‐eligible girls.7
The population‐level impact of HPV vaccination programs also includes possible indirect effects, such as herd protection. So far, the population‐level impact of HPV vaccination programs on the HPV prevalence has mainly been studied among women. Surveillance studies have shown a decrease in the HPV16/18 prevalence since the introduction of vaccination.8 Some studies have also shown decreases in the HPV16/18 prevalence among unvaccinated women.9, 10, 11 This decrease among unvaccinated women is attributed to herd protection in men, yet there is limited information about trends in HPV prevalence among men, especially after bivalent HPV vaccination. One study has shown that the HPV16/18 prevalence in urine samples from men decreased from 5.0% prevaccination to 1.1% 2–4 years post girls‐only bivalent vaccination.12 However, since the method of sample collection had changed, the authors were cautious in drawing conclusions. Because HPV16/18 are associated with the majority of HPV‐related cancers in men,1 demonstrating herd protection for these types in heterosexual men is important for assessing the overall health gain from a girls‐only HPV vaccination program.13, 14
We assessed the population‐level impact of the girls‐only bivalent HPV vaccination program in the Netherlands by studying trends in the prevalence of HPV vaccine and cross protective types from prevaccination up to 6 years postvaccination. We included women as well as heterosexual men, and focused on unvaccinated women and heterosexual men with vaccine‐eligible partners to study herd protection. We used data from the PASSYON (PApillomavirus Surveillance among STI clinic YOungsters in the Netherlands) study, a biennial cross‐sectional study among visitors to STI clinics that had been designed to monitor the HPV vaccination program in the Netherlands.
Materials and Methods
Study design and population
The PASSYON study started in 2009 when HPV vaccination was implemented in the Netherlands. Young (16‐ to 24‐year‐old) people who visited STI clinics throughout the Netherlands were asked to participate in the study. In addition to the routine STI consultation, participants were asked to fill out a questionnaire regarding demographics, sexual behavior and vaccination status. Moreover, they were asked to provide a self‐collected genital swab for HPV testing. Women were instructed to insert a swab (Copan Diagnostics, Italy) about 4 cm into the vagina until resistance was felt and to turn it around along the walls of the vagina. Men were instructed to firmly move the swab up and down the entire shaft, the glans, the coronal sulcus and under the foreskin of the penis. More details about the PASSYON study have been published previously.15 To explore trends in the HPV prevalence after implementation of HPV vaccination, the PASSYON study was repeated in 2011, 2013 and 2015 using the same study protocol. Participants could be included in multiple study rounds, but the probability of repeated consultations is low as we sampled for only 2 months in the same period every other year (Fig. 1). The Dutch Medical Research Involving Human Subjects Act (Dutch acronym: WMO) does not apply for our study, because only for‐the‐researchers‐anonymized‐data were used and there were no (medical) interventions other than routine care. The Medical Ethical Committee of the University of Utrecht, the Netherlands, provided a waiver for full medical ethical review (protocol number 08/397). Data were obtained using a unique code per person and all participants gave informed consent.
Figure 1.
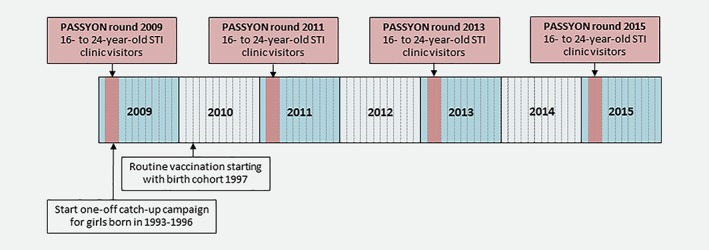
Human papillomavirus (HPV) vaccination in the Netherlands and the PASSYON study design. Abbreviation: STI: sexually transmitted infection. [Color figure can be viewed at wileyonlinelibrary.com]
Laboratory methods
HPV testing protocols were constant across all years and described in detail elsewere.15 Briefly, DNA was extracted using the MagnaPure platform (Total Nucleic Acid Isolation Kit, Roche, the Netherlands) and HPV‐DNA was amplified using the SPF10 primer set and detected using the DNA enzyme‐linked immunoassay (HPV‐DEIA, DDL Diagnostics Laboratory, the Netherlands). Positive samples were genotyped with line‐probe assay (HPV‐LiPA25, DDL Diagnostics Laboratory, the Netherlands), which is able to detect 25 HPV types, including HPV16 and HPV18.
Statistical analyses
Only participants with a genital swab were included in the analyses. All analyses were performed separately for all women (irrespective of vaccination status), heterosexual men (based on self‐identified sexual preference) and unvaccinated women (based on self‐reported vaccination status).
We calculated the prevalence and Wilson score 95% confidence interval (95% CI) of HPV16 and HPV18 (combined and separately) for each PASSYON year and performed a crude Cochran‐Armitage Trend Test. Next, we compared the HPV prevalence of the postvaccination periods (2011, 2013 and 2015) with the prevaccination period (2009) and calculated prevalence ratios (PRs) using a Poisson model with robust error variance. This results in comparable estimates as compared to log‐binomial regression, and improves numerical convergence.16 Additionally, because we assumed identical effects of covariates on the prevalence of HPV types included in the analyses, we made use of a generalized estimating equation (GEE) model with an exchangeable correlation structure. This allows efficient estimation of coefficients and calculation of the population‐averaged effect of study year on the HPV prevalence, either type‐specific or pooled (as a weighted average).17 Linear trends over time were assessed by including PASSYON year as a continuous variable in the model. These analyses were adjusted for age (16–20 and 21–24 years) and possible confounders, presented in Table 1. The variables age at sexual debut and number of sex partners in the past 6 months and lifetime were categorized for analyses purposes based on knowledge about the HPV risk and size of each category. The selection of confounders was based on the following procedure. First, we explored the association with PASSYON year and high‐risk HPV (hrHPV) positivity (being positive for HPV16/18/31/33/35/39/45/51/52/56/58/59), using Chi‐square tests. Using hrHPV instead of HPV16/18 positivity for the selection of confounders gave more power to detect possible associations. Variables associated with PASSYON year and hrHPV positivity (p < 0.05) in univariable analyses were selected. Second, because sexual risk behavior variables were highly correlated, we used computerized selection models (stepwise with p < 0.05 as entry and stay criteria) with hrHPV positivity as an outcome and the sexual risk behavior variables that were selected as independent variables. Variables that were included in the final selection model were evaluated as possible confounders to adjust for in the Poisson GEE models for comparing HPV prevalence between study rounds. For all women, we also adjusted for self‐reported vaccination status to assess if possible trends in HPV prevalence over time was explained by an increasing proportion of vaccinated women in our study population. Although HPV vaccination was not offered to men in the Dutch NIP, it is possible that men were vaccinated elsewhere. In sensitivity analyses, we excluded heterosexual men who reported to have been HPV vaccinated. To study the population‐level impact and herd protection for the cross‐protective types HPV31/33/459, we repeated the analyses by also including these types.
Table 1.
Characteristics of the study population of all PASSYON years combined for all women, heterosexual men and unvaccinated women
| All women | Heterosexual men | Unvaccinated women | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total | 4,996 | 1,901 | 3,594 |
| Age | |||
| 16–20 years | 2,012 (40.3) | 557 (29.3) | 1,186 (33.0) |
| 21–24 years | 2,984 (59.7) | 1,344 (70.7) | 2,408 (67.0) |
| Self‐defined ethnicity | |||
| Dutch | 4,319 (86.5) | 1,522 (80.1) | 3,127 (87.1) |
| Not Dutch | 675 (13.5) | 377 (19.9) | 465 (12.9) |
| Education level 1 | |||
| Low/middle | 1,246 (25.1) | 591 (31.2) | 835 (23.3) |
| High | 3,719 (74.9) | 1,303 (68.8) | 2,745 (76.7) |
| Sexual preference | |||
| Heterosexual | 4,804 (96.2) | 1,901 (100) | 3,457 (96.2) |
| Gay or bisexual | 192 (3.8) | ‐ | 137 (3.8) |
| Age sexual debut 2 | |||
| ≤14 years | 647 (13.1) | 322 (17.1) | 439 (12.3) |
| 15–16 years | 2,396 (48.5) | 762 (40.5) | 1,697 (47.7) |
| ≥17 years | 1,898 (38.4) | 799 (42.4) | 1,423 (40.0) |
| Sex partners past 6 months 3 | |||
| 0–1 partner | 1,627 (32.6) | 418 (22.0) | 1,203 (33.5) |
| 2–3 partners | 2,412 (48.3) | 715 (37.6) | 1,721 (47.9) |
| 4–5 partners | 687 (13.8) | 390 (20.5) | 499 (13.9) |
| ≥6 partners | 265 (5.3) | 378 (19.9) | 169 (4.7) |
| Lifetime sex partners 3 | |||
| ≤2 partners | 570 (11.6) | 105 (5.8) | 396 (11.2) |
| 3–4 partners | 973 (19.8) | 202 (11.1) | 688 (19.4) |
| 5–6 partners | 966 (19.7) | 240 (13.2) | 694 (19.6) |
| 7–14 partners | 1,702 (34.7) | 585 (32.2) | 1,245 (35.2) |
| ≥15 partners | 693 (14.1) | 687 (37.8) | 516 (14.6) |
| Anal sex past 6 months | |||
| No | 4,351 (87.6) | 1,590 (84.8) | 3,122 (87.3) |
| Yes | 614 (12.4) | 284 (15.2) | 455 (12.7) |
| Notified for STI 4 | |||
| No | 4,511 (90.6) | 1,608 (85.0) | 3,263 (91.1) |
| Yes | 467 (9.4) | 284 (15.0) | 319 (8.9) |
| STI related symptoms 4 | |||
| No | 3,799 (76.5) | 1,367 (72.4) | 2,721 (76.1) |
| Yes | 1,170 (23.5) | 521 (27.6) | 853 (23.9) |
| Self‐reported history of any STI | |||
| No | 2,852 (57.4) | 1,055 (55.7) | 2,101 (58.7) |
| Yes | 1,266 (25.5) | 377 (19.9) | 920 (25.7) |
| Never tested | 851 (17.1) | 462 (24.4) | 558 (15.6) |
| Genital chlamydia infection 4 | |||
| No | 4,283 (86.1) | 1,594 (84.4) | 3,098 (86.5) |
| Yes | 694 (13.9) | 294 (15.6) | 482 (13.5) |
| Steady partner | |||
| No | 2,961 (60.7) | 1,037 (56.5) | 2,127 (60.6) |
| Yes, for 0–6 months | 1,102 (22.6) | 475 (25.9) | 801 (22.8) |
| Yes, for ≥6 months | 813 (16.7) | 324 (17.6) | 583 (16.6) |
| Condom use past 6 months, casual partner 5 | |||
| Inconsistent | 1,950 (39.2) | 851 (44.9) | 1,344 (37.5) |
| Consistent | 1,806 (36.3) | 658 (34.7) | 1,361 (37.9) |
| No casual partners | 1,224 (24.6) | 385 (20.3) | 882 (24.6) |
Abbreviations: STI: sexually transmitted infection.
Numbers vary because of missing values.
High educational level included school of higher general secondary education, pre‐university education, university of applied sciences and university. Low/middle educational level included all other levels of education.
Categorized for analyses purposes. Minimum‐maximum age reported: 9–24 years among (unvaccinated) women and heterosexual men.
Categorized for analyses purposes. Maximum partners reported: 540 past 6 months and 900 lifetime partners among (unvaccinated) women; 50 past 6 months and 400 lifetime partners among heterosexual men.
Based on information of the STI clinic visit.
Inconsistent included reporting never, rarely and “sometimes I do, sometimes I do not” condom use. Consistent included reporting often or always condom use.
Because women who were offered vaccination in the Netherlands (women born in 1993 or later), were aging over the span of the PASSYON study, the vaccination coverage by age category differed over the years. We assessed for effect modification by age category by including an interaction term between PASSYON year and age category. For all women, we again additionally adjusted for self‐reported vaccination status to assess if the possible difference in trends by age category were explained by differences in vaccination coverage. We also calculated the adjusted PRs (aPRs) and the trend for the age categories separately.
If participants reported being in a relationship, the age of the steady partner was asked. For heterosexual men, we assessed if the steady partner had been eligible for HPV vaccination in the Dutch NIP based on the reported age of the steady partner. We assumed the steady partner had been eligible for vaccination if she was ≤17 years in PASSYON round 2011, ≤19 years in PASSYON round 2013 and ≤ 21 years in PASSYON round 2015. If the steady partner was older is a specific PASSYON round, we assumed she had not been eligible for HPV vaccination in the NIP. Also for all heterosexual men included in PASSYON round 2009, we assumed the steady partner had not been eligible for HPV vaccination. To consider herd effects, we calculated the combined HPV16/18 prevalence among heterosexual men by age of the steady partner and vaccine‐eligibility of the steady partner. Next, we assessed the difference in HPV16/18 prevalence between heterosexual men with or without a vaccine‐eligible steady partner by using a GEE model with HPV16/18 as an outcome and vaccine‐eligibility of the steady partner as an independent variable. This analysis was adjusted for age of the men and age of the steady partner.
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). We used a significance level of p < 0.05. We did complete case analyses, as none of the variables had more than 5% missing.
Results
Study population
A total of 7,108 women and heterosexual men participated in the PASSYON study, of whom 6,897 (4,996 women and 1,901 heterosexual men) delivered a genital swab and were included in the current analysis; 1,524 in 2009, 1,775 in 2011, 1,816 in 2013 and 1,782 in 2015. The proportion of women who had been eligible for vaccination increased from 0.4% in 2009 to 5.3% in 2011, 27% in 2013 and to 57% in 2015. Of the women who had been eligible for vaccination, 55% (n = 650) reported to be vaccinated at least once (30 reported to be vaccinated with 1 dose, 42 with 2 doses, 456 with 3 doses and 122 did not know the number of doses). The proportion of all women reporting to be vaccinated at least once increased from 2.3% in 2009 to 37% in 2015. In total 27 heterosexual men (1.4%) reported to be HPV vaccinated.
Characteristics of the study population of all PASSYON years combined are presented in Table 1. In general, the indicators for sexual risk behavior increased over the years among women, heterosexual men and unvaccinated women (Supporting Information Tables 1–3). For example, we observed an association between lifetime sex partners and PASSYON year; with proportions reporting ≥15 lifetime sex partners of 12% in 2009 and 18% in 2015 among all women; 31% in 2009 and 44% in 2015 among heterosexual men; and 12% in 2009 and 19% in 2015 among unvaccinated women. The genital chlamydia prevalence was associated with PASSYON study year among heterosexual men only. Supporting Information Tables 1–3 also show the association between the characteristics and hrHPV positivity. In general, people with higher sexual risk behavior were more often hrHPV positive.
HPV prevalence over time
Figure 2 presents the HPV16 and HPV18 prevalence over time and the crude trend test among all women, heterosexual men and unvaccinated women. Among all women, the HPV16/18 prevalence decreased from 23% in 2009 to 15% in 2015 (aPR 0.62, Table 2). Also for HPV16 and HPV18 separately, there was a significant decrease over time (aPR 0.59 and 0.69 respectively). When we additionally adjusted for vaccination status, the prevalences in 2015 were no longer significantly different from 2009. Among heterosexual men, the combined HPV16/18 prevalence decreased from 17% in 2009 to 11% in 2015 (aPR 0.52). Also separately, HPV16 and HPV18 prevalences were significantly lower in 2015 compared to 2009 (aPR 0.64 and 0.33 respectively). Excluding the 27 heterosexual men who reported to be vaccinated did not lead to different results (Supporting Information Table 4). Among unvaccinated women, we observed no trends in the HPV16 or HPV18 prevalence.
Figure 2.
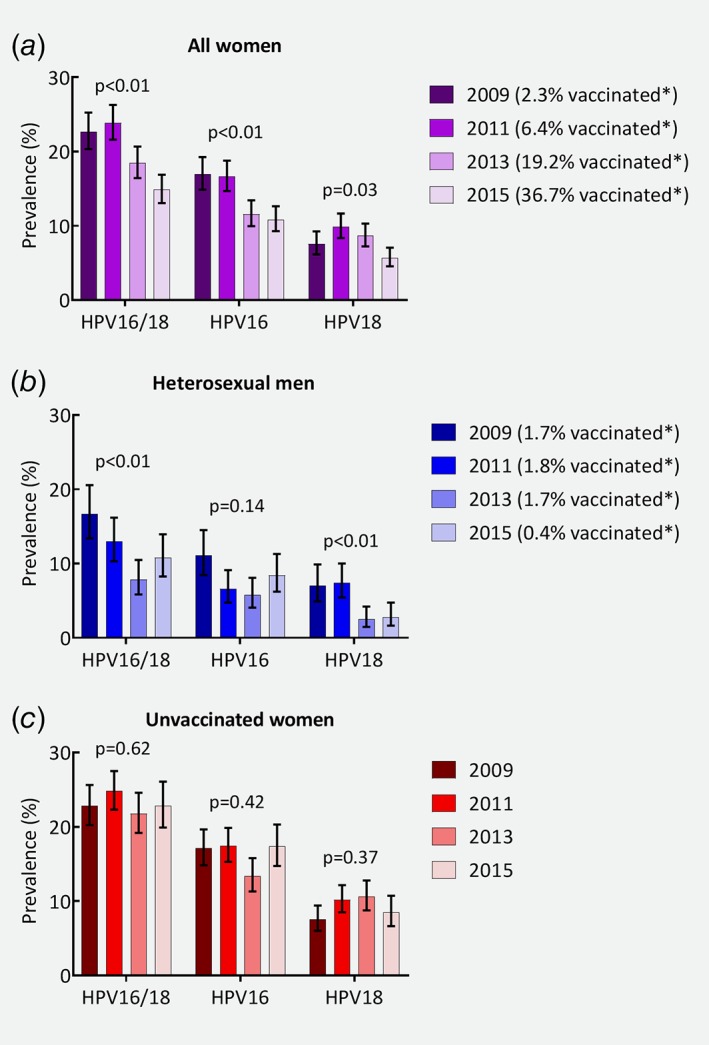
Prevalence of human papillomavirus (HPV) types 16 or 18, HPV16 and HPV18 over time and p values for the crude trend test, among (a) all women; (b) heterosexual men; (c) unvaccinated women. Note: the p value presents the Cochran‐Armitage Trend Test. *Percentage of women and heterosexual men who reported to be vaccinated at least once. [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Comparing postvaccination human papillomavirus (HPV) prevalence with prevaccination prevalence (2009) and assessing the trend among all women, heterosexual men and unvaccinated women
| All women | Heterosexual men | Unvaccinated women | ||||
|---|---|---|---|---|---|---|
| % positive (95% CI) | aPR (95% CI)1 | % positive (95% CI) | aPR (95% CI)2 | % positive (95% CI) | aPR (95% CI)3 | |
| HPV16/18 4 | ||||||
| 2009 | 22.7 (20.3–25.3) | Reference | 16.7 (13.4–20.6) | Reference | 22.8 (20.2–25.6) | Reference |
| 2011 | 23.9 (21.6–26.3) | 1.08 (0.92–1.26) | 13.0 (10.3–16.2) | 0.75 (0.54–1.05) | 24.8 (22.3–27.5) | 1.11 (0.94–1.31) |
| 2013 | 18.5 (16.4–20.7) | 0.79 (0.67–0.94) | 7.9 (5.8–10.5) | 0.44 (0.30–0.64) | 21.8 (19.2–24.6) | 0.90 (0.76–1.08) |
| 2015 | 14.9 (13.1–16.9) | 0.62 (0.52–0.74) | 10.8 (8.3–13.9) | 0.52 (0.36–0.75) | 22.8 (19.9–26.1) | 0.94 (0.78–1.14) |
| p trend value5 | <0.01 | <0.01 | <0.01 | <0.01 | 0.62 | 0.16 |
| HPV16 | ||||||
| 2009 | 16.9 (14.8–19.3) | Reference | 11.1 (8.4–14.5) | Reference | 17.1 (14.8–19.7) | Reference |
| 2011 | 16.6 (14.7–18.8) | 0.98 (0.81–1.17) | 6.6 (4.7–9.1) | 0.57 (0.36–0.89) | 17.5 (15.3–19.9) | 1.01 (0.83–1.23) |
| 2013 | 11.6 (10.0–13.5) | 0.66 (0.54–0.81) | 5.7 (4.1–8.1) | 0.49 (0.31–0.78) | 13.4 (11.3–15.8) | 0.73 (0.59–0.91) |
| 2015 | 10.8 (9.3–12.6) | 0.59 (0.48–0.73) | 8.4 (6.2–11.3) | 0.64 (0.42–0.98) | 17.3 (14.7–20.3) | 0.92 (0.74–1.14) |
| p trend value5 | <0.01 | <0.01 | 0.14 | 0.06 | 0.42 | 0.08 |
| HPV18 | ||||||
| 2009 | 7.6 (6.2–9.3) | Reference | 7.0 (4.9–9.9) | Reference | 7.5 (6.0–9.4) | Reference |
| 2011 | 9.9 (8.4–11.7) | 1.31 (1.00–1.71) | 7.4 (5.4–10.0) | 1.04 (0.64–1.67) | 10.2 (8.5–12.2) | 1.35 (1.01–1.79) |
| 2013 | 8.7 (7.2–10.3) | 1.09 (0.83–1.43) | 2.5 (1.5–4.2) | 0.35 (0.18–0.66) | 10.6 (8.8–12.8) | 1.31 (0.97–1.75) |
| 2015 | 5.7 (4.6–7.1) | 0.69 (0.51–0.94) | 2.8 (1.6–4.7) | 0.33 (0.17–0.65) | 8.5 (6.6–10.7) | 0.98 (0.70–1.37) |
| p trend value5 | 0.03 | <0.01 | <0.01 | <0.01 | 0.37 | 0.95 |
Abbreviations: aPR: adjusted prevalence ratio; 95% CI: 95% confidence interval.
Adjusted for: age, lifetime sex partners, history of any sexually transmitted infection, steady partner and condom use with casual partners.
Adjusted for: age, lifetime sex partners, history of any sexually transmitted infection and steady partner.
Adjusted for: age, lifetime sex partners, history of any sexually transmitted infection, and condom use with casual partners.
Defined as positive for HPV16 or HPV18 in the percentage positive, and as a pooled estimate to calculate the aPR.
The crude p trend values were calculated using the Cochran‐Armitage Trend Test. The adjusted p trend values were calculated by including PASSYON year as a continuous variable.
For HPV31, HPV33 and HPV45, we only observed a declining trend in the HPV31 prevalence among all women (adjusted ptrend 0.01), but not among heterosexual men or unvaccinated women. For the other HPV types, no trends were observed except for an increasing trend of HPV45 among unvaccinated women (Supporting Information Table 5).
The vaccination coverage over time differed by age category; for example in 2013, 40% of the 16‐ to 20‐year‐old women reported to be vaccinated (≥1 dose), while 4.6% of the 21‐ to 24‐year‐old women reported to be vaccinated. We observed that the HPV16/18 prevalence among 16‐ to 20‐year‐old women decreased faster as compared to 21‐ to 24‐year‐old women (aPR 0.41 and 0.74 respectively for 2015 compared to 2009, Supporting Information Fig. 1 and Supporting Information Table 6). The difference in the effect of year by age group was statistically significant (p < 0.01). After additional adjustment for vaccination status, the difference between ages was no longer statistically significant. Among heterosexual men and unvaccinated women, there was no statistically significant interaction with age. Among unvaccinated women, there were no statistically significant trends in the HPV16 or HPV18 prevalence for both age categories (Supporting Information Table 6).
Vaccine‐eligible steady partner
The proportion of heterosexual men reporting a steady partner who had been eligible for HPV vaccination increased from 2.2% in 2011 to 15% in 2013 and 19% in 2015. Figure 3 shows the combined HPV16/18 prevalence among heterosexual men according to the age of the steady partner and vaccine‐eligibility of the steady partner. Overall, heterosexual men whose steady partner had been vaccine‐eligible were less often HPV16/18 positive compared to heterosexual men whose steady partner had not been vaccine‐eligible in the NIP (aPR 0.13 [95% CI 0.04–0.41]).
Figure 3.
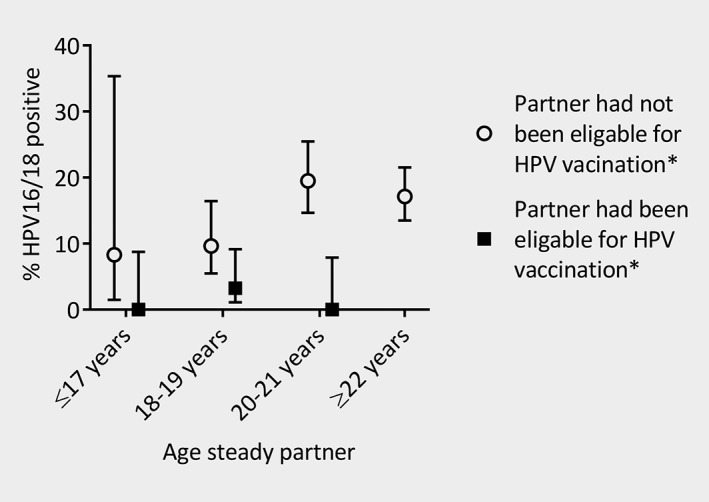
Prevalence of human papillomavirus (HPV) types 16 or 18 among heterosexual men who reported to have a steady partner, by age of the steady partner and vaccine‐eligibility of the steady partner. *Vaccine‐eligibility of the steady partner was based on the reported age of the steady partner in a specific PASSYON study round and the Dutch national immunization program. None of the steady partners of ≥22 years had been eligible according to the national immunization program in the Netherlands.
Discussion
We estimated the population‐level impact of the national girls‐only bivalent HPV vaccination program in the Netherlands by comparing HPV prevalence from prevaccination to postvaccination periods among male and female visitors to STI clinics. We showed decreasing trends in the HPV16 and HPV18 prevalence among all women and heterosexual men, but not among unvaccinated women separately. Of the heterosexual men who reported to have a steady partner, HPV16/18 prevalence was lower among those whose steady partner had been vaccine‐eligible.
Our results provide compelling evidence for herd protection for the vaccine‐types among men in the aftermath of girls‐only HPV16/18 vaccination and show that herd effects among heterosexual men will likely precede those among unvaccinated women. Our data offer empirical support for the population‐level impact of vaccination against the two most oncogenic HPV types as previously predicted by transmission‐dynamic models.18
We do acknowledge some limitations. First, STI clinics became stricter in prioritizing high‐risk individuals especially since 2015, when the funding of the STI clinics had changed.19 We indeed observed increased sexual risk behavior over time possibly related to changes in the access policy of STI clinics. Although we adjusted for known changes, unknown changes in the study population may have resulted in changes in the HPV prevalence unrelated to HPV vaccination. This could for instance explain the observed increase in HPV16 prevalence in 2015 compared to 2013 among heterosexual men. If participants in the postvaccination study periods were at higher HPV risk, we may have underestimated the impact of vaccination, including declines in HPV16/18 prevalence among unvaccinated women. However, the chlamydia prevalence did not increase among (unvaccinated) women, suggesting that unrecorded sexual risk behavior likely did not chance that much among female study participants. Analyses restricted to chlamydia positive unvaccinated women did not lead to different results (results not shown). Second, the use of self‐reported vaccination status may have induced bias. Among women, we believe the bias will be minimal as we previously showed that the HPV16 and HPV18 antibody concentrations agreed well with the self‐reported vaccination status.6 Of the heterosexual men 1.4% reported to be HPV vaccinated. If these men were truly vaccinated against HPV, this would lead to an overestimation of the herd effects. However, excluding the heterosexual men who reported to be HPV vaccinated did not lead to different results. We also used self‐reported sexual preference to identify heterosexual men. It might be that some men did (also) have sex with men. Such bias would have underestimated the impact of vaccination. Third, only one prevaccination measurement was available. If there were natural fluctuations in the HPV prevalence in the absence of vaccination, multiple prevaccination measurements would have been preferred to obtain an accurate estimate of the average prevaccination prevalence and to assess possible prevaccination trends. Last, we used a population of visitors to STI clinics who are at higher HPV risk as compared to the general population. The results are therefore not representative of the general Dutch population, probably underestimating the impact of vaccination.20
The decrease in the HPV16/18 prevalence among women in our study, coincided with an increase in the percentage of women who reported to be vaccinated. After adjustment for vaccination status, the HPV16/18 prevalence did not differ in 2015 as compared to 2009, indicating that the increasing proportion of vaccinated women explained the decreasing HPV prevalence. In other countries, also a decline in the HPV16/18 prevalence among women was observed after introduction of bivalent HPV vaccination. In England, the HPV16/18 prevalence decreased from 18% prevaccination to 4.0% 4–5 years postvaccination among 16 to 18‐year‐old sexually active women.21 In Scotland, the HPV16/18 prevalence decreased from 30% prevaccination to 4.5% 7 years postvaccination among 20 to 21‐year‐old women who underwent their first cervical screening.9 The larger declines in these countries as compared to our study could be explained by an overall lower percentage of women vaccinated in our study (37% ≥1 dose in 2015). This reflects both a lower percentage of women who had been eligible for vaccination (57% in 2015) and a lower vaccination uptake among vaccine‐eligible women (55% ≥1 dose). Among 16‐ to 20‐year‐old women, with a higher percentage vaccinated, we observed larger declines in the HPV16/18 prevalence.
We also observed a decrease in the HPV16/18 prevalence among heterosexual men since the introduction of girls‐only bivalent HPV vaccination. Our results are comparable to Australia where a declining trend in the HPV16/18 prevalence among heterosexual men was observed after girls‐only quadrivalent HPV vaccination.22 In Australia, also a decline in the HPV16/18 prevalence was observed among foreign‐born heterosexual men who had arrived from countries with a bivalent HPV vaccination program within 2 years of study inclusion. However, effects of exposure within those countries might have been negligible because the majority of the HPV16/18 infections clear within 2 years.23
While the decreasing HPV16/18 prevalence among heterosexual men in our study strongly suggests herd protection, causality cannot be concluded based on ecological analyses. Nonetheless, the decreasing prevalence in combination with a lower HPV16/18 prevalence among men whose steady partner had been vaccine‐eligible strongly indicates that heterosexual men receive indirect protection. With most HPV‐related penile cancers attributed to HPV16,24 cancer reductions are also expected to occur for heterosexual men in the aftermath of girls‐only HPV vaccination. Among men who have sex with men, large reductions in HPV‐related cancers are not expected, because they benefit less from herd protection after girls‐only vaccination.25 While it is anticipated that HPV prevalence will also decline at other anatomical sites among heterosexual men, this has not yet been demonstrated. Given that oropharyngeal cancers constitute the largest HPV‐related burden in men,26 showing herd effects against oral HPV is valuable to acknowledge the ultimate impact of HPV vaccination.
Among heterosexual men, the decline in HPV prevalence was larger for HPV18 than for HPV16, which is in line with data from Finland where also larger herd effects were observed for HPV18.10 Higher herd effects for HPV18 could be explained by a lower basic reproduction number (as a consequence of a higher clearance relative to HPV16).23, 27, 28 Even though HPV31/33/45 could also be expected to have a lower basic reproduction number than HPV16, there were no signs of herd effects for these types. This could be related to a relatively low background prevalence in combination with reduced vaccine effectiveness, resulting in limited power to detect herd effects against cross‐protective types compared to the vaccine types.
Because the vaccination coverage of completed schedule among vaccine‐eligible women in the Netherlands is 50% to 60%,5 herd protection will not have reached its full potential.13, 14 This is particularly true for herd protection in unvaccinated heterosexual women, which is derived from herd protection in heterosexual men, and thus constitutes a second‐order effect. With suboptimal girls‐only vaccination coverage, vaccinating boys along with girls will not only protect boys themselves, but could also increase herd protection to unvaccinated women.29, 30 Based on modeling studies, 80% vaccination coverage in both men and women, but not in either sex, could eradicate the vaccine types.18
We did not find signs of herd effects among unvaccinated women, also not when stratified by age. Nonetheless, other studies have observed a declining prevalence of the HPV vaccine types among unvaccinated women suggestive of herd protection. In Scotland, unvaccinated women born in 1995 were less often HPV16/18 positive at their first cervical screening compared to women born in 1988 who were not eligible for vaccination (5.3% versus 30%).9 Also in Australia and the United States, decreases in the vaccine type prevalence have been recorded among unvaccinated women.31, 32 There are several possible explanations for the absence of a declining trend among unvaccinated women in our study. First, in high‐risk populations with frequent changes in sex partners, people are more likely to encounter a HPV‐positive man or women, limiting herd effects.20 However, in Australia declines in vaccine‐type prevalence were also observed among the high‐risk group of chlamydia‐positive unvaccinated women.33 Second, in Australia and Scotland, the vaccination initiation rate was much higher: over 80% in Australia and over 90% in Scotland.2 Third, the time horizon of our study (6 years postvaccination) might be too short to observe second‐order herd effects. In the United States, where vaccination coverage was also limited, decreases in vaccine type prevalence among unvaccinated women were noted 5–8 years after vaccine introduction and not yet after 3–6 years.32, 34
In conclusion, the declining HPV16/18 prevalence among women is consistent with previous studies, but our findings also provide evidence for herd protection in heterosexual men after girls‐only HPV16/18 vaccination. Due to the reduction in the HPV16/18 prevalence among women and heterosexual men, HPV‐related cancers are expected to decline in both sexes after girls‐only HPV vaccination. The absence of measurable herd effects among unvaccinated women 6 years postvaccination highlights once again the importance of high vaccination coverage to optimally reduce HPV‐related cancer morbidity.
Author Contribution
MS developed the PASSYON study design. The Public Health Services collected the data. PW coordinated the data collection. SL and the Medical Microbiological Laboratories obtained, identified, stored the samples and performed the laboratory analyses. AK coordinated the laboratory analyses. PW led on the statistical analyses and data interpretation with oversight from JB. BB and CH assisted with data interpretation. PW drafted the paper. All authors contributed to drafting and revision of the paper and all authors read and approved the final version. The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and final responsibility to submit for publication.
Supporting information
Supplementary Table 1: Characteristics over time and relation with high‐risk human papillomavirus (hrHPV) positivity among all women.
Supplementary Table 2: Characteristics over time and relation with high‐risk human papillomavirus (hrHPV) positivity among heterosexual men.
Supplementary Table 3: Characteristics over time and relation with high‐risk human papillomavirus (hrHPV) positivity among unvaccinated women.
Supplementary Table 4: Comparing postvaccination human papillomavirus (HPV) prevalence with prevaccination prevalence (2009) and assessing the trend among heterosexual men, excluding the men who reported to be HPV vaccinated.
Supplementary Table 5: Comparing postvaccination human papillomavirus (HPV) prevalence with prevaccination prevalence (2009) and assessing the trend for HPV31, HPV33 and HPV45 among all women, heterosexual men, and unvaccinated women.
Supplementary Table 6: Comparing postvaccination HPV prevalence with prevaccination prevalence (2009) and assessing the trend among all women, heterosexual men, and unvaccinated women, stratified by age category.
Supplementary Figure 1: Prevalence of human papillomavirus (HPV) types 16 or 18, HPV16, and HPV18 over time and p vales for the crude trend test, among: A) 16‐ to 20‐year‐old women; B) 21‐ to 24‐year‐old women; C) 16‐ to 20‐year‐old heterosexual men; D) 21‐ to 24‐year‐old heterosexual men; E) 16‐ to 20‐year‐old unvaccinated women; F) 21‐ to 24‐year‐old unvaccinated women.
Acknowledgements
The authors thank Hein Boot (deceased), Elske van Logchem, Naomi van Marm and Rianne Vriend for their valuable contributions to the design or execution of the study and Susan T. Landry for editorial assistance in the preparation of this report. Furthermore, the STI clinics, including all nurses and physicians, within the Public Health Services and the hospitals are acknowledged for their permission to collect data from their patients and their effort. The authors acknowledge the medical microbiologic laboratories and the analysts for storage and testing of the samples. Medical Microbiological Laboratories: Certe: D Adema, R Buist‐Arkema, D Luijt, S Meijer, J Schirm. ETZ Hospital Tilburg: A Buiting, H Verbakel, P van Esch, J Verweij. Erasmus Medical Center: A van der Eijk. University Medical Center Utrecht: F Verduyn Lunel, S Lakbiach, R Schuurman. Public Health Laboratory Amsterdam: D Abma, K Adams, S Bruisten, I Linde, P Oostvogel, C Touwen, W Vermeulen. Maastricht University Medical Center: J Nelissen, P Wolffs. Jeroen Bosch Hospital: N van Duijvendijk, P Schneeberger. Radboud University Medical center: M Dinnissen ‐ van Poppel, W Melchers. Izore: M Hooghiemstra, H Huisman, J Weel. LabMicTA: F. Bosma, F. Geeraedts, I.Polman. Isala: P van Goor, M Wolfhagen. Rijnstate: C de Mooij, E van Koolwijk, M Peters, C Swanink, R Tiemessen. Medical laboratory dr. Stein & Collegae: J Janssen, M Pelsers. Canisius Wilhelmina Hospital: W de Waal. Public Health Services: PHS Drenthe: G Aalfs. PHS IJsselland: H van Buel. PHS Gelderland‐Zuid: C van Bokhoven‐Rombouts, P Cornelissen, M Kersten, C van Ruitenbeek, I Molenaar. University Medical Center Utrecht: E Doorn. PHS Rotterdam‐Rijnmond: H Götz, M Illidge, J Stam, E Swaders. PHS Groningen: F Postma. PHS Zuid Limburg: AM Niekamp, M Smit. PHS Fryslân: D Bukasa, M Chirandjilal, T Taconis. PHS Twente: M de Graas, I Hondelink, C Kampman. PHS Hart voor Brabant: M van de Pas. PHS Amsterdam: T Heijman, A Hogewoning, M van Rooijen. PHS Gelderland‐Midden: F Neienhuijsen, M Pelgrim.
Conflict of interest: All authors declare no competing interests.
References
- 1. Chaturvedi AK. Beyond cervical cancer: burden of other HPV‐related cancers among men and women. J Adolesc Health 2010;46:S20–6. [DOI] [PubMed] [Google Scholar]
- 2. Bruni L, Diaz M, Barrionuevo‐Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 2016;4:e453–63. [DOI] [PubMed] [Google Scholar]
- 3. de Melker HE, Conyn‐Van Spaendonck MA, Boot HJ, et al. Introduction to vaccination against cervical cancer. Ned Tijdschr Geneeskd 2009;153:658–61. [PubMed] [Google Scholar]
- 4. van Lier EA, Oomen PJ, Giesbers H, et al. Vaccination coverage National Immunization Program The Netherlands, report year 2011. Dutch. Bilthoven: RIVM 2011; 210021014/2011. https://www.rivm.nl/bibliotheek/rapporten/210021014.pdf [Google Scholar]
- 5. van Lier EA, Geraedts JLE, Oomen PJ, et al. Vaccination coverage and year report of the National Immunization Dental Program in The Netherlands 2016. Dutch. Bilthoven: RIVM 2017; 2017–0010. https://www.rivm.nl/bibliotheek/rapporten/2017-0010.pdf [Google Scholar]
- 6. Woestenberg PJ, King AJ, van Benthem BHB, et al. Bivalent vaccine effectiveness against type‐specific HPV positivity: evidence for cross‐protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis 2018;217:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donken R, King AJ, Bogaards JA, et al. High effectiveness of the bivalent human papillomavirus (HPV) vaccine against incident and persistent HPV infections up to 6 years after vaccination in young Dutch women. J Infect Dis 2018;217:1579–89. [DOI] [PubMed] [Google Scholar]
- 8. Drolet M, Benard E, Boily MC, et al. Population‐level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta‐analysis. Lancet Infect Dis 2015;15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7‐year cross‐sectional study. Lancet Infect Dis 2017;17:1293–302. [DOI] [PubMed] [Google Scholar]
- 10. Lehtinen M, Soderlund‐Strand A, Vanska S, et al. Impact of gender‐neutral or girls‐only vaccination against human papillomavirus‐results of a community‐randomized clinical trial (I). Int J Cancer 2018;142:949–58. [DOI] [PubMed] [Google Scholar]
- 11. Mesher D, Panwar K, Thomas SL, et al. The impact of the national HPV vaccination program in England using the bivalent HPV vaccine: surveillance of type‐specific HPV in young females, 2010‐2016. J Infect Dis 2018;218:911–21. [DOI] [PubMed] [Google Scholar]
- 12. Sonnenberg P, Clifton S, Beddows S, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of sexual attitudes and lifestyles (Natsal). Lancet 2013;382:1795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bogaards JA, Wallinga J, Brakenhoff RH, et al. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: bayesian evidence synthesis. BMJ 2015;350:h2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qendri V, Bogaards JA, Berkhof J. Health and economic impact of a tender‐based, sex‐neutral human papillomavirus 16/18 vaccination program in The Netherlands. J Infect Dis 2017;216:210–9. [DOI] [PubMed] [Google Scholar]
- 15. Vriend HJ, Boot HJ, van der Sande MA. Type‐specific human papillomavirus infections among young heterosexual male and female STI clinic attendees. Sex Transm Dis 2012;39:72–8. [DOI] [PubMed] [Google Scholar]
- 16. Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 2013;22:661–70. [DOI] [PubMed] [Google Scholar]
- 17. Xue X, Gange SJ, Zhong Y, et al. Marginal and mixed‐effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev 2010;19:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brisson M, Benard E, Drolet M, et al. Population‐level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta‐analysis of predictions from transmission‐dynamic models. Lancet Public Health 2016;1:e8–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Visser M, van Aar F, Van Aam Oeffelen, et al. Sexually tranmitted infections in The Netherlands in 2016. Bitlhoven: RIVM, 2017. 2017–0003. [Google Scholar]
- 20. Baussano I, Lazzarato F, Ronco G, et al. Impacts of human papillomavirus vaccination for different populations: a modeling study. Int J Cancer 2018;143:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mesher D, Panwar K, Thomas SL, et al. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: an ongoing cross‐sectional study. BMJ Open 2016;6:e009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chow EPF, Machalek DA, Tabrizi SN, et al. Quadrivalent vaccine‐targeted human papillomavirus genotypes in heterosexual men after the Australian female human papillomavirus vaccination programme: a retrospective observational study. Lancet Infect Dis 2017;17:68–77. [DOI] [PubMed] [Google Scholar]
- 23. Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet 2011;377:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 2009;20:449–57. [DOI] [PubMed] [Google Scholar]
- 25. Chow EP, Read TR, Wigan R, Donovan B, Chen MY, Bradshaw CS, Fairley CK. Ongoing decline in genital warts among young heterosexuals 7 years after the Australian human papillomavirus (HPV) vaccination programme. Sex Transm Infect 2015;91:214–9. [DOI] [PubMed] [Google Scholar]
- 26. McDonald SA, Qendri V, Berkhof J, et al. Disease burden of human papillomavirus infection in The Netherlands, 1989‐2014: the gap between females and males is diminishing. Cancer Causes Control 2017;28:203–14. [DOI] [PubMed] [Google Scholar]
- 27. Brisson M, Van de Velde N, Boily MC. Different population‐level vaccination effectiveness for HPV types 16, 18, 6 and 11. Sex Transm Infect 2011;87:41–3. [DOI] [PubMed] [Google Scholar]
- 28. Ramanakumar AV, Naud P, Roteli‐Martins CM, et al. Incidence and duration of type‐specific human papillomavirus infection in high‐risk HPV‐naive women: results from the control arm of a phase II HPV‐16/18 vaccine trial. BMJ Open 2016;6:e011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brisson M, van de Velde N, Franco EL, et al. Incremental impact of adding boys to current human papillomavirus vaccination programs: role of herd immunity. J Infect Dis 2011;204:372–6. [DOI] [PubMed] [Google Scholar]
- 30. Lehtinen M, Luostarinen T, Vanska S, et al. Gender‐neutral vaccination provides improved control of human papillomavirus types 18/31/33/35 through herd immunity: results of a community randomised trial (III). Int J Cancer 2018;143:2299–310. [DOI] [PubMed] [Google Scholar]
- 31. Tabrizi SN, Brotherton JM, Kaldor JM, et al. Assessment of herd immunity and cross‐protection after a human papillomavirus vaccination programme in Australia: a repeat cross‐sectional study. Lancet Infect Dis 2014;14:958–66. [DOI] [PubMed] [Google Scholar]
- 32. Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction‐National Health and nutrition examination survey, United States, 2003‐2014. J Infect Dis 2017;216:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chow EP, Danielewski JA, Fehler G, et al. Human papillomavirus in young women with chlamydia trachomatis infection 7 years after the Australian human papillomavirus vaccination programme: a cross‐sectional study. Lancet Infect Dis 2015;15:1314–23. [DOI] [PubMed] [Google Scholar]
- 34. Markowitz LE, Liu G, Hariri S, et al. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 2016;137:e20151968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Characteristics over time and relation with high‐risk human papillomavirus (hrHPV) positivity among all women.
Supplementary Table 2: Characteristics over time and relation with high‐risk human papillomavirus (hrHPV) positivity among heterosexual men.
Supplementary Table 3: Characteristics over time and relation with high‐risk human papillomavirus (hrHPV) positivity among unvaccinated women.
Supplementary Table 4: Comparing postvaccination human papillomavirus (HPV) prevalence with prevaccination prevalence (2009) and assessing the trend among heterosexual men, excluding the men who reported to be HPV vaccinated.
Supplementary Table 5: Comparing postvaccination human papillomavirus (HPV) prevalence with prevaccination prevalence (2009) and assessing the trend for HPV31, HPV33 and HPV45 among all women, heterosexual men, and unvaccinated women.
Supplementary Table 6: Comparing postvaccination HPV prevalence with prevaccination prevalence (2009) and assessing the trend among all women, heterosexual men, and unvaccinated women, stratified by age category.
Supplementary Figure 1: Prevalence of human papillomavirus (HPV) types 16 or 18, HPV16, and HPV18 over time and p vales for the crude trend test, among: A) 16‐ to 20‐year‐old women; B) 21‐ to 24‐year‐old women; C) 16‐ to 20‐year‐old heterosexual men; D) 21‐ to 24‐year‐old heterosexual men; E) 16‐ to 20‐year‐old unvaccinated women; F) 21‐ to 24‐year‐old unvaccinated women.


