Abstract
Objective
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, including recommended treatments such as oxygen, commonly used medications such as opioids, and emerging medications such as intranasal ketamine. Particular focus is paid to a large subset of respondents 65 years of age or older.
Background
Large international surveys of cluster headache are rare, as are examinations of treatments and side effects in older cluster headache patients. This article presents data from the Cluster Headache Questionnaire, with respondents from over 50 countries and with the vast majority from the United States, the United Kingdom, and Canada.
Methods
This internet‐based survey included questions on cluster headache diagnostic criteria, which were used as part of the inclusion/exclusion criteria for the study, as well as effectiveness of medications, physical and medical complications, psychological and emotional complications, mood scores, and difficulty obtaining medications. The diagnostic questions were also used to create a separate group of respondents with probable cluster headache. Limitations to the methods include the use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of some medications (eg, all triptans as opposed to sumatriptan subcutaneous alone).
Results
A total of 3251 subjects participated in the questionnaire, and 2193 respondents met criteria for this study (1604 cluster headache and 589 probable cluster headache). Of the respondents with cluster headache, 68.8% (1104/1604) were male and 78.0% (1245/1596) had episodic cluster headache. Over half of respondents reported complete or very effective treatment for triptans (54%, 639/1139) and oxygen (54%, 582/1082). Between 14 and 25% of respondents reported complete or very effective treatment for ergot derivatives (dihydroergotamine 25%, 42/170; cafergot/ergotamine 17%, 50/303), caffeine and energy drinks (17%, 7/41), and intranasal ketamine (14%, 5/37). Less than 10% reported complete or very effective treatment for opioids (6%, 30/541), intranasal capsaicin (5%, 7/151), and intranasal lidocaine (2%, 5/241). Adverse events were especially low for oxygen (no or minimal physical and medical complications 99%, 1077/1093; no or minimal psychological and emotional complications 97%, 1065/1093), intranasal lidocaine (no or minimal physical and medical complications 97%, 248/257; no or minimal psychological and emotional complications 98%, 251/257), intranasal ketamine (no or minimal physical and medical complications 95%, 38/40; no or minimal psychological and emotional complications 98%, 39/40), intranasal capsaicin (no or minimal physical and medical complications 91%, 145/159; no or minimal psychological and emotional complications 94%, 150/159), and caffeine and energy drinks (no or minimal physical and medical complications 89%, 39/44; no or minimal psychological and emotional complications 91%, 40/44). This is in comparison to ergotamine/cafergot (no or minimal physical and medical complications 83%, 273/327; no or minimal psychological and emotional complications 89%, 290/327), dihydroergotamine (no or minimal physical and medical complications 81%, 143/176; no or minimal psychological and emotional complications 91%, 160/176), opioids (no or minimal physical and medical complications 76%, 416/549; no or minimal psychological and emotional complications 77%, 423/549), or triptans (no or minimal physical and medical complications 73%, 883/1218; no or minimal psychological and emotional complications 85%, 1032/1218). A total of 139 of 1604 cluster headache respondents (8.7%) were age 65 and older and reported similar effectiveness and adverse events to the general population. The 589 respondents with probable cluster headache reported similar medication effectiveness to respondents with a full diagnosis of cluster headache.
Conclusions
Oxygen is reported by survey respondents to be a highly effective treatment with few complications in cluster headache in a large international sample, including those 65 years or over. Triptans are also very effective with some side effects, and newer medications deserve additional study. Patients with probable cluster headache may respond similarly to acute medications as patients with a full diagnosis of cluster headache.
Keywords: cluster headache, trigeminal autonomic cephalalgia, probable cluster headache, oxygen, triptan, Medicare
Abbreviations
- BDI
Beck Depression Inventory
- CHQ
Cluster Headache Questionnaire
- HDSQ
Hopelessness Depression Symptom Questionnaire
- ICHD
International Classification of Headache Disorders
Introduction
Cluster headache is a primary headache disorder characterized by severe unilateral pain lasting 15–180 minutes, occurring up to 8 times daily, that is associated with cranial autonomic features and/or restlessness.1 In the acute treatment of cluster headache, options are limited and high‐flow oxygen has received much attention for several reasons. First, oxygen is 1 of only 3 acute treatments with level A evidence from either American or European guidelines, along with sumatriptan (subcutaneous and nasal formulations) and zolmitriptan (nasal formulation).2, 3 Oxygen is also the preferred acute treatment in pregnancy and lactation.4, 5 Second, oxygen has minimal side effects, contraindications, and limitations whereas triptans such as sumatriptan and zolmitriptan have side effects, vascular contraindications, and limitations for the number of times they can be used daily.6 Third, oxygen is not always reimbursed by insurance carriers for cluster headache. In the United States, oxygen for cluster headache was covered by at least 4 private commercial health insurance companies but not all,7 and more insurance companies covered sumatriptan than oxygen.8 In addition, oxygen was not covered by the US Centers for Medicare and Medicaid Services,7 which includes coverage for many patients 65 years and older. In a survey of headache societies worldwide, which did not include the United States, oxygen was reimbursed for cluster headache in 50% of the 22 countries that responded, with only 3 countries having restrictions for patients 65 years and older.9
We aim to investigate how oxygen compares to other acute medications recommended by current guidelines, such as the triptans and intranasal lidocaine, as well as to other frequently used medications such as opioids, caffeine, and, more recently, intranasal ketamine. We also aim to investigate treatments and complications in the subgroup of respondents 65 years and older. This dataset has previously been presented as an abstract.10
Methods
The Cluster Headache Questionnaire (CHQ) is a self‐administered internet‐based survey conceived and constructed by authors SMP and LIS, with authors MJB and RES asked to provide input as neurologists and assist in analysis and interpretation, and author YY asked to provide statistical analysis. The CHQ consists of 152 items organized into 8 separate sections: (1) Sign up and Verification; (2) Symptom Screening; (3) Demographics; (4) Experience; (5) Medications/Treatment; (6) Beck Depression Inventory; (7) Hopelessness Depression Symptom Questionnaire; and (8) End of Survey – Contact Options. Sections 1–5 were newly created by the authors and were tested on 10 cluster headache respondents and reviewed by 1 neurologist prior to release of the final version; however, these questions were not otherwise validated. The scope of this manuscript focuses primarily on “Medications/Treatment” in Section 5. Several of the questions for Sections 2 and 5 are shown in Supplemental Figure 1. The study was performed in Qualtrics, which enabled the authors to construct, distribute, collect, and securely store responses. Qualtrics is an online survey company that provides web‐based survey software, encrypted cloud‐based data storage, and controlled user access. The University of West Georgia holds a Qualtrics software license and datacenter.
Informed Consent. Respondents were given a summary of the intent and purpose of the research as well as a brief summary of each section. In the frame following, respondents were required to verify their age (18 years or older) as well as agree to participate in the survey. Due to concerns about suicidality, international suicide prevention resources were embedded in the CHQ and respondents could skip some questions that were deemed to be potential triggers. At the end of the survey, respondents could elect to share their contact information for further follow‐up, but this was not required.
Distribution and Data Collection. Recruitment, distribution, and data collection consisted of 3 concurrent efforts: direct email through the Clusterbusters member listserv, web‐site hosting through Clusterbusters and the International Headache Society, and advertising on Google via Google AdWords as well as Reddit forum. Clusterbusters is a nonprofit organization with a mission statement that includes “research, education, support, and advocacy related to cluster headache”11 and thus their website would be expected to select for respondents interested in more information on several aspects of cluster headache. The survey was open without a password. It was voluntary and accessible internationally by anyone with internet access; however, the survey utilized cookies and recorded IP addresses to identify unique survey respondents and prevent multiple submissions. The survey was presented in the same order to all respondents and displayed a progress bar; respondents could use a “back button” to review their responses before submission, and progress was saved (based on IP address) allowing respondents to close their browser or navigate away. No incentives were offered for taking the survey. IRB approval was obtained in January 2016 from the University of West Georgia, the survey was piloted in February 2016 with 10 cluster headache respondents and a neurologist, and the survey was open online from March 2016 to April 2018.
Participants. For inclusion, participants must have: (1) stated that they were at least 18 years of age; (2) stated that they had been diagnosed with cluster headache by a medical professional; (3) completed at least 90% of the survey including all inclusion/exclusion questions; and (4) filled out the English version (other versions were generated in Google translate but have not been fully verified by native speakers). For exclusion, participants answered several questions that addressed the full International Classification of Headache Disorders (ICHD) 3‐beta criteria for cluster headache and probable cluster headache,12 including all autonomic features except for rhinorrhea, and all other criteria except criterion E (“not better accounted for by another ICHD‐3‐beta diagnosis”). Because we asked respondents about their longest period of remission in the last year, the definitions of episodic and chronic cluster headache reflect the new ICHD‐3 criteria that were released during this study (ie, 3 months of headache freedom for episodic cluster headache).1 Chronic cluster headache was defined as a remission period lasting less than 3 months in the last year; episodic cluster headache was defined as all respondents who stated that they were episodic, as well as all respondents who stated they were chronic but the headache remission period was 3 months or longer. Authors MJB and RES reviewed these questions and excluded all respondents who did not meet the criteria for cluster headache or probable cluster headache. However, the authors did not corroborate a formal clinical diagnosis of cluster headache. The diagnoses of cluster headache and probable cluster headache were never combined in the analysis and were always examined independently.
Development. Qualtrics provides an adaptive/responsive display framework in conjunction with response validations, thus not all respondents received all 152 questions. Questions were grouped on each screen so that an individual screen could contain between 1 and 30 questions. At a minimum, the survey prompted and required all respondents to answer “yes,” “no,” or “decline to answer” for each treatment subcategory. Section 5 (Medications/Treatment) divided interventions into 4 subsections: preventive medications, abortive medications, unregulated treatments, and surgical/neuromodulation treatments. For the abortive medication section, the first question displayed a list of common abortive medications in “Check Box” format: (1) Triptans; (2) 100% Oxygen; (3) Cafergot/Ergotamine; (4) Intranasal Ketamine; (5) Lidocaine Nasal Drops; (6) DHE–IV (Migranal); (7) Intranasal Capsaicin; and (8) Opiates, as well as 3 additional “other” boxes for respondent write‐in (see Supplemental Fig. 1). Questions about triptans referred not to specific medications or specific routes of delivery but to the class of triptans (almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan). Questions about oxygen mentioned 100% oxygen but did not specify a flow rate, flow duration, or type of delivery mask or cannula used. For the purposes of the survey, oxygen was included as a “medication.” Intranasal capsaicin was included as an abortive despite being recommended as a preventive medication that may take several days for effect13 because some patients have found immediate relief and use it in an abortive fashion. Questions for each medication were asked as follows:
Effectiveness: This question carried forward and displayed only the previously checked medications. Respondents then evaluated the effectiveness of each medication as: (1) completely ineffective; (2) minimally effective; (3) somewhat effective; (4) very effective; and (5) completely effective. No further explanation or definitions of these choices were provided.
Access: This question asked respondents “how difficult it was to obtain” each medication as: (1) no difficulty; (2) slight difficulty; (3) some difficulty; (4) extreme difficulty; and (5) unable to get. No further explanation or definitions of these choices were provided.
Adverse events: Respondents were subsequently asked to evaluate the psychological or emotional complications as well as the physical or medical complications of each medication in 2 separate questions. For both questions, respondents chose from (1) none; (2) minimal complications; (3) some complications; or (4) severe complications. These questions also displayed text/write‐in option allowing respondents to describe if they marked “severe” complications. No further explanation or definitions of these choices were provided.
One category of abortive medications – caffeine and energy drinks – was created after the survey was closed because a free text box was allowed and there were a high number of entries for caffeine, coffee, espresso, and energy drinks. We excluded combination medications such as caffeine plus aspirin or acetaminophen plus caffeine plus butalbital. While the study did ask about abortive neuromodulation devices such as sphenopalatine ganglion stimulation and vagus nerve stimulation as acute treatments, there were less than 25 responses for each and the type of device used could not be verified, thus they were not included in the analysis.
Statistical analysis: All statistical analyses were performed in R, version 3.4.2 (www.r-project.org). For categorical variables, a multinomial test was used to test the null hypothesis of equal value. To test the relationship between nominal and ordinal variables, we calculated Freeman’s Theta and performed a Cochran–Armitage test for trend. A Bonferroni correction was used to adjust P values, and adjusted P values less than .05 were considered statistically significant. A separate Bonferroni correction was calculated for each analysis (ie, a specific Bonferroni correction was calculated for each figure). Two‐tailed tests were used throughout the study.
To compare medications, we reduced the Likert scales from 5 categories to 2 categories to increase statistical strength, limited our analysis to the 3 most commonly used treatments in the survey (oxygen, triptans, and opioids), then performed a generalized linear mixed‐effects model using these 3 medications as the predictors. The significance was then evaluated by likelihood ratio tests; post hoc pairwise comparisons of oxygen, triptans, and opioids were examined using Tukey’s method through the “lsmeans” package in R version 3.4.2.14 The categories were reduced from 5 to 2 before analyzing the data after discussion between authors. The reduced categories were as follows: (1) high effectiveness (completely effective and very effective) vs low effectiveness (somewhat effective, minimally effective, and completely ineffective); and (2) high complications (severe complications and some complications) vs low complications (minimal complications and no complications).
No statistical calculation of power was performed prior to the study. Sample size was based on a previous study.15 Due to the rarity of the disorder, the study was open for 2 years to ensure sufficient time for the widest reach internationally. In this study, we analyzed 2 subsets of respondents: (1) probable cluster headache, decided after the survey started but before analysis began, and (2) respondents 65 years and older, a subset decided before the survey began in part to investigate the difference in insurance coverage in this age group. All other analyses were planned either before the survey began (by authors SMP and LIS) or after the survey started but before analysis began (by authors MJB, RES, and YY).
Missing data are as follows. For cluster headache respondents, 8 respondents did not provide an answer for chronic vs episodic (n = 1596), 3 respondents did not answer duration of headache (n = 1601), and 1 respondent did not answer the Beck’s Depression Inventory II (n = 1603). There were several respondents that answered questions on complications and access to medications that did not answer the question about effectiveness, suggesting missing data on effectiveness for 25 triptans, 11 oxygen, 6 dihydroergotamine, 24 cafergot/ergotamine, 3 ketamine, 8 opioid, 8 capsaicin, 3 caffeine and energy drinks, and 16 lidocaine. The full range of missing data for medications, however, is unknown: in our survey design, a blank response could mean the respondent did not try a medication, but could also mean that they forgot that they tried a medication. For probable cluster headache respondents, 1 respondent did not provide an answer for age of onset (n = 588), and 2 respondents did not provide an answer for chronic vs episodic (n = 587).
Results
A total of 4876 IP addresses were recorded on the website representing 4876 potential subjects. A total of 3251 subjects agreed to participate in the questionnaire, and 2193 (1604 cluster headache and 589 probable cluster headache) met inclusion and exclusion criteria for the study (Fig. 1). Demographics and locations of included respondents with cluster headache are shown in Table 1. Compared to published reports,15, 16 the participants represent a typical sample of cluster headache patients in terms of sex, age of onset, duration of headaches, and proportion with restlessness; however, they reported a slightly lower proportion of episodic cluster headache and slightly higher average frequency of attacks per day. They include a wide range of ages, with 139 respondents age 65 and older. The participants reported from 6 continents and 56 countries/territories (Supplemental Table 1), with the majority (80.5%, 1292/1604) from 3 countries: the United States, the United Kingdom, and Canada. Compared to the cluster headache sample, respondents with probable cluster headache had a similar age of onset and a similar proportion of restlessness, with a higher proportion of women, a lower proportion of episodic cluster headache, a longer duration of headaches, and a higher frequency of attacks (Supplemental Table 2).
Figure 1.
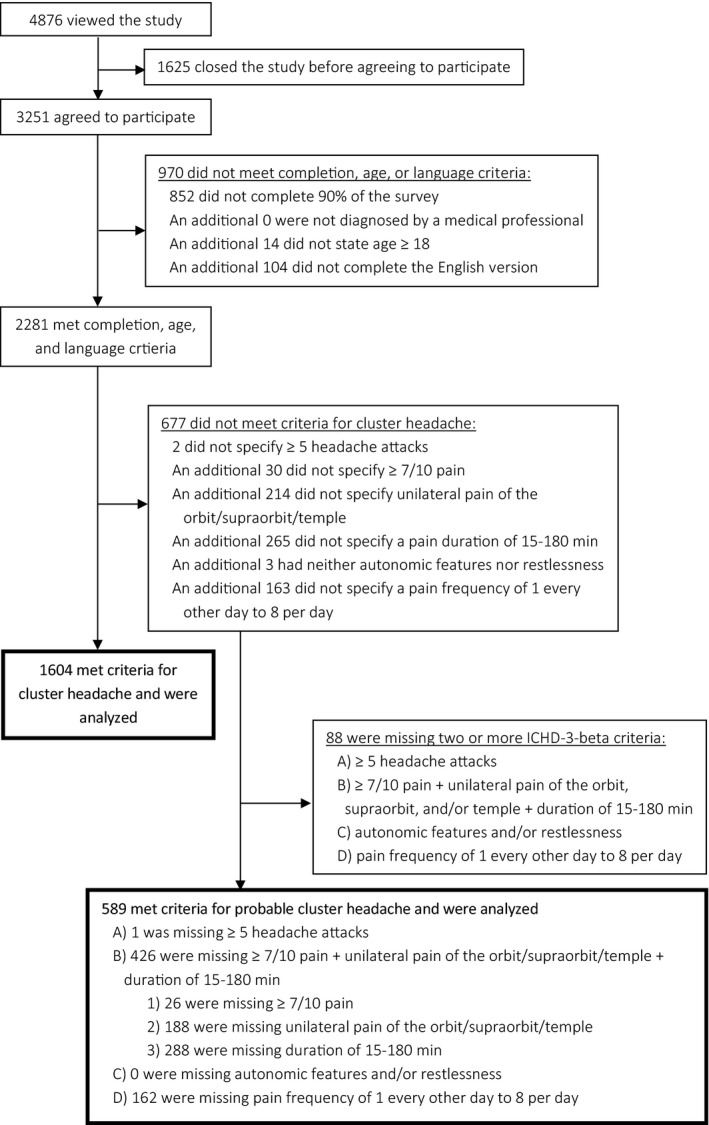
Flow diagram of inclusion and exclusion. Respondents who opened the study were identified by IP addresses; for all other portions of the flow chart, respondents were identified based on answers to screening questions. Probable cluster headache was defined as respondents with all but one of the ICHD‐3‐beta criteria for cluster headache; of note the study did not ask about rhinorrhea or about criterion E, “not better accounted for by another ICHD‐3‐beta diagnosis.”
Table 1.
Demographics of Survey Respondents With Cluster Headache
| Cluster headache (total n = 1604) | |
| Basic demographics | |
| Age in years | 46 (13) |
| Age 65 and older in years | 139 (8.7%) |
| Sex | 1104 male (68.8%), 497 female (31%), 3 other (0.2%) |
| Headache characteristics | |
| Episodic vs chronic diagnosis | 1245 episodic (78.0%), 351 chronic (22.0%) |
| Age of onset of cluster headache in years | 27 (13) |
| Duration of headache in hours | 1.4 (0.7) |
| Frequency of headache in attacks/day | 3.9 (2.1) |
| Respondents with restlessness | 1550 (96.6%) |
| Continent of residence | |
| North America: 7 countries/territories responding | 1074 (67%) |
| Europe: 30 countries responding | 382 (23.8%) |
| Australia/Pacific Islands: 2 countries responding | 77 (4.8%) |
| Africa: 4 countries responding | 33 (2.1%) |
| Asia: 9 countries responding | 31 (1.9%) |
| South America: 4 countries responding | 7 (0.4%) |
Data reported as either “average (standard deviation)” or as “total number (% of total).” For frequency of headache, episodic cluster headache respondents were asked about the frequency during the peak of their headache cycle. Respondents with probable cluster headache are not included in this Table (see Supplemental Table 2) nor is a full list of countries (see Supplemental Table 1). Chronic cluster headache was defined as a remission period lasting less than 3 months in the last year; all other patients were considered episodic, including those with chronic cluster headaches in previous years.
Figure 2 shows responses for the effectiveness of acute medications in all respondents (Fig. 2A) and in respondents 65 years and older (Fig. 2B) (data also reported in table format in Supplemental Table 3). In all respondents, triptans and oxygen were reported as completely effective or very effective in 54% each, dihydroergotamine in 25%, cafergot/ergotamine in 17%, caffeine and energy drinks in 17%, opioids in 6%, intranasal capsaicin in 5%, and intranasal lidocaine in 2%. Respondents 65 years and older reported similar effectiveness: triptans were reported as completely effective or very effective in 61%, and oxygen in 56%. There was a small sample size of respondents 65 and older taking other treatments, and findings were not significant.
Figure 2.
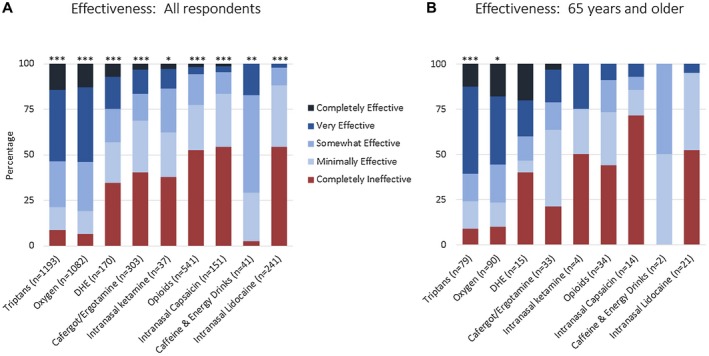
Effectiveness of acute medications in cluster headache based on an international survey. Figure shows all respondents (A) and respondents age 65 and older (B). Not all respondents trialed every medication, thus the number of responses for each medication is shown. Adjusted P values compare completely effective, very effective, somewhat effective, minimally effective, and completely ineffective for individual medications; asterisks denote adjusted P value <.05 (*), <.001 (**), and <.0001 (***). Values for this figure are listed in Supplemental Table 3.
We specifically compared the effectiveness of oxygen, triptans, and opioids and observed a statistical significance of the association (P < .001). We further tested which medication differed in effectiveness and found: (1) triptans and oxygen were not statistically different in effectiveness (P = .99); (2) triptans were more likely to be effective than opioids (odds ratio 19.77, 95% confidence interval [CI] 16.18‐24.16, P < .0001); and (3) oxygen was more likely to be effective than opioids (odds ratio: 19.94 (95% CI 16.32‐24.38), P < .0001). The subgroup of respondents age 65 and older had a smaller sample size and was not investigated in this comparative analysis.
Further analysis was performed on respondents with complete effectiveness to 1 or more medications to see if any categories might be predictive of an excellent response to cluster headache medications. Complete effectiveness did not vary significantly by sex, age, country, or cluster headache features; however, complete effectiveness of triptans interestingly did associate with the effectiveness of calcium channel blockers and corticosteroids, while no other acute medication had any significant associations (Supplemental Table 4).
Further analysis was also performed after dividing respondents into episodic and chronic cluster headache. Oxygen gas was more effective in episodic cluster headache than in chronic cluster headache (adjusted P = .0007, see Supplemental Fig. 2). No significantly different responses between episodic and chronic cluster headache were seen for triptans, dihydroergotamine, cafergot/ergotamines, intranasal ketamine, opioids, intranasal capsaicin, caffeine and energy drinks, or intranasal lidocaine (data not shown).
Respondents were also asked about any side effects of acute medications. Figure 3 shows physical and medical complications. In all respondents (Fig. 3A), there were no or minimal physical and medical complications for oxygen at 99%, intranasal lidocaine at 97%, ketamine at 95%, and intranasal capsaicin at 92%. There were also no or minimal physical and medical complications for caffeine and energy drinks at 89%, cafergot/ergotamine at 83%, dihydroergotamine at 81%, opioids at 76%, and triptans at 73%. Complications did not vary significantly by sex or age, though triptan complications did vary by country (Supplemental Table 5). Physical and medical complications generally associated with psychological and emotional complications for the same medication. Complications did not vary with depression or hopelessness inventories for any medication. In respondents 65 years and older (Fig. 3B), no or minimal physical and medical complications were seen for oxygen at 97%, intranasal lidocaine at 96%, triptans at 79%, cafergot/ergotamine at 69%, and opioids at 68%.
Figure 3.
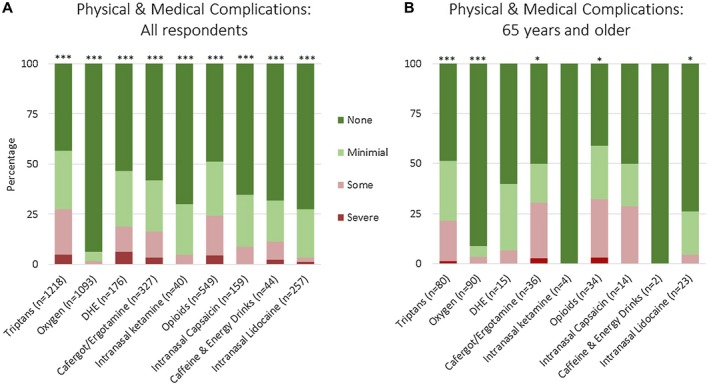
Physical and medical adverse effects of acute medications in cluster headache based on an international survey. Figure shows all respondents (A) and respondents age 65 and older (B). Not all respondents trialed every medication, thus the number of responses for each medication is shown. Adjusted P values compare no, mild, some, and severe adverse effects for individual medications; asterisks denote adjusted P value <.05 (*), <.001 (**), and <.0001 (***). Values for this figure are listed in Supplemental Table 3.
Figure 4 shows psychological and emotional complications, which show similar findings to physical and medical complications. In all respondents (Fig. 4A), there were no or minimal psychological and emotional complications for intranasal lidocaine at 98%, ketamine at 98%, oxygen at 97%, intranasal capsaicin at 94%, dihydroergotamine at 91%, and caffeine and energy drinks at 91%. There were also no or minimal psychological and emotional complications for cafergot/ergotamine at 89%, triptans at 85%, and opioids at 77%. In respondents 65 years and older (Fig. 4B), no or minimal psychological and emotional complications were seen for oxygen at 100%, triptans at 89%, cafergot/ergotamine at 86%, and opioids at 82%. Respondents 65 years and older reported very few complications from oxygen overall, with 91% (82/90) showing no, 6% (5/90) showing minimal, and 3% (3/90) showing some physical and medical complications, and 97% (87/90) showing no and 3% (3/90) showing minimal psychological and emotional complications.
Figure 4.
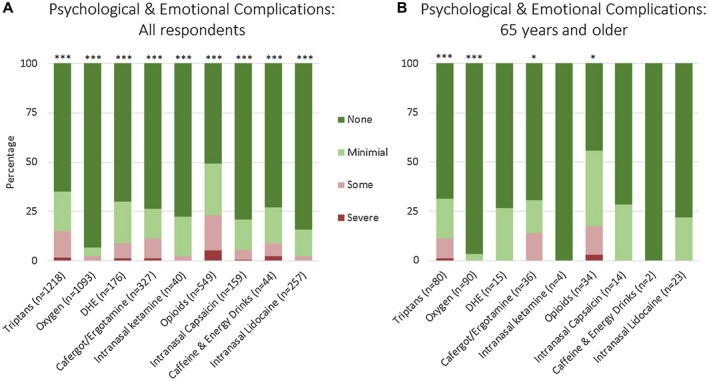
Psychological and emotional adverse effects of acute medications in cluster headache based on an international survey. Figure shows all respondents (A) and respondents age 65 and older (B). Not all respondents trialed every medication, thus the number of responses for each medication is shown. Adjusted P values compare no, mild, some, and severe adverse effects for individual medications; asterisks denote adjusted P value <.05 (*), <.001 (**), and <.0001 (***). Values for this figure are listed in Supplemental Table 3.
We also tested the association of oxygen, triptans, and opioids with physical and medical complications and observed a statistical significant association (P < .001). The post hoc testing to evaluate which medication differed in complications revealed: (1) oxygen was less likely to have physical or medical complications than triptans (odds ratio 464.43, 95% CI 300.94‐716.73, P < .0001); (2) oxygen was less likely to have physical or medical complications than opioids (odds ratio 1628.18, 95% CI: 981.78‐2700.15, P < .0001); and (3) triptans were less likely to have physical or medical complications than opioids (odds ratio 3.51, 95% CI 2.68‐4.59, P < .0001). In this study, the extremely high odds ratios were due to missing medication information: only 27.7% of respondents trialed all of oxygen, triptans, and opioids; furthermore, there were very few respondents with complications from oxygen (28 respondents). We then analyzed the data of the 27.7% of respondents who had trialed all 3 of oxygen, triptans, and opioids (394 respondents) and again found similar patterns: oxygen had less complications than triptans or opioids, and triptans had less complications than opioids. Specifically, there was a significant association between physical or medical complications and medications (P < .0001) with odds ratios as follows: triptans vs opiates (odds ratio 2.31, 95% CI 1.85‐2.90, P < .0001); oxygen vs opiates (odds ratio 15.54, 95% CI 10.70‐22.57, P < .0001); oxygen vs triptans (odds ratio 6.71, 95% CI 4.67‐9.65, P < .0001). The findings were identical for psychological or emotional complications: oxygen was less likely to have psychological or emotional complications than either triptans (odds ratio 464.43, 95% CI 300.94‐716.73, P < .0001) or opioids (odds ratio 1628.18, 95% CI 981.78‐2700.15, P < .0001), and triptans were also less likely to have psychological or emotional complications than opioids (odds ratio 3.51, 95% CI 2.68‐4.59, P < .0001). The subgroup of respondents age 65 and older had a smaller sample size and was not investigated in this comparative analysis.
The questionnaire explored access to acute treatments for cluster headache. Figure 5 shows the ability to obtain medications in all respondents and in those 65 years and older. For all respondents, no difficulty or slight difficulty was seen for caffeine and energy drinks at 100%, intranasal capsaicin at 91%, intranasal lidocaine at 81%, cafergot/ergotamine at 77%, intranasal ketamine at 73%, dihydroergotamine at 64%, opioids and triptans at 60% each, and oxygen at 49%. For oxygen, an additional question was added for time to prescription (Supplemental Fig. 3A) and data were available for 566 respondents: 36% of respondents were able to obtain oxygen within 1 month of their diagnosis of cluster headache, 25% within 1‐6 months, 11% within 6‐12 months, 15% within 1‐2 years, and 13% within 2‐5 years. For respondents over age 65, data on 46 respondents were available: 37% were able to obtain oxygen within 1 month of their diagnosis of cluster headache, 24% within 1‐6 months, 9% within 6‐12 months, 15% within 1‐2 years, and 15% within 2‐5 years. For respondents with difficulty obtaining oxygen, reasons included that physicians did not believe it would be effective or covered by insurance, insurance would not cover it, there were problems obtaining the medication, the respondent was a smoker, and practicality (Supplemental Fig. 3B). However, access was ultimately available for all medications: very few respondents were unable to get any of the treatments.
Figure 5.
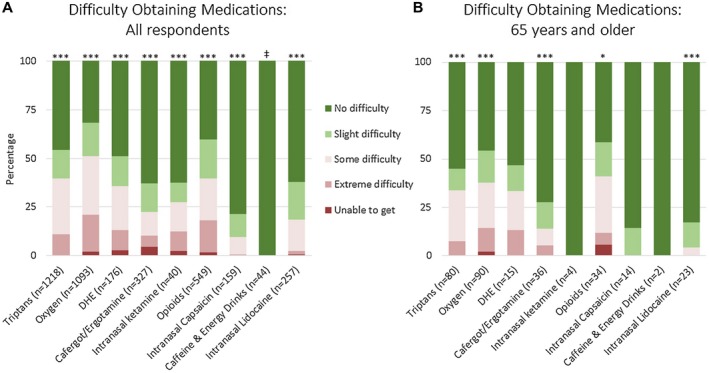
Difficulty in obtaining acute medications in cluster headache based on an international survey. Figure shows all respondents (A) and respondents age 65 and older (B). Not all respondents trialed every medication, thus the number of responses for each medication is shown. Adjusted P values compare no, mild, some, and severe adverse effects for individual medications; asterisks denote adjusted P value <.05 (*), <.001 (**), and <.0001 (***). ‡For “Caffeine & Energy Drinks” in all respondents, no respondent reported a side effect and therefore no statistical comparisons were necessary. Values for this figure are listed in Supplemental Table 3.
Finally, the questionnaire examined respondents with probable cluster headache (Supplemental Table 2). Respondents must have fulfilled criteria for 3 of Criteria A‐D1: only 1/589 missed Criterion A (“at least 5 attacks”), 72% or 426/589 missed Criterion B (“severe or very severe unilateral orbital, supraorbital and/or temporal pain lasting 15‐180 minutes”), none missed Criterion C (cranial autonomic features and/or restlessness or agitation), and 28% or 162/589 missed Criterion D (“occurring with a frequency between one every other day and 8 per day”). For respondents with a duration outside of 15‐180 minutes, the majority (89% or 256/288) reported headaches greater than 3 hours and the longest headache duration was 5 hours. For respondents with a frequency outside of 1 every other day and 8 per day, the majority (94.4% or 153/162) reported more than 8 headaches per day with the most attacks per day at 12. The effectiveness of acute medications in respondents with probable cluster headache (Fig. 6) were generally similar to respondents with a full diagnosis of cluster headache. Oxygen was reported as completely effective or very effective in 44% (178/411), triptans in 43% (178/411), dihydroergotamine in 24% (18/76), cafergot/ergotamine in 12% (13/112), opioids in 8% (19/226), intranasal capsaicin in 2% (1/55), and intranasal lidocaine in 2% (2/107). Intranasal ketamine and caffeine and energy drinks did not meet significance and had small sample sizes.
Figure 6.

Effectiveness of acute medications in probable cluster headache based on an international survey. Not all respondents trialed every medication, thus the number of responses for each medication is shown. Adjusted P values compare completely effective, very effective, somewhat effective, minimally effective, and completely ineffective for individual medications; asterisks denote adjusted P value <.05 (*), <.001 (**), and <.0001 (***). Values for this figure are listed in Supplemental Table 3.
Discussion
This is the largest cluster headache survey performed to date with respect to number of respondents to investigate the effect of acute medications in cluster headache. Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects. However, respondents reported that it was difficult to obtain. Triptans also had a high rate of effectiveness but also had high rates of complications. Dihydroergotamine and cafergot/ergotamine had intermediate effectiveness and intermediate side effects, while intranasal capsaicin and intranasal lidocaine were easy to access with limited complications, but also limited effectiveness. This study is the first to investigate the effects of intranasal ketamine, opioids, and caffeine in a large sample. Intranasal ketamine has an intermediate rate of effectiveness and few side effects. It has primarily been used by our American respondents (31 of 40 from the United States, 7 of 40 from Canada, and 1 each from the United Kingdom and Spain). Opioids, in contrast, are completely ineffective in more than half of respondents, with only 1% finding them completely effective and 4% finding them very effective, and with physical, medical, psychological, and emotional complications reported in some respondents. This study does not differentiate between types of opioids, and it is not clear if 1 type may be more effective than another. Interestingly, caffeine and energy drinks did have some degree of effectiveness in the majority of respondents with low levels of complications. A recent study in Denmark showed that cluster headache subjects were more likely to drink energy drinks but not coffee compared to controls,17 and energy drinks often have higher doses of caffeine. The effects of caffeine in our study were not collected systematically as they were obtained after the study from free text entries, but are interesting and require further examination.
When comparing the most commonly used medications, oxygen was more likely to be effective than opioids but not triptans. Oxygen was less likely to have complications than either opioids or triptans.
This study is also the first to investigate the effectiveness of acute medications in probable cluster headache in a large sample. Probable cluster headache may respond similarly to cluster headache, with triptans and oxygen having high levels of effectiveness. It should be noted, however, that the ICHD‐3‐beta Criterion E (“not better accounted for by another ICHD‐3 diagnosis”) was not included in this study, thus the definition of probable cluster headache requires meeting all but one of criteria A‐D.
The effectiveness of oxygen for cluster headache attacks ranges between 56% and 82% across multiple controlled and open‐label trials, as well as clinic and non‐clinic based questionnaires.8, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 This study is a non‐clinic based questionnaire showing oxygen as completely effective in 13%, very effective in 41%, somewhat effective in 27%, minimally effective in 12%, and completely ineffective in 7%. This study adds to the current literature in several ways. First, this is a large international survey, and again confirms that oxygen is reported by respondents to be highly effective for cluster headache. Second, given the size of the study we are able to comment on a large subgroup of older respondents. Respondents 65 years and older, like other respondents, find oxygen to be highly effective with minimal complications. The older respondents generally replicated the responses of all respondents: oxygen and triptans were effective but difficult to obtain, with triptans having some complications and oxygen having few complications. Opioids had intermediate levels of effectiveness and high levels of complications.
As the study did not specify the oxygen flow rate or mask type, the results in this study may in fact underestimate the effectiveness of the guideline recommendations for oxygen 6‐7 L/min or higher.2, 3 While oxygen is highly effective, it may not be the most effective treatment because all triptans were grouped together. Subcutaneous sumatriptan may be more effective than oxygen based on some previous questionnaires,21, 28 though a questionnaire that specifically compared oxygen >10 L/min to injectable sumatriptan found no difference in effectiveness.27 Thus higher doses of oxygen at 10‐15 L/min or more, which are used in clinical practice,29 may be the most effective doses of oxygen. Similarly, cafergots and ergotamine were grouped together, as were all routes of administration for dihydroergotamine.
Previous studies have looked at factors associated with oxygen in an attempt to predict who might respond. Oxygen responsiveness has been positively associated with shorter attacks and a lack of interictal pain,23 and negatively associated with photophobia or phonophobia during an attack,30 nausea and vomiting during an attack,26 or restlessness.26 Previous studies have found conflicting results for associations with age,18, 23, 26, 27 sex,18, 23, 31 and history of smoking.23, 26, 27, 30, 32 Our findings show no association of responses to any acute therapies with any cranial autonomic features examined (rhinorrhea was not examined) and no association with restlessness, photophobia/phonophobia, nausea/vomiting, age (current age or age of onset of cluster headaches), sex, or any other feature examined. However, this study does find that respondents who respond to triptans are also more likely to respond to calcium channel blockers and steroids. While a subgroup of treatment‐responsive patients may exist, another explanation is that these treatments all have the highest level recommendation in European guidelines2 and thus it may not be surprising that patients respond to all of these medications. The current literature does not suggest a genetic subgroup of patients that respond to triptans, calcium channel blockers, and steroids: a report has linked the rs5443 polymorphism of the GNB3 gene to a positive triptan response in cluster headache, but this polymorphism was not related to verapamil or steroid response.33 Our study had very few respondents that were completely refractory to all medications and our study did not collect information on cluster‐like headaches as a result of intracranial lesions, carotid endarterectomies, or other disorders; therefore, we cannot comment on these aspects of cluster headache.
Recent studies have suggested that some treatments are more efficacious in episodic cluster headache than in chronic cluster headache, including 1 acute treatment (noninvasive vagal nerve stimulation34, 35) and, in preliminary news releases, 2 preventive treatments (galcanezumab, fremanezumab36, 37). Our study found that oxygen is significantly more effective in episodic cluster headache than chronic cluster headache, but there were no differences for other acute medications. The differential responses of vagal nerve simulation, galcanezumab, fremanezumab, and oxygen between episodic and chronic cluster headache require further investigation, as it is not clear what these 4 treatments share that is not shared by triptans, ergotamines, ketamine, capsaicin, caffeine and energy drinks, or lidocaine.
Most respondents were able to obtain all treatments in the study, but a higher percentage had difficulty obtaining oxygen. There is a variety of possible reasons. First, there are insurance barriers to obtaining oxygen. However, among US respondents 65 years or older, few were completely unable to get the medication; this could be because some respondents pay out of pocket for oxygen or have other types of insurance than Medicare. Further, respondents were not asked at what age they sought therapies, and some respondents may have obtained oxygen or other treatments prior to age 65 years. Physician barriers to access might also exist. In one previous study, 12% of providers refused to prescribe oxygen, and the respondents stated that the providers’ reasoning was that either they did not think it would work (44%), they did not know about oxygen for cluster headache (32%), or they were not convinced by the medical literature on oxygen effectiveness (16%).8 Another proposed medical concern of oxygen includes mucosal damage and thinning of the temporal retinal nerve fiber layer;38 furthermore there is a proposed safety concern with a flammable gas in a disease with a high rate of smokers.39 There are also logistical barriers, as one study found that less than half of prescriptions specified a flow rate or mask type, and half of patients never received proper training.8 Finally, there may be patient preferences, as patients may simply not prefer oxygen because it is expensive or inconvenient.24 Oxygen may take longer for full effect than other treatments8 or the headaches may return when the oxygen is stopped.18, 22, 40 This study does not investigate these concerns specifically. However, the study does suggest that oxygen has a lower rate of complications than other acute medications used for cluster headache.
There are several limitations to this study. First, this is a self‐administered questionnaire with an inherent recall bias. Furthermore, questions about physical, medical, psychological, and emotional complications may be interpreted differently by different respondents. Second, the study did not confirm a diagnosis of cluster headache. Several questions related to the ICHD‐3‐beta criteria were asked in an attempt to increase accuracy; however, all ICHD‐3‐beta criteria were not included. There is significant symptomatic overlap between cluster headache and other headache disorders, in particular paroxysmal hemicrania and hemicrania continua. Both of these headache disorders are completely responsive to indomethacin, and this study did not inquire about indomethacin effectiveness. However, the population prevalence of these disorders is substantially less than cluster headache. Third, as the oxygen flow rate was not specified and all triptans were grouped together, the study may have misestimated the side effects and access to these medications. However, in clinical trials there were no serious adverse effects of oxygen at 12 L/min,20 and in a study of different oxygen masks for cluster headache with oxygen at 15 L/min, all adverse events were determined to be unrelated to the study.39 Also, oxygen may be easier to obtain at lower flow rates, and certain triptans may be easier to obtain than others. Fourth, this study did not include all recommended acute treatments for cluster headache, notably it did not ask about octreotide and did not have sufficient numbers of responses for sphenopalatine ganglion stimulation or vagus nerve stimulation. And finally, the study did not specify when respondents had tried various treatments: for some, treatment response may have changed over time; in the subgroup of patients over 65, some of the treatments likely had been tried before the age of 65 for some respondents.
In conclusion, oxygen is reported by survey respondents to be a highly effective treatment with few complications in cluster headache in a large international sample. When choosing among acute treatments, this study suggests that oxygen be considered first‐line therapy for cluster headache patients regardless of age, as supported by recent clinical trials20 and current guidelines.2, 3
Statement of Authorship
Category 1
(a) Conception and Design
Stuart M. Pearson, Larry I. Schor
(b) Acquisition of Data
Stuart M. Pearson, Larry I. Schor
(c) Analysis and Interpretation of Data
Stuart M. Pearson, Mark J. Burish, Robert E. Shapiro, Yuanqing Yan, Larry I. Schor
Category 2
(a) Drafting the Manuscript
Stuart M. Pearson, Mark J. Burish, Yuanqing Yan
(b) Revising It for Intellectual Content
Stuart M. Pearson, Mark J. Burish, Robert E. Shapiro, Yuanqing Yan, Larry I. Schor
Category 3
(a) Final Approval of the Completed Manuscript
Stuart M. Pearson, Mark J. Burish, Robert E. Shapiro, Yuanqing Yan, Larry I. Schor
Supporting information
Acknowledgments
We thank Robert Wold and Stewart Tepper for insights into study design. We thank Clusterbusters and the International Headache Society for promoting the questionnaire. This study received funding support from Autonomic Technologies, Inc. and Clusterbusters. Clusterbusters did not have a direct role in analysis or interpretation but the following should be noted: (1) Robert Wold is a founding member of Clusterbusters; (2) two of the authors (RES and LIS) have served in advisory roles for Clusterbusters; (3) preliminary data from this study were presented at a Clusterbusters annual conference.
Conflict of Interest: The authors SM Pearson, MJ Burish, Y Yan, and LI Schor have no conflicts of interest. RE Shapiro has served as a paid consultant to Eli Lilly as a member of the Data Monitoring Committee for galcanezumab multi‐center clinical trials for both cluster headache and migraine.
Funding: This study received funding support from Autonomic Technologies, Inc. and Clusterbusters.
Stuart M. Pearson and Mark J. Burish contributed equally to this work as first authors.
References
- 1. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 2. May A, Leone M, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal‐autonomic cephalalgias. Eur J Neurol. 2006;13:1066‐1077. [DOI] [PubMed] [Google Scholar]
- 3. Robbins MS, Starling AJ, Pringsheim TM, Becker WJ, Schwedt TJ. Treatment of cluster headache: The American Headache Society evidence‐based guidelines. Headache. 2016;56:1093‐1106. [DOI] [PubMed] [Google Scholar]
- 4. Calhoun AH, Peterlin BL. Treatment of cluster headache in pregnancy and lactation. Curr Pain Headache Rep. 2010;14:164‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jürgens T, Schaefer C, May A. Treatment of cluster headache in pregnancy and lactation. Cephalalgia. 2009;29:391‐400. [DOI] [PubMed] [Google Scholar]
- 6. May A, Schwedt TJ, Magis D, Pozo‐rosich P, Evers S. Cluster headache. Nat Rev Dis Prim. 2018;4:1‐17. [DOI] [PubMed] [Google Scholar]
- 7. O’Brien M, Ford JH, Aurora SK, Govindan S, Tepper DE, Tepper SJ. Economics of inhaled oxygen use as an acute therapy for cluster headache in the United States of America. Headache. 2017;57:1416‐1427. [DOI] [PubMed] [Google Scholar]
- 8. Rozen TD, Fishman RS. Inhaled oxygen and cluster headache sufferers in the United States: Use, efficacy and economics: Results from the United States cluster headache survey. Headache. 2011;51:191‐200. [DOI] [PubMed] [Google Scholar]
- 9. Evers S, Rapoport A, International Headache Society . The use of oxygen in cluster headache treatment worldwide – A survey of the International Headache Society (IHS). Cephalalgia. 2017;37:396‐398. [DOI] [PubMed] [Google Scholar]
- 10. Schor L. EP‐02‐001: Cluster headache: Investigating severity of pain, suicidality, personal burden, access to effective treatment, and demographics among a large international survey sample. Cephalalgia. 2017;37(Suppl. 1):172‐208.28880582 [Google Scholar]
- 11. Inc C. Clusterbusters – About us [Internet]. 2018:1 [cited 2018 Oct 21]. Available from: https://clusterbusters.org/about-us/ [Google Scholar]
- 12. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 13. Marks DR, Rapoport A, Padla D, et al. A double‐blind placebo‐controlled trial of intranasal capsaicin for cluster headache. Cephalalgia. 1993;13:114‐116. [DOI] [PubMed] [Google Scholar]
- 14. Lenth RV. Least‐squares means: The R package lsmeans. J Stat Softw. 2016;69:1‐33. [Google Scholar]
- 15. Rozen TD, Fishman RS. Cluster headache in the United States of America: Demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 2012;52:99‐113. [DOI] [PubMed] [Google Scholar]
- 16. Goadsby PJ. Trigeminal autonomic cephalalgias. Continuum (Minneap Minn). 2012;18:883‐895. [DOI] [PubMed] [Google Scholar]
- 17. Lund N, Petersen A, Snoer A, Jensen RH, Barloese M. Cluster headache is associated with unhealthy lifestyle and lifestyle‐related comorbid diseases: Results from the Danish Cluster Headache Survey. Cephalalgia. 2018; doi: 10.1177/0333102418784751. [DOI] [PubMed] [Google Scholar]
- 18. Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache. 1981;21:1‐4. [DOI] [PubMed] [Google Scholar]
- 19. Fogan L. Treatment of cluster headache. A double‐blind comparison of oxygen v air inhalation. Arch Neurol. 1985;42:362‐363. [DOI] [PubMed] [Google Scholar]
- 20. Cohen AS, Burns B, Goadsby PJ. High‐flow oxygen for treatment of cluster headache: A randomized trial. JAMA. 2009;302:2451‐2457. [DOI] [PubMed] [Google Scholar]
- 21. Lademann V, Jansen J‐P, Evers S, Frese A. Evaluation of guideline‐adherent treatment in cluster headache. Cephalalgia. 2015;36:760‐764. [DOI] [PubMed] [Google Scholar]
- 22. Gallagher RM, Mueller L, Ciervo CA. Analgesic use in cluster headache. Headache. 1996;36:105‐107. [DOI] [PubMed] [Google Scholar]
- 23. Backx APM, Haane DYP, De Ceuster L, Koehler PJ. Cluster headache and oxygen: Is it possible to predict which patients will be relieved? A retrospective cross‐sectional correlation study. J Neurol. 2010;257:1533‐1542. [DOI] [PubMed] [Google Scholar]
- 24. Riess CM, Becker WJ, Robertson M. Episodic cluster headache in a community: Clinical features and treatment. Can J Neurol Sci. 1998;25:141‐145. [DOI] [PubMed] [Google Scholar]
- 25. Zhao JM, Schaanning J, Sjaastad O. Cluster headache: The effect of low oxygen saturation. Headache. 1990;30:656‐659. [DOI] [PubMed] [Google Scholar]
- 26. Schürks M, Rosskopf D, de Jesus J, Jonjic M, Diener H‐C, Kurth T. Predictors of acute treatment response among patients with cluster headache. Headache. 2007;47:1079‐1084. [DOI] [PubMed] [Google Scholar]
- 27. Schindler EAD, Wright DA, Weil MJ, Gottschalk CH, Pittman BP, Sico JJ. Survey analysis of the use, effectiveness, and patient‐reported tolerability of inhaled oxygen compared with injectable sumatriptan for the acute treatment of cluster headache. Headache. 2018;58:1568‐1578. [DOI] [PubMed] [Google Scholar]
- 28. Schürks M, Kurth T, De Jesus J, Jonjic M, Rosskopf D, Diener H‐CC. Cluster headache: Clinical presentation, lifestyle features, and medical treatment. Headache. 2006;46:1246‐1254. [DOI] [PubMed] [Google Scholar]
- 29. Tepper SJ, Duplin J, Nye B, Tepper DE. Prescribing oxygen for cluster headache: A guide for the provider. Headache. 2017;57:1428‐1430. [DOI] [PubMed] [Google Scholar]
- 30. Haane DYP, De Ceuster LME, Geerlings RPJ, Dirkx THT, Koehler PJ. Cluster headache and oxygen: Is it possible to predict which patients will be relieved? A prospective cross‐sectional correlation study. J Neurol. 2013;260:2596‐2605. [DOI] [PubMed] [Google Scholar]
- 31. Rozen TD, Fishman RS. Female cluster headache in the United States of America: What are the gender differences? J Neurol Sci. 2012;317:17‐28. [DOI] [PubMed] [Google Scholar]
- 32. Rozen TD. Cluster headache clinical phenotypes: Tobacco nonexposed (never smoker and no parental secondary smoke exposure as a child) versus tobacco‐exposed: Results from the United States Cluster Headache Survey. Headache. 2018;58:688‐699. [DOI] [PubMed] [Google Scholar]
- 33. Schürks M, Kurth T, Stude P, et al. G protein beta3 polymorphism and triptan response in cluster headache. Clin Pharmacol Ther. 2007;82:396‐401. [DOI] [PubMed] [Google Scholar]
- 34. Silberstein SD, Mechtler LL, Kudrow DB, et al. Non‐invasive vagus nerve stimulation for the acute treatment of cluster headache: Findings from the randomized, double‐blind, sham‐controlled ACT1 study. Headache. 2016;56:1317‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goadsby PJ, de Coo IF, Silver N, et al. Non‐invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: A randomized, double‐blind, sham‐controlled ACT2 study. Cephalalgia. 2018;38:959‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lilly’s Galcanezumab meets primary endpoint in phase 3 study evaluating galcanezumab for the prevention of episodic cluster headache [Internet]. 2018. [cited 2018 Sep 3]. Available from: https://investor.lilly.com/news-releases/news-release-details/lillysgalcanezumab-meets-primary-endpoint-phase-3-study
- 37.Teva provides update on clinical trial of fremanezumab for use in chronic cluster headache [Internet]. 2018. [cited 2018 Sep 6]. Available from: http://www.tevapharm.com/news/teva_provides_update_on_clinical_trial_of_fremanezumab_for_use_in_chronic_cluster_headache_06_18.aspx
- 38. Ewering C, Haşal N, Alten F, et al. Temporal retinal nerve fibre layer thinning in cluster headache patients detected by optical coherence tomography. Cephalalgia. 2015;35:946‐958. [DOI] [PubMed] [Google Scholar]
- 39. Petersen AS, Barloese MC, Lund NL, Jensen RH. Oxygen therapy for cluster headache. A mask comparison trial. A single‐blinded, placebo‐controlled, crossover study. Cephalalgia. 2017;37:214‐224. [DOI] [PubMed] [Google Scholar]
- 40. Geerlings RP, Haane DY, Koehler PJ. Rebound following oxygen therapy in cluster headache. Cephalalgia. 2011;31:1145‐1149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


