Abstract
Aims
To explore molecular mechanisms that link peri-implantitis and type 2 diabetes mellitus (T2DM) by bioinformatic analysis of publicly available experimental transcriptomic data.
Materials and methods
Gene expression data from peri-implantitis were downloaded from the Gene Expression Omnibus database, integrated and differentially expressed genes (DEGs) in peri-implantitis were identified. Next, experimentally validated and computationally predicted genes related to T2DM were downloaded from the DisGeNET database. Protein–protein interaction network (PPI) pairs of DEGs related to peri-implantitis and T2DM related genes were constructed, “hub” genes and overlapping DEG were determined. Functional enrichment analysis was used to identify significant shared biological processes and signaling pathways. The PPI networks were subjected to cluster and specific class analysis for identifying “leader” genes. Module network analysis of the merged PPI network identified common or cross-talk genes connecting the two networks.
Results
A total of 92 DEGs overlapped between peri-implantitis and T2DM datasets. Three hub genes (IL-6, NFKB1, and PIK3CG) had the highest degree in PPI networks of both peri-implantitis and T2DM. Three leader genes (PSMD10, SOS1, WASF3), eight cross-talk genes (PSMD10, PSMD6, EIF2S1, GSTP1, DNAJC3, SEC61A1, MAPT, and NME1), and one signaling pathway (IL-17 signaling) emerged as peri-implantitis and T2DM linkage mechanisms.
Conclusions
Exploration of available transcriptomic datasets revealed IL-6, NFKB1, and PIK3CG expression along with the IL-17 signaling pathway as top candidate molecular linkage mechanisms between peri-implantitis and T2DM.
Keywords: Type 2 diabetes, Peri-implantitis, Gene, Pathway, Bioinformatics
Introduction
With the increasing use of dental implants in oral rehabilitation, peri-implant inflammatory diseases have risen in incidence and comprise a significant clinical challenge. Peri-implant disease constitutes of peri-implant mucositis, characterized by reversible inflammation restricted to soft tissues around an implant and peri-implantitis, characterized by peri-implant alveolar bone loss due to progression of the inflammatory lesion (Renvert et al., 2018). Being multi-factorial, peri-implantitis is associated with a number of risk factors (Dreyer et al., 2018). Diabetes mellitus, a common metabolic disorder, is characterized by hyperglycemia resulting from insulin resistance, inadequate insulin secretion, or excessive glucagon secretion (Blair, 2016). Type 2 diabetes (T2DM), the main form of diabetes affecting 90–95% of diabetics, is a risk factor for peri-implantitis (Dreyer et al., 2018; Monje, Catena & Borgnakke, 2017), with a 50% higher risk reported in a meta-analysis (Monje, Catena & Borgnakke, 2017). Others have noted disease severity of peri-implantitis increases with worse glycemic control in T2DM (Al Amri et al., 2016; Gómez-Moreno et al., 2015).
Pathogenic mechanisms underlying T2DM mediated aggravation of peri-implant diseases are not as well-investigated and remain poorly understood. A common understanding is that impairment of vascularization and angiogenesis inherent to a T2DM microenvironment impairs bone healing by delaying wound healing, reducing bone formation, and impairing osteogenesis (Marin et al., 2018). Notably, diabetes mellitus alters host-immune responses, skewing these toward pro-inflammatory dominance (Nielsen et al., 2017). As a result, in diabetics, the cytokine production in response to peri-implant biofilm can be altered, when compared to that in healthy subjects. Among individuals with peri-implantitis, those having diabetes show an overproduction of multiple peri-implant fluid pro-inflammatory cytokines; IL-1, IL-6, IL-8, TNF-alpha, chemokine receptors CCR5 and CXR3 (Venza et al., 2010; Al-Askar et al., 2018), where the severity of dysregulation appears to be worsen with poor diabetes control. Such unfavorable immune modulation is likely to cause the higher susceptibility to peri-implantitis noted in diabetics (Monje, Catena & Borgnakke, 2017). Other mechanisms whereby peri-implantitis is aggravated in a poorly controlled diabetic mileu include potential alterations in biofilm composition (Gulia et al., 2018) and accumulation of advanced glycation end products (Al-Sowygh et al., 2018; Alrabiah et al., 2018). Nevertheless, to the authors’ knowledge, not much is known about the scale of molecular relationships between both diseases. The identification of linkage molecular mechanisms could translate to potential therapeutic targets for individualized treatment of peri-implantitis in T2DM affected subjects.
Integrated analysis of bioinformatic data has several advantages in scoping poorly understood disease associations. Relevant genomic data from multiple microarray and next-generation sequencing studies of single diseases can be used to determine molecular linkages through bioinformatic analyses. Several microarray or sequencing studies have examined global gene-expression patterns relevant to peri-implantitis (Becker et al., 2014; Schminke et al., 2015) and to T2DM (Berisha et al., 2011; Pihlajamäki et al., 2009; Taneera et al., 2013). An overview of these studies suggests some similarities in the molecular mechanisms implicated in both diseases. These include inflammatory cytokines (Interleukin-1β, Interleukin-6), vascular endothelial growth factor, and reactive oxygen species-related genes. By integrative bioinformatics, genomic data from T2DM and peri-implantitis documented in previous studies can facilitate the identification of potential molecular links. Therefore, a bioinformatic study of existing experimental transcriptome datasets was designed to investigate putative molecular links between peri-implantitis and T2DM by identifying cross-talk genes, biological processes (BPs), and signaling pathways involved in both diseases.
Materials and Methods
Procurement of data
Two transcriptome datasets pertaining to peri-implantitis (GSE33774 and GSE57631) were downloaded from the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/projects/geo/). The diagnostic criteria of peri-implantitis in each study was aligned with the current consensus case-definition of peri-implantitis: radiographic evidence of bone loss ≥3 mm and/or probing depths ≥6 mm in conjunction with profuse bleeding (Renvert et al., 2018). For the dataset GSE33774, a probing depth ≥5 mm in combination with radiographic bone loss around implants >3 mm were indicative of peri-implantitis (Becker et al., 2014). For the dataset GSE57631, the diagnostic criteria of peri-implantitis were not specified in the relevant text (Schminke et al., 2015) and by contacting the authors (Schminke et al., 2015), these were determined to be consistent with the current consensus criteria (Renvert et al., 2018). Experimentally validated and computationally predicted genes associated with T2DM were downloaded from the DisGeNET database (http://www.disgenet.org/home/). The DisGeNET database is a comprehensive platform that integrates information concerning human disease-associated genes and their variants (Piñero et al., 2016). This database integrates data from expert curated repositories, text mining data extracted from scientific literature, experimentally validated data, and referred data (Piñero et al., 2016). By using this database, a total of 1,274 T2DM-associated genes were determined.
Differential gene expression and functional enrichment analysis
The two peri-implantitis-related transcriptome datasets were subjected to differential expression analysis using the “limma” package in R (Ritchie et al., 2015). Peri-implantitis-related genes with p-value < 0.05 and |logFC| ≥ 1 were screened and defined as differentially expressed genes (DEGs). Next, enrichment analysis was performed with the R package “clusterProfiler” to describe the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional profiles of the DEGs related to peri-implantitis.
Protein–protein interaction network analysis
Protein–protein interaction (PPI) pairs of DEGs related to peri-implantitis and genes related to T2DM were each determined using the free web-available database Search Tool for the Retrieval of Interacting Genes (Release 9.1, http://string-db.org/), as it has medium to high confidence scores. Based on these PPI pairs, PPI networks were constructed for peri-implantitis and T2DM, respectively, and the topological characteristics of the two PPI networks were analyzed.
Clustering analysis
A “combined association score” (CAS) of each PPI pair, representing the interacting pair’s degree of confidence, was determined. The CAS for each gene over that of its neighbors was summed and defined as “comscore,” which represented the number of weighted links for that gene. K-means clustering analysis was applied to these “comscores” and gene nodes were classified into five classes according to their weighted number of links. Specific class analysis was performed by determination of K-means based classes specific to and significantly associated with peri-implantitis and T2DM each, by using analysis of variance and multiple-testing with Tukey–Kramer test (at p < 0.01). Genes in these classes were further analyzed. Genes belonging to the highest rank were defined as “leader genes,” as these genes had the highest weighted number of links as compared to others in that network. The “leader gene” approach enables the identification of a small number of potentially most relevant candidate genes from the experimental dataset.
Cross-talk gene analysis
Protein–protein interaction networks for peri-implantitis and T2DM were merged to construct a global network. T2DM related genes among the DEGs associated to peri-implantitis were identified as cross-talk genes. Module networks of these cross-talk genes were constructed using a graph theoretic clustering algorithm “Molecular Complex Detection.” Modules represent genes that are densely connected in a PPI network are likely to represent highly related protein complexes or modules.
Results
Identification of DEGs
The DEGs identified in peri-implantitis are listed in Table S1. A total of 224 DEGs were identified in GSE33774 and 813 DEGs were identified in GSE57631. Combining these, a total of 1,028 genes emerged as associated with peri-implantitis. For T2DM, a total of 1,274 genes were obtained. A total of 92 of the DEGs were shared by peri-implantitis and T2DM (Fig. S1).
Functional enrichment analysis
Differentially expressed genes in peri-implantitis were mainly involved in the BPs of neutrophil activation, neutrophil mediated immunity, antigen processing and presentation of peptide antigen via MHC class I, and positive regulation of protein catabolic process (Fig. S2). Genes associated with T2DM were mainly enriched in BPs of response to nutrient levels and response to oxidative stress (Fig. S3). No BP emerged as overlapped between peri-implantitis and T2DM. In terms of KEGG signaling pathways, DEGs associated with peri-implantitis were seen as mainly related to protein processing in endoplasmic reticulum (ER), IL-17 signaling pathway, and Leukocyte transendothelial migration (Fig. S4). Genes related to T2DM were significantly enriched in pathways related to insulin resistance and FoxO signaling (Fig. S5). Combining these, a single signaling pathway; IL-17 signaling, was seen as shared by peri-implantitis and T2DM.
PPI network analysis
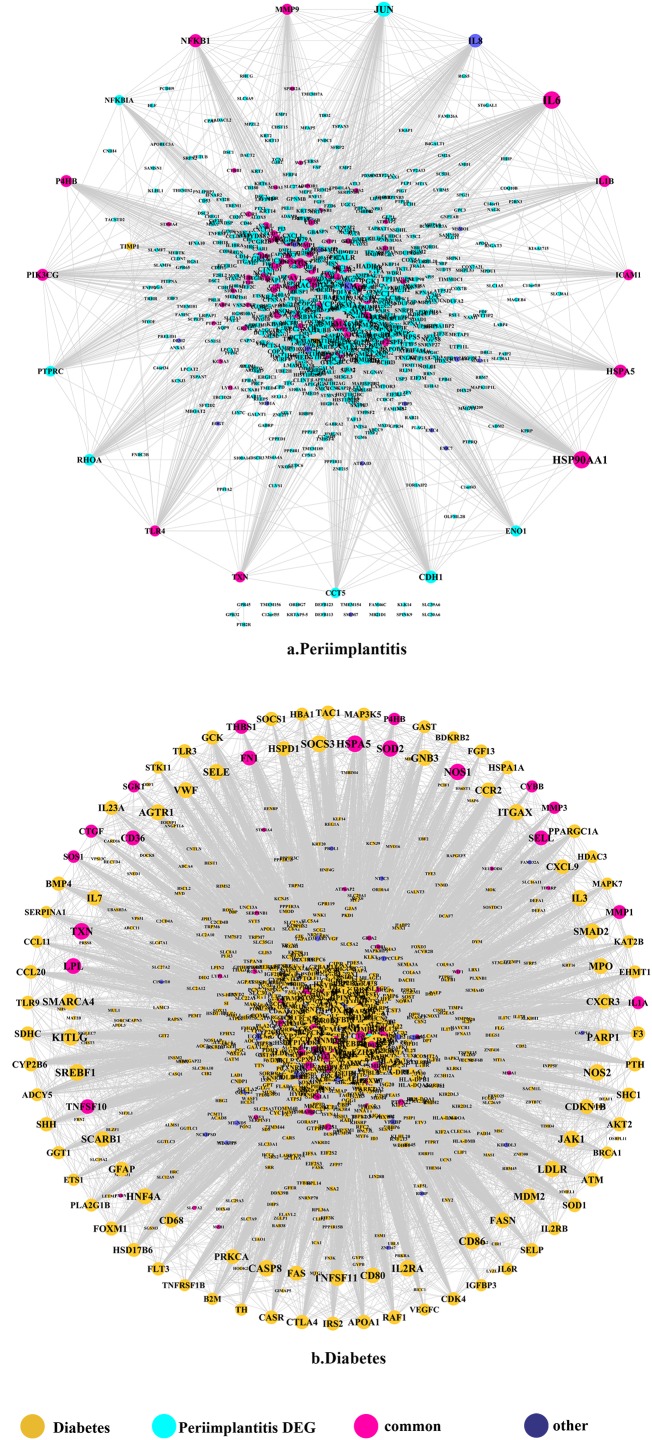
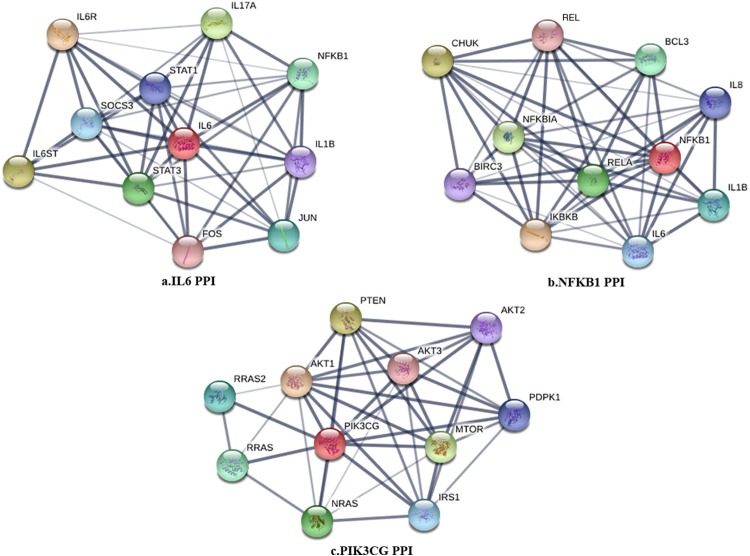
The PPI network in peri-implantitis showed 786 nodes and 5,278 interaction pairs (Fig. 1A) whereas, that in T2DM showed 1,171 nodes and 26,862 interaction pairs (Fig. 1B). The topological characteristics of the two PPI networks are summarized in Table S2, where gene nodes are ranked in descending order of their degree, and the top 20 nodes or “hub genes” were determined. Among these top 20 hub genes, three genes (IL6, NFKB1, and PIK3CG) were common to peri-implantitis and T2DM, thus can be regarded as the potential cross-talk genes. Considering these hub genes appeared to be central to the regulation of and may prominently impact the biological network, PPI subnetworks of these three genes were extracted (Fig. 2). In the leader gene approach, cluster analyses for peri-implantitis showed class 2 as the most significantly associated class (Fig. S6A) and for T2DM, class 3 was the most significant (Fig. S6B). Extracting the genes in these classes, three leader genes (PSMD10, SOS1, and WASF3) were found to be overlapping (Table S3).
Figure 1. The PPI network of DEGs expressed in peri-implantitis (A) and PPI network of genes related to type 2 diabetes (B).
Figure 2. The PPI subnetwork of IL6 (A), NFKB1 (B), and PIK3CG (C).
Module network analysis
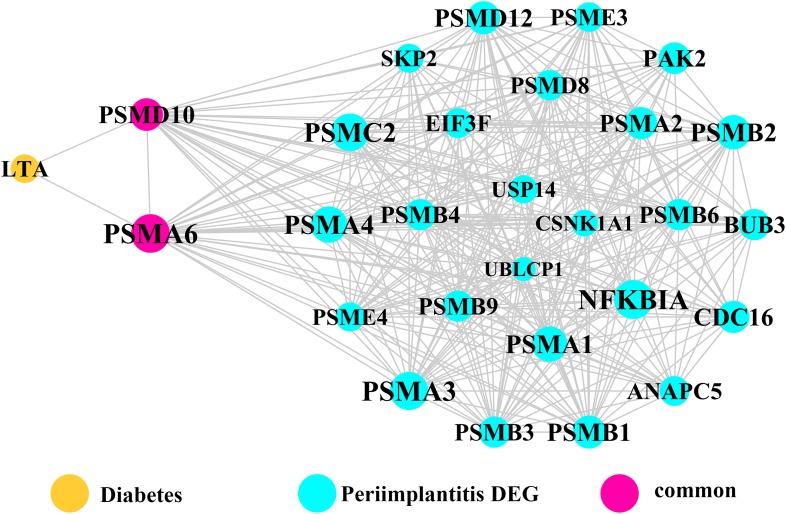
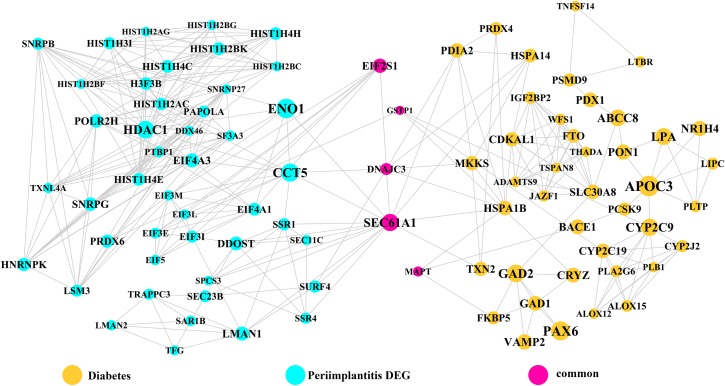
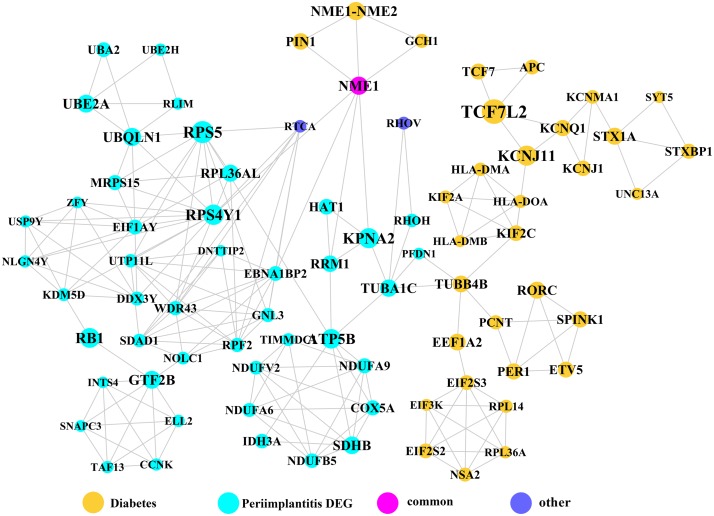
Module network analysis of the cross-talk genes showed three subnetworks. Module subnetwork 1, where red nodes represent the common genes, green nodes represent T2DM genes and blue nodes are DEGs associated with peri-implantitis, showed PSMD10 and PSMD6 as cross-talk genes, which directly interacted with one gene closely related to diabetes (LTA), as well as with DEGs in peri-implantitis (PSM family genes (PSMC2, PSMA4, PSME4, etc.), SKP2, EIF3F, USP14, UBLCP1, etc.) (Fig. 3). In module subnetwork 2 (Fig. 4), five cross-talk genes; EIF2S1, GSTP1, DNAJC3, SEC61A1, and MAPT were noted, which directly interacted with a few T2DM genes and DEGs of peri-implantitis. Module subnetwork 3 (Fig. 5), showed only one common gene (NME1), interacting with small number of genes involved in both networks.
Figure 3. The module network 1 identified two cross-talk genes (PSMD1 and PSMD6).
Figure 4. The module network 2 identified five cross-talk genes (EIF2S1, GSTP1, DNAJC3, SEC61A1, and MAPT).
Figure 5. The module subnetwork 3 identified only one cross-talk gene (NME1).
Known functions of the key hub genes and cross talk genes in T2DM and peri-implantitis each are described in Table 1.
Table 1. The functions of genes identified in the pathogenesis of T2DM and peri-implantitis, respectively.
| Genes | General functions | Functions in T2DM | Functions in peri-implantitis |
|---|---|---|---|
| IL-6 | Interleukin 6 (IL-6) is an interleukin that acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine |
|
|
| NFKB1 | NFKB1 (Nuclear Factor Kappa B Subunit 1) encodes a 105 kD protein which is a DNA binding subunit of the NF-kappa-B (NFKB) protein complex |
|
|
| PIK3CG (also called PI3K) |
|
Its reduction impairs insulin signal transduction, resulting in the impaired translocation of glucose transporter protein GLUT4 and insulin resistance |
|
| SOS1 | SOS1 (SOS Ras/Rac guanine nucleotide exchange factor 1) regulates Ras (Rat sarcoma) proteins by aiding the exchange of GTP for GDP | Regulates Ras proteins, which implicates in the development of diabetic vascular dysfunction by inducing abnormal vascular reactivity | Regulates Ras proteins, which is an early signal for osteogenesis |
| WASF3 | WASF3 (Wiskott–Aldrich syndrome protein family member 3) plays a role in the regulation of cell morphology and cytoskeletal organization |
|
Actin binding was shown to be significant biological process in peri-implantitis, thus WASF3 may be involved in peri-implantitis by controlling actin binding |
| PSMD10 | PSMD10 (Proteasome 26S Subunit, Non-ATPase 10), PSMD6 (Proteasome 26S Subunit, Non-ATPase 6) is regulatory component for the 26S proteasome, which is central to protein regulation by ubiquitination-degradation |
|
|
| EIF2S1 | EIF2S1 (Eukaryotic translation initiation factor 2 subunit 1) is a component of the PI3K pathway, encoding for eukaryotic initiation factor 2α(eIF2α), the phosphorylation of which can reduce protein synthesis | Dysregulation of eIF2α phosphorylation is poorly tolerated by pancreatic β cells, leading to dysfunction | Downregulates infection-induced cytokine expression, thus involved in the immune inflammatory response |
| GSTP1 | GSTP1 (Glutathione S-Transferase Pi 1) is strongly associated with the metabolic efficiency, detoxification, and inflammatory diseases and cancer susceptibility |
|
|
| DNAJC3 | P58IPK (DNAJ Heat Shock Protein Family (Hsp40) Member C3, also called P58IPK) functions as a signal for the downregulation of endoplasmic reticulum (ER)-associated proteins involved in the initial ER stress response | Be involved in the development of T2DM since the disruption of its mediated ER can cause the dysfunction of insulin-secreted beta cells | Be involved in peri-implantitis by activating the unfolded protein response (UPR) pathway associated with inflammation and alveolar bone resorption |
| SEC61A1 | SEC61A1 (Sec61 Translocon Alpha 1 Subunit) plays a crucial role in the insertion of secretory and membrane polypeptides into the endoplasmic reticulum (ER) | Has the similar mechanism with DNAJC3: be involved in the development of T2DM since the disruption of its mediated ER can cause the dysfunction of insulin-secreted beta cells | Has the similar mechanism with DNAJC3: be involved in peri-implantitis by activating the unfolded protein response (UPR) pathway associated with inflammation and alveolar bone resorption |
| MAPT | MAPT (microtubule associated protein Tau), a neural phosphoprotein member of the MAP family, is implicated in microtubule function within the cell cytoskeleton | Disturbance in MAPT phosphorylation is shown to decrease insulin production from pancreatic beta cells | Be implicated in bone mineral density regulation |
| NME1 | NME1 (NME/NM23 Nucleoside Diphosphate Kinase 1) is a negative regulator of nuclear factor-κB (NF-kB) signaling which is a critical player in immune responses | The inhibited activity of NF-kB can improve conductance artery function in T2DM, thus NME1 may be involved in the T2DM by impairing the artery function |
|
Discussion
The current study included both peri-implant bone and peri-implant soft tissue transcriptomes and the findings can be considered as an integrated or non-specific view of peri-implantitis-T2DM molecular linkages. Three common or cross-talk genes; IL-6, NFKB1, and PIK3CG, were noted among the top 20 hub genes in the PPI networks of T2DM and peri-implantitis. Raised interleukin (IL)-6 has been demonstrated as an independent predictor of T2DM (Akbari & Hassan-Zadeh, 2018). IL-6 promotes inflammation and can induce insulin resistance (Rehman et al., 2017). IL-6 expression is also increased in peri-implantitis, reflecting its role in destruction of peri-implant tissue and bone resorption by exerting pro-inflammatory effects (Candel-Martí et al., 2011). Raised IL-6 in the peri-implant crevicular fluid has been proposed as a marker of peri-implantitis (Yaghobee et al., 2014). The NFKB1 gene is a transcription factor which encodes the nuclear-factor kappa beta (NF-kB) p105/p50 isoforms (Héron, Deloukas & Van Loon, 1995). NF-kB inflammatory signaling is implicated in the development of T2DM (Baker, Hayden & Ghosh, 2011) and experimental evidence shows interfering with NF-kB signaling can decrease hyperglycemia and insulin resistance. In peri-implantitis, NFKB1 has been previously identified as a key candidate gene (Zhang et al., 2017) and is shown to regulate inflammation-induced osteoclastogenesis by regulating receptor activator of NF-κB ligand (RANKL)—mediated osteoclast formation and activation in peri-implantitis (Boyce et al., 2015). The phosphoinositide-3 kinase (PI3K, also called PIK3CG) is an important regulator of cell response to extra-cellular stimuli. PI3KCG gene encodes a class I catalytic subunit of PI3K protein, which can bind a p85 regulatory subunit to form PI3K (Amzel et al., 2008). In T2DM, PI3K activity reduction impairs insulin signal transduction and impairs translocation of glucose transporter protein GLUT4 leading to insulin resistance (Niswender et al., 2003). PI3K signaling is also shown to increase the osteogenic differentiation of periodontal ligament stem cells (Lee et al., 2014a), thus, could be implicated in peri-implantitis via regulation of osteogenesis. Thus, experimental evidence supports the notion that the in silico determined shared genes could be significant mechanistic links between T2DM and peri-implantitis.
Three common leader genes; PSMD10, SOS1, and WASF3, were identified from the gene clusters linked to peri-implantitis and T2DM each. Proteasomes are involved in intracellular protein degradation and implicated in several diseases. The PSMD10 or Gankyrin gene (proteasome 26S subunit, non-ATPase 10) encodes subunits of the 26S proteasome, a component of the ubiquitin–proteasome (UPS) system (Hochstrasser, 1996). It has been found enriched in inflammation (Lecker, Goldberg & Mitch, 2006) and may be induced by pro-inflammatory Interleukin-1 beta stimulation (Qureshi, Morrison & Reis, 2012). It is upregulated in T2DM (Costes et al., 2011), and was also implicated previously in peri-implantitis (Zhang et al., 2017) but no experimental evidence exists. SOS1 (SOS Ras/Rac guanine nucleotide exchange factor 1) regulates Rat sarcoma proteins and facilitates exchange of GTP for GDP. In T2DM, Ras-GTPase has been implicated in inducing aberrant vascular reactivity and dysfunction (Yousif et al., 2004). In peri-implantitis, while SOS1 was previously identified as a hub gene (Zhang et al., 2017) but experimental evidence is similarly lacking. Ras activation is an early signal for osteogenesis in human bone marrow stromal cells (Wang et al., 2001). The Ras superfamily is also suggested to regulate inflammation by acting as regulators of NF-κB and Ral pathways (Oeckinghaus et al., 2014). WASF3 (WAS protein family member 3) controls actin binding, thus regulating cell shape and motility, and has been frequently implicated in cancer cell motility and metastasis (Teng et al., 2016). As glucose transporter recruitment is actin dependent, a plausible role of WASF3 signaling in T2DM pathology is suggestible (Tunduguru et al., 2017). In addition, actin binding was found as a significantly dysregulated process by a whole-exome sequencing study of peri-implantitis (Lee et al., 2014b), but no experimental study has yet characterized WASF3’s expression in peri-implantitis.
Module network analysis identified eight cross-talk genes; PSMD10, PSMD6, EIF2S1, DNAJC3, SEC61A1, GSTP1, MAPT, and NME1. Like PSMD10, PSMD6 is regulatory for the 26S proteasome, which is central to protein regulation by ubiquitination-degradation. UPS dysregulation might be a molecular mechanism underlying insulin resistance (Balasubramanyam, Sampathkumar & Mohan, 2005) and has also been implicated in microvascular complications of T2DM (Aghdam & Sheibani, 2013). In peri-implantitis, proteasomal ubiquitin-dependent protein catabolic process (GO term: 0043161) was found to be significantly enriched (Zhang et al., 2017). Based on these perspectives, it may be hypothesized that UPS dysregulation in a T2DM state contributes to aggravated peri-implantitis via a positive feedback loop, wherein pro-inflammatory cytokine responses to peri-implant biofilm may further its dysregulation. EIF2S1 is a component of the PI3K pathway, encoding for the eukaryotic initiation factor 2α (eIF2α) involved in regulating protein synthesis (Jiang & Wek, 2005) and ER stress responses. Activation of eIF2α-mediated signaling by bacterial pathogens downregulates infection-induced cytokine expression (Shrestha et al., 2012) and may propagate the inflammatory processes. It is also implicated in T2DM, as dysregulation of eIF2α leads to pancreatic β cells dysfunction (Cnop et al., 2017). The expression pattern or effects of eIF2α in peri-implantitis are not specifically investigated. Among the genes noted in module subnetwork 2, DNAJC3 (DnaJ Heat Shock Protein Family (Hsp40) Member C3 or P58IPK) inhibits eIF-2α signaling, thereby attenuating the later phases of the ER stress response (Ladiges et al., 2005). DNAJC3 mutation is implicated in the development of T2DM via dysfunction of insulin-secreting beta cells (Ladiges et al., 2005). In peri-implantitis it can activate the unfolded protein response pathway associated with inflammation and alveolar bone resorption (Yamada et al., 2015). The possible mechanisms of SEC61A1 (Sec61 Translocon Alpha 1 Subunit) in T2DM and peri-implantitis appear to be similar to those of the DNAJC3 gene. SEC61A1 is also related to ER stress response by control of polypeptide transport into ER (Lang et al., 2012). Glutathione S-Transferase Pi 1 (GSTP1) is suggested to protect cells against oxidative stress (Savic-Radojevic et al., 2007), which is involved in both peri-implantitis (Sánchez-Siles et al., 2016) and T2DM (Wright, Scism-Bacon & Glass, 2006) pathology. GSTP1 polymorphism is associated with susceptibility to type II diabetes mellitus (Saadat, 2017) and the risk for developing chronic periodontitis (Camargo Ortega et al., 2014) and could similarly imply risk for its analogue peri-implantitis (Dhir et al., 2013). The microtubule associated protein Tau (MAPT), a member of the MAP family regulated microtubule function within the cell cytoskeleton (Sündermann, Fernandez & Morgan, 2016). Disturbance in MAPT phosphorylation is shown to decrease insulin production from pancreatic beta cells, supporting its role in T2DM pathology (Maj et al., 2016). MAPT gene polymorphism is also implicated in bone mineral density regulation (Dengler-Crish, Smith & Wilson, 2017). NME1 (NME/NM23 Nucleoside Diphosphate Kinase 1) is a known negative regulator of nuclear factor-κB signaling (You et al., 2014). Considering nuclear factor-κB signaling has been shown to be dysregulated and involved in pathogenesis of both T2DM (Andreasen et al., 2011) and peri-implantitis (Rakić et al., 2013), NME1 may be a relevant upstream molecular link between T2DM and peri-implantitis.
IL-17 signaling emerged as the sole pathway significantly shared between T2DM and peri-implantitis. IL-17 plays critical regulatory roles in host defense and inflammatory diseases showing both protective and destructive effects (Zou et al., 2013). Although IL-17 protects against pathogen invasion at epithelial or mucosal barriers, its dysregulation can stimulate overexpression of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) leading to continued tissue damage (Jin & Dong, 2013). IL-17 overexpression is noted in peri-implant crevicular fluid of peri-implantitis (Severino et al., 2016) and serum of T2DM (Nadeem et al., 2013). IL-17 negatively impacts osteogenesis in peri-implantitis affected tissue (Kim et al., 2014). In T2MD, IL-17 can contribute to the exacerbation of insulin resistance by inducing apoptosis of pancreatic β-cells (Yousefidaredor et al., 2014). Considering that IL-17 antagonist molecules have recently emerged as therapeutics, this finding may suggest a basis to explore their therapeutic potential in T2DM affected peri-implantitis patients (Abdel-Moneim, Bakery & Allam, 2018).
Taken together, perspectives from prior literature support the biological basis for many of the significant genes and pathways that emerged as putative mechanistic links between peri-implantitis and T2DM. The major limitation of this study is the lack of experimental validation of the genes highlighted in the in silico analyses which was beyond the scope of the current investigation. Thus, these findings have significant implications for future research. Most of the linkage genes revealed by the study lack experimental evidence in context of peri-implantitis-T2DM disease association. In theory, these molecular entities could be valuable as potential targets for individualized gene therapy, risk stratification, and therapy of peri-implantitis in type 2 diabetes. Further validation of key molecular mechanisms could promote the development of targeted drugs for blocking or aiding their expression and modulating related pathways. Most importantly, the present findings may be considered as hypotheses for future validation experiments and direction for research. As such, bioinformatic data mining of experimental transcriptomes is exploratory and at best considered as a source of well-supported hypotheses. Validation experiments could include comparison of the highlighted genes’ expression levels in the peripheral blood and peri-implant crevicular fluid of peri-implantitis affected individuals with and without T2DM and explore their roles in vitro/animal disease models.
Conclusion
Bioinformatics analysis combining experimental transcriptomic data from T2DM and peri-implantitis revealed potentially shared molecular linkages. Three hub genes (IL-6, NFKB1, and PIK3CG) identified in PPI networks, three cross-talk genes (PSMD10, SOS1, and WASF3) identified by specific class analysis and eight cross-talk genes (PSMD10, PSMD6, EIF2S1, GSTP1, DNAJC3, SEC61A1, MAPT, and NME1) obtained by module network analysis, and IL-17 signaling emerged as top candidate shared molecular linkages. Future studies should explore their roles in context of T2DM-peri-implantitis disease association.
Supplemental Information
Table S1. The number of DEGs identified in two datasets (GSE33774 and GSE57631) of peri-implantitis.
Table S2. Top 20 nodes in PPI networks of both peri-implantitis and T2MD.
Table S3. Three leader genes shared in two selected significant classes.
Acknowledgments
We are grateful to Ms. Xiangqiong Liu and Mr. Yupei Deng, who are bioinformatics engineers at Shanghai Genomap Technologies, Shanghai, China. We have to express our appreciation to them for providing us technological assistance during the analysis of this research.
Funding Statement
The authors received doctoral study support from the China Scholarship Council (CSC) for Simin Li (CSC No: 201608080010) at University Leipzig. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare no potential conflict of interests with respect to the authorship and publication of this paper.
Author Contributions
Tianliang Yu analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Aneesha Acharya prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, proof-reading.
Nikos Mattheos approved the final draft, proof-reading.
Simin Li analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Dirk Ziebolz analyzed the data, approved the final draft, proof-reading.
Gerhard Schmalz analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Rainer Haak contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Jana Schmidt contributed reagents/materials/analysis tools, approved the final draft.
Yu Sun authored or reviewed drafts of the paper, approved the final draft.
Data Availability
References
- Abdel-Moneim, Bakery & Allam (2018).Abdel-Moneim A, Bakery HH, Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomedicine & Pharmacotherapy. 2018;101:287–292. doi: 10.1016/j.biopha.2018.02.103. [DOI] [PubMed] [Google Scholar]
- Aghdam & Sheibani (2013).Aghdam SY, Sheibani N. The ubiquitin-proteasome system and microvascular complications of diabetes. Journal of Ophthalmic & Vision Research. 2013;8(3):244–256. [PMC free article] [PubMed] [Google Scholar]
- Akbari & Hassan-Zadeh (2018).Akbari M, Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. 2018;26(3):685–698. doi: 10.1007/s10787-018-0458-0. [DOI] [PubMed] [Google Scholar]
- Al Amri et al. (2016).Al Amri MD, Kellesarian SV, Al-Kheraif AA, Malmstrom H, Javed F, Romanos GE. Effect of oral hygiene maintenance on HbA1c levels and peri-implant parameters around immediately-loaded dental implants placed in type-2 diabetic patients: 2 years follow-up. Clinical Oral Implants Research. 2016;27(11):1439–1443. doi: 10.1111/clr.12758. [DOI] [PubMed] [Google Scholar]
- Al-Askar et al. (2018).Al-Askar M, Ajlan S, Alomar N, Al-Daghri NM. Clinical and radiographic peri-implant parameters and whole salivary interleukin-1β and interleukin-6 levels among type-2 diabetic and nondiabetic patients with and without peri-implantitis. Medical Principles and Practice. 2018;27(2):133–138. doi: 10.1159/000488032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrabiah et al. (2018).Alrabiah M, Al-Aali KA, Al-Sowygh ZH, Binmahfooz AM, Mokeem SA, Abduljabbar T. Association of advanced glycation end products with peri-implant inflammation in prediabetes and type 2 diabetes mellitus patients. Clinical Implant Dentistry and Related Research. 2018;20(4):535–540. doi: 10.1111/cid.12607. [DOI] [PubMed] [Google Scholar]
- Al-Sowygh et al. (2018).Al-Sowygh ZH, Ghani SMA, Sergis K, Vohra F, Akram Z. Peri-implant conditions and levels of advanced glycation end products among patients with different glycemic control. Clinical Implant Dentistry and Related Research. 2018;20(3):345–351. doi: 10.1111/cid.12584. [DOI] [PubMed] [Google Scholar]
- Amzel et al. (2008).Amzel LM, Huang C-H, Mandelker D, Lengauer C, Gabelli SB, Vogelstein B. Structural comparisons of class I phosphoinositide 3-kinases. Nature Reviews Cancer. 2008;8(9):665–669. doi: 10.1038/nrc2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen et al. (2011).Andreasen AS, Kelly M, Berg RMG, Møller K, Pedersen BK. Type 2 diabetes is associated with altered NF-κB DNA binding activity, JNK phosphorylation, and AMPK phosphorylation in skeletal muscle after LPS. PLOS ONE. 2011;6(9):e23999. doi: 10.1371/journal.pone.0023999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, Hayden & Ghosh (2011).Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metabolism. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanyam, Sampathkumar & Mohan (2005).Balasubramanyam M, Sampathkumar R, Mohan V. Is insulin signaling molecules misguided in diabetes for ubiquitin-proteasome mediated degradation? Molecular and Cellular Biochemistry. 2005;275(1-2):117–125. doi: 10.1007/s11010-005-1083-y. [DOI] [PubMed] [Google Scholar]
- Becker et al. (2014).Becker ST, Beck-Broichsitter BE, Graetz C, Dörfer CE, Wiltfang J, Häsler R. Peri-implantitis versus periodontitis: functional differences indicated by transcriptome profiling. Clinical Implant Dentistry and Related Research. 2014;16(3):401–411. doi: 10.1111/cid.12001. [DOI] [PubMed] [Google Scholar]
- Berisha et al. (2011).Berisha SZ, Serre D, Schauer P, Kashyap SR, Smith JD. Changes in whole blood gene expression in obese subjects with type 2 diabetes following bariatric surgery: a pilot study. PLOS ONE. 2011;6(3):e16729. doi: 10.1371/journal.pone.0016729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair (2016).Blair M. Diabetes mellitus review. Urologic Nursing. 2016;36(1):27–36. [PubMed] [Google Scholar]
- Boyce et al. (2015).Boyce BF, Xiu Y, Li J, Xing L, Yao Z. NF-κB-mediated regulation of osteoclastogenesis. Endocrinology and Metabolism. 2015;30(1):35–44. doi: 10.3803/EnM.2015.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo Ortega et al. (2014).Camargo Ortega VR, Bravo López LD, Visoso Salgado A, Mejia Sanchez F, Castillo Cadena J. Polymorphisms in glutathione S-transferase M1, T1, and P1 in patients with chronic periodontitis: a pilot study. International Scholarly Research Notices. 2014;2014(1):135368. doi: 10.1155/2014/135368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candel-Martí et al. (2011).Candel-Martí ME, Flichy-Fernández AJ, Alegre-Domingo T, Ata-Ali J, Peñarrocha-Diago MA. Interleukins IL-6, IL-8, IL-10, IL-12 and periimplant disease. An update. Medicina Oral Patología Oral y Cirugia Bucal. 2011;16:e518–e521. doi: 10.4317/medoral.16.e518. [DOI] [PubMed] [Google Scholar]
- Cnop et al. (2017).Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P. Endoplasmic reticulum stress and eIF2α phosphorylation: the Achilles heel of pancreatic β cells. Molecular Metabolism. 2017;6(9):1024–1039. doi: 10.1016/j.molmet.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes et al. (2011).Costes S, Huang C-J, Gurlo T, Daval M, Matveyenko AV, Rizza RA, Butler AE, Butler PC. β-cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency. Diabetes. 2011;60(1):227–238. doi: 10.2337/db10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler-Crish, Smith & Wilson (2017).Dengler-Crish CM, Smith MA, Wilson GN. Early evidence of low bone density and decreased serotonergic synthesis in the dorsal raphe of a tauopathy model of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2017;55(4):1605–1619. doi: 10.3233/JAD-160658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir et al. (2013).Dhir S, Mahesh L, Kurtzman GM, Vandana KL. Peri-implant and periodontal tissues: a review of differences and similarities. Compendium of Continuing Education in Dentistry. 2013;34(7):e69–e75. [PubMed] [Google Scholar]
- Dreyer et al. (2018).Dreyer H, Grischke J, Tiede C, Eberhard J, Schweitzer A, Toikkanen SE, Glöckner S, Krause G, Stiesch M. Epidemiology and risk factors of peri-implantitis: a systematic review. Journal of Periodontal Research. 2018;53(5):657–681. doi: 10.1111/jre.12562. [DOI] [PubMed] [Google Scholar]
- Gómez-Moreno et al. (2015).Gómez-Moreno G, Aguilar-Salvatierra A, Rubio Roldán J, Guardia J, Gargallo J, Calvo-Guirado JL. Peri-implant evaluation in type 2 diabetes mellitus patients: a 3-year study. Clinical Oral Implants Research. 2015;26(9):1031–1035. doi: 10.1111/clr.12391. [DOI] [PubMed] [Google Scholar]
- Gulia et al. (2018).Gulia S, Bhatt V, Shetty M, Prasad KD, Gupta P. Effect of type II diabetes mellitus, candida albicans and streptococcus mutans on the biofilm formation on prosthetic materials. Journal of Contemporary Dental Practice. 2018;19(12):1538–1545. [PubMed] [Google Scholar]
- Héron, Deloukas & Van Loon (1995).Héron E, Deloukas P, Van Loon AP. The complete exon-intron structure of the 156-kb human gene NFKB1, which encodes the p105 and p50 proteins of transcription factors NF-κB and IκB-γ: implications for NF-κB-mediated signal transduction. Genomics. 1995;30(3):493–505. doi: 10.1006/geno.1995.1270. [DOI] [PubMed] [Google Scholar]
- Hochstrasser (1996).Hochstrasser M. Ubiquitin-dependent protein degradation. Annual Review of Genetics. 1996;30(1):405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- Jiang & Wek (2005).Jiang H-Y, Wek RC. Phosphorylation of the α-subunit of the eukaryotic initiation factor-2 (eIF2α) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. Journal of Biological Chemistry. 2005;280(14):14189–14202. doi: 10.1074/jbc.M413660200. [DOI] [PubMed] [Google Scholar]
- Jin & Dong (2013).Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerging Microbes & Infections. 2013;2(1):e60. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2014).Kim YG, Park JW, Lee JM, Suh JY, Lee JK, Chang BS, Um HS, Kim JY, Lee Y. IL-17 inhibits osteoblast differentiation and bone regeneration in rat. Archives of Oral Biology. 2014;59(9):897–905. doi: 10.1016/j.archoralbio.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Ladiges et al. (2005).Ladiges WC, Knoblaugh SE, Morton JF, Korth MJ, Sopher BL, Baskin CR, MacAuley A, Goodman AG, LeBoeuf RC, Katze MG. Pancreatic β-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54(4):1074–1081. doi: 10.2337/diabetes.54.4.1074. [DOI] [PubMed] [Google Scholar]
- Lang et al. (2012).Lang S, Benedix J, Fedeles SV, Schorr S, Schirra C, Schäuble N, Jalal C, Greiner M, Hassdenteufel S, Tatzelt J, Kreutzer B, Edelmann L, Krause E, Rettig J, Somlo S, Zimmermann R, Dudek J. Different effects of Sec61α, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. Journal of Cell Science. 2012;125(8):1958–1969. doi: 10.1242/jcs.096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker, Goldberg & Mitch (2006).Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. Journal of the American Society of Nephrology. 2006;17(7):1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2014b).Lee S, Kim JY, Hwang J, Kim S, Lee JH, Han DH. Investigation of pathogenic genes in peri-implantitis from implant clustering failure patients: a whole-exome sequencing pilot study. PLOS ONE. 2014b;9(6):e99360. doi: 10.1371/journal.pone.0099360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2014a).Lee JS, Yi J-K, An SY, Heo JS. Increased osteogenic differentiation of periodontal ligament stem cells on polydopamine film occurs via activation of integrin and PI3K signaling pathways. Cellular Physiology and Biochemistry. 2014a;34(5):1824–1834. doi: 10.1159/000366381. [DOI] [PubMed] [Google Scholar]
- Maj et al. (2016).Maj M, Hoermann G, Rasul S, Base W, Wagner L, Attems J. The microtubule-associated protein tau and its relevance for pancreatic beta cells. Journal of Diabetes Research. 2016;2016(3):1–12. doi: 10.1155/2016/1964634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin et al. (2018).Marin C, Luyten FP, Van Der Schueren B, Kerckhofs G, Vandamme K. The impact of type 2 diabetes on bone fracture healing. Frontiers in Endocrinology. 2018;9:6. doi: 10.3389/fendo.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje, Catena & Borgnakke (2017).Monje A, Catena A, Borgnakke WS. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: systematic review and meta-analysis. Journal of Clinical Periodontology. 2017;44(6):636–648. doi: 10.1111/jcpe.12724. [DOI] [PubMed] [Google Scholar]
- Nadeem et al. (2013).Nadeem A, Javaid K, Sami W, Zafar A, Jahan S, Zaman S, Nagi A. Inverse relationship of serum IL-17 with type-II diabetes retinopathy. Clinical Laboratory. 2013;59:1311–1317. doi: 10.7754/clin.lab.2013.121140. [DOI] [PubMed] [Google Scholar]
- Nielsen et al. (2017).Nielsen TB, Pantapalangkoor P, Yan J, Luna BM, Dekitani K, Bruhn K, Tan B, Junus J, Bonomo RA, Schmidt AM, Everson M, Duncanson F, Doherty TM, Lin L, Spellberg B. Diabetes exacerbates infection via hyperinflammation by signaling through TLR4 and RAGE. mBio. 2017;8(4):e00818-17. doi: 10.1128/mBio.00818-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender et al. (2003).Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52(2):227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus et al. (2014).Oeckinghaus A, Postler TS, Rao P, Schmitt H, Schmitt V, Grinberg-Bleyer Y, Kühn LI, Gruber CW, Lienhard GE, Ghosh S. κB-Ras proteins regulate both NF-κB-dependent inflammation and Ral-dependent proliferation. Cell Reports. 2014;8(6):1793–1807. doi: 10.1016/j.celrep.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamäki et al. (2009).Pihlajamäki J, Boes T, Kim E-Y, Dearie F, Kim BW, Schroeder J, Mun E, Nasser I, Park PJ, Bianco AC, Goldfine AB, Patti ME. Thyroid hormone-related regulation of gene expression in human fatty liver. Journal of Clinical Endocrinology & Metabolism. 2009;94(9):3521–3529. doi: 10.1210/jc.2009-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñero et al. (2016).Piñero J, Bravo À, Queralt-Rosinach N, Gutiérrez-Sacristán A, Deu-Pons J, Centeno E, García-García J, Sanz F, Furlong LI. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Research. 2016;45(D1):D833–D839. doi: 10.1093/nar/gkw943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi, Morrison & Reis (2012).Qureshi N, Morrison DC, Reis J. Proteasome protease mediated regulation of cytokine induction and inflammation. Biochimica Et Biophysica Acta. 2012;1823(11):2087–2093. doi: 10.1016/j.bbamcr.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakić et al. (2013).Rakić M, Nikolić-Jakoba N, Struillout X, Petković-Curcin A, Stamatović N, Matić S, Janković S, Aleksić Z, Vasilić D, Leković V, Vojvodić D. Receptor activator of nuclear factor kappa B (RANK) as a determinant of peri-implantitis. Vojnosanitetski Pregled. 2013;70(4):346–351. doi: 10.2298/VSP1304346R. [DOI] [PubMed] [Google Scholar]
- Rehman et al. (2017).Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, Rasul A. Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus. Critical Reviews in Eukaryotic Gene Expression. 2017;27(3):229–236. doi: 10.1615/CritRevEukaryotGeneExpr.2017019712. [DOI] [PubMed] [Google Scholar]
- Renvert et al. (2018).Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. Journal of Periodontology. 2018;89(Suppl 1):S304–S312. doi: 10.1002/JPER.17-0588. [DOI] [PubMed] [Google Scholar]
- Ritchie et al. (2015).Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat (2017).Saadat M. Evaluation of glutathione S-transferase P1 (GSTP1) Ile105Val polymorphism and susceptibility to type 2 diabetes mellitus, a meta-analysis. EXCLI Journal. 2017;16:1188–1197. doi: 10.17179/excli2017-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Siles et al. (2016).Sánchez-Siles M, Lucas-Azorin J, Salazar-Sánchez N, Carbonell-Meseguer L, Camacho-Alonso F. Salivary concentration of oxidative stress biomarkers in a group of patients with peri-implantitis: a transversal study. Clinical Implant Dentistry and Related Research. 2016;18(5):1015–1022. doi: 10.1111/cid.12367. [DOI] [PubMed] [Google Scholar]
- Savic-Radojevic et al. (2007).Savic-Radojevic A, Mimic-Oka J, Pljesa-Ercegovac M, Opacic M, Dragicevic D, Kravic T, Djokic M, Micic S, Simic T. Glutathione S-transferase-P1 expression correlates with increased antioxidant capacity in transitional cell carcinoma of the urinary bladder. European Urology. 2007;52(2):470–477. doi: 10.1016/j.eururo.2007.01.046. [DOI] [PubMed] [Google Scholar]
- Schminke et al. (2015).Schminke B, Vom Orde F, Gruber R, Schliephake H, Bürgers R, Miosge N. The pathology of bone tissue during peri-implantitis. Journal of Dental Research. 2015;94(2):354–361. doi: 10.1177/0022034514559128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino et al. (2016).Severino VO, Beghini M, De Araújo MF, De Melo MLR, Miguel CB, Rodrigues WF, De Lima Pereira SA. Expression of IL-6, IL-10, IL-17 and IL-33 in the peri-implant crevicular fluid of patients with peri-implant mucositis and peri-implantitis. Archives of Oral Biology. 2016;72:194–199. doi: 10.1016/j.archoralbio.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Shrestha et al. (2012).Shrestha N, Bahnan W, Wiley DJ, Barber G, Fields KA, Schesser K. Eukaryotic initiation factor 2 (eIF2) signaling regulates proinflammatory cytokine expression and bacterial invasion. Journal of Biological Chemistry. 2012;287(34):28738–28744. doi: 10.1074/jbc.M112.375915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sündermann, Fernandez & Morgan (2016).Sündermann F, Fernandez M-P, Morgan RO. An evolutionary roadmap to the microtubule-associated protein MAP Tau. BMC Genomics. 2016;17(1):264. doi: 10.1186/s12864-016-2590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneera et al. (2013).Taneera J, Fadista J, Ahlqvist E, Zhang M, Wierup N, Renström E, Groop L. Expression profiling of cell cycle genes in human pancreatic islets with and without type 2 diabetes. Molecular and Cellular Endocrinology. 2013;375(1-2):35–42. doi: 10.1016/j.mce.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Teng et al. (2016).Teng Y, Qin H, Bahassan A, Bendzunas NG, Kennedy EJ, Cowell JK. The WASF3-NCKAP1-CYFIP1 complex is essential for breast cancer metastasis. Cancer Research. 2016;76(17):5133–5142. doi: 10.1158/0008-5472.CAN-16-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunduguru et al. (2017).Tunduguru R, Zhang J, Aslamy A, Salunkhe VA, Brozinick JT, Elmendorf JS, Thurmond DC. The actin-related p41ARC subunit contributes to p21-activated kinase-1 (PAK1)–mediated glucose uptake into skeletal muscle cells. Journal of Biological Chemistry. 2017;292(46):19034–19043. doi: 10.1074/jbc.M117.801340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venza et al. (2010).Venza I, Visalli M, Cucinotta M, De Grazia G, Teti D, Venza M. Proinflammatory gene expression at chronic periodontitis and peri-implantitis sites in patients with or without type 2 diabetes. Journal of Periodontology. 2010;81(1):99–108. doi: 10.1902/jop.2009.090358. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2001).Wang FS, Wang CJ, Huang HJ, Chung H, Chen RF, Yang KD. Physical shock wave mediates membrane hyperpolarization and Ras activation for osteogenesis in human bone marrow stromal cells. Biochemical and Biophysical Research Communications. 2001;287(3):648–655. doi: 10.1006/bbrc.2001.5654. [DOI] [PubMed] [Google Scholar]
- Wright, Scism-Bacon & Glass (2006).Wright E, Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. International Journal of Clinical Practice. 2006;60(3):308–314. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghobee et al. (2014).Yaghobee S, Khorsand A, Ghohroudi AAR, Sanjari K, Kadkhodazadeh M. Assessment of interleukin-1beta and interleukin-6 in the crevicular fluid around healthy implants, implants with peri-implantitis, and healthy teeth: a cross-sectional study. Journal of the Korean Association of Oral and Maxillofacial Surgeons. 2014;40(5):220–224. doi: 10.5125/jkaoms.2014.40.5.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada et al. (2015).Yamada H, Nakajima T, Domon H, Honda T, Yamazaki K. Endoplasmic reticulum stress response and bone loss in experimental periodontitis in mice. Journal of Periodontal Research. 2015;50(4):500–508. doi: 10.1111/jre.12232. [DOI] [PubMed] [Google Scholar]
- You et al. (2014).You DJ, Park CR, Lee HB, Moon MJ, Kang JH, Lee C, Oh SH, Ahn C, Seong JY, Hwang JI. A splicing variant of NME1 negatively regulates NF-κB signaling and inhibits cancer metastasis by interacting with IKKβ. Journal of Biological Chemistry. 2014;289(25):17709–17720. doi: 10.1074/jbc.M114.553552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefidaredor et al. (2014).Yousefidaredor H, Zare-Bidaki M, Hakimi H, Assar S, Bagheri V, Arababadi MK. IL-17A plays an important role in induction of type 2 diabetes and its complications. Asian Pacific Journal of Tropical Disease. 2014;4(5):412–415. doi: 10.1016/S2222-1808(14)60598-3. [DOI] [Google Scholar]
- Yousif et al. (2004).Yousif MHM, Benter IF, Abraham S, Akhtar S. Inhibition of Ras-GTPase improves diabetes-induced abnormal vascular reactivity in the rat perfused mesenteric vascular bed. Medical Principles and Practice. 2004;13(2):57–62. doi: 10.1159/000075629. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang H, Zhang X, Huang J, Fan X. Identification of key genes and pathways for peri-implantitis through the analysis of gene expression data. Experimental and Therapeutic Medicine. 2017;13(5):1832–1840. doi: 10.3892/etm.2017.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou et al. (2013).Zou W, Greenblatt MB, Brady N, Lotinun S, Zhai B, De Rivera H, Singh A, Sun J, Gygi SP, Baron R, Glimcher LH, Jones DC. The microtubule-associated protein DCAMKL1 regulates osteoblast function via repression of Runx2. Journal of Experimental Medicine. 2013;210(9):1793–1806. doi: 10.1084/jem.20111790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The number of DEGs identified in two datasets (GSE33774 and GSE57631) of peri-implantitis.
Table S2. Top 20 nodes in PPI networks of both peri-implantitis and T2MD.
Table S3. Three leader genes shared in two selected significant classes.
Data Availability Statement
The following information was supplied regarding data availability:
No primary data was generated in this study. The publicly available microarray datasets (GSE33774 and GSE57631) were downloaded from Gene Expression Omnibus (GEO) database.