Abstract
From 2015, Norway has implemented high‐risk human papilloma virus (hrHPV) testing in primary screening for cervical cancer. Women aged 34–69 years, living in four counties, have been pseudo‐randomly assigned (1:1 randomization) to either hrHPV testing every 5 years (followed by cytology if hrHPV is positive), or cytology testing every 3 years (followed by hrHPV testing if low‐grade cytology is detected). We compared anxiety and depression scores among participants by screening arm and results. In total, 1,008 women answered a structured questionnaire that included the validated Patient Health Questionnaire‐4 (PHQ‐4). The Relative Risk Ratio (RRR) of mild vs. normal anxiety and depression scores, and moderate/severe vs. normal anxiety and depression scores, were estimated by multinomial logistic regression with 95% confidence intervals (95% CIs). Compared to women who were screened with cytology, women randomized to hrHPV testing were not more likely to have mild anxiety and depression scores (RRR 0.96, CI 0.70–1.31) nor more likely to have moderate/severe anxiety and depression scores (RRR 1.14, CI 0.65–2.02). Women with five different combinations of abnormal screening test results were not more likely to have mild or moderate/severe vs. normal anxiety and depression scores than women with normal screening results. The likelihood of having abnormal long‐term (4–24 months after the screening) anxiety or depression scores among women 34 years and older was not affected by screening method or screening results. The results of our study suggest that a change to hrHPV testing in primary screening would not increase psychological distress among participants.
Keywords: cervical cancer screening, high‐risk human papilloma virus testing, HPV, cancer registry, anxiety and depression, epidemiology, cancer, women, health‐care system, prevention, Norway, Scandinavia
Short abstract
What's new?
Norway is one of the first countries to implement high‐risk human papilloma virus (hrHPV) testing in primary cervical‐cancer screening. Does this newer type of testing impact the emotional well‐being of patients? In this study, the authors found no significant difference in either anxiety or depression scores between the viral‐screening arm and standard cytology screening. These findings could be useful for other countries considering implementing hrHPV testing, and are reassuring for the ongoing implementation process in Norway.
Abbreviations
- NCCSP
The Norwegian Cervical Cancer Screening Programme
- CRN
Cancer Registry of Norway—Institute of Population based Cancer Research
- hrHPV
high‐risk human papilloma virus
- ASC‐US
atypical squamous cells of undetermined sgnificance
- LSIL
low‐grade squamous intraepithelial lesion
- AGUS
atypical glandular cells of undetermined significance
- ASC‐H
atypical squamous cells—cannot exclude HSIL
- HSIL
high‐grade squamous intraepithelial lesion
- ACIS
adeno carcinoma in situ
- Ca
cancer
- HG
high grade
- RRR
Relative risk ratio
- CI
Confidence interval
Introduction
The Norwegian Cervical Cancer Screening Programme (NCCSP) has been national since 1995. It is managed by the Cancer Registry of Norway—Institute of Population based Cancer Research (CRN), which reminds women aged 25–69 years to have a cervical cytology taken every 3 years.1 From 2005, high‐risk human papilloma virus (hrHPV) testing has been used as a triage test in the screening programme as part of follow‐up among women with abnormal low‐grade cytology.2
Between 2015 and 2018, a national project implemented hrHPV testing in primary screening3 (i.e. as the first screening test taken) among women in four counties (Rogaland, Hordaland, Sør‐Trøndelag, and Nord‐Trøndelag). A prerequisite from the National Council for Priority Setting prior to the introduction of the implementation project was that hrHPV testing would not lead to increased anxiety and depression among screening participants.4 The most common mental health conditions in the general population are anxiety and depression.5, 6, 7 The prevalence of these conditions in Norway is similar to other countries in Europe and the United States:8 about 25% of the Norwegian women will be affected by an anxiety disorder at some point in life,9 and around 20% will experience depression.8 Women may experience increased anxiety and depression at the time of any cervical examination,10 but it is unknown whether a change from cytology to hrHPV testing in primary screening will increase anxiety and depression levels among screening participants.
A study conducted in Norway prior to the implementation project indicated that a switch to hrHPV testing was unlikely to affect women's anxiety nor to reduce their participation in NCCSP.11 A limitation of the study was that it was not carried out in a real‐life situation but merely asked participants how they assumed they would experience the two different screening methods. Research carried out in Ireland12 found that the emotional impact of hrHPV testing in screening participants was modest and that women's concerns were related to screening test results more than to the screening method used. Yet, the conclusion drawn by these two studies are at odds with those drawn by other researchers, suggesting that hrHPV‐based screening programmes may be associated with increased anxiety and stress and to increased concerns about having a sexually transmitted infection.13, 14 Hence, the relationship between hrHPV testing in primary screening and women's mental health is unresolved. Moreover, the majority of studies addressing this issue have been performed in the setting of secondary hrHPV testing (the second test taken after cytology).15, 16 We took advantage of the ongoing implementation project in the NCCSP to perform a cross‐sectional study comparing anxiety and depression scores among women screened with hrHPV testing or cytology in primary screening.
Objectives
Our objectives were to compare long‐term (4–24 months after the screening) anxiety and depression scores between women allocated to the two screening arms, cytology or hrHPV test.
Subjects and Methods
The implementation project of hrHPV testing in primary screening
The implementation project of hrHPV testing in primary screening is pseudo‐randomized, that is, allocation to screening method is decided by the participant's date of birth being an odd or an even day. Women aged 34–69 years living in one of the four implementation counties were allocated either to (i) hrHPV testing with a 5‐year screening interval (followed by cytology if hrHPV positive) or to (ii) cytology with a 3‐year screening interval (followed by hrHPV testing in case of low‐grade cytology).3, 17 hrHPV testing was performed on liquid‐based cytology samples. Figure 1 summarizes the algorithm for hrHPV and cytology screening used in the implementation project. Women with a negative hrHPV test in the hrHPV arm, received an additional letter from the NCCSP with information about the extended screening interval that followed a hrHPV negative test result. Women aged 25–33 years were not included in the implementation project but continued to be screened with cytology every 3 years due to the generally high hrHPV prevalence with no clinical relevance in this age category.18
Figure 1.
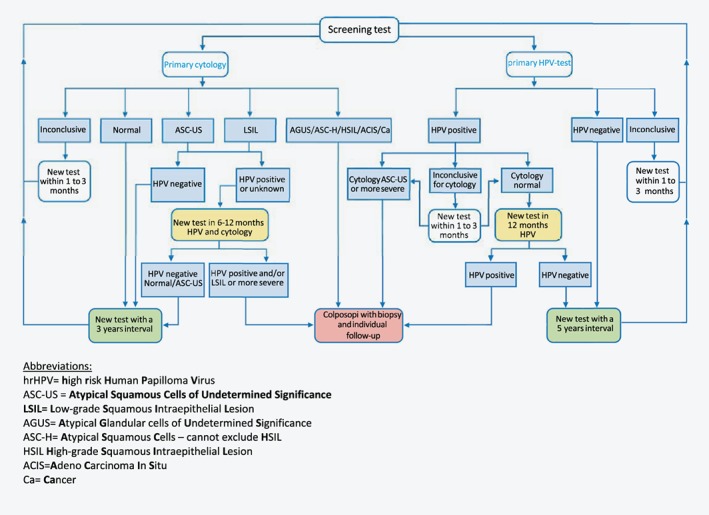
The Norwegian cervical cancer screening algorithm for primary cytology and primary hrHPV screening used in the period from 2015 to 2018 when implementing hrHPV testing in four counties among women aged 34–69 years. [Color figure can be viewed at wileyonlinelibrary.com]
Study population
As of September 2017, 168,201 women had participated in the implementation project according to the Cancer Registry of Norway, which records all screening tests taken as part of the NCCSP unless participating women have actively objected to their screening data being registered.19 Among these, we identified women who had undergone cervical cancer screening between February 15, 2015 and September 15, 2016 and randomly sampled 500 women from the cytology arm and 500 women from the hrHPV arm. In addition, we randomly oversampled 500 women who had had a positive cytology in the cytology arm, and 500 women who had had a positive hrHPV test in the hrHPV arm. We did so to compare anxiety and depression scores among women with positive screening test results in the two arms. Thus, a total of 2,000 women were sampled for our study (Fig. 2).
Figure 2.
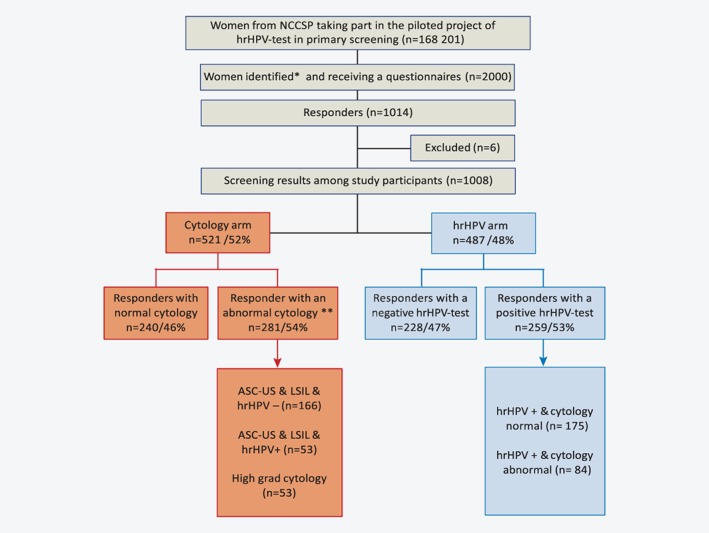
Flow diagram of study participants and their previous screening results. *We identified women from the pilot project with the following strata: 500 women from the cytology arm, 500 women from the hrHPV arm, 500 with a positive cytology results from the cytology arm and 500 women with a positive hrHPV test results from the hrHPV arm. Total 2000 women: **nine women with inconclusive screening test results in the cytology arm are excluded from the analysis. [Color figure can be viewed at wileyonlinelibrary.com]
These women received a structured questionnaire by postal mail in January or February 2017, 4–24 months after they had been informed about their last screening test result. An information leaflet and a prepaid return envelope were enclosed with the questionnaire. The initial response rate was 39% (n = 789). The questionnaire was resent to nonresponders after one month, resulting in a total response rate of 51% (n = 1,014). We excluded six women who had answered the same questionnaire twice with dissimilar answers, leading to a 50% response rate (n = 1,008). Nine women with unsatisfactory cytology results in the cytology arm were also excluded from the analysis (Fig. 2). The registration of screening activity and screening results in the NCCSP made it feasible to link completed questionnaires to objective data on screening test results.
Questionnaire
The questionnaire (in Norwegian language [shown in the Supporting Information 1]) included the Patient Health Questionnaire‐4 (PHQ‐4) for anxiety and depression. 5, 20 The PHQ‐4 has been developed from two larger and validated anxiety and depression measure instruments: the 7‐itemed GAD‐7 measuring anxiety,21 and the 9‐itemed PHQ‐9 measuring depression.22 PHQ‐4 incorporates the core criteria for anxiety and depression from these instruments and has been validated for use as a screening tool.5, 20 A meta‐analysis23 has found that combining anxiety and depression items into one tool gives better results than single‐item screening tools. The PHQ‐4 consists of two questions regarding anxiety and two regarding depression. It asks: “Over the last two weeks, how often have you been bothered by the following problems” and then specifies these as “feeling nervous, anxious, or on the edge,”” not being able to stop control worrying,” “feeling down, depressed or hopeless” and “having little interest or pleasure in doing things”. For each of these items, responses were scored as: 0 (“not at all”), 1 (“several days”), 2 (“more than half the days”) or 3 (“nearly every day”), with a total score range from 0 to 12. Anxiety and depression was graded as none (total score from 0 to 2), mild (3–5), moderate (6–8) and severe (9–12).
The questionnaire also asked about age, marital status and educational level. In addition, nonvalidated questions were asked about the study participants' experience with the NCCSP (e.g. have you ever received a letter from the Cancer Registry reminding you to take a screening test?), knowledge related to cervical cancer (e.g. before reading the attached information letter, did you know that a cervical cytology test can reveal cellular changes that untreated can lead to cervical cancer?) knowledge related to HPV (e.g. before you read about human papillomavirus (HPV) in the attached information letter, did you know that HPV can cause cancer?), their last screening test results (e.g. what was the results of your last screening test?), and the type of screening method that had been used at their last screening attendance (e.g. what screening method was used when your last screening test was taken?) The questionnaire was piloted in two rounds in 2016 among women working at CRN.
Ethical considerations
The Privacy Ombudsman and Data Protection Officer at Oslo University Hospital in Norway, assessed and recommended the study (case number 2016/15743). The information leaflet explained the study, including the registry linkages that would take place, and that answering and returning the questionnaire would be considered informed consent to participate in the study. Each questionnaire was assigned a unique ID number*, from 1 to 2000, and was linked to the objective clinical screening data at the NCCSP.
Statistical analysis
Statistical differences between women in the two screening arms (cytology and hrHPV) were assessed using χ2 test for categorical outcomes and Student's t‐test for the continuous variable, age. Anxiety and depression scores were categorized as normal (values ≤2), mild (values 3–5), or moderate/severe (values >5), and compared between the two screening arms and among persons with different screening test results. We estimated the relative risk ratio (RRR) of scoring (a) mild vs. normal and (b) moderate/severe vs. normal on anxiety and depression by multinomial logistic regression with 95% confidence intervals (95% CI).
The association between the two screening arms and between the specific screening results and anxiety and depression were estimated in two separate models. These models were adjusted for demographic characteristics that differed between women in the two screening arms (Table 1): marital status (married/cohabiting, single/divorced/widowed) and place of birth (Norway, all other countries). Based on findings of prior studies of the association between screening method and psychological distress,11, 24, 25 we also included age group (34–44, 45–54, ≥55) and educational level (≤13 years, > 13 years) as covariates in the two multinomial logistic regression models. The goodness of fit of the multinomial crude models was evaluated by testing the goodness of fit in the two corresponding logistic regression models for mild vs. normal and moderate/severe vs. normal using the Hosmer and Lemeshow test.26
Table 1.
Characteristics of 1,008 women participating in the study
| Total | Cytology | hrHPV | P‐values by χ2 | ||
|---|---|---|---|---|---|
| n = 1,008 | n = 521(52%) | n = 487 (48%) | |||
| Age | 34–44 years | 301 (30%) | 159 (30%) | 142 (29%) | 0.33 |
| 45–54 years | 343 (34%) | 185 (36%) | 158 (32%) | ||
| 55 years and older | 364 (36%) | 177 (34%) | 187 (39%) | ||
| Marital status1 | Married/Cohabiting/ | 830 (82%) | 441 (85%) | 389 (80%) | 0.04 |
| Single/divorced/widow | 175 (18%) | 78 (15%) | 97 (20%) | ||
| Attained education2 | Up to 13 years | 421 (42%) | 210 (40%) | 211 (43%) | 0.34 |
| More than 13 years | 582 (58%) | 308 (60%) | 274 (57%) | ||
| Years lived in Norway3 | Less than all life | 148 (15%) | 83 (16%) | 65 (13%) | 0.25 |
| All life | 858 (85%) | 437 (84%) | 421 (87%) | ||
| Birthplace | Norway | 917 (91%) | 465 (89%) | 452 (93%) | 0.05 |
| Other countries | 91 (9%) | 56 (11%) | 35 (7%) | ||
| Screening results | Normal screen results | 468 (52%) | 240 (46%) | 228 (47%) | 0.81 |
| Abnormal screen results | 540 (48%) | 281 (54%) | 259 (53%) | ||
| Anxiety and depression4 | Normal scores | 716 (73%) | 370 (73%) | 346 (73%) | 0.88 |
| Mild scores | 206 (21%) | 109 (22%) | 97 (21%) | ||
| Moderate/severe scores | 53 (6%) | 26(5%) | 27 (6%) | ||
| Time between screening and answering questionnaire | 4 months to 1 year | 521 (52%) | 216 (41%) | 227 (47%) | 0.10 |
| 1–2 years | 487 (48%) | 305 (59%) | 260 (53%) | ||
| Knowledge that screening can prevent cervical cancer5 | Yes | 980 (98%) | 503 (97%) | 477 (98%) | 0.30 |
| No | 19 (2%) | 16 (3%) | 10 (2%) | ||
| Knowledge of the link between hrHPV and cervical cancer | Yes | 695 (69%) | 342 (66%) | 353 (72%) | 0.02 |
| No | 313 (31%) | 179 (34%) | 134 (28%) | ||
| Knowledge related to last screening method used6 | Correct | 364 (37%) | 181(35%) | 183 (39%) | 0.00 |
| Not correct | 112(12%) | 18 (4%) | 94 (20%) | ||
| I do not know | 502 (51%) | 307 (61%) | 195 (41%) | ||
| Knowledge related to last screening test results7 | Correct | 698 (74%) | 316 (66%) | 382 (82%) | 0.00 |
| Not correct | 251 (26%) | 166 (34%) | 85 (18%) | ||
| If the screening interval is extended from 3 to 5 years, I would take an addition screening test on my own initiative: | More often (>5 years) | 557 (56%) | 277 (53%) | 280 (58%) | 0.292 |
| Rarer (<5 years) | 15 (1%) | 6 (1%) | 9 (2%) | ||
| When reminder from CRN | 352 (35%) | 189 (36%) | 163 (33%) | ||
| Never or not sure | 84 (8%) | 49 (10%) | 35 (7%) | ||
3 missing.
5 missing.
2 missing.
33 missing.
9 missing.
30 missing.
59 missing.
Abbreviations: CRN: Cancer Registry of Norway; hrHPV: high‐risk human papilloma virus.
The elapsed time from information about screening results to answering the questionnaire was categorized (4 months to 1 year, 1–2 years). We tested for interactions between the elapsed time and the two screening arms and between elapsed time and the specific screening results by including interaction term in the models, in addition to performing stratified analyses.
The sample in the univariate and multivariate analyses vary from 966 to 1,008 women, due to missing values. All tests were two‐sided with a 5% significance level. All statistical analyses were conducted using the Stata statistical software package (version 14.2).
Definitions of cervical cancer screening test results
Women taking part in the implementation project can, when the NCCSP's guidelines 27 are followed, receive seven† different combinations of screening test results:
In the cytology arm:
Cytology normal
ASC‐US or LSIL and hrHPV positive
High‐grade cytology
In the hrHPV arm:
hrHPV negative
hrHPV positive and normal cytology
hrHPV positive and ASC‐US or more severe
Results
Among the 1,008 women who answered the questionnaire, 521 (52%) were from the cytology arm and 487 (48%) from the hrHPV arm. The mean age was 50.5 years (SD ± 9.7) in the cytology arm and 51.2 years (SD ± 10.1) in the hrHPV arm. The frequency of abnormal primary cytology and positive primary hrHPV tests results were 54% and 53%,** respectively (Table 1).
Age, attained education, duration of residency in Norway, previous screening test results and the time that had elapsed from a woman received her last screening test result until she answered the questionnaire, were similar in the two arms (Table 1). However, women in the hrHPV arm were significantly more often single/divorced/widowed (p = 0.04) and more often born in Norway (p = 0.05) than women in the cytology arm.
The knowledge that participation in screening can detect precancerous lesions and prevent cervical cancer was similar and very high in both study arms (97% and 98% of women in the cytology and the hrHPV arm, respectively [p = 0.30]). Fewer (66%) in the cytology arm than in the hrHPV arm (72%) knew that an hrHPV infection can cause cervical cancer (p = 0.02). When women were asked what screening‐method had been used the last time they were screened, the answers differed by arms: 35% of those in the cytology arm, and 39% of those in the hrHPV arm, answered correctly, whereas 4% in the cytology arm and 20% in the hrHPV arm answered this question incorrectly (p < 0.0001). Hence, the majority of women were unable to identify the analysis method used the last time they were screened (61% of women in the cytology arm and 41% in the hrHPV arm [p < 0.0001]). When asked about the results of their last screening test, fewer in the cytology arm (66%) reported this correctly than in the hrHPV arm (82%) (p < 0.0001). 53% of women in the cytology arm and 58% of women in the hrHPV arm indicated that they would have had a screening test performed on their own initiative if the screening interval were to be expanded from 3 to 5 years (p = 0.29) (Table 1).
Anxiety and depression
The distribution of PHQ‐4 scores for anxiety and depression were nearly identical in the cytology and the hrHPV arm: 73% had normal scores, 22% and 21% had mild scores and 5% and 6% had moderate/severe scores, respectively (Fig. 3 a). Furthermore, the distribution of anxiety and depression scores among women with different diagnoses is shown in Figure 3 b. Most women with normal anxiety and depression scores were to be found among women with normal cytology in the cytology arm (34%) and among women with negative hrHPV test in the hrHPV arm (36%). Anxiety and depression scores among women testing positive on the primary screening test but negative on the second screening test were similar in the two screening arms (women in the cytology arm having ASC‐US or LSIL but being hrHPV negative, and women in the hrHPV arm being hrHPV positive but having normal cytology: 23%–25% had normal scores, 7%–9% had mild scores and 2%–1% had moderate/severe anxiety and depression scores, respectively). Anxiety and depression among women being double positive were also similar in the two screening arms (women in the cytology arm having ASC‐US or LSIL and being hrHPV positive, and women in the hrHPV arm being hrHPV positive in addition to having ASC‐US or more severe cytological diagnoses: 8%–12% had normal scores, 3%–4% had mild scores, and 1%–2% had moderate/severe anxiety and depression scores, respectively) (Fig. 3 b).
Figure 3.
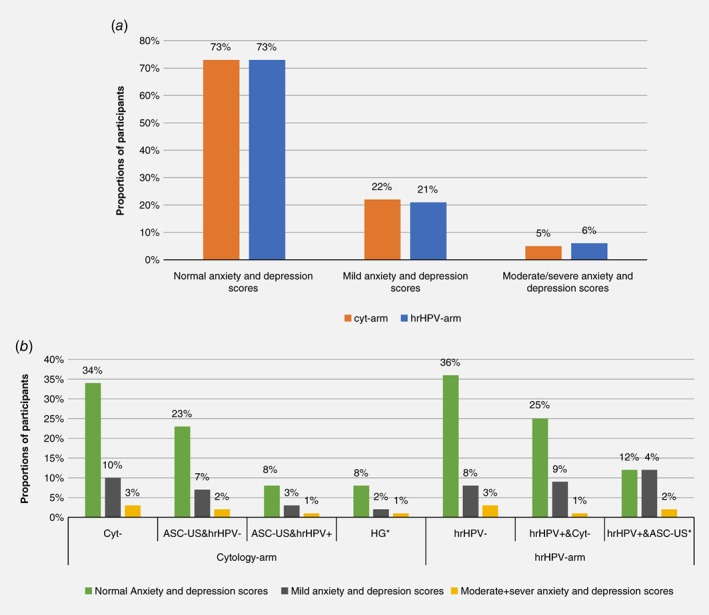
Scores of anxiety and depression among 966 women with different screening test results (496 from the cytology arm and 470 from the hrHPV arm) (a) Grouped presentation of anxiety and depression scores between screening arm (b) Distribution of anxiety and depression scores between seven different screening diagnoses and between comparable diagnosis in the cytology and the hrHPV arm respectively. The percentage are calculated in the cytology and the hrHPV‐arm, respectively, *HG = high‐grade cytology. [Color figure can be viewed at wileyonlinelibrary.com]
Two final multivariate logistic regression models confirmed the results from Figure 3 a,b with crude percentage distribution of anxiety and depression scores being similar between screening arms and between screening outcomes (Table 2). Univariate models showed no difference in anxiety and depression scores between women in the cytology arm and women in the hrHPV arm (mild vs. normal anxiety and depression scores [RRR 0.95, 95% CI 0.70–1.30] and moderate/severe vs. normal anxiety and depression [1.11, 0.64–1.94]). Adjustment for age, marital status, education level and birthplace, gave virtually identical results (mild vs. normal anxiety and depression scores [0.96, 0.70–1.31] and moderate/severe vs normal anxiety and depression scores [1.14, 0.65–2.02]), that is, there were no differences in anxiety and depression scores and screening arms. When we compared the different screening test outcomes with normal cytology (being the most common screening test results in NCCSP), we found no statistically significant differences neither in univariate nor in multivariate models between any screening test results and mild vs. normal anxiety depression scores nor between moderate/severe vs. normal anxiety and depression scores. Women in the cytology arm having the diagnosis ASC‐US and in addition being hrHPV negative had results in univariate models and multivariate models, respectively: (mild vs. normal anxiety and depression scores [1.11, 0.68–1.81; 1.05, 0.64–1.74] and moderate/severe vs. normal anxiety and depression scores [0.91, 0.36–2.26; 0.93, 0.37–2.38]). Women in the cytology arm having the diagnosis ASC‐US and in addition being hrHPV positive had in univariate and multivariate models results, respectively: (mild vs. normal anxiety and depression scores [1.22, 0.60–2.47; 1.10, 0.53–2.25] and moderate/severe vs. normal anxiety and depression scores [0.69, 0.15–3.20; 0.62 0.13–2.94]). Women in the cytology arm having a high‐grade cytology had in the univariate and multivariate models, respectively (mild vs. normal anxiety and depression scores, (0.94, 0.44–2.02; 0.73, 0.33–1.60) and moderate/severe vs. normal anxiety and depression scores (1.04, 0.28–3.82; 0.95, 0.25–3.63)). Women in the hrHPV arm being hrHPV negative had in univariate and multivariate models, respectively (mild vs. normal anxiety and depression scores [0.77, 0.48–1.24; 0.79, 0.48–1.28] and moderate/severe vs. normal anxiety and depression scores [0.99, 0.45–2.21; 1.07, 0.47–2.43]). Women in the hrHPV arm being hrHPV positive and having normal cytology had results in univariate and multivariate models, respectively (mild vs. normal anxiety and depression scores [1.27, 0.79–2.04; 1.15, 0.70–1.87] and moderate/severe vs. normal anxiety and depression scores [0.89, 0.36–2.22; 0.87, 0.34–2.23]). Finally, women in the hrHPV arm being hrHPV positive and having ASC‐US or more severe cytology diagnoses had in univariate and multivariate models, respectively: (mild vs. normal anxiety and depression scores [1.15, 0.62–2.13, 1.00, 0.53–1.90] and moderate/severe vs. normal anxiety and depression scores [1.41, 0.51–3.88, 1.31, 0.46–3.78]). To sum up, we found no difference in anxiety and depression scores neither between women in the two screening arms nor between women with different screening diagnosis.
Table 2.
Multinomial logistic regression for anxiety and depression scores among 966 women (496 in the cytology and 470 in the hrHPV arm) with different screening test results, reporting RRRs
| Anxiety and depression scores | Univariate | Multivariable1 | Univariate | Multivariable1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Mild | Moderate/severe | RRR | 95% CI | RRR | 95 %CI | RRR | 95% CI | RRR | 95 %CI | ||
| Mild vs. normal | Moderate/sever vs. normal | |||||||||||
| Screening arm | Cytology arm | 363(73%) | 107 (22%) | 26 (5%) | 1 | 1 | 1 | 1 | ||||
| hrHPV arm | 346 (73%) | 97 (21%) | 27 (6%) | 0.95 | 0.70–1.30 | 0.96 | 0.70–1.31 | 1.11 | 0.64–1.94 | 1.14 | 0.65–2.02 | |
| Screening results | I. Cytology− | 171 (74%) | 48 (21%) | 13 (5%) | 1 | 1 | 1 | 1 | ||||
| II. ASC‐US/hrHPV− | 116 (73%) | 36 (22%) | 8 (5%) | 1.11 | 0.68–1.81 | 1.05 | 0.64–1.74 | 0.91 | 0.36–2.26 | 0.93 | 0.37–2.38 | |
| III. ASC‐US/hrHPV+ | 38 (72%) | 13 (25%) | 2 (3%) | 1.22 | 0.60–2.47 | 1.10 | 0.53–2.25 | 0.69 | 0.15–3.20 | 0.62 | 0.13–2.94 | |
| IV. High‐grade | 38 (74%) | 10 (20%) | 3 (6%) | 0.94 | 0.44–2.02 | 0.73 | 0.33–1.60 | 1.04 | 0.28–3.82 | 0.95 | 0.25–3.63 | |
| V. hrHPV− | 172 (77%) | 37 (17%) | 13 (6%) | 0.77 | 0.48–1.24 | 0.79 | 0.48–1.28 | 0.99 | 0.45–2.21 | 1.07 | 0.47–2.43 | |
| VI. hrHPV+/Cytology− | 118 (70%) | 42 (25%) | 8(5%) | 1.27 | 0.79–2.04 | 1.15 | 0.70–1.87 | 0.89 | 0.36–2.22 | 0.87 | 0.34–2.23 | |
| VII. hrHPV+/High‐grade | 56 (70%) | 18 (22%) | 6 (8%) | 1.15 | 0.62–2.13 | 1.00 | 0.53–1.90 | 1.41 | 0.51–3.88 | 1.31 | 0.46–3.78 | |
Adjusted for age, marital status, education level and place of birth.
Abbreviations: RRR: relative risk ratio; hrHPV: high risk human papilloma virus; ASC‐US: Atypical squamous cells of undetermined significance.
We performed stratified analyses to further investigate whether anxiety and depression scores were associated with time elapsed between when the women were informed about their last screening test results and when they answered the questionnaire. There was no interaction between elapsed time and screening arm (p = 0.11) nor between elapsed time and screening results (p = 0.39). Stratified analyses by elapsed time can be found in the Supporting Information Table S1.
Separate analyses of the anxiety and the depression dimensions of the PHQ‐4‐score showed no difference by screening arm or by screening test result on anxiety scores, or on depression scores (Supporting Information Table S2).
Discussion
In this cross‐sectional study, we compared mild vs. normal anxiety and depression scores as well as moderate/severe anxiety and depression scores vs. normal, among women, 34 years and older, tested by cytology or hrHPV in primary screening. We found no difference in long‐term (4–24 months after the screening) anxiety and depression scores as measured by the PHQ‐4 scale between the two groups. Seven different combinations of screening test results were possible outcomes in the implementation project. There were no statistically significant differences in long‐term anxiety and depression scores between women receiving each of these different screening results.
A concern when changing test method in a screening programme is whether the new test could influence screening participation negatively and psychological distress might potentially lead to reduced participation.28 The literature is inconsistent regarding anxiety and depression related to hrHPV testing in cervical cancer screening and its impact on screening participation. The results from our study are coinciding with a large randomized trial from Manchester in 2008 enrolling 24,510 women. our study found no adverse significant psychological effect on routine hrHPV screening as compared to cytology screening.29 This lack of association between HPV screening and psychological distress might be explained by what is found in three other studies30, 31, 32 showing that anxiety and depression is first and foremost linked to having abnormal screening results and not to the screening method used. In contrast to this, a systematic review of 17 studies in 2012 concluded that hrHPV testing could indeed increase anxiety, impact relationships and provoke fear of stigmatization.33 This review and several other studies have linked women's experiences of anxiety to reflections around stigma as HPV infections are sexually transmitted.13, 34 In concordance to our study, where hrHPV was the primary screening test, the women in the above‐mentioned studies13, 34 knew that they had an abnormal cytology, and the hrHPV test was part of follow‐up.
The vast majority of women taking part in our study knew that screening can prevent—and that an HPV infection can cause—cervical cancer. However, most women did not know which method was used in their last screening test. This was the case even though information campaigns in mass media and at health centers had been carried out prior to the initiation of the implementation project of hrHPV as primary screening method, suggesting that the information strategy did not fully succeed in enlightening the women targeted for screening. This should be taken into consideration when interpreting these results.
Half of the participants in the present study said they would seek screening tests more often than every year if the programme's screening interval were to be expanded from 3 to 5 years. This may reflect women's fear that a 5‐year interval might be unsafe. However, to expand the screening interval from 3 to 5 years using hrHPV testing in primary screening is considered safer than a 3‐year interval with conventional primary cytology screening according to different studies.18, 35 However, this fact is not currently known among women targeted by the national cervical cancer screening programme in Norway. Our findings are in line with those from a modeling study in Australia. 36 The Australian study indicated that extended screening intervals when adopting hrHPV testing as primary screening method is expected to lead to fluctuation in screening participation of about ±50% in the first 5‐year period. A screening programme where over half of the women seek opportunistic screening would entail unnecessary use of healthcare services and costs. Therefore, further efforts are needed to provide women with accurate and understandable information about the HPV infection and the safely of expanding screening intervals with the introduction of hrHPV testing in primary screening.18, 25 Information must necessarily be tailored to match women's needs to be effective.37 Moreover, knowledge has the potential to reduce negative psychological outcomes.38, 39, 40 Interestingly, in our study women in the hrHPV arm had significantly more knowledge about their own screening test results than women in the cytology arm. This may be because women in the hrHPV arm who were hrHPV negative received an additional information letter from the CRN,3 including information about the method used and the extended screening interval. The difference in knowledge could also be because women who are tested with hrHPV and women who are hrHPV positive tend to seek information about screening test results more actively than women who are tested with cytology.12, 33 This may also indicate that the information given related to screening test results raised awareness about the screening procedures and outcomes.
Strengths and limitations
Our study has several limitations. The PHQ‐4 scale is a general tool for detecting symptoms of anxiety and depression; as such it does not include questions related to cervical cancer screening or its procedures. Women answered the questionnaire 4 months to 2 years after receiving their last screening result, and any anxiety and depression they might have felt closer in time to screening participation could have faded by the time they took part in the study. Another possible limitation in our study is non‐response bias, as individuals who chose to answer the questionnaire may differ from nonresponders. Nonresponse is unlikely to differ between arms but may have reduced the representability of the study's sample.
We did not find any significant associations in the study, but some RRR's were somewhat elevated for example, women in the hrHPV arm had a nonsignificant 14% increased risk of experiencing moderate/severe anxiety and depression scores as compared to women in the cytology arm (Table 2). Due to the limited number of study participants, we cannot entirely rule out that a difference exists but was not revealed due to lack of statistical power.
The entire questionnaire was piloted only among women working at the CRN, a select group likely more aware of health‐related issues than the general population. However, the women involved in the pilot were asked to try to look at the questionnaire as if they did not have their professional knowledge, and many good suggestions and subsequent changes were made as a result of this piloting process. Thus, we do not know whether some of the questions were difficult to understand among other women. The PHQ‐4 score was, however, a validated screening tool for measuring anxiety and depression.
Despite randomization, there were some differences between screening arms regarding marital status and birthplace, and these differences were therefore adjusted for in the two multinomial logistic regression models. Finally, the survey questionnaire was answered at a single point in time, and any changes in anxiety and depression over time were not assessed.
Prior to the onset of the hrHPV pilot project, information campaigns regarding HPV had been delivered in mass media and at doctors’ offices, and additional information was delivered from the CRN to women randomized into the hrHPV testing arm only. This may have contributed to raise the level of knowledge among women in the hrHPV testing arm, thus making these women more prepared to deal with an abnormal screening test result than women in the cytology arm. This might at least partially explain the difference in knowledge we found between arms in our study.
The study also has several strengths. It was conducted on a large sample, and anxiety and depression scores were measured in a real‐life situation (and not only hypothetically as in the previously published study.9) Women with positive screening results were oversampled by design, which might have led to higher anxiety and depression scores compared to that in the general population. However, the lifetime occurrence of anxiety and depression among participants in our study are fairly similar to the prevalence of anxiety and depression found among Norwegian women.
Conclusions
In our study, we have compared long‐term (4–24 months after the screening) anxiety and depression scores between women 34 years and older undergoing primary cervical cancer screening with cytology and hrHPV testing. We found no differences in anxiety and depression scores between the two groups measured from 4 months to 2 years after women had received their last screening test result. We conclude that there are no indications that screening participation will be adversely affected by anxiety and depression nor that hrHPV testing in primary screening would make women have more anxious and/or depressed symptoms than conventional cytology screening.
Supporting information
Appendix S1: Kvinners erfaringer med screening mot livmorhalskreft
Supplementary Table 1 Multinomial logistic regression for anxiety and depression scores among 966 women (496 in the cytology and 470 in the hrHPV arm) with different screening test results, measured between 4 months and 1 year and between 1 and 2 years since women had received their last screening test results. Reporting Relative Risk Ratios (RRR)
Supplementary Table 2. Multinomial logistic regression for anxiety scores only and depression scores only among 966 women (496 in the cytology an and 470 in the hrHPV arm) with different screening test results, reporting Relative Risk Ratios (RRR)
Acknowledgements
The authors would like to thank the CRN for financing our study and Ms Gry Baadstrand Skare for data management assistance.
Footnotes
In Norway, all citizens are given a unique personal identification number when born. To ensure the anonymity of the study participants, we did not use this personal identification number on the questionnaires, rather a unique ID number.
In addition, it is also possible to receive inconclusive cytology and hrHPV test result.
Atypical squamous cells of undetermined significance (ASC‐US).
Low‐grade squamous Intraepithelial lesions (LSIL).
The high percentage of abnormal screening results is due to oversampling women with positive test results in the study cohort.
References
- 1. Nygård JF, Skare GB, Thoresen SO. The cervical cancer screening programme in Norway, 1992‐2000: changes in pap smear coverage and incidence of cervical cancer. J Med Screen 2002;9:86–91. [DOI] [PubMed] [Google Scholar]
- 2. Haldorsen T, Skare GB, Ursin G, et al. Results of delayed triage by HPV testing and cytology in the Norwegian Cervical Cancer Screening Programme. Acta oncologica (Stockholm, Sweden) 2015;54:200–9. [DOI] [PubMed] [Google Scholar]
- 3. Tropè A, Engesæter B, Nygård M, et al. Trygg implementering av HPV‐testing i Livmorhalsprogrammet. [Safe implementation of HPV testing in the Norwegian Cerical Cancer screening Programme]. Tidsskr Nor Laegeforen 2017;137. [DOI] [PubMed] [Google Scholar]
- 4.Saker behandlet av Prioriteringsrådet 2007–2017 [Cases dealt with by the National Council for Priority Setting 2007–2017]. The Norwegian Directorate of Health, 2018;137. Available at https://helsedirektoratet.no/prioritering/saker-behandlet-av-prioriteringsradet-2007-2017 Accessed August 31, 2018. doi:10.4045/tidsskr.17.0306
- 5. Kroenke K, Spitzer RL, Williams JB, et al. An ultra‐brief screening scale for anxiety and depression: the PHQ‐4. Psychosomatics 2009;50:613–21. [DOI] [PubMed] [Google Scholar]
- 6. Demyttenaere K, Bruffaerts R, Posada‐Villa J, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization world mental health surveys. JAMA 2004;291:2581–90. [DOI] [PubMed] [Google Scholar]
- 7. Ansseau M, Dierick M, Buntinkx F, et al. High prevalence of mental disorders in primary care. J Affect Disord 2004;78:49–55. [DOI] [PubMed] [Google Scholar]
- 8. Bang Nes R, Clench‐Aas J. Psykisk helse i Norge: Tilstandsrapport med internasjonale sammenligninger [mental health in Norway: condition report with international comparisons]. Oslo: Folkehelseinstituttet, Norwegian Institue of Public Health, 2011. Available at www.fhi.no. [Google Scholar]
- 9. Reneflot A, Aarø L, Aase H, et al. Psykisk helse i Norge [Mental health in Norway]. Oslo, Norway: Folkehelseinstituttet, Norwegian Institute of Public Health, 2018. Available at www.fhi.no. [Google Scholar]
- 10. Eardley A, Elkind AK, Spencer B, et al. Attendance for cervical screening—Whose problem? Soc Sci Med 1985;20:955–62. [DOI] [PubMed] [Google Scholar]
- 11. Burger EA, Nygard M, Gyrd‐Hansen D, et al. Does the primary screening test influence women's anxiety and intention to screen for cervical cancer? A randomized survey of Norwegian women. BMC Public Health 2014;14:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Connor M, Costello L, Murphy J, et al. I don't care whether it's HPV or ABC, I just want to know if I have cancer.’ Factors influencing women's emotional responses to undergoing human papillomavirus testing in routine management in cervical screening: a qualitative study. BJOG 2014;121:1421–9. [DOI] [PubMed] [Google Scholar]
- 13. McCaffery K, Waller J, Nazroo J, et al. Social and psychological impact of HPV testing in cervical screening: a qualitative study. Sex Transm Infect 2006;82:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCaffery K, Waller J, Forrest S, et al. Testing positive for human papillomavirus in routine cervical screening: examination of psychosocial impact. BJOG 2004;111:1437–43. [DOI] [PubMed] [Google Scholar]
- 15. Kwan TT, Cheung AN, Lo SS, et al. Psychological burden of testing positive for high‐risk human papillomavirus on women with atypical cervical cytology: a prospective study. Acta Obstet Gynecol Scand 2011;90:445–51. [DOI] [PubMed] [Google Scholar]
- 16. Maissi E, Marteau TM, Hankins M, et al. Psychological impact of human papillomavirus testing in women with borderline or mildly dyskaryotic cervical smear test results: cross sectional questionnaire study. BMJ 2004;328:1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engesæter B, Nygård M, Tropè A. Implentering av HPV‐test i primærscreening [implementing HPV test in primaryscreening]. Oslo, Norway: The Cancer Registry of Norway, 2017. [Google Scholar]
- 18. Ronco G, Dillner J, Elfstrom KM, et al. Efficacy of HPV‐based screening for prevention of invasive cervical cancer: follow‐up of four European randomised controlled trials. Lancet (London, England) 2014;383:524–32. [DOI] [PubMed] [Google Scholar]
- 19. Cancer Registry of Norway . Regulation on the collection and processing of personal health data in the Cancer Registry of Norway (Cancer Registry Regulations) [January 2013 update]. Oslo, Norway; Cancer Registry of Norway, 2001. Available at: www.ub.uio.ujur. http://app.uio.no/ujur/oversatte-lover/data/for-20011221-1477-eng.pdf. Accessed April 20, 2018.
- 20. Löwe B, Wahl I, Rose M, et al. A 4‐item measure of depression and anxiety: validation and standardization of the patient health Questionnaire‐4 (PHQ‐4) in the general population. J Affect Disord 2010;122:86–95. [DOI] [PubMed] [Google Scholar]
- 21. Spitzer R, Kroenke K, Williams J, et al. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Arch Intern Med 2006;166:1092–7. [DOI] [PubMed] [Google Scholar]
- 22. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitchell AJ, Coyne J. Do ultra‐short screening instruments accurately detect depression in primary care? A pooled analysis and meta‐analysis of 22 studies. Br J Gen Pract. 2007;57:144–51. [PMC free article] [PubMed] [Google Scholar]
- 24. Ideström M, Milsom I, Andersson‐Ellstrom A. Knowledge and attitudes about the pap‐smear screening program: a population‐based study of women aged 20‐59 years. Acta Obstet Gynecol Scand 2002;81:962–7. [DOI] [PubMed] [Google Scholar]
- 25. Anhang R, Goodman A, Goldie SJ. HPV communication: review of existing research and recommendations for patient education. CA Cancer J Clin 2004;54:248–59. [DOI] [PubMed] [Google Scholar]
- 26. Hosmer L. Applied logistic regression. New York, NY: John Wiley & Sons, 2000. [Google Scholar]
- 27. Cancer Registry of Norway . Triage algorithm. The Cancer Registry, Oslo, Norway: available at: https://www.kreftregisteret.no/screening/livmorhalsprogrammet/Helsepersonell/HPV-i-primarscreening/. Accessed September3, 2018.
- 28. Basen‐Engquist K, Fouladi RT, Cantor SB, et al. Patient assessment of tests to detect cervical cancer. Int J Technol Assess Health Care 2007;23:240–7. [DOI] [PubMed] [Google Scholar]
- 29. Kitchener HC, Almonte M, Gilham C, et al. ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening. Health Technol Assess (Winchester, England) 2009;13:1–150. iii‐iv. [DOI] [PubMed] [Google Scholar]
- 30. Rask M, Swahnberg K, Lindell G, et al. Women's experiences of abnormal pap smear results ‐ a qualitative study. Sex Reprod Healthc 2017;12:3–8. [DOI] [PubMed] [Google Scholar]
- 31. Markovic‐Denic L, Popovac S, Djuric O, et al. Psychological effects of concurrent cytology and colposcopy testing in women referred to cancer counseling outpatient clinic in Belgrade. J. BUON 2017;22:214–23. [PubMed] [Google Scholar]
- 32. Maissi E, Marteau TM, Hankins M, et al. The psychological impact of human papillomavirus testing in women with borderline or mildly dyskaryotic cervical smear test results: 6‐month follow‐up. Br J Cancer 2005;92:990–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hendry M, Pasterfield D, Lewis R, et al. Are women ready for the new cervical screening protocol in England? A systematic review and qualitative synthesis of views about human papillomavirus testing. Br J Cancer 2012;107:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waller J, Marlow LA, Wardle J. The association between knowledge of HPV and feelings of stigma, shame and anxiety. Sex Transm Infect 2007;83:155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arbyn M, Anttila A, Jordan J, et al. European guidelines for quality Assurance in Cervical Cancer Screening. Second edition‐‐summary document. Ann Oncol 2015;21:448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith MA, Gertig D, Hall M, et al. Transitioning from cytology‐based screening to HPV‐based screening at longer intervals: implications for resource use. BMC Health Serv Res 2016;16:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bensing JM, Verhaak PF, van Dulmen AM, et al. Communication: the royal pathway to patient‐centered medicine. Patient Educ Couns 2000;39:1–3. [DOI] [PubMed] [Google Scholar]
- 38. O'Connor M, Costello L, Murphy J, et al. Influences on human papillomavirus (HPV)‐related information needs among women having HPV tests for follow‐up of abnormal cervical cytology. J Fam Plann Reprod Health Care 2015;41:134–41. [DOI] [PubMed] [Google Scholar]
- 39. Rosen NO, Knauper B, Di Dio P, et al. The impact of intolerance of uncertainty on anxiety after receiving an informational intervention about HPV: a randomised controlled study. Psychol Health 2010;25:651–68. [DOI] [PubMed] [Google Scholar]
- 40. Williams‐Piehota P, Schneider TR, Pizarro J, et al. Matching health messages to information‐processing styles: need for cognition and mammography utilization. Health Commun 2003;15:375–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Kvinners erfaringer med screening mot livmorhalskreft
Supplementary Table 1 Multinomial logistic regression for anxiety and depression scores among 966 women (496 in the cytology and 470 in the hrHPV arm) with different screening test results, measured between 4 months and 1 year and between 1 and 2 years since women had received their last screening test results. Reporting Relative Risk Ratios (RRR)
Supplementary Table 2. Multinomial logistic regression for anxiety scores only and depression scores only among 966 women (496 in the cytology an and 470 in the hrHPV arm) with different screening test results, reporting Relative Risk Ratios (RRR)


