Summary
The ability to monitor micropipette injections with a high-resolution fluorescent microscope has utility for a variety of applications. Herein, different approaches were tested for creating broad-band fluorescently labelled glass micropipettes including: UV cured glass glues, baked glass enamel containing fluorescent dyes as well as nanodiamonds attached during pipette formation in the microforge. The most robust and simplest approach was to use labelled baked enamel on the exterior of the pipette. This approach was tested using pipettes designed to mimic a mosquito proboscis for the injection of the malaria parasite, Plasmodium spp., into the dermis of a living mouse ear. The pipette (~30 micron diameter) was easily detected in the microscopy field of view and tolerated multiple insertions through the skin. This simple inexpensive approach to fluorescently labelling micropipettes will aid in the development of procedures under the fluorescent microscope.
Keywords: Intravital imaging, malaria, sporozoites, 2-photon excitation, mouse, skin
Introduction
The glass micropipette is a basic tool in numerous types of microscopy studies: injection of drugs, markers, and cells as well as cellular manipulations. A particularly challenging procedure is to conduct intravital micropipette manipulations under microscopic guidance, because the optical monitoring is limited by the optical density of the tissue. Intravital confocal or multiphoton fluorescence techniques are utilised for numerous applications in cancer biology, neuroscience, cell physiology and infectious disease. However, the typical glass micropipette is undetectable with fluorescence, making positioning under the microscope difficult. Previous studies have tried to solve this problem with quantum dot coated micropipettes (Andrasfalvy et al., 2014). However, this method has proved limited as it requires specialised chemistry and the quantum dots can be partially lost during insertion into tissue. We sought to improve upon this technique to make a more robust, inexpensive and simple method for a diverse range of laboratory applications. To this end, we undertook a broad survey of methods for labelling glass micropipettes with fluorescent probes commonly used for intravital studies and compatible with multiphoton/confocal microscopy.
In our case, these experiments were driven by the goal of mimicking mosquito inoculation of malaria parasites into a mouse and observing these parasites directly after inoculation. Previous intravital imaging of the malaria parasite utilised multiple intradermal injections of parasites with a commercial microsyringe (Nanofil syringe #nanofil with 36-gauge needle, # NF36BV; World Precision Instruments, Sarasota, FL, USA) (Amino et al., 2006; Hopp et al., 2015). These needles are about five times larger than the mosquito proboscis (Fig. 1), and even with small volumes of inoculation, such as 0.2 μL, a nonphysiological amount of fluid is injected into the tissue, which is likely to impact tissue architecture and parasite behaviour. Temporal information is also lost with this technique as injections are conducted prior to placing the mouse on the microscope stage, necessitating searching for injection sites with the microscope and, resulting in a delay between injection and imaging (Amino et al., 2006; Hopp et al., 2015). The goal of this study was to use appropriately sized fluorescently labelled glass micropipette for the injection, localise the injection site within the field of view (FOV) of the fluorescent scope, and then directly observe the injection and dissemination of the parasites within the skin. Although our focus was on the application of this approach for understanding parasite transmission in the skin, this simple, inexpensive, reusable, and versatile fluorescent glass micropipette can be used to target injections to a specific location and simultaneously visualise injections using fluorescent excitation microscopy providing functionality in a breath of fields as described in the discussion.
Fig. 1.
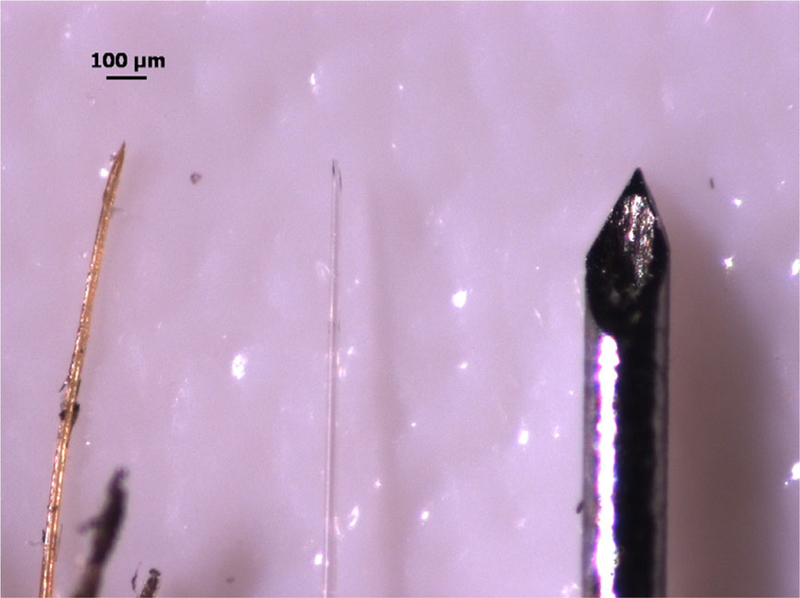
Mimicking the mosquito proboscis with glass pipettes. From left to right: mosquito proboscis, engineered glass pipette, and 36G needle previously used for intradermal inoculations. Scale bar = 100 μm.
Methods and experimental results
Labelling strategies
We sought to attach or incorporate fluorescent moieties to glass micropipettes. Three strategies were evaluated for labelling glass pipettes: fluorescent diamond incorporation into molten glass, ultraviolet (UV) curing glues, and heat curing enamels.
Fluorescent nanodiamonds
Fluorescent nanodiamonds are an attractive fluorescent agent due to their high fluorescent efficiency as well as nonbleaching, nonblinking behaviour (Yu et al., 2005). Diamonds (80–90 nm diameter) for this study were obtained from Adamas Nanotechnologies with no chemical modifications. Difficulty arose in getting the diamonds to a sufficient density to use in a suspension of glue or enamel for application to the pipette. Because the diamonds are relatively heat stable we attempted another strategy, namely to attach the diamonds to the glass in the molten state. For this method, diamonds were concentrated in water and loaded into a microcapillary tube by drawing the diamond solution into the capillary tube. Tubes with diamond solution were then dried at 100 °C for several days until all water evaporated leaving a diamond coating on the glass. The microforge element was placed over the dried diamond region within the pipette and pulled on a standard microforge (microforge setting and glass type below). In the molten state the diamonds attached to the glass and were retained after vigorous washings with distilled water that removed the diamonds in the nonheated region of the pipette. This resulted in good but inconsistent internal labelling of the pipette as judged by confocal microscopy using 638 nm excitation and 2-photon microscopy using 980 nm excitation (Fig. 2). Thus, this approach might be most applicable for very small glass pipettes or microelectrodes where the probe efficiency is critical for detection, or where internal labelling is desired. This approach was not developed further for these pipettes.
Fig. 2.
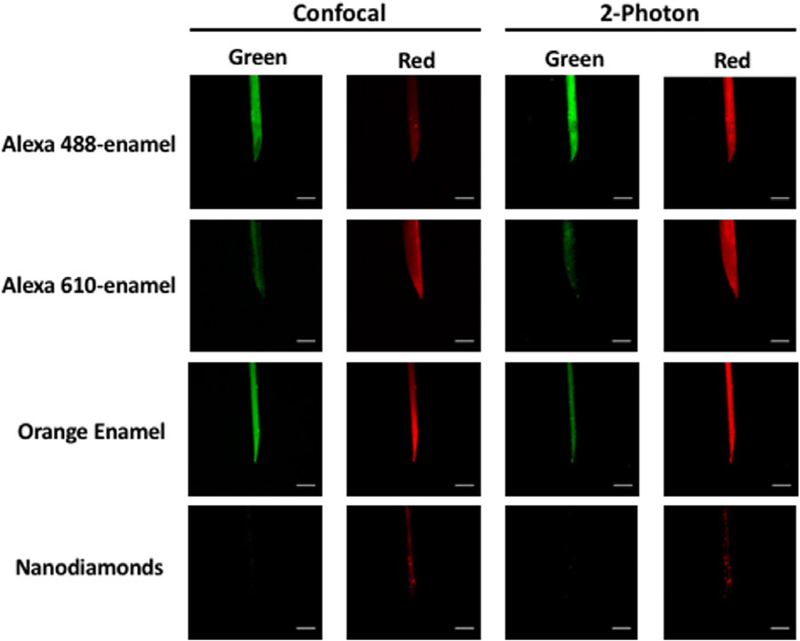
Fluorescently labelled pipettes. Representative labelled pipettes imaged by confocal and 2-photon microscopy. Pipettes are labelled with either Alexa Fluor 488 or 610 in enamel, orange-enamel (1:50 dilution in clear enamel), or nanodiamonds. For confocal imaging, Alexa 488-enamel pipette was excited at 488 nm, whereas Alexa 610-enamel, orange-enamel and nanodiamond pipettes were excited at 638 nm. For 2-photon imaging, all pipettes were imaged using 980nm excitation. Emission spectra for all excitations were collected in the green (523–593 nm confocal; 500–528 nm 2-photon) and red (695–765 nm confocal, 555–694 nm 2-photon) channels. Scale bar = 50 μm.
UV curable glues
UV curable glues are used to bond glass structures via a UV catalysed photoinitiator that generates local free radicals to initiate a polymerisation process (Goss, 2002). It was assumed that this approach could provide a robust attachment between the trapped fluorophore and the outer surface of the pipette (Goss, 2002). Using UV curable glues varying from low (C.R. Laurence #UV601) to medium (C.R. Laurence #UV604L25) viscosities, as well as different UV curing conditions involving manipulation of coating methods, UV light source and coating times, we did not find these agents to provide a robust hard coating on the pipettes. In addition, the UV curing bleached many of the fluorophores mixed into the glue. For these reasons this approach was abandoned.
Heat cured enamel
Heat cured enamels are commonly used to paint glass. We tried existing coloured enamel that had intrinsic fluorescent properties as well as clear enamel to which we added well-characterised fluorophores. These enamels were then cured by heating the coated micropipette (190 °C for 45 min). Overall, we found that this method was simple, gave uniform robust coating, was inexpensive and enabled a wide dynamic range of fluorescent emissions. It is described in more detail below.
Fluorescent enamel
Clear enamel (DecoArt, #DS89–30, Stanford, KY, USA) was mixed with Alexa 488 (ThermoFisher, #A20000, Waltham, MA, USA), 514 (ThermoFisher, #A30002), 532 (ThermoFisher, #A20001) or 610 (ThermoFisher, #A30050) succinimidyl ester dyes that were pretreated to cleave the ester bonds. These dyes are commercially available as hydrophobic esters for lipid membrane permeation that reduce maximum water solubility. To increase water permeability, the ester bonds were cleaved by hydrolysing 1 mg dye in 0.5 mL 0.1 M HCl followed by 0.5 mL 0.1 M NaOH to neutralise the pH. Ester cleaved Alexa dyes were added to clear enamel (DecoArt, #DS89–30) at a 1:1 ratio by volume and vortexed, resulting in a 0.5 mg dye/mL enamel:water. Enamel-Alexa solutions were stored at room temperature in the dark.
Some coloured enamel paints have intrinsic fluorescence. The best of those tested was the orange paint from the DecoArt Glass Paint Marker Multi-Pack (#13443247). It was brightly fluorescent and demonstrated a broad range of excitation and emission spectra (Fig. S1). The orange enamel paint was diluted 1:50 or 1:100 in clear enamel paint prior to coating the pipettes to optimise fluorescence intensity.
Pipette coating with enamels
Pipettes were pulled from borosilicate glass capillary tubes (Sutter Instruments, #BF100–50-15, Novato, CA, USA) using a microforge (Sutter Instruments, #P-9 7) at the following settings: Heat = 666, Pull = 30, Velocity = 120, Time = 200, Pressure = 200. Pulled pipettes were lightly coated in the different enamel mixtures by gently rolling the tip of the pipette in the paint. Coated pipettes were baked at 190 °C for 45 min using a conventional oven and then allowed to cool at room temperature.
After cooling, the coated pipettes were bevelled as previously described (Canfield, 2006). Briefly, pipettes were held at a 20° angle using a micromanipulator and brought down to a spinning hard drive that had been repurposed by covering the hard drive with silicon carbide coated paper (Norton T402, Worcester, MA). A 20° angle was found to be best suited for injection into the skin; however, other bevel angles can easily be made. Pipette tip size was confirmed to have a bevelled opening between 60–65 μm and 30 μm wide, which was comparable to the mosquito proboscis and significantly smaller than the previously used 36-gauge needle (Fig. 1). The robust nature of the coating was also demonstrated by the ability of the coated pipette to be bevelled with no apparent damage to the fluorescent coating.
Pipettes coated with the heat cured enamels were imaged by confocal and 2-photon microscopy (Figs. 2, S2). In confocal, Alexa 488-enamel was excited using 488 nm excitation, Alexa 514-, 532- and 555-enamels were exited at 514 nm excitation, and Alexa 610-enamel as well as the commercially available orange enamel were excited using 638 excitation. In 2-photon, 980 nm excitation was used for all fluorophores. Emission in both the green (523–593 nm confocal; 500–528 nm 2-photon) and red (695–765 nm confocal, 555–694 nm 2-photon) spectra were collected. Although for our purposes, confocal and 2-photon visualisation were desired, it is also possible to visualise fluorescent pipettes through the oculars in conventional single photon excitation or dissecting scopes with the proper filter set (Fig. S3).
To confirm durability of the fluorescent coating on the pipettes, we inserted them into the dermis of a mouse ear 10 times monitoring the integrity of the coating (clear enamel with Alexa 514) between each trial. As seen in Figure 3, the coating was modestly compromised by the insertions and was easily detected in the tissue after 10 trials. This effect of multiple insertions in the skin was estimated from the change in fluorescence intensity as a function of 10 individual injections in Figure S4A. Some loss (~40%) in fluorescence was observed that likely had multiple sources: (1) damage to the coating, (2) debris from the dermis attached to the pipette as we did not wash the pipette after each injection to attempt to keep the same optical plane, but debris was observed by eye and in the heterogeneity of the fluorescence on the pipette and (3) photobleaching of the probe during the observations. Photobleaching over 10 min of continuous 2-photon excitation reduced the fluorescence intensity by ~50% (Fig. S4B). Thus, some of the decline in fluorescence signal after multiple injections could be due to unavoidable fluorescence bleaching. The loss of signal with usage did not impact the repeated use of pipettes as the signal to noise was well above the utility threshold, even after 10 insertions into the skin. Pipettes were usually abandoned due to breakage before inadequate fluorescence signal was realised.
Fig. 3.
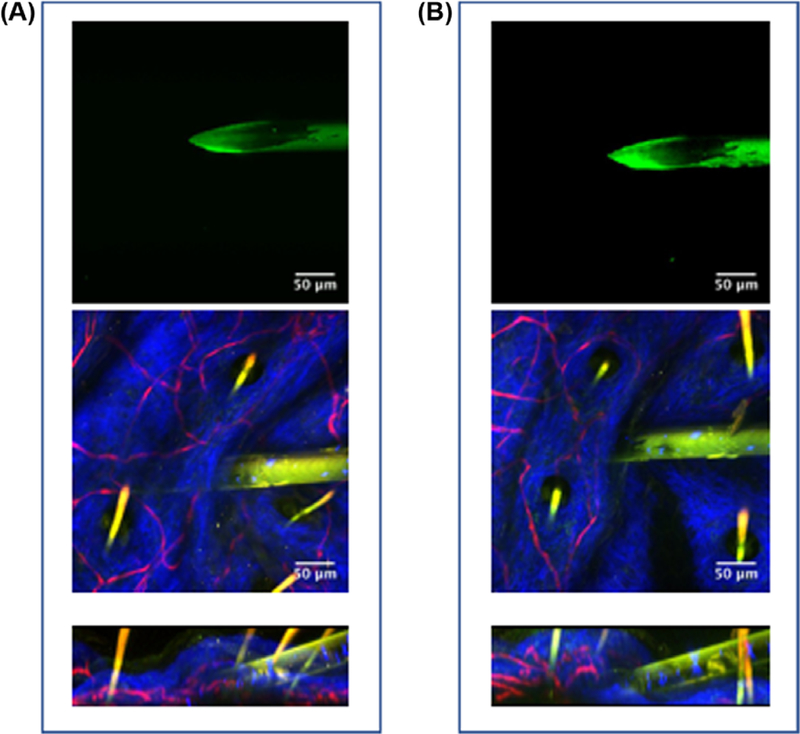
Durability of fluorescent labelling. (A) Two-photon image of Alexa 514-enamel coated pipette before injections into skin (top) and during 1st injection into skin (XY and XZ planes, middle and bottom panels, respectively). (B) 2-photon image of same pipette after ten injections into skin (top) and during the 10th injection into skin (XY and XZ planes, middle and bottom panels, respectively).
Mice and parasites
Fms-like tyrosine kinase-dsRed (Flt:dsRed) mice from Jackson Labs (strain 400513), which have previously been shown to have fluorescently labelled blood vessels, were used for these studies (Matsumoto et al., 2012). During experiments mice were kept under isoflurane anaesthesia using the Narishinge SGM-4 (Tokyo, Japan) head holder system on top of a 37 °C water heated surgical bed (E-Z Anesthesia, EZ-211; Palmer, PA, USA) and monitored throughout the course of the experiment. Studies were conducted following animal research protocol NHLBI H-0299.
Transgenic parasites expressing green fluorescent protein (GFP) under the hsp70 promoter (Amino et al., 2008) were used for intravital imaging. The sporozoite stage of the parasite was generated in Anopheles mosquitoes as previously described (Vanderberg & Gwadz, 1980). Prior to imaging, mosquito salivary glands were dissected in Lebowitz media (L-15, Sigma #L4386), homogenised to release sporozoites and then subject to two 100 × g spins at 4 °C to remove mosquito debris. The sporozoite suspension was then loaded into the pipette by negative pressure utilising a hand pump (Pneumatic Microinjector, #IM-11–2; Narishige, Tokyo, Japan).
Parasite injection
To demonstrate the utility of this approach we attempted to monitor the initial inoculation of fluorescently labelled sporozoites using a 2-photon microscope. Injections were performed under the objective (25 × 1.1 NA water emersion) of the 2-photon microscope (Leica DM 6 CFS: Wetzlar, Germany), using a customised stage with an attached custom designed micromanipulator and commercial mouse head holder/anaesthesia system (Narishinge, SGM-4: Tokyo, Japan). A dissecting microscope (Leica M80: Wetzlar) on a swinging arm was used to guide the micropipette to the focal column of the microscope as well as guide the initial stages of the insertion (Fig. 4A). Illumination of the stage for the dissecting scope and reflected light for initial positioning was provided by a flexible light source (Heine HL 1200: Herrsching, Germany). The mouse ear was fixed to the head holder by use of adhesive putty on top of the ear pins (Fig. 4B). Bevelled pipettes preloaded with sporozoite suspension were mounted on the micromanipulator such that the needle was at a 20° angle with the dermis with the bevel facing up (Fig. 4C). After dissecting scope guided penetration of the dermis by the bevelled pipette (Fig. 4D), the stage was brought up to the 2-photon objective. The transverse positioning of the pipette was adjusted initially by bright field through the oculars of the 2-photon using the flexible light source. Once observed to be in the proper location, 2-photon microscopy was utilised with collagen (via second harmonic generation: 470–488 nm) and blood vessels (fluorescence: 555–694 nm) as a guide for tissue depth, and the position of the pipette was finalised (Figs. 3 and 5). This hybrid injection method, using the dissecting scope to guide the initial gross localisation of the pipette, overcame the limitations of FOV in high-resolution fluorescence microscopy by assuring the alignment of the pipettes with focal plane of the high-resolution objective. Sporozoites were then injected utilising the same hand-pump used to load the pipette (Fig. 5, Movie S1) and monitored by their genetically inserted GFP (fluorescence: 500–528 nm).
Fig. 4.
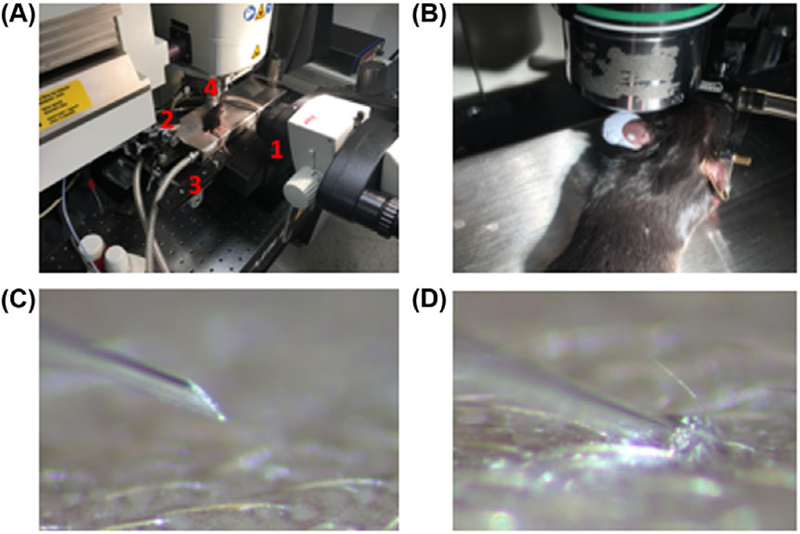
Injection system. (A) Dissecting scope injection system with: 1=dissecting scope, 2=pipette, 3=2-photon stage, and 4=2-photon objective. (B) Ear mounted for imaging using putty on ear pins of the mouse restrainer. (C) Observation of pipette through the dissecting scope just prior to penetration. (D) Observation of pipette through the dissecting scope after insertion into the skin.
Fig. 5.
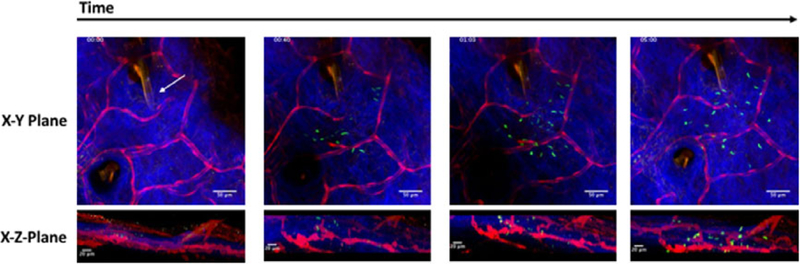
Sporozoite inoculation into dermis. Two-photon time series of injection into skin. The time series begins with pipette (coated in 1:50 orange enamel, indicated with white arrow) in the dermis before injection. Observation of sporozoite inoculation into dermis with just a few sporozoites trickling into the tissue at40s followed by bulk injection of sporozoites. XY maximum projection shown in top panel with corresponding XZ maximum projection shown beneath. Red channel = vasculature and pipette; blue channel = collagen; green channel = parasites and pipette. Timestamp in upper left corner. Scale bar in top panels = 50 μm; scale bar in bottom panels = 20 μm.
As seen in Figures 5 and 3, the micropipette tip was placed in the collagen layer of the skin, which was used as a marker for the dermis, the site mosquito inoculation. This was accomplished using the outline of the pipette which was readily identified with its fluorescent coating, with dye dilutions optimised to not overwhelm the other chromophores. Naturally, this dilution will depend on a given application. An example of the time course inoculation of sporozoites is shown in Figure 5 and Movie S1, which illustrates injection into the desired tissue space. As observed in Figure 5 and Movie S1, sporozoites were alive and motile after inoculation with all parasite movement after 01:26 on the time stamp attributed to active parasite motility and not from propulsion by the pipette injection. Parasites were found to exhibit expected peak velocities (approximately 6 μm/s) as well as invasion of blood vessels demonstrating parasite viability.
Discussion
We have developed a method for generating fluorescently labelled micropipettes. The fluorescent labelling of the pipettes is durable and withstand multiple rounds of insertion into tissue (Fig. 3). Additionally, pipettes can be tailored to any desired size or shape, and can be fluorescently labelled for a wide array of applications. Here we validate our system utilising malaria parasites, which are normally inoculated into the dermal layer of the skin by an infected mosquito. Our fluorescent micropipette system proved optimal for mimicking a mosquito bite into the dermis and significantly improved procedures for intravital imaging of these parasites in the skin (Figs. 1 and 5). Using these fluorescent pipettes, we can now precisely monitor the depth and position of the inoculation, and observe the behaviour of the parasites immediately upon their injection, providing useful information on the earliest stages of infection.
We explored several methods to fluorescently label glass pipettes so that they could be monitored during microscopy-guided procedures. Of these methods, we found the heat-treated glass enamels were robust, technically easy and versatile. The fluorescent labelling was accomplished using commercially coloured enamels or with the addition of Alexa dyes that maintained their fluorescence in the baked enamel on the pipette tips. It is likely that other optical dyes would also be suitable and retain their optical properties embedded in the enamel cement. The fluorescence efficiencies appeared to be similar in the enamel along with the photobleaching properties of the dye.
While our interests were focused on the malaria parasite, many other infectious disease studies will benefit from utilisation of the described pipette system. Other pathogens transmitted by insect vectors such as Leishmania spp. (parasite responsible for leishmaniasis), Borrelia spp. (bacteria responsible for Lyme disease), and dengue (virus responsible for dengue fever) can now be studied in vivo at the instant of inoculation into the skin. Studying vector borne diseases in this fashion will expand our understanding of transmission and dissemination of these pathogens, enabling the design of new vaccines and control mechanisms. Nonvector borne pathogens will also benefit from this approach due to enhanced control of inoculum. Additionally, this technique will prove valuable for the neuroscience and cancer biology communities. Using the fluorescent pipettes created herein, one can target a drug, compound, dye or cell suspension to an area of interest while under direct microscopic observation, permitting instantaneous observation of behaviour or response to inoculated elements. The capacity to do so provides an opening to answer yet unexplored temporal and special questions in these fields.
Supplementary Material
Emission spectra of orange enamel. Emission spectra of the DecoArt orange enamel (#13443247) measured with a Shimadzu spectro fluorophometer (#RF-6000).
Fluorescently labelled pipettes. Two-photon microscopy of Alexa 514-, 532-, 555-enamel coated pipettes using optimal emission spectra: 500–528 nm for Alexa 514-enamel, 555–694 nm for Alexa 532- and 555-enamels. Scale bars = 50 μm
Visualisation of fluorescent pipettes under dissecting scope. Paint-enamel coated pipette excited withmercury bulb and FITC filter set. Scale bar = 50 μm
Quantification of fluorescence durability and bleaching. (A) An Alexa 532-enamel coated pipette was injected into the dermis of a mouse ear 10 times. Before any injections (injection 0) and following every injection, the pipette was imaged using 2-photon excitation as described in the methods. An ROI was created, over the same area of the pipette, on a maximum projection and mean ± SD fluorescence intensity was quantified in ImageJ.
(B) Mean pixel intensity of one linear area along the length of an Alexa-488-enamel coated pipette throughout a 10-min time series of 2-photon excitation. Quantification was performed in ImageJ.
The movie beginswith the pipette (coated in 1:50 orange enamel) in the dermis before injection andis followed by a few sporozoites trickling into the tissue at 40 s, then injection ofmost of the sporozoites. Stills from this movie were used in Figure 5. Red channel=vasculature and pipette; blue channel = collagen; green channel = parasites and pipette. Timestamp in upper left corner. Scale bar = 50 μm.
Acknowledgements
We are grateful to the Insectary and Parasitology Core Facilities at the Johns Hopkins Malaria Research Institute, expertly staffed by Chris Kizito, Godfree Mlambo and Abai Tripathi, and to the Bloomberg Family Foundation for their support of this facility. We are also grateful to Dr. Fidel Zavala of the Johns Hopkins Malaria Research Institute for sharing parasite lines used in these studies. This study was supported by a fellowship from the Johns Hopkins Malaria Research Institute (AB) and National Institutes of Health grant R01AI056840 (PS). We are grateful to Dr. Nihal Altan-Bonnet of the National Heart Blood and Lung Institute for support with mouse work and reading of the manuscript. We would also like to thank Bertrand Lucotte for his technical expertise and generous assistance with the 2-photon microscope.
Footnotes
Supporting Information
Additional Supporting informationmay be found in the online version of this article at the publisher’s website:
References
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F & Ménard R (2006) Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 12, 220–224. [DOI] [PubMed] [Google Scholar]
- Amino R, Giovannini D, Thiberge S, et al. (2008) Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3, 88–96. [DOI] [PubMed] [Google Scholar]
- Andrásfalvy BK, Galinanes GL, Huber D, et al. (2014) Quantum dot-based multiphoton fluorescent pipettes for targeted neuronal electrophysiology. Nat. Meth. 11, 1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield JG (2006) Dry beveling micropipettes using a computer hard drive. J. Neurosci. Meth. 158, 19–21. [DOI] [PubMed] [Google Scholar]
- Goss B (2002) Bonding glass and other substrates with UV curing adhesives. Int. J. Adhes. Adhes. 22, 405–408. [Google Scholar]
- Hopp CS, Chiou K, Ragheb DR, Salman AM, Khan SM, Liu AJ & Sinnis P (2015) Longitudinal analysis of Plasmodium sporozoite motility in the dermis reveals component of blood vessel recognition. eLife 4, e07789, 10.7554/eLife.07789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Azami T, Otsu A, et al. (2012) Study of normal and pathological blood vessel morphogenesis in Flt1-tdsRed BAC Tg mice. Genesis 50, 561–571. [DOI] [PubMed] [Google Scholar]
- Vanderberg JP & Gwadz RW (1980) The transmission by mosquitoes of plasmodia in the laboratory. Malaria 2, 153–234. [Google Scholar]
- Yu SJ, Kang MW, Chang HC, Chen KM & Yu YC (2005) Bright fluorescent nanodiamonds: no photobleaching and low cytotoxicity. J. Am. Chem. Soc. 127, 17604–17605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Emission spectra of orange enamel. Emission spectra of the DecoArt orange enamel (#13443247) measured with a Shimadzu spectro fluorophometer (#RF-6000).
Fluorescently labelled pipettes. Two-photon microscopy of Alexa 514-, 532-, 555-enamel coated pipettes using optimal emission spectra: 500–528 nm for Alexa 514-enamel, 555–694 nm for Alexa 532- and 555-enamels. Scale bars = 50 μm
Visualisation of fluorescent pipettes under dissecting scope. Paint-enamel coated pipette excited withmercury bulb and FITC filter set. Scale bar = 50 μm
Quantification of fluorescence durability and bleaching. (A) An Alexa 532-enamel coated pipette was injected into the dermis of a mouse ear 10 times. Before any injections (injection 0) and following every injection, the pipette was imaged using 2-photon excitation as described in the methods. An ROI was created, over the same area of the pipette, on a maximum projection and mean ± SD fluorescence intensity was quantified in ImageJ.
(B) Mean pixel intensity of one linear area along the length of an Alexa-488-enamel coated pipette throughout a 10-min time series of 2-photon excitation. Quantification was performed in ImageJ.
The movie beginswith the pipette (coated in 1:50 orange enamel) in the dermis before injection andis followed by a few sporozoites trickling into the tissue at 40 s, then injection ofmost of the sporozoites. Stills from this movie were used in Figure 5. Red channel=vasculature and pipette; blue channel = collagen; green channel = parasites and pipette. Timestamp in upper left corner. Scale bar = 50 μm.


