Abstract
BACKGROUND
Thrombectomy is currently recommended for eligible patients with stroke who are treated within 6 hours after the onset of symptoms.
METHODS
We conducted a multicenter, randomized, open-label trial, with blinded outcome assessment, of thrombectomy in patients 6 to 16 hours after they were last known to be well and who had remaining ischemic brain tissue that was not yet infarcted. Patients with proximal middle-cerebral-artery or internal-carotid-artery occlusion, an initial infarct size of less than 70 ml, and a ratio of the volume of ischemic tissue on perfusion imaging to infarct volume of 1.8 or more were randomly assigned to endovascular therapy (thrombectomy) plus standard medical therapy (endovascular-therapy group) or standard medical therapy alone (medical-therapy group). The primary outcome was the ordinal score on the modified Rankin scale (range, 0 to 6, with higher scores indicating greater disability) at day 90.
RESULTS
The trial was conducted at 38 U.S. centers and terminated early for efficacy after 182 patients had undergone randomization (92 to the endovascular-therapy group and 90 to the medical-therapy group). Endovascular therapy plus medical therapy, as compared with medical therapy alone, was associated with a favorable shift in the distribution of functional outcomes on the modified Rankin scale at 90 days (odds ratio, 2.77; P<0.001) and a higher percentage of patients who were functionally independent, defined as a score on the modified Rankin scale of 0 to 2 (45% vs. 17%, P<0.001). The 90-day mortality rate was 14% in the endovascular-therapy group and 26% in the medical-therapy group (P = 0.05), and there was no significant between-group difference in the frequency of symptomatic intracranial hemorrhage (7% and 4%, respectively; P = 0.75) or of serious adverse events (43% and 53%, respectively; P = 0.18).
CONCLUSIONS
Endovascular thrombectomy for ischemic stroke 6 to 16 hours after a patient was last known to be well plus standard medical therapy resulted in better functional outcomes than standard medical therapy alone among patients with proximal middle-cerebral-artery or internal-carotid-artery occlusion and a region of tissue that was ischemic but not yet infarcted. (Funded by the National Institute of Neurological Disorders and Stroke; DEFUSE 3 ClinicalTrials.gov number, NCT02586415.)
Endovascular thrombectomy has been shown to be effective for the treatment of acute ischemic stroke in patients with occlusion of the first segment of the middle cerebral artery or occlusion of the internal carotid artery if treatment is initiated within 6 hours.1,2 The results of the recently reported DAWN (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo) trial showed that the time window for endovascular treatment may be extended to 24 hours after the patient was last known to be well if patients are carefully selected on the basis of a disproportionately severe clinical deficit in comparison with the size of the stroke on imaging.3 Other trials have suggested that computed tomographic (CT) perfusion imaging, as well as the combination of diffusion and perfusion magnetic resonance imaging (MRI), can estimate the volume of irreversibly injured ischemic tissue and the volume of brain tissue that is ischemic but not yet infarcted.4,5 These techniques may identify patients who will have a favorable response to endovascular reperfusion therapy, even when the therapy is initiated longer after the onset of stroke symptoms than is typical in clinical practice.6,7
The Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trial was designed to test the hypothesis that patients who were likely to have salvageable ischemic brain tissue as identified by perfusion imaging and who underwent endovascular therapy 6 to 16 hours after they were last known to have been well would have better functional outcomes than patients treated with standard medical therapy.
METHODS
TRIAL OVERSIGHT
The DEFUSE 3 trial was funded by the National Institutes of Health (NIH) through StrokeNet, a network of more than 300 U.S. hospitals. The trial was approved by the StrokeNet central institutional review board and the Food and Drug Administration (FDA) for an investigational device exemption (IDE G150028). The trial was designed by the first three authors and the last author. A steering committee provided guidance during monthly telephone conferences. Data management, oversight of site monitoring, and interim statistical analysis were performed by the NIH StrokeNet National Data Management Center at the Medical University of South Carolina. The trial was monitored by an NIH-appointed independent data and safety monitoring board. All the authors attest to the trial integrity and the completeness and accuracy of the reported data and adverse events. The first draft of the manuscript was written by the first author.
PATIENTS AND PARTICIPATING CENTERS
The trial was performed at 38 centers in the United States. Neurointerventionalists were preapproved to participate on the basis of training and experience. (For approval requirements, see the Supplementary Appendix, available with the full text of this article at NEJM.org.) Enrolled patients or their surrogates provided written informed consent. Patients were enrolled if they met clinical and imaging eligibility requirements and could undergo initiation of endovascular therapy between 6 and 16 hours after the time that they had last been known to be well, including if they had awakened from sleep with symptoms of a stroke. Perfusion imaging had to be performed at the trial-site hospital in which endovascular therapy was planned.
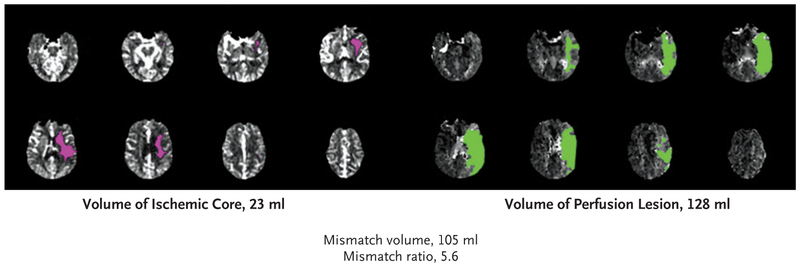
Patients were eligible if they had an initial infarct volume (ischemic core) of less than 70 ml, a ratio of volume of ischemic tissue to initial infarct volume of 1.8 or more, and an absolute volume of potentially reversible ischemia (penumbra) of 15 ml or more. Estimates of the volume of the ischemic core and penumbral regions from CT perfusion or MRI diffusion and perfusion scans were calculated with the use of RAPID software (iSchemaView), an automated image postprocessing system. The size of the penumbra was estimated from the volume of tissue for which there was delayed arrival of an injected tracer agent (time to maximum of the residue function [Tmax]) exceeding 6 seconds.8 (An example is given in Fig. 1.) Patients were required to have an occlusion of the cervical or intracranial internal carotid artery or the proximal middle cerebral artery on CT angiography (CTA) or magnetic resonance angiography (MRA). Detailed inclusion and exclusion criteria for the trial are provided in the Supplementary Appendix.
Figure 1. Example of Perfusion Imaging Showing a Disproportionately Large Region of Hypoperfusion as Compared with the Size of Early Infarction.
A 59-year-old man presented with a “wake-up stroke” (having awakened with symptoms of stroke) 13 hours after he was last known to be well. The score on the National Institutes of Health Stroke Scale (NIHSS; range, 0 to 42, with higher scores indicating a greater deficit) was 23. A baseline CT perfusion scan that was obtained with the use of RAPID software shows a region of severely reduced cerebral blood flow (<30% of that in normal tissue), which represents the early infarct (ischemic core), of 23 ml (pink) and a region of perfusion delay of more than 6 seconds, which represents hypoperfused tissue, of 128 ml (green).
TRIAL DESIGN AND RANDOMIZATION
The DEFUSE 3 trial was a randomized, open-label trial, with blinded outcome assessment, that compared endovascular therapy plus standard medical therapy with standard medical therapy alone in patients with acute ischemic stroke. The trial design has been published,9 and the protocol and statistical analysis plan are available at NEJM.org. Patients who met all eligibility criteria were randomly assigned in a 1:1 ratio to endovascular therapy plus medical therapy or medical therapy alone with the use of a Web-based dynamic randomization system (Table S1 in the Supplementary Appendix). Randomization was stratified according to age, core infarct volume, time from symptom onset to enrollment, baseline score on the National Institutes of Health Stroke Scale (NIHSS; range, 0 to 42, with higher scores indicating a greater deficit), and trial site.
ENDOVASCULAR THROMBECTOMY AND MEDICAL THERAPY
Thrombectomy was performed with any FDA-approved thrombectomy device, at the discretion of the neurointerventionalist. For patients with stenosis or occlusion of the cervical internal carotid artery due to atherosclerosis, carotid angioplasty with or without stenting was permitted as part of the intervention. The protocol required that femoral puncture occur within 90 minutes after the end of qualifying imaging. The use of general anesthesia was discouraged, and intraarterial tissue plasminogen activator (t-PA) was not allowed (intravenous t-PA was allowed if begun within 4.5 hours after symptom onset). The protocol specified that standard medical therapy, based on current American Heart Association guidelines, would be administered to patients in both groups of the trial.10
OUTCOMES
The primary efficacy outcome was the ordinal score on the modified Rankin scale (range, 0 [no symptoms] to 6 [death]) at day 90; the score was assessed in person, or by telephone if an in-person visit was not feasible, by a certified rater who was not aware of the trial-group assignments. The secondary efficacy outcome was functional independence (defined as a score on the modified Rankin scale of 0 to 2) at day 90. The primary safety outcomes were death within 90 days and the occurrence of symptomatic intracranial hemorrhage within 36 hours, defined as an increase of at least 4 points in the NIHSS score that was associated with brain hemorrhage on imaging within 36 hours after symptom onset.
Imaging outcomes were infarct volume measured at 24 hours (with a window of ±6 hours) after randomization; lesion growth (increase in volume of the infarct) between baseline imaging and 24 hours; reperfusion, defined as a greater than 90% reduction in the region of perfusion delay (Tmax of >6 seconds) between baseline and 24 hours; and complete recanalization of the primary arterial occlusive lesion at 24 hours on CTA or MRA. The technical efficacy of the endovascular procedure in establishing reperfusion was defined in the endovascular-therapy group by a modified Thrombolysis in Cerebral Infarction (TICI) score of 2b (50 to 99% reperfusion) or 3 (complete reperfusion).11
CLINICAL AND RADIOLOGIC ASSESSMENT
Clinical assessments were performed at baseline, 24 hours after randomization, at hospital discharge, and at 30 and 90 days; assessments included the score on the modified Rankin scale and the NIHSS score, both determined by certified assessors who were unaware of the trial group assignments at 30 and 90 days. Baseline and follow-up MRI and CT images were assessed independent of each other at the core imaging laboratory at Stanford University by assessors who were unaware of the trial-group assignments. Angiographic studies from the endovascular procedure were assessed for reperfusion by two independent raters, who were unaware of any other imaging and clinical data, and a consensus was reached in cases of disagreement.
STATISTICAL ANALYSIS
We planned for this trial to use an adaptive enrichment design.12 The maximal sample size was calculated to be 476, with two planned interim analyses after 200 and 350 patients had data on 90-day outcomes that could be evaluated. The plan was modified in June 2017 to accommodate the results of the DAWN trial, which involved patients and treatments similar to those in our trial and which showed clinical benefit of endovascular thrombectomy over medical therapy when treatment was initiated 6 to 24 hours after the onset of stroke symptoms. Enrollment in the DEFUSE 3 trial was placed on hold, and an early interim analysis, including subgroup analysis of the primary and secondary efficacy outcomes in patients who would have been eligible for the DAWN trial, was requested by the sponsor (the NIH).
As a result of that interim analysis, the trial was halted because the prespecified efficacy boundary (P<0.0025) had been exceeded. The statistical analysis plan specified one-sided hypothesis testing for the Wilcoxon rank-sum test and a P value of less than 0.025 as a measure of statistical significance, but we report two-sided results and use a P value of less than 0.05 as a measure of statistical significance. Adjusted treatment effects and P values for the primary efficacy outcome were calculated with the use of ordinal regression on the full modified Rankin scale and stratified Cochran–Mantel–Haenszel tests, with the randomization stratification variables split at their medians as the covariates. For patients lost to follow-up at 90 days, the missing 90-day score on the modified Rankin scale was imputed from the 30-day score by the last-observation-carried-forward method.
RESULTS
PATIENT CHARACTERISTICS
From May 2016 through May 2017, a total of 182 patients underwent randomization (92 to the endovascular-therapy group and 90 to the medical-therapy group) at 38 centers in the United States. Baseline demographic and clinical characteristics, imaging methods, and time to randomization of the two trial groups were balanced (Table 1, and Table S4 in the Supplementary Appendix). A total of 3 patients were lost to follow-up at day 90 (Fig. S1 in the Supplementary Appendix). All the patients who were assigned to endovascular therapy had an endovascular procedure initiated.
Table 1.
Baseline Characteristics of the Patients and Features of Thrombectomy.*
| Characteristic | Endovascular Therapy (N = 92) |
Medical Therapy (N = 90) |
|---|---|---|
| Median age (IQR) — yr | 70 (59–79) | 71 (59–80) |
| Female sex — no. (%) | 46 (50) | 46 (51) |
| Median NIHSS score (IQR)† | 16 (10–20) | 16 (12–21) |
| Stroke onset witnessed — no. (%) | ||
| Yes‡ | 31 (34) | 35 (39) |
| No | ||
| Symptoms were present on awakening | 49 (53) | 42 (47) |
| Symptoms began during wakefulness | 12 (13) | 13 (14) |
| Treatment with intravenous t-PA — no. (%)§ | 10 (11) | 8 (9) |
| Imaging characteristics¶ | ||
| Qualifying imaging — no. (%) | ||
| CT perfusion imaging | 69 (75) | 64 (71) |
| Diffusion and perfusion MRI | 23 (25) | 26 (29) |
| Median volume of ischemic core (IQR) — ml | 9.4 (2.3–25.6) | 10.1 (2.1–24.3) |
| Median volume of perfusion lesion (IQR) — ml∥ | 114.7 (79.3–146.3) | 116.1 (73.4–158.2) |
| Occlusion site on baseline CTA or MRA — no. (%) | ||
| Internal carotid artery | 32 (35) | 36 (40) |
| Middle cerebral artery** | 60 (65) | 54 (60) |
| Median ASPECTS on baseline CT (IQR)†† | 8 (7–9) | 8 (7–9) |
| Process measures — hr:min | ||
| Median time from stroke onset to qualifying imaging (IQR) | 10:29 (8:09–11:40) | 9:55 (7:59–12:20) |
| Median time from stroke onset to randomization (IQR) | 10:53 (8:46–12:21) | 10:44 (8:42–13:04) |
| Median time from qualifying imaging to femoral puncture (IQR) | 0:59 (0:39–1:27) | NA |
| Median time from femoral puncture to reperfusion (IQR) | 0:38 (0:26- 0:59) | NA |
Patients in the endovascular-therapy group received endovascular therapy plus standard medical therapy. Patients in the medical-therapy group received standard medical therapy alone. There were no significant differences between the two groups for any baseline characteristic. CT denotes computed tomography, CTA computed tomographic angiography, IQR interquartile range, MRA magnetic resonance angiography, and MRI magnetic resonance imaging.
Scores on the National Institutes of Health Stroke Scale (NIHSS) range from 0 to 42, with higher scores indicating a greater deficit.
Patients with witnessed onset underwent randomization a median of 9.5 hours after stroke onset.
Treatment with intravenous tissue plasminogen activator (t-PA) was allowed if begun within 4.5 hours after symptom onset.
Values are those reported by the central imaging laboratory.
Shown is the volume of tissue for which there was delayed arrival of an injected tracer agent exceeding 6 seconds. Data were missing for two patients in the medical-therapy group owing to technically inadequate perfusion studies. These two patients were enrolled on the basis of alternative neuroimaging inclusion criteria (see the Supplementary Appendix).
All middle-cerebral-artery occlusions involved the M1 segment, except in one patient in the medical-therapy group who had an occlusion involving the M2 segment.
The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) is a tool that is used to estimate the volume of infarcted tissue. Scores range from 0 to 10, with lower scores indicating a larger area. Baseline scores were available for 76 patients in the endovascular-therapy group and 66 patients in the medical-therapy group. Baseline scores are not reported for patients in whom the qualifying imaging study was an MRI.
PRIMARY AND SECONDARY EFFICACY OUTCOMES
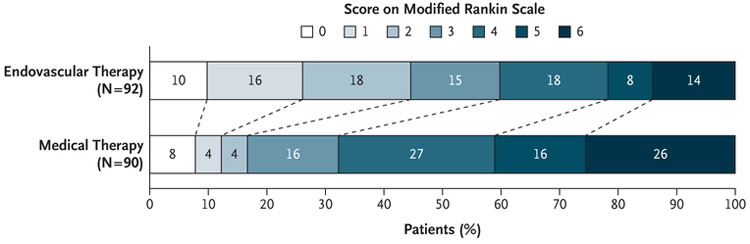
Endovascular therapy plus standard medical therapy was associated with a more favorable distribution of disability scores on the modified Rankin scale at 90 days than standard medical therapy alone (unadjusted common odds ratio, 2.77; 95% confidence interval [CI], 1.63 to 4.70; P<0.001) (Fig. 2 and Table 2). The odds ratio after adjustment for stratification factors was 3.36 (95% CI, 1.96 to 5.77; P<0.001). The percentage of patients who were functionally independent, defined as a score on the modified Rankin scale of 0 to 2, at 90 days was 45% in the endovascular-therapy group, as compared with 17% in the medical-therapy group (risk ratio, 2.67; 95% CI, 1.60 to 4.48; P<0.001).
Figure 2. Scores on the Modified Rankin Scale at 90 Days.
Patients in the endovascular-therapy group received endovascular therapy plus standard medical therapy. Patients in the medical-therapy group received standard medical therapy alone. Scores on the modified Rankin scale range from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death. There was a significant difference favoring the endovascular-therapy group over the medical-therapy group in the overall distribution of scores (unadjusted common odds ratio, 2.77; 95% CI, 1.63 to 4.70; P<0.001).
Table 2.
Clinical and Imaging Outcomes.
| Outcome | Endovascular Therapy (N = 92)* |
Medical Therapy (N = 90) |
Odds Ratio or Risk Ratio (95% CI)† |
P Value |
|---|---|---|---|---|
| Primary efficacy outcome: median score on modified Rankin scale at 90 days (IQR)‡ | 3 (1–4) | 4 (3–6) | 2.77 (1.63–4.70)§ | <0.001 |
| Secondary efficacy outcome: functional independence at 90 days — no. (%)¶ | 41 (45) | 15 (17) | 2.67 (1.60–4.48) | <0.001 |
| Safety outcomes — no. (%) | ||||
| Death at 90 days | 13 (14) | 23 (26) | 0.55 (0.30–1.02) | 0.05 |
| Symptomatic intracranial hemorrhage∥ | 6 (7) | 4 (4) | 1.47 (0.40–6.55) | 0.75 |
| Early neurologic deterioration | 8 (9) | 11 (12) | 0.71 (0.30–1.69) | 0.44 |
| Parenchymal hematoma type 2 | 8 (9) | 3 (3) | 2.61 (0.73–14.69) | 0.21 |
| Imaging outcomes** | ||||
| Median infarct volume at 24 hr (IQR) — ml | 35 (18–82) | 41 (25–106) | — | 0.19 |
| Median infarct growth at 24 hr (IQR) — ml | 23 (10–75) | 33 (18–75) | — | 0.08 |
| Reperfusion >90% at 24 hr — no./total no. (%) | 59/75 (79) | 12/67 (18) | 4.39 (2.60–7.43) | <0.001 |
| Complete recanalization at 24 hr — no./total no. (%) | 65/83 (78) | 14/77 (18) | 4.31 (2.65–7.01) | <0.001 |
| TICI score of 2b or 3 — no./total no. (%) | 69/91 (76) | — | — |
An intervention was attempted in 90 patients (98%), of whom 88 had an attempted mechanical thrombectomy and 2 had carotid stenting alone. In one of these two cases, the interventionalist elected not to perform a thrombectomy. The other patient did not have an occlusion on the baseline angiogram but was treated with carotid stenting for presumed dissection. The 2 patients with no intervention had carotid-artery occlusions, one in the common carotid and the other in the internal carotid, and the interventionalist decided that treatment was not feasible. Revascularization of the carotid artery with angioplasty, stenting, or both was performed in 13 patients (14%).
The odds ratio is shown for the primary efficacy outcome, and risk ratio is shown for the other outcomes.
Scores on the modified Rankin scale range from 0 to 6, with higher scores indicating greater disability. The protocol required the score to be assessed by a person who was not aware of the trial-group assignments. However, three patients in the endovascular-therapy group and one patient in the medical-therapy group had an assessor who was aware of the trial-group assignments.
Shown is the unadjusted common odds ratio. The odds ratio with adjustment for stratification factors is 3.36 (95% CI, 1.96 to 5.77; P<0.001). The proportional-odds assumption was not met when core volume was included in the fully adjusted model; without core volume included, the adjusted odds ratio is 3.24 (95% CI, 1.89 to 5.55).
Functional independence was defined as a score on the modified Rankin scale of 0 to 2.
Among the patients with symptomatic intracranial hemorrhage, the hemorrhage was rated as parenchymal hematoma type 2 (dense blood clot exceeding 30% of the infarct volume with substantial space-occupying effect; in two patients in the endovascular-therapy group and three patients in the medical-therapy group), parenchymal hematoma type 1 (blood clot not exceeding 30% of the infarcted area with some mild space-occupying effect; in one patient in the endovascular-therapy group), hemorrhagic infarction type 2 (confluent petechiae within the infarcted area, but without space-occupying effect; in three patients in the endovascular-therapy group), or hemorrhagic infarction type 1 (small petechiae along the margins of the infarct; in one patient in the medical-therapy group).
Infarct volume at 24 hours was assessed on diffusion-weighted MRI (or CT if MRI was not feasible). Infarct volume and infarct growth at 24 hours were assessed in 90 patients in the endovascular-therapy group and 89 patients in the medical-therapy group (2 patients in the endovascular-therapy group and 1 patient in the medical-therapy group died before imaging).
SAFETY OUTCOMES
Mortality at 90 days was 14% in the endovascular-therapy group and 26% in the medical-therapy group (P = 0.05). The rate of symptomatic intracranial hemorrhage did not differ significantly between the two groups (7% and 4%, respectively; P = 0.75). Five patients with symptomatic intracranial hemorrhages in the endovascular-therapy group died, as compared with two with symptomatic intracranial hemorrhages in the medical-therapy group. Parenchymal hematoma type 2 (dense blood clot exceeding 30% of the infarct volume with substantial space-occupying effect) occurred in 9% of the patients in the endovascular-therapy group and 3% of those in the medical-therapy group (P = 0.21). Thrombectomy-related complications occurred in two patients: a vessel perforation resulting in subarachnoid hemorrhage that was associated with a 3-point increase in the NIHSS score (90-day score on the modified Rankin scale, 5), and device-related vasospasm that did not lead to neurologic worsening. Serious adverse effects were reported in 43% of the patients in the endovascular-therapy group and 53% of those in the medical-therapy group (P = 0.18) (Table S2 in the Supplementary Appendix).
CHARACTERISTICS OF ENDOVASCULAR THROMBECTOMY
In the endovascular-therapy group, the median time from symptom onset to baseline imaging was 10 hours 29 minutes (interquartile range, 8 hours 9 minutes to 11 hours 40 minutes) (Table 1), and the median time from randomization to femoral puncture was 28 minutes. An intervention was attempted in 90 patients (98%). Of the 91 patients with an occlusion on the baseline angiogram, 10 (11%) had a TICI score of 0 on the final angiogram, 12 (13%) had a score of 2a, 52 (57%) had a score of 2b, and 17 (19%) had a score of 3.
Protocol violations occurred in five patients (5%) during the interventions. Two patients received intraarterial t-PA: one had a symptomatic intracerebral hemorrhage and died, and the other had a large ischemic infarct (without hemorrhagic transformation) and a small contralateral subdural hematoma (90-day score on the modified Rankin scale, 5). Two patients had intracranial stents placed: one had a vessel perforation (as described above), and the other had parenchymal hemorrhage without neurologic worsening. One patient had an intracranial angioplasty, without stenting, and did not have neurologic worsening. General anesthesia was used in 26 patients (28%). The devices and concomitant therapies that were used are summarized in Table S3 in the Supplementary Appendix.
IMAGING OUTCOMES
Imaging outcomes are summarized in Table 2. The median growth of the volume of the infarct region between baseline and 24 hours was 23 ml in the endovascular-therapy group and 33 ml in the medical-therapy group (P=0.08). Reperfusion of more than 90% of the initial perfusion lesion at 24 hours was more common in the endovascular-therapy group than in the medical-therapy group (79% vs. 18%, P<0.001). Similarly, the percentage of patients with complete recanalization of the primary arterial occlusive lesion at 24 hours on CTA or MRA was higher in the endovascular-therapy group than in the medical-therapy group (78% vs. 18%, P<0.001).
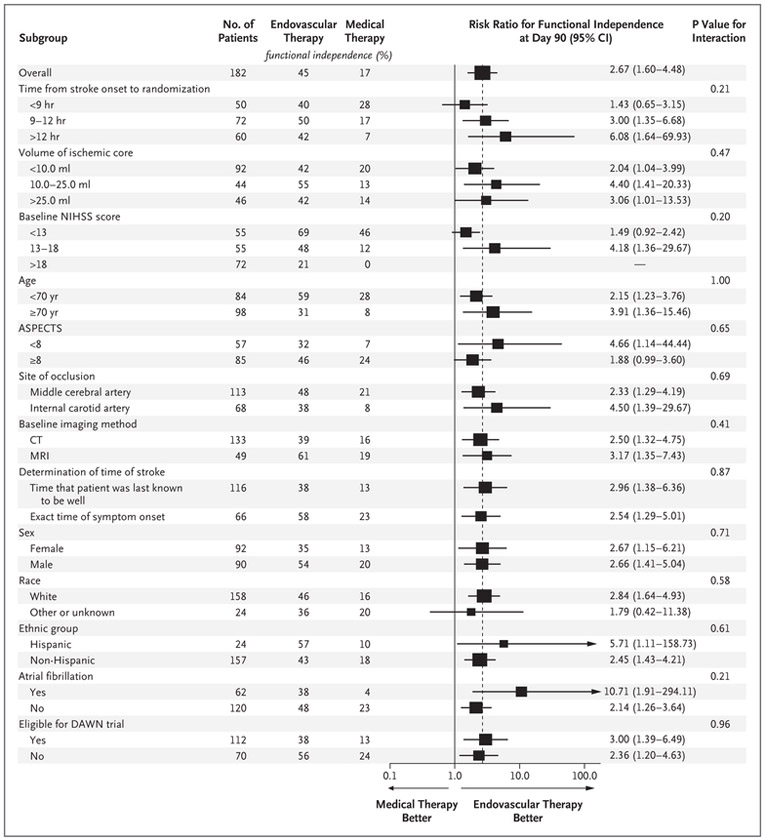
SUBGROUP ANALYSES
The power to assess the response to therapy in subgroups was limited owing to the lower-than-anticipated number of patients enrolled as a result of early termination of the trial. There was no heterogeneity of the treatment effect in any of the prespecified subgroups (Fig. 3). Within the limitations of reduced statistical power, the beneficial effect of endovascular therapy was consistent in patients with a known time of stroke onset and those with an unknown time of onset, in patients treated 9 to 12 hours after onset and those treated more than 12 hours after onset (but not those treated <9 hours after onset), and in patients selected on the basis of diffusion and perfusion MRI and those selected on the basis of CT perfusion imaging. Results of the primary efficacy outcome (Fig. S2 in the Supplementary Appendix) and the secondary efficacy outcome (Fig. 3) were significant for patients who met the DAWN enrollment criteria and those who did not meet those criteria. For the primary efficacy outcome, the odds ratio was 2.66 (95% CI, 1.36 to 5.23) among patients eligible for the DAWN trial and 2.96 (95% CI, 1.26 to 6.97) among those ineligible for the DAWN trial.
Figure 3 (facing page). Subgroup Analyses.
The forest plot shows that the difference in the risk ratio for functional independence (defined as a score on the modified Rankin scale of 0 to 2) at 90 days favored the endovascular-therapy group across all prespecified subgroups. The size of the squares is proportional to the number of patients in the subgroup. Arrows indicate that the limits of the confidence interval are not shown. The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) is a tool that is used to estimate the volume of infarcted tissue. Scores range from 0 to 10, with lower scores indicating a larger area. Race and ethnic group were reported by the patient or a family member. Patients who were considered to be ineligible for the DAWN (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo) trial3 had one or more of the following characteristics: a prestroke score on the modified Rankin scale of 2 (13 patients); a baseline NIHSS score of less than 10 (31 patients); a baseline volume of the ischemic core of 51 ml or more (13 patients); an age of 80 years or older with a baseline volume of the ischemic core of 21 ml or more (14 patients); and an age of less than 80 years, an NIHSS score of less than 20, and a baseline volume of the ischemic core of 31 ml or more (14 patients) (some patients met multiple criteria). All other patients were considered to be eligible for the DAWN trial. A risk ratio was not calculated for a baseline NIHSS score of more than 18 because of zero outcomes in the medical-therapy group.
DISCUSSION
In this trial, endovascular thrombectomy for ischemic stroke 6 to 16 hours after a patient was last known to be well plus standard medical therapy resulted in better 90-day functional outcomes than standard medical therapy alone among patients who had evidence of salvageable tissue on the basis of a formula that incorporated early infarct size and the volume of hypoperfused tissue on perfusion imaging. There was an absolute difference of 28 percentage points in the rate of functional independence at 90 days in favor of the endovascular-therapy group. The incidence of symptomatic cerebral hemorrhage was numerically higher in the endovascular-therapy group than in the medical-therapy group, but the difference was not significant. Five patients with symptomatic cerebral hemorrhages in the endovascular-therapy group died, as compared with two with symptomatic cerebral hemorrhages in the medical-therapy group. The overall mortality rate was numerically lower in the endovascular-therapy group.
Previous trials of endovascular treatment of acute stroke have suggested that a limited proportion of patients had a favorable clinical outcome when reperfusion was instituted later than 7 or 8 hours after stroke onset.13-15 A difference between the DEFUSE 3 trial and most of those trials was the inclusion in our trial of only patients who had findings on perfusion imaging of a penumbral (potentially salvageable) region of tissue. Two thrombectomy trials that treated patients within 6 hours after the onset of stroke (EXTEND-IA [Extending the Time for Thrombolysis in Emergency Neurological Deficits — Intra-Arterial]16 and SWIFT PRIME [Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment]17) used perfusion-imaging–based selection algorithms similar to those in our trial and showed higher rates of good functional outcome than trials that used other strategies to select patients.
The outcomes of thrombectomy in our trial were paradoxically better than those in many trials that treated patients within 6 hours after the onset of stroke. This finding may have been due to the selection of patients in the DEFUSE 3 trial with favorable collateral circulation and slower infarct growth. Subgroup analyses in our trial were underpowered but did not suggest a reduction in the treatment effect in patients who underwent randomization more than 12 hours after symptom onset.
The larger treatment effects in the DAWN and DEFUSE 3 trials than in the 6-hour trials are also attributable to the low rate of favorable outcomes in the medical control group, which may be related to the small proportion of patients in our trial who presented in time to receive intravenous t-PA. In contrast, in trials of thrombectomy that were conducted in the first several hours after stroke, a meta-analysis has indicated that intravenous t-PA was given to 87% of the patients in the medical-therapy group,1 leading to reperfusion of approximately one third of middle-cerebral-artery occlusions, a circumstance that typically is associated with good clinical outcomes.18
Our findings confirm and extend those of the DAWN trial, which used the same automated perfusion software (RAPID) as in our trial to measure the volume of the early infarct and to measure hypoperfused volume.3 The DAWN trial enrolled patients between 6 and 24 hours after the onset of stroke symptoms using selection criteria that required a discrepancy between the severity of the clinical deficit and the size of the early infarct on imaging. Our trial enrolled patients who had larger core infarctions than those in the DAWN trial and also included patients with milder stroke symptoms. Because of these differences, the DEFUSE 3 trial enrolled a broader patient population than the DAWN trial (approximately 40% of the patients in the DEFUSE 3 trial would not have met the DAWN selection criteria). However, the efficacy of endovascular therapy in our trial was similar in patients who did meet the eligibility criteria used in the DAWN trial and in patients who did not meet those criteria.
Rates of reperfusion and vessel recanalization were higher in the endovascular-therapy group than in the medical-therapy group (the latter group nevertheless had an 18% rate of spontaneous recanalization). Despite clinical benefit with thrombectomy, between-group differences in 24-hour infarct volume and growth after thrombectomy were not significant. Recent studies suggest that infarct growth can occur over a period of several days in patients who do not have reperfusion of ischemic regions.19,20 In future trials, later time points could be considered to assess changes in infarct volume.
In conclusion, among patients with acute ischemic stroke due to large-vessel occlusion who had favorable findings on perfusion imaging, endovascular therapy 6 to 16 hours after stroke onset plus standard medical therapy resulted in less disability and a higher rate of functional independence at 3 months than standard medical therapy alone.
Supplementary Material
Acknowledgments
Supported by grants (U10NS086487 and U01NS092076) from the National Institute of Neurological Disorders and Stroke. The RAPID software platform was provided to all sites by iSchemaView.
Footnotes
Contributor Information
Gregory W. Albers, Departments of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, California
Michael P. Marks, Department of Diagnostic Radiology Stanford University School of Medicine, Stanford, California
Stephanie Kemp, Departments of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, California
Soren Christensen, Departments of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, California
Jenny P. Tsai, Departments of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, California
Santiago Ortega-Gutierrez, Departments of Neurology, Anesthesia, Neurosurgery, and Radiology, University of Iowa, Ames
Ryan A. McTaggart, Departments of Diagnostic Imaging, Neurology, and Neurosurgery, Warren Alpert School of Medicine at Brown University and Rhode Island Hospital, Providence
Michel T. Torbey, Departments of Neurology and Neurosurgery, Ohio State University, Columbus, Ohio
May Kim-Tenser, Department of Neurology, Keck School of Medicine, University of Southern California, Los Angeles, California
Thabele Leslie-Mazwi, Departments of Neurosurgery and Neurology, Massachusetts General Hospital, Boston
Amrou Sarraj, Department of Neurology, University of Texas Health Science Center, Houston
Scott E. Kasner, Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia
Sameer A. Ansari, Departments of Radiology, Neurology, and Neurological Surgery, Northwestern University, Feinberg School of Medicine, Chicago
Sharon D. Yeatts, Department of Public Health Sciences, Medical University of South Carolina, Charleston
Scott Hamilton, Departments of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, California
Michael Mlynash, Departments of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, California
Jeremy J. Heit, Department of Diagnostic Radiology Stanford University School of Medicine, Stanford, California
Greg Zaharchuk, Department of Diagnostic Radiology Stanford University School of Medicine, Stanford, California
Sun Kim, Department of Neurology, New York University School of Medicine, New York
Janice Carrozzella, University of Cincinnati Gardner Neuroscience Institute and the Department of Neurology and Rehabilitation Medicine, University of Cincinnati College of Medicine, Cincinnati, Ohio
Yuko Y. Palesch, Department of Public Health Sciences, Medical University of South Carolina, Charleston
Andrew M. Demchuk, Departments of Clinical Neurosciences and Radiology, Hotchkiss Brain Institute, University of Calgary Cumming School of Medicine, Calgary, AB, Canada
Roland Bammer, Department of Radiology, Stanford University School of Medicine, Stanford, California
Philip W. Lavori, Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, California
Joseph P. Broderick, University of Cincinnati Gardner Neuroscience Institute and the Department of Neurology and Rehabilitation Medicine, University of Cincinnati College of Medicine, Cincinnati, Ohio
Maarten G. Lansberg, Departments of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford
REFERENCES
- 1.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387: 1723–31. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46:3020–35. [DOI] [PubMed] [Google Scholar]
- 3.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Goyal M, Jahan R, et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol 2016;79:76–89. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler HM, Mlynash M, Inoue M, et al. Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke 2013;44:681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012; 11:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lansberg MG, Christensen S, Kemp S, et al. Computed tomographic perfusion to predict response to recanalization in ischemic stroke. Ann Neurol 2017;81:849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 2010;32:1024–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albers GW, Lansberg MG, Kemp S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke 2017;12:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947. [DOI] [PubMed] [Google Scholar]
- 11.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai TL, Lavori PW, Liao OY. Adaptive choice of patient subgroup for comparing two treatments. Contemp Clin Trials 2014; 39:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khatri P, Yeatts SD, Mazighi M, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol 2014;13:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. [DOI] [PubMed] [Google Scholar]
- 15.Fransen PS, Berkhemer OA, Lingsma HF, et al. Time to reperfusion and treatment effect for acute ischemic stroke: a randomized clinical trial. JAMA Neurol 2016;73:190–6. [DOI] [PubMed] [Google Scholar]
- 16.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. [DOI] [PubMed] [Google Scholar]
- 17.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. [DOI] [PubMed] [Google Scholar]
- 18.Albers GW, Goyal M, Jahan R, et al. Relationships between imaging assessments and outcomes in Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment for acute ischemic stroke. Stroke 2015;46:2786–94. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler HM, Mlynash M, Inoue M, et al. The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int J Stroke 2015;10:723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federau C, Mlynash M, Christensen S, et al. Evolution of volume and signal intensity on fluid-attenuated inversion recovery MR images after endovascular stroke therapy. Radiology 2016;280:184–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.