Abstract
Background:
Pathology induced by metabolic disorders, eg. Type 2 Diabetes, has recently been linked to both sensory and motor deficits in the absence of a formal clinical diagnosis of peripheral neuropathy. Studies have demonstrated mild cognitive impairment in diabetic patients, which also plays a role in one’s loss of ability to successfully perform basic motor activities. This project focused on evaluating cognitive function while maintaining balance. We hypothesized that simultaneous cognitive and motor deficits would occur among adults with Type 2 Diabetes versus healthy age- and sex-matched control during a balance task.
Methods:
A sample of ten (10) Type 2 Diabetes patients and ten (10) age- and sex-matched controls underwent a series of sensory, motor, cognitive, and cognitive-motor evaluations. Blood pressure and A1c levels were assessed.
Results:
Significantly lower cognitive function scores, particularly in the domain of working memory, were exhibited in the diabetic group than controls. Balance in the diabetic group was overall poorer in both single- and dual-tasks than controls. When diabetic patients were asked to verbally recall different words while maintaining their balance, their accuracy rate was significantly lower than controls. Some health state measures were found to co-vary with motor function. Increased body mass index in the diabetic group did not account for motor function deficits exhibited.
Significance:
Our data suggest (1) systemic deficits beyond tactile dysfunction and increased body mass index contribute to reduced motor function in diabetes, and (2) both balance and working memory functions are simultaneously impaired in patients with Type 2 Diabetes.
Keywords: Type 2 Diabetes, motor function, cognitive function, working memory, BMI, cognitive-motor impairment
Introduction
Currently, over 422 million (8.5%) individuals worldwide over the age of 18 are living with Type 2 Diabetes1. Recent evidence suggests that in addition to the well-known complications of diabetes (coronary heart disease, large vessel atherosclerosis, stroke, nephropathy, and neuropathy), adults with Type 2 Diabetes experience declines in cognitive functions as compared to individuals without Type 2 Diabetes. Specifically, these individuals are at high risk of developing mild cognitive impairment (MCI); defined as a slight yet noticeable and measurable decline in cognitive abilities2. The risk of MCI development with diabetes is increased with age, particularly in those over the age of 603–8. A combination of underlying vascular damage combined with poor glucose control has been cited as the source of MCI development in patients with Type 2 Diabetes9–11.
Cognitive decline has been defined by impairment in a variety of mental tasks12,13. Failure in specific cognitive domains, such as working memory has been recently documented in diabetic patients3,4,14. Poor glucose control in diabetics has been linked specifically with verbal memory deficits, reduced processing speed, and motor slowing10,15. Despite these changes, other domains of cognitive function appear to be preserved in patients with Type 2 Diabetes16.
Impaired memory adversely affects the ability to manage complex daily diabetes self-management tasks such as meal preparation, taking medications, and exercise14,17,18. Many of these self-care tasks require balance maintenance in the environment; any difficulties with balance may result in significant difficulty in maintaining self-care.
In addition to the cognitive complications of diabetes, sensorimotor functions of all four extremities are known to be impaired with Type 2 Diabetes19–22. Specifically, poor tactile sensation and presence of diabetic peripheral neuropathy have been linked to poorer balance control and increased fall risk in patients with Type 2 Diabetes23–27. Recent evidence suggest that the increased fall risk in this population is due to more than just peripheral neuropathy, indicating subtle systemic changes all play a role in balance deficits28.
Currently, few studies have assessed concurrent cognitive-motor deficits (also known as cognitive-motor interference, CMI) in patients with Type 2 Diabetes. CMI occurs when the simultaneous performance of a cognitive and a motor task (known as dual-tasking) results in deterioration of performance in one or both tasks relative to performance of each task separately (known as single-tasking)29. Previous work in diabetic CMI has not considered a number of features important to CMI characterization30 including: standardized balance measures, consideration of sensory deficit contributions, and long term glucose control in both diabetic patients and matched controls. Specifically within the study by Smith et al.30, postural control was measured via degree of angle arc in sway, not with respect to movement of the center of pressure (the point of application of the ground reaction force) as is traditionally measured in biomechanics and motor control (e.g.31,32). The data presented in the study also did not consider the contribution of tactile dysfunction (including diagnosis of peripheral neuropathy) to the results. The authors also did not include assessment of long term glucose control (as measured by glycated hemoglobin, A1c) in the control/healthy group. Only A1c values for the diabetic group were used in correlations with measures of interest. Given the known rise in A1c with advanced age, this approach may have confounded the reported results.
Dual-task deficits have been shown in several patient populations, particularly in cases in which only motor deficits were initially clinically assumed (egs. Stroke, Parkinson’s disease)33–36. In these cases, CMI has been associated with loss of functionality in daily life33,37. Previously, we have shown subtle motor impairment in patients living with Type 2 Diabetes, however, the purpose of this study is to probe for CMI losses due to a combination of cognitive impairment and mild motor impairment in diabetic patients during the maintenance of upright balance.
Accordingly, the goal of this study is to assess the effects of Type 2 Diabetes on basic cognitive-motor activities during working memory evaluation (cognitive task) and a motor task involving upright balance. Specifically, we hypothesize that: (1) diabetic patients will demonstrate impairment in baseline evaluations and in cognitive, sensory, and motor single-tasks compared to healthy age- and sex-matched controls, and (2) diabetic patients will demonstrate further impairment in one or both the cognitive and motor domains during dual-tasks compared to their own single-task performance as well as performance by healthy age- and sex-matched controls. No hypotheses regarding changes in diabetic function with disease state were developed a priori, as exploration of function deterioration with disease state was an exploratory aim of this study.
Methods & Materials
Participants
Ten individuals with Type 2 Diabetes voluntarily participated in this study (4 men, 6 women). Ten healthy age- and sex-matched controls also participated voluntarily. Table 1 contains demographics for the sample groups. Study participants were excluded if they had a history of any other confounding neurological disorder (e.g. stroke, Parkinson’s disease, other sensorimotor dysfunction not related to diabetes, etc.). Diabetic participants had a confirmed diagnosis of Type 2 Diabetes either with or without diagnosis of peripheral neuropathy per physician.
Table 1.
Demographic and Clinical Characteristics of Type II Diabetes Patients.
| Patient # |
Age (years) |
Sex | T2D Duration (months) |
% A1c | Systole (mmHg) |
Diastole (mmHg) |
|---|---|---|---|---|---|---|
| 1 | 65 | M | 65 | 8.5 | 159 | 95 |
| 2* | 64 | F | 215 | 8.7 | 130 | 92 |
| 3* | 60 | F | 48 | 6.7 | 160 | 84 |
| 4 | 55 | F | 169 | 9.3 | 109 | 77 |
| 5 | 50 | F | 248 | 8.1 | 124 | 79 |
| 6* | 72 | M | 48 | 7.2 | 155 | 106 |
| 7 | 62 | F | 24 | 7.8 | 149 | 100 |
| 8 | 55 | M | 147 | 9 | 149 | 87 |
| 9 | 65 | F | 188 | 8.3 | 109 | 73 |
| 10* | 55 | M | 60 | 7.7 | 145 | 88 |
| Mean | 60 | -- | 121.2 | 8.1 | 139 | 88 |
| SD | 7 | -- | 81 | 0.8 | 20 | 10 |
| Controls | 60 ± 7 | -- | 0 | 5.4 ± 0.2 | 133 ± 9 | 88 ± 8 |
Indicates a clinical diagnosis of diabetic peripheral neuropathy. F, female; M, male; SD, standard deviation
Study Procedures
All study evaluations were performed in a single visit. Health status and tactile sensation data were collected prior to all cognitive and motor assessments. All participants provided written informed consent in accordance with the procedures provided by the Committees for the Protection of Human Subjects at the University of Houston and the Institutional Review Board of the University of Texas Health Science Center at Houston, in line with the Declaration of Helsinki.
Health Status Information
Blood pressure was measured using a commercially available device (Omron Intellisense, Model BP785, Bannockburn, IL, USA). A1c values were assessed from capillary blood samples using a commercially available point of care kit (A1c Now+, PTS Diagnostics, Indianapolis, IN, USA). The presence of peripheral neuropathy (PN status) was determined by abnormalities on either clinical examination or EMG/NCS testing (per physician).
Tactile Evaluation
Tactile sensation of the feet was tested using the Semmes-Weinstein Monofilament Test 38 for the three nerves of the feet (medial plantar, lateral plantar, and tibial nerves). During the test, participants kept their eyes closed and verbally indicated if and where they perceived monofilament touch. The monofilament size was increased until the subject was able to detect its touch a minimum of two times at the same location.
Cognitive Evaluation
Montreal Cognitive Assessment (MoCA)
Cognitive function of each participant was screened using the Montreal Cognitive Assessment (MoCA)39. This is a brief examination of the cognitive domains: attention and concentration, executive functions, working memory/recall, language, visuo-constructional skills, conceptual thinking, calculations, and orientation. The number of years of patient education is accounted for within the MoCA scoring structure.
Working Memory (N-Back) Evaluation
Working memory of each participant was probed using the working memory (N-back) evaluation. This test required participants to repeat the “nth” word back in a list of random words. The difficulty level is controlled by requiring participants to remember words further back in the series. Three conditions of the N-back task were assigned to each subject (easiest to most difficult: 0-, 1-, and 2-back conditions) in a block randomized manner. Participants wore a headset with headphone and microphone capabilities (Plantronics Inc., Santa Cruz, California), through which they heard a randomized sequence of words (Visual C++, Microsoft Corp., Redmond, Washington). The software program generated randomized words through the headphones at an interval of 4s per word. Participants were instructed to repeat the words into the headset in the correct sequence for a task duration of 60s. Working memory function was probed at a baseline (single-task) as well as during motor function evaluations (dual-task). Single-task working memory was assessed while participants were seated in a quiet location. All single-tasks occurred prior to dual-tasks in order to avoid confusion. The rate of correct responses and verbal reaction time were recorded by the software and extracted to evaluate performance. Three trials were collected in each of the N-back conditions for both single- and dual-task evaluation. N-back conditions were block randomized across all participants in both single- and dual-task conditions.
Motor Evaluations
Center of pressure (COP) data were collected via computerized dynamic posturography system (NeuroCom International, Inc., Clackamas, OR) at 100Hz. A rectangular stability boundary was estimated by the outer extremes of the feet for each subject; boundaries were marked and maintained in all conditions. In all conditions, participants stood upright with feet and body properly positioned, fitted with a safety harness and arms crossed in front of chest. Participants were tested under two conditions: (1) quiet stance, and (2) postural-cognitive evaluation (dual-task). All time series COP data were filtered using Butterworth low-pass filters with a cutoff frequency of 2 Hz.
Quiet stance testing
At the start of each motor evaluation testing session, evaluation of quiet stance occurred. In the quiet stance condition, participants were instructed to cross their arms in front of their chest and keep eyes open. Participants underwent three trials, lasting 60s each.
Postural-Cognitive Evaluation
During testing, participants underwent evaluation of working memory. Participants were given a series of random words through a headphone-microphone, instructed to repeat the words, and at the same time maintain upright stance on the platform. Participants underwent three trials in each of the N-back conditions, lasting 60s each.
Kinetic Data Analysis
COP time series data were directly obtained via NeuroCom. The following measures were calculated directly from COP data: AP path length, AP velocity, COP migration area, and minimum time to boundary (TTB).
Statistics
The data are presented in the text and figures as means ± standard errors. Repeated measures analyses of variance (RM-ANOVAs) were performed on all data with the main factors of: Group (two levels; Diabetic or Control). Evaluation of test-specific RM-ANOVA analyses included: Condition (four levels for postural evaluation: quiet stance, 0-, 1-, and 2-back; three levels for cognitive evaluation: 0-, 1-, and 2-back), TaskType (for cognitive function, two levels: single- and dual-task), and Nerve (three levels: one level each for the medial plantar, lateral plantar, and tibial nerves). For monofilament data, Foot (two levels: right and left) was included in the analysis to probe for possible asymmetry. In the event of significant Group findings in RM-ANOVAs, subsequent analysis of covariance (ANCOVA) was performed using A1c, duration of diagnosis, diagnosis of peripheral neuropathy (via indicator variable, PN status), body mass index (BMI), and monofilament data as co-variates to investigate the relationship between disease indicators and observed behaviors, all in one model. In the event of significant co-variates, follow-up correlation analyses were performed between the health state co-variate and the measured behavior. All significant co-variates can be found in Table 2. Monofilament data were log transformed due to non-linearity. Non-transformed data are shown in figures to avoid reader confusion. In multiple comparisons, Bonferonni corrected post-hocs were used.
Table 2.
Significant ANCOVA covariate output and regression results.
| Measure | Covariate | F | p | r | p |
|---|---|---|---|---|---|
| Tactile Evaluation | A1c | 32.44 | < 0.001 | 0.326 | < 0.001 |
| Total MoCA Score | Systole | 20.43 | < 0.05 | −0.587 | < 0.005 |
| Recall MoCA score | Diastole | 6.71 | < 0.05 | -- | -- |
| ML Path Length | BMI | 15.2 | < 0.001 | 0.294 | < 0.01 |
| Systole | 10.57 | < 0.005 | -- | -- | |
| Duration | 6.13 | < 0.05 | 0.293 | < 0.01 | |
| COP Area | BMI | 18.01 | < 0.001 | -- | -- |
| Systole | 27.56 | < 0.001 | -- | -- | |
| Diastole | 13.6 | < 0.005 | -- | -- | |
| Duration | 16.93 | < 0.001 | -- | -- | |
| Med. Plant. Sens. | 10.36 | < 0.005 | 0.319 | < 0.005 | |
| Tibial Sens. | 4.67 | < 0.05 | -- | -- | |
| AP Path Length | Diastole | 14.21 | < 0.001 | -- | -- |
| Lat. Plant. Sens. | 4.52 | < 0.05 | 0.297 | < 0.01 | |
| Tibial Sens. | 4.73 | < 0.05 | 0.514 | < 0.001 | |
| AP iTTB | A1c | 5.23 | < 0.05 | -- | -- |
| Strategy Score | Diastole | 7.35 | < 0.01 | -- | -- |
| A1c | 4.2 | < 0.05 | −0.232 | < 0.05 | |
Results
Tactile Evaluation
Main Effects: Significant impairment in tactile detection thresholds was found in the diabetic group compared to controls via RM-ANOVA (Group: F1,100 = 9.29, p < 0.005), Fig 1A. Tactile detection threshold values were significantly higher in the tibial nerve compared to the medial and lateral plantar nerves (Nerve: F2,100 = 32.44, p < 0.001), Fig. 1A. No differences were found between the right and left feet. Of note, PN status did not impact tactile detection thresholds.
Figure 1. Mean and standard error for tactile detection thresholds and MoCA data.
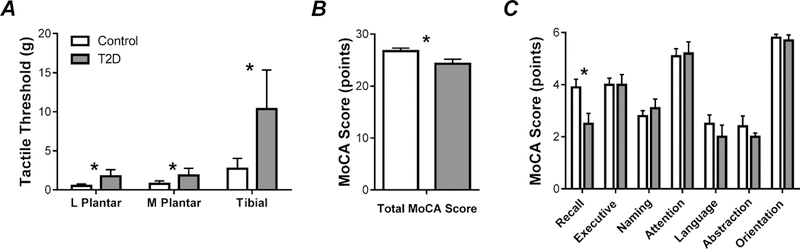
(A) Group averages of tactile detection thresholds for the medial plantar, lateral plantar, and tibial nerves. (B) Group averages of total MoCA scores. (C) Group averages of domain specific MoCA scores.
Cognitive Function
MoCA
Main Effects: RM-ANOVA of MoCA results indicated a significant Group difference (F1,9 = 6.56, p < 0.05), such that total MoCA scores were lower on average in the diabetic group versus controls, Fig 1B. Subsequent RM-ANOVA of each MoCA domain revealed Group differences in the recall/working memory domain of the evaluation (F1,9 = 10.74, p < 0.05); diabetic scores in working memory/recall were significantly lower than controls. Total MoCA and domain specific MoCA scores can be found in Fig. 1B-1C.
N-back/Working Memory
Main Effects (reaction time): No significant differences between Group were observed in RM-ANOVA of reaction time of the N-back evaluation (p > 0.5); only a Condition effect was found (F2,89 = 25.56, p < 0.001), such that reaction times increased with task difficulty. Post-hoc analysis indicated that reaction time were longest in the 2-back Condition, as compared to 0- and 1-back, Fig 2A-2B.
Figure 2. Mean and standard error for working memory function data.
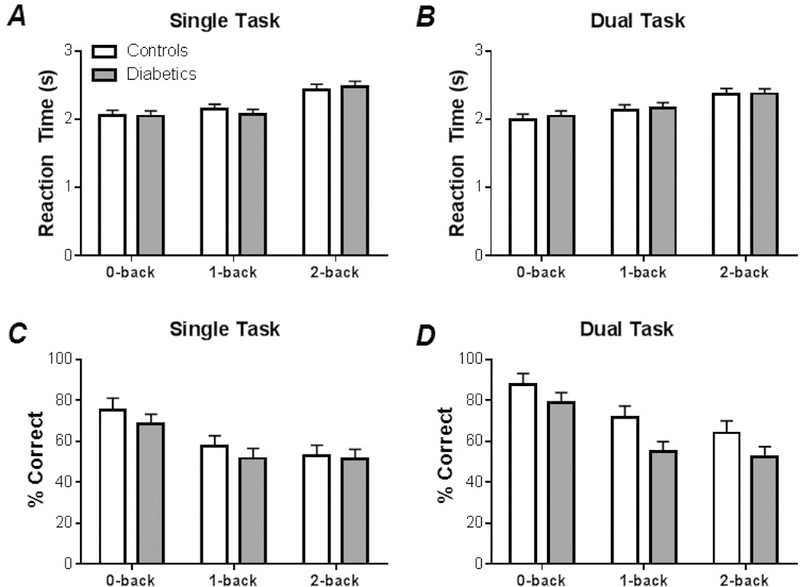
(A) Group averages of reaction times in single-task (cognitive only) N-back evaluations. (B) Group averages of reaction times in dual-task (simultaneous cognitive and motor) N-back evaluations. (C) Group averages of correct response rates in single-task (cognitive only) N-back evaluations. (D) Group averages of correct response rates in dual-task (simultaneous cognitive and motor) N-back evaluations.
Main Effects (correct response rate): Correct response rate did show between Group differences (F1,87 = 7.76, p < 0.01) in the initial RM-ANOVA, along with a Condition effect (F2,87 = 20.87, p < 0.001), and TaskType effect (F1,87 = 8.78, p < 0.01). The number of correct responses were lower for the diabetic group compared to controls (Fig. 2C-2CD). The number of errors produced per trial was significantly lower in the 0-back condition, compared to both the 1- and 2-back conditions. The correct response rate was lower in single-tasks versus dual-tasks, Fig. 2C-2D.
Postural Data
Main Effects: Assessment of postural characteristics revealed a number of Group effects. Specifically, the AP path length (F1,63 = 33.95, p < 0.001), AP TTB (F1,63 = 11.18, p < 0.005), ML path length (F1,63 = 26.40, p < 0.001), ML TTB (F1,63 = 4.26, p < 0.05) and COP migration area (F1,63 = 19.00, p < 0.001) were all significantly higher in the diabetic Group, Figs. 3A-3E. Strategy Score (F1,63 = 17.27, p < 0.001) was significantly lower in the diabetic Group, Fig. 3F. Assessment of Condition effects indicated that Condition had little impact on postural control measures. Only AP velocity was affected by Condition (F3,63 = 2.88, p < 0.05), such that AP velocity was larger in the anterior direction in the 2-back condition as compared to other N-back conditions, Fig. 3G.
Figure 3. Mean and standard error of motor function data in single- (Quiet) versus dual-task conditions (0-, 1-, and 2-back).
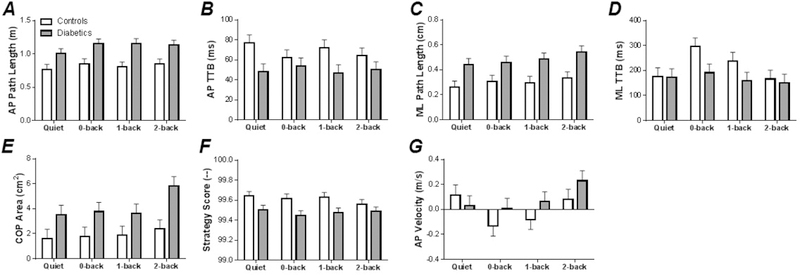
(A) Group versus Condition AP path length data. (B) Group versus Condition AP TTB data. (C) Group versus Condition ML path length data. (D) Group versus Condition ML TTB data. (E) Group versus Condition COP area data. (F) Group versus Condition strategy score data. (G) Group versus Condition AP velocity data.
Covariates: ANCOVA indicated that the Group effect in some of the postural measures were magnified by the inclusion of health state markers in the statistical models. This included ML path length and COP migration area, see Table 2 for details. In contrast, ANCOVA indicated that the Group effect in some of the postural measures were accounted for by health state markers. This included AP path length, AP TTB, and Strategy Score.
Discussion
The purpose of the current study was to assess the effects of Type 2 Diabetes on cognitive-motor activities during a postural maintenance task. Our findings on all of the baseline and single-task evaluations support the first hypothesis, such that patients with Type 2 Diabetes demonstrate impairment in baseline evaluations and in each of the cognitive, tactile, and motor single-tasks evaluated in this study compared to controls. In contrast, the data do not support the second hypothesis. The data indicate that patients with Type 2 Diabetes do demonstrate impairment in the cognitive and motor domains during dual-tasks compared to controls; however, these impairments were comparable with single-task losses in both the cognitive and motor domains. Health state covariates did impact these relationships, such that some features of posture that were worsened in the diabetic group (eg. AP sway) were associated with the general poor health state of the sample. In other measures, such as COP sway area, consideration of health state data enhanced the sway effects shown in the diabetic sample. In the following paragraphs, we discuss the results of this study in regards to the published literature as it relates to impaired balance and self-care as well as possible mechanisms responsible for behaviors exhibited.
T2D-induced cognitive and motor deficits, balance, and self-care
Baseline evaluation of tactile sensory function, motor function, and cognitive function all revealed significant losses in the diabetic group. Unsurprisingly, tactile detection thresholds in the feet of the diabetic group were in the range of diminished protective sensation to loss of protective sensation (< 2g), even in participants without a clinical diagnosis of peripheral neuropathy40–42. Tactile thresholds for the control group were significantly lower, indicating impaired sensation in the diabetic cohort. All measures of motor function (assessed via balance), indicated worsened control of balance in the diabetic group consistently across both motor and cognitive-motor tasks, suggesting global motor impairment in postural control in this population42. Some, but not all, measures of motor performance deficits were found to correlate with poor health status. This suggests that the occurrence of diabetes, in addition to poor overall cardiovascular health and higher body mass (or body mass index, BMI), drives motor dysfunction in this population. This finding is significant as body mass index has been found to correlate with motor deficits in obese participants without diabetes43,44. Thus, previous assumptions that motor deficits in diabetics are primarily due to mechanical issues induced solely by increased body mass are not supported in the current data set.
Baseline cognitive function, assessed via MoCA, was also found to be reduced in the diabetic group, particularly in the domain of recall/working memory, consistent with recent reports of MCI and amnesiac MCI in this particular patient population3,4,14,45. Working memory deficits emerged in the correct response rates produced by the diabetic group in both single- and dual-tasks in the current study, while reaction time was not affected. This suggests that diabetic individuals respond verbally using similar response times to audio stimuli as healthy controls, however, the accuracy of their responses is negatively impacted by diabetes in general. This may indicate that diabetic individuals require more attentional resources for successful task completion during cognitive tasks. Similar outcomes have been found in individuals with increased body mass during postural tasks43, however, our data indicate that BMI does not affect working memory function in diabetics. The lack of correlation between measures of working memory (reaction time and correct response rate) with health status measures indicates that no single measure of health may be indicative of short term memory performance in this population, beyond a diagnosis of diabetes.
While baseline functions were impaired in all tasks in the diabetic group, it is important to note that these declines were not exacerbated by the simultaneous performance of cognitive-motor tasks, indicating a stable form of CMI. This may mean that diabetic patients may exhibit functional declines in cognitive and motor functions separately; their ability to perform activities of daily living that require the close coupling of cognitive and motor functions may not be as significantly impaired as other populations with cardiovascular and neurovascular compromise (eg. stroke)37. Given the length of time for diabetes to progress in most patients, the slow metabolic changes experienced may permit the development of coping strategies by the neuromuscular system concurrently with the development of the disease, unlike the acute neuromuscular deficits found in patients after stroke.
Despite the possibility of developing coping mechanisms, the significant tactile, motor, and cognitive deficits in diabetic patients should not be downplayed. Clinicians should take these deficits into account when developing medical management strategies, including physical activity interventions, and patient-environment interactions.
Potential mechanisms
In our exploratory co-variate evaluations, several disease state markers were found correlate with tactile, motor, and cognitive functions. In some cases, these health state markers accounted for the observed behavioral differences in diabetic patients; in others, they magnified the group differences between diabetics and controls. Consistent with previous literature19,20, tactile dysfunction positively correlated with A1c, suggesting that worsened glucose control is globally associated with worsened sensory function. Baseline cognitive function was indicated to be negatively correlated with blood pressure46. The novel health state covariation indications of this study are found in a combination of several health states (such as BMI, disease duration, and systole) to enhance the group effects found in the motor function data. In contrast, other health state variables (such as A1c and tactile sensation thresholds) appear to account for the group effects found in the motor function data. Looking at this data, even though our diabetic cohort may have larger BMI values, this alone does not account for the motor function differences exhibited by this population. Instead, it appears that a constellation of health factors play into the motor (as well as other) deficits exhibited by this population, consistent with the development of the common soil hypothesis47. As a result and contrary to traditional clinical opinion, reduced motor function in the diabetic group may not be due solely to peripheral nerve damage, but to multi-system changes in the body21,28. We do acknowledge that this work is exploratory in nature. Accordingly, this initial study has been useful to our group in designing subsequent studies on cognitive and motor dysfunction in diabetic patients. We are currently pursuing multiple projects to evaluate the contribution of health state covariates as well as cortical and corticospinal contributions to CMI. It is our hope that results from these upcoming studies may be used to better understand the full scope of how self-care is impacted by systemic cardiovascular and neurological impairment in diabetic patients.
Based on the results of the current study, we urge clinicians to consider the following when developing medical management strategies, particularly physical activity interventions, for older adults with Type II Diabetes: older adults with Type 2 Diabetes present with global impairments in postural stability that are not specifically driven by BMI or peripheral neuropathy; and that older adults with Type 2 Diabetes require increased attentional resources to perform both cognitive and motor tasks. Thus, interventions focusing on weight loss are unlikely to mitigate postural instability in this population. Clinicians should also consider designing interventions utilizing reduced distraction environments given the increased attentional resource demands of this particular population. Keeping both of these issues in mind may help in designing impactful interventions for this growing population.
Limitations
In the current study, we acknowledge three limitations: (1) a small sample size, (2) exploratory analyses of health state variables, and (3) collection of primarily behavioral data. Despite these limitations, the current data set has been informative in shaping our future research directions in the area of diabetic CMI. Our upcoming projects address all three limitations of the current project.
Acknowledgements:
We are thankful for the help with data collection from our team of research interns: Sahifah Ansari, Beatriz Thames, Lena Younes, Neryeda Ochoa, Taft Knowles, Zahrah Mohamed, Taylor Peabody, and Aisha Khan. This work was supported by American Heart Association Grant #16BGIA27250047 (Gorniak).
Footnotes
Conflict of Interest: None of the authors have conflicts of interest to report related to this work.
References
- 1.Diabetes. World Health Organization. http://www.who.int/news-room/fact-sheets/detail/diabetes Accessed August 29, 2018.
- 2.Mild Cognitive Impairment (MCI) | Signs, Symptoms, & Diagnosis. Dementia. http://www.alz.org/dementia/mild-cognitive-impairment-mci.asp Accessed October 3, 2016.
- 3.Arvanitakis Z, Wilson RS, Bennett DA. Diabetes mellitus, dementia, and cognitive function in older persons. J Nutr Health Aging. 2006;10(4):287–291. [PubMed] [Google Scholar]
- 4.Creavin ST, Gallacher J, Bayer A, Fish M, Ebrahim S, Ben-Shlomo Y. Metabolic Syndrome, Diabetes, Poor Cognition, and Dementia in the Caerphilly Prospective Study. J Alzheimers Dis. 2011;28(4):931–939. [DOI] [PubMed] [Google Scholar]
- 5.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7(2):184–190. [DOI] [PubMed] [Google Scholar]
- 6.van den Berg E, Dekker JM, Nijpels G, et al. Cognitive Functioning in Elderly Persons with Type 2 Diabetes and Metabolic Syndrome: the Hoorn Study. Dement Geriatr Cogn Disord. 2008;26(3):261–269. [DOI] [PubMed] [Google Scholar]
- 7.Strachan MWJ, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7(2):108–114. [DOI] [PubMed] [Google Scholar]
- 8.Fontbonne A, Berr C, Ducimetière P, Alpérovitch A. Changes in Cognitive Abilities Over a 4-Year Period Are Unfavorably Affected in Elderly Diabetic Subjects: Results of the Epidemiology of Vascular Aging Study. Diabetes Care. 2001;24(2):366–370. [DOI] [PubMed] [Google Scholar]
- 9.Biessels GJ, Strachan MWJ, Visseren FLJ, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246–255. [DOI] [PubMed] [Google Scholar]
- 10.McNay EC, Recknagel AK. Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem. 2011;96(3):432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol Aging. 2009;24(1):154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer JS, Xu G, Thornby J, Chowdhury M, Quach M. Longitudinal analysis of abnormal domains comprising mild cognitive impairment (MCI) during aging. J Neurol Sci. 2002;201(1–2):19–25. [DOI] [PubMed] [Google Scholar]
- 13.Bennett DA, Wilson RS, Schneider JA, et al. Natural History of Mild Cognitive Impairment in Older Persons. Neurology. 2002;59(2):198–205. [DOI] [PubMed] [Google Scholar]
- 14.Christman AL, Vannorsdall TD, Pearlson GD, Hill-Briggs F, Schretlen DJ. Cranial Volume, Mild Cognitive Deficits, and Functional Limitations Associated with Diabetes in a Community Sample. Arch Clin Neuropsychol. 2009;25(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. [DOI] [PubMed] [Google Scholar]
- 16.Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26(8):1044–1080. [DOI] [PubMed] [Google Scholar]
- 17.Gold DA. An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. J Clin Exp Neuropsychol. 2012;34(1):11–34. [DOI] [PubMed] [Google Scholar]
- 18.Vance D, Larsen KI, Eagerton G, Wright MA. Comorbidities and Cognitive Functioning. J Neurosci Nurs. 2011;43(4):215–224. [DOI] [PubMed] [Google Scholar]
- 19.Gorniak SL, Khan A, Ochoa N, Sharma MD, Phan CL. Detecting subtle fingertip sensory and motor dysfunction in adults with type II diabetes. Exp Brain Res. 2014;232(4):1283–1291. [DOI] [PubMed] [Google Scholar]
- 20.Ochoa N, Gorniak SL. Changes in sensory function and force production in adults with Type II Diabetes. Muscle Nerve. 2014;50(6):984–990. [DOI] [PubMed] [Google Scholar]
- 21.Ochoa N, Gogola GR, Gorniak SL. Contribution of tactile dysfunction to manual motor dysfunction in type II diabetes. Muscle Nerve. 2016;54:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison S, Colberg SR, Parson HK, Vinik AI. Relation between risk of falling and postural sway complexity in diabetes. Gait Posture. 2012;35(4):662–668. [DOI] [PubMed] [Google Scholar]
- 23.Najafi B, Horn D, Marclay S, Crews RT, Wu S, Wrobel JS. Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. J Diabetes Sci Technol. 2010;4(4):780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison S, Colberg SR, Mariano M, Parson HK, Vinik AI. Balance training reduces falls risk in older individuals with type 2 diabetes. Diabetes Care. 2010;33(4):748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macgilchrist C, Paul L, Ellis BM, Howe TE, Kennon B, Godwin J. Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med J Br Diabet Assoc. 2010;27(2):162–168. [DOI] [PubMed] [Google Scholar]
- 26.Fulk GD, Robinson CJ, Mondal S, Storey CM, Hollister AM. The effects of diabetes and/or peripheral neuropathy in detecting short postural perturbations in mature adults. J Neuroengineering Rehabil. 2010;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turcot K, Allet L, Golay A, Hoffmeyer P, Armand S. Investigation of standing balance in diabetic patients with and without peripheral neuropathy using accelerometers. Clin Biomech Bristol Avon. 2009;24(9):716–721. [DOI] [PubMed] [Google Scholar]
- 28.Hewston P, Deshpande N. Falls and Balance Impairments in Older Adults with Type 2 Diabetes: Thinking Beyond Diabetic Peripheral Neuropathy. Can J Diabetes. 2016;40(1):6–9. [DOI] [PubMed] [Google Scholar]
- 29.Plummer P, Eskes G, Wallace S, et al. Cognitive-motor interference during functional mobility after stroke: state of the science and implications for future research. Arch Phys Med Rehabil. 2013;94(12):2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MA, Else JE, Paul L, et al. Functional Living in Older Adults With Type 2 Diabetes: Executive Functioning, Dual Task Performance, and the Impact on Postural Stability and Motor Control. J Aging Health. June 2014. [DOI] [PubMed] [Google Scholar]
- 31.Zatsiorsky V Kinetics of Human Motion. 1st ed. Human Kinetics; 2002. [Google Scholar]
- 32.Winter D Biomechanics and Motor Control of Human Movement. 3rd ed. Hoboken, New Jersey: John Wiley & Sons, Inc.; 2005. [Google Scholar]
- 33.Chen C, Leys D, Esquenazi A. The interaction between neuropsychological and motor deficits in patients after stroke. Neurology. 2013;80(3 Suppl 2):S27–34. [DOI] [PubMed] [Google Scholar]
- 34.Dennis A, Bosnell R, Dawes H, et al. Cognitive context determines premotor and prefrontal brain activity during hand movement in patients after stroke. Stroke J Cereb Circ. 2011;42(4):1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Yahya E, Johansen-Berg H, Kischka U, Zarei M, Cockburn J, Dawes H. Prefrontal Cortex Activation While Walking Under Dual-Task Conditions in Stroke: A Multimodal Imaging Study. Neurorehabil Neural Repair. 2016;30(6):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plotnik M, Giladi N, Dagan Y, Hausdorff JM. Postural instability and fall risk in Parkinson’s disease: impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp Brain Res. 2011;210(3–4):529–538. [DOI] [PubMed] [Google Scholar]
- 37.Haggard P, Cockburn J, Cock J, Fordham C, Wade D. Interference between gait and cognitive tasks in a rehabilitating neurological population. J Neurol Neurosurg Psychiatry. 2000;69(4):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell-Krotoski JA. Light touch-deep pressure testing using Semmes-Weinstein monofilaments In: Hunter Mackin, Schneider Callahan, eds. Rehabilitation of the Hand. 4th ed. St. Louis: CV Mosby Co; 1990:585–593. [Google Scholar]
- 39.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 40.Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. 2009;50(3):675–682. [DOI] [PubMed] [Google Scholar]
- 41.Nather A, Neo SH, Chionh SB, Liew SCF, Sim EY, Chew JLL. Assessment of sensory neuropathy in diabetic patients without diabetic foot problems. J Diabetes Complications. 2008;22(2):126–131. [DOI] [PubMed] [Google Scholar]
- 42.Lin S, Chen Y, Liao C, Chou C. Association between sensorimotor function and forward reach in patients with diabetes. Gait Posture. 2010;32(4):581–585. [DOI] [PubMed] [Google Scholar]
- 43.Mignardot J-B, Olivier I, Promayon E, Nougier V. Obesity Impact on the Attentional Cost for Controlling Posture. Sorensen TIA, ed. PLoS ONE. 2010;5(12):e14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng H, O’Connor DP, Lee BC, Layne CS, Gorniak SL Effects of adiposity on postural control and cognition. Gait Posture. 2016;43:31–37. [DOI] [PubMed] [Google Scholar]
- 45.Manschot SM, Biessels GJ, Valk H, et al. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50(11):2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sierra C Cerebral small vessel disease, cognitive impairment and vascular dementia. Panminerva Med. 2012;54(3):179–188. [PubMed] [Google Scholar]
- 47.Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44(4):369–374. [DOI] [PubMed] [Google Scholar]


