Abstract
Cardiovascular diseases (CVD) remain the leading cause of mortality and a major contributor to preventable deaths worldwide. The dominant modifiable risk factors and the social and environmental determinants that increase cardiovascular risk are known, and collectively, are as important in racial and ethnic minority populations as they are in majority populations. Their prevention and treatment remain the foundation for cardiovascular health promotion and disease prevention. Genetic and epigenetic factors are increasingly recognized as important contributors to cardiovascular risk and provide an opportunity for advancing precision cardiovascular medicine. In this review, we explore emerging concepts at the interface of precision medicine and CVD in racial and ethnic minority populations. Important among these are the lack of racial and ethnic diversity in genomics studies and biorepositories; the resulting misclassification of benign variants as pathogenic in minorities; and the importance of ensuring ancestry-matched controls in variant interpretation. We address the relevance of epigenetics, pharmacogenomics, genetic testing and counseling, and their social and cultural implications. We also examine the potential impact of precision medicine on racial and ethnic disparities. The National Institutes of Health’s All of Us Research Program and the National Heart, Lung, and Blood Institute’s Trans-Omics for Precision Medicine Initiative (TOPMed) are presented as examples of research programs at the forefront of precision medicine and diversity to explore research implications in minorities. We conclude with an overview of implementation research challenges in precision medicine and the ethical implications in minority populations. Successful implementation of precision medicine in CVD in minority populations will benefit from strategies that directly address diversity and inclusion in genomics research and go beyond race and ethnicity to explore ancestry-matched controls, as well as geographical, cultural, social, and environmental determinants of health.
Keywords: Ancestry, Bioethics, Epigenetics, Ethnicity, Genetics, Genomics, Precision Medicine, Pharmacogenetics, Race, Tuskegee
Introduction
Cardiovascular diseases (CVD), principally ischemic heart disease and stroke, constitute the leading cause of mortality worldwide.1 In 2017, CVD had an estimated global prevalence of 73 million,2 causing nearly 18 million deaths.1 One-third of these deaths occurred in persons younger than 70 years old. In the U.S. alone, CVD caused 793,840 deaths in 2017.3 The dominant modifiable CVD risk factors include hypertension, dyslipidemia, tobacco use, physical inactivity, obesity, and poor nutrition. Together with the social and environmental determinants of health, these risk factors account for over 90 percent of the population-attributable risk of a first myocardial infarction.4 Collectively, CVD risk factors are as important in racial and ethnic minority populations as they are in majority populations. Thus, risk factor prevention, treatment, and control remain the foundation for cardiovascular health promotion and disease prevention worldwide.5
Increasingly, however, the role of genetics, epigenetics, and heredity in the pathogenesis of CVD is being recognized.6 This provides an opportunity for advancing precision medicine (prevention and treatment strategies that take individual variability into account) for all populations.7 This review explores the emerging concepts at the interface of precision medicine and CVD in racial and ethnic minority populations, beginning with the conceptualization of race, ethnicity, and ancestry in precision medicine, and concluding with the social, cultural, and ethical implications for biomedical research.
Race, Ethnicity, and Ancestry
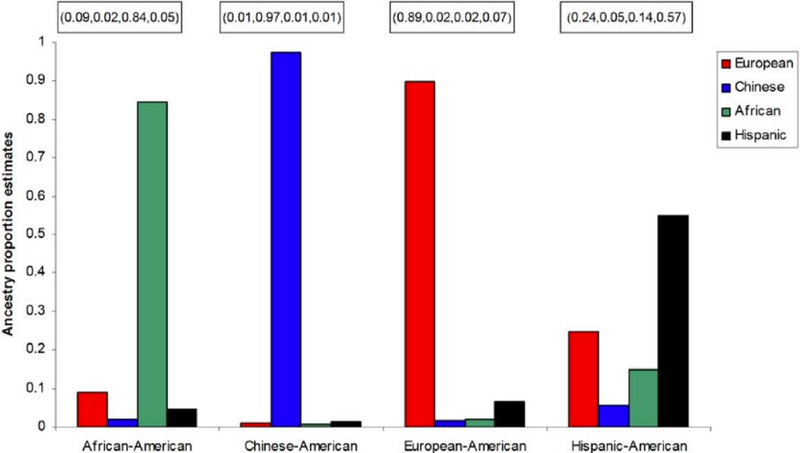
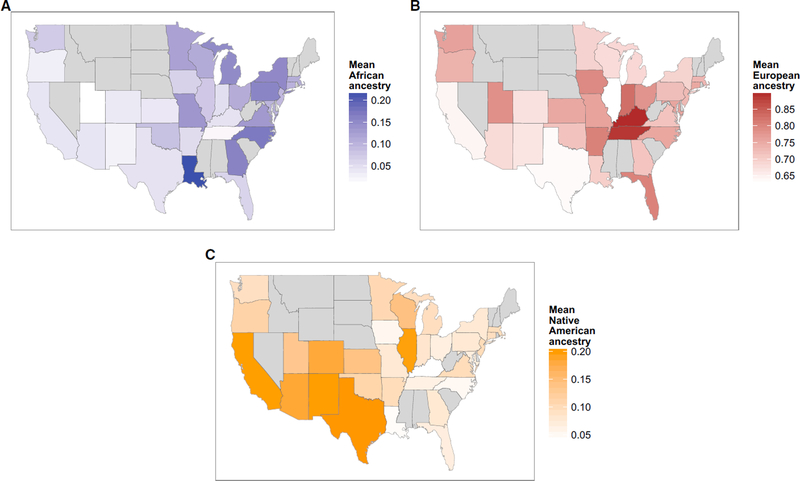
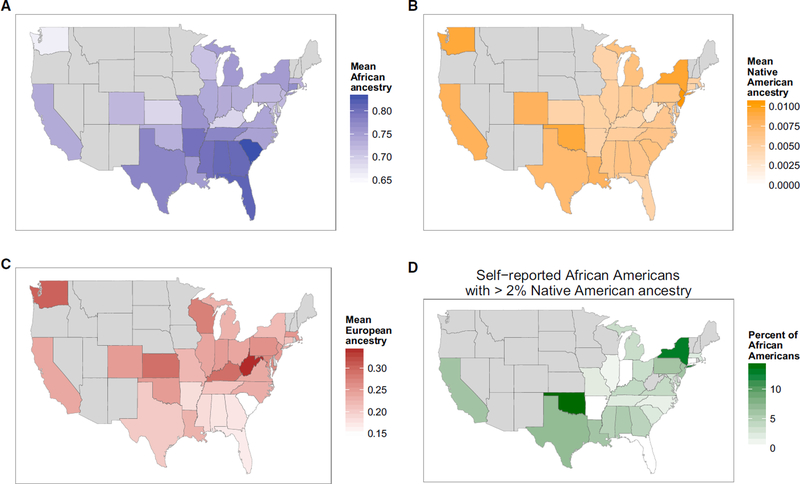
Categories of race and ethnicity are social, political, and cultural constructs that are invaluable in clinical and public health research.8 However, they are flawed as biological constructs for genomics and precision medicine.9 Whether self-reported or assigned, racial and ethnic categories are imprecise, and their definitions change over time.10 Although self-reported race/ethnicity can help group individuals from geographically distant regions, it is less successful in distinguishing persons who have mixed origins.11 Self-reported race/ethnicity is less reliable for the Hispanic-American population when compared to ancestry-informative genetic markers (Figure 1).12 Additional evidence of the fine-scale differences in ancestry within and across the U.S. is provided by Bryc et al. (Figures 2A and 2B).12
Figure 1:
Comparison between self-reported ancestry and ancestry proportion estimates (among European, Chinese, African, and Hispanic). Reproduced from Divers J, et al.11
Figure 2A:
The Distribution of Ancestry of Self-Reported African Americans across the US, Differences, by state, in levels of (A) African ancestry in African Americans (blue); (B) Native American ancestry in African Americans (orange); (C) European ancestry of African Americans (red). States with fewer than ten individuals are excluded in gray; (D) The geographic distribution of self-reported African Americans with Native American ancestry. The proportion of African Americans in each state who have 2% or more Native American ancestry is shown by shade of green. States with fewer than 20 individuals are excluded in gray. Reproduced from Bryc K, et al.12
Figure 2B:
The Distribution of Ancestry of Self-Reported Latinos across the US. Differences in mean levels of African (A), European (B), and Native American (C) ancestry in Latinos from each state is shown by shade of blue, red, and orange, respectively. States with fewer than ten individuals are excluded in gray. Reproduced from Bryc K, et al.12
A second important concept is that racial/ethnic categories are heavily confounded by socio-economic status, income, education, neighborhood characteristics, perceived racism, environmental exposures, access to healthcare, and other social determinants of health.13,14 Progress in precision medicine will be accelerated when population subgrouping goes beyond racial/ethnic categories to include these factors as well as ancestry information. For example, it is far more informative to describe a person as a 50-year-old, college-educated woman of West-African ancestry employed as a school teacher in New York City than it is to describe her as simply a 50-year-old non-Hispanic black woman.
Diversity and Inclusion in Genomics
Although the importance of diversity in genomics and precision medicine is well recognized, the majority of published studies are from European ancestry populations. As of 2009, approximately 96% of participants in genome-wide association studies (GWAS) were of European ancestry. By 2016, this figure had declined to 80%, primarily due to an increase in the study of East Asian populations. The number of GWAS studies involving participants of African or Amerindian ancestry and Hispanic or Latino ethnicity had not substantially increased.15 This is now changing with the introduction of large scale genomics studies such as the Trans-Omics for Precision Medicine (TOPMed) program,16 the Human Health and Heredity in Africa (H3Africa),17 and Population Architecture using Genomics and Epidemiology (PAGE).18
Cases where benign variants are misclassified as pathogenic due to lack of diversity in control data sets highlight the crucial need for diverse genomic data and biospecimen resources. For example, Manrai et al. showed that common, benign variants in black Americans had previously been misclassified in patients undergoing genetic testing for hypertrophic cardiomyopathy.19 Differences in allele frequencies between groups may lead to misdiagnosis and contribute to health disparities because of the lack of racial and ethnic diversity in control populations.19
Genetics, Epigenetics, and Pharmacogenetics
Recent genome-based advances now offer the opportunity to measure individual variability in genes, environment, and lifestyle that constitute an important basis for precision medicine. Next generation sequencing approaches also allow us to look for genetic patterns or variants, gene expression, and regulatory networks in subpopulations that correlate with disease phenotypes.20 For example, a recent study shows a greater risk for CVD in African Americans harboring certain variants of the LPA gene.21 Deep coverage whole genome sequencing in 8932 individuals of European and African Ancestry revealed similar heritability and shared variants in SORT1 and KIV2 CNV modifier loci, despite inter-ethnic differences in circulating Lp(a). However, LPAL2 intronic variants were identified that had significant but opposing effects in each ancestry group.21 Additionally, LPA locus variants that were largely private to African Americans conferred greater absolute effect on Lp(a) levels when compared to the shared variants.21 This discovery emphasizes the importance of rarer variants found primarily in specific ancestry groups for the prediction of risk in those groups. It also provides further context for the localization of epistatic effects that could be missed in single ancestry studies or those where minority population inclusion was minimal.
Important Role of Social and Environmental Determinants
The crucial role of social and environmental determinants of health (SDOH) is summed up in the aphorism that health status may be better determined by one’s zip code than genetic code. For racial and ethnic minority populations, non-genetic factors including social, economic, cultural, behavioral, lifestyle, community, neighborhood, and shared physical environment have important influences on cardiovascular health and related disparities across the lifespan. Current research suggests that these factors may lead to alterations in DNA methylation, accelerated loss of telomeres, and other epigenetic mechanisms that may provide the causal link between SDOH and the development of CVD.6,22 For example, the chronic stress of neighborhood deprivation, lack of social cohesion, joblessness, food insecurity, and racial discrimination adversely impact cardiovascular health and may provide the opportunity for hypothesis-driven research in health disparities.
NHLBI has identified SDOH as the first of six scientific focus areas for implementing its strategic vision in cardiovascular sciences.23 SDOH is also prime for hypothesis-driven studies, especially within the context of our cohort studies (Table), the NIH All of Us Research program, and NHLBI’s TOPMed program. For example, if the environmental and behavioral exposures in racial and ethnic minority communities are well characterized, could they be examined in interaction with genomic data to better understand the variation in the epigenome, transcriptome, proteome and metabolome? What are the biological pathways through which these factors interact in different ancestry populations to influence DNA methylation patterns and gene expression, perhaps with a focus on glucose and insulin homeostasis to impact cardiometabolic risk? How does exposure to ambient air pollution, interact with genomic variation to influence other epigenetic mechanisms to cause CVD and related disparities?
Racial and Ethnic Distribution of Participants in Large Epidemiologic Cohort Studies Funded by the National Heart, Lung, and Blood Institute
| Name of Cohort Study | Year of First Examination | Number of Participants | Percentage Female | Participant Distribution by Race or Ethnicity | Cohort Study Web Site |
|---|---|---|---|---|---|
| Framingham Heart Study Original Cohort | 1948 | 5,209 | 55% | White (100%) | www.framinghamheartstudy.org |
| Framingham Heart Study Offspring Cohort | 1971 | 5,124 | 52% | White (100%) | www.framinghamheartstudy.org |
| Coronary Artery Risk Development in Young Adults | 1985 | 5,115 | 54.5% | White (48.5%); Black (51.5%) | www.cardia.dopm.uab.edu |
| Atherosclerosis Risk in Communities | 1987 | 15,792 | 55% | White (73%); Black (27%) | www2.cscc.unc.edu/aric/ |
| Cardiovascular Health Study | 1989 | 5,888 | 42% | White (84%); Black (16%) | www.chs-nhlbi.org |
| Strong Heart Study | 1989 | 4,549 | 41% | American Indians (100%) | https://strongheartstudy.org/ |
| Women’s Health Initiative* | 1991 | 161,808 | 100% | White (83%); Black (9%); Asian (3%) | www.nhlbi.nih.gov/whi/ |
| Jackson Heart Study | 2000 | 5,306 | 63% | Black (100%) | https://www.jacksonheartstudy.org/ |
| Multi-ethnic Study of Atherosclerosis | 2000 | 6,814 | 53% | White (39%); Black (28%): Hispanic (22%); Chinese (12%) | www.mesa-nhlbi.org |
| Framingham Heart Study Generation 3 | 2002 | 4,095 | 47% | White (100%) | www.framinghamheartstudy.org |
| Hispanic Community Health Study/Study of Latinos | 2008 | 16,415 | 60% | Hispanic (100%) | www.cscc.unc.edu/hchs |
The Women’s Health Initiative initially had both observational and clinical trial components; the clinical trial was later converted to a follow-up observational study after trial termination; limited to female participants (all other studies on this list recruited both male and female subject
All of Us Research Program
The All of Us Research Program is a component of the National Precision Medicine Initiative launched in 2016 with the goal of understanding how genetics, environment, and lifestyle influence the best approaches for preserving health and preventing disease.7 It will recruit and follow over one million volunteers with an emphasis on diversity of people (age, race/ethnicity, sexual orientation, and socioeconomic status), geography, health statuses, and data types captured.24 Importantly, All of Us Research Program’s myriad data types collected on large numbers of participants who are under-represented in biomedical research offers exciting opportunities to advance precision medicine and mitigate health disparities.
Trans-Omics for Precision Medicine (TOPMed) Program
TOPMed was established by NHLBI to characterize the genetic architecture and phenotypic variation of heart, lung, blood, and sleep disorders, with the ultimate aim of improving disease prevention, diagnosis, and treatment. A founding principle was to generate a diverse resource with genomics in multiple non-European ancestry participants. The nearly 145,000 mainly U.S. participants include 40% European, 32% African, 16% Hispanic/Latino, 10% Asian, and 2% in “other” category.25 This level of participant diversity will provide much needed molecular biological information for these often under-represented and under-served populations.
Implementation Research Challenges
Major contributors to healthcare disparities include unequal access to care, unequal treatment, and often sub-optimal quality care delivery. Precision medicine will neither cure these problems nor be immune to their adverse impact on care delivery. However, designing strategies to enable precision medicine interventions to have broad reach, affordability, sustainability, and especially, social and cultural acceptability, and ensure respect in racial and ethnic populations and tribal communities will be crucial. Carefully-designed community-engaged implementation research within the complex settings of racial, ethnic, and tribal communities will be invaluable.26 Additionally, the use of evidence-based tools to guide genetic testing, can be useful in fostering precision medicine interventions that are proven effective and ready for individual- and population-level implementation.27
Cardiovascular Disparities in the Era of Precision Medicine
Despite remarkable declines in cardiovascular mortality in the U.S. over the last half-century, racial and ethnic disparities in CVD have remained pervasive.28 These disparities have been well-documented and their contributing factors have also been extensively reviewed.29,30 As NIH leadership recently emphasized, “it is not enough to identify factors that contribute to health disparities: intervention science must be applied in full force to seek solutions”.31 The question often asked is whether precision medicine will be part of the solution or does it have the potential to exacerbate these disparities?
The emerging consensus suggests that precision medicine may exacerbate disparities unless concerted efforts are made to prioritize diversity in GWAS and other large-scale genomics datasets and biorepositories.19 Additionally, differential access to the benefits of precision medicine could exacerbate racial and ethnic disparities in cardiovascular health. The myriad, complex, multilevel factors that contribute to unequal treatment are all plausible in the era of precision medicine and could also contribute to cardiovascular disparities.29 The challenge is to embrace these complexities and develop and test interventions that lead to equitable access to the benefits of precision medicine.32
The good news is that lessons from TOPMed, PAGE, and H3Africa suggest that diversity and inclusion in genomics studies is feasible. When appropriately informed, racial and ethnic minority populations are willing to participate in genetic studies.33 Studies with large numbers of racial and ethnic minority populations have already yielded important information to advance precision medicine. Examples include findings in sickle cell trait,34 APOL1,35 and lipid mutations.36 These findings provide new information for more personalized evaluation and prevention of chronic renal and atherosclerotic cardiovascular disease in persons who carry the related trait, risk alleles, or mutations, as part of the effort to advance precision medicine.
Other important considerations include recognition that racial and ethnic populations are not monolithic. Strategies to enrich participant diversity must be mindful of the cultural, linguistic, geographic, and genetic diversity, especially in Asian American and Hispanic populations. Gaining ethnic, tribal, or community trust is also crucial for full and active participation in genetic studies as well as in acceptance of precision medicine interventions.
Ethical Implications
Rapidly emerging areas of biomedical research always pose a variety of ethical issues, but particularly when research involves vulnerable populations, including underserved racial and ethnic minorities. The historical legacy of the Tuskegee study of untreated syphilis and other unethical human experimentation have generated mistrust.37 Other important considerations include issues of cultural disrespect, discrimination, and stigmatization. For example, to make treatment more precise, the use of genetic and epigenetic data requires stratification of patients into groups and subgroups.38 Inherent in this “division” is the risk of stigmatization and discrimination at individual, community, and population levels.39 Meanwhile, identification of a genetic “reason” for poor health in a given population could create a disincentive to address social and economic factors that contribute (sometimes substantially) to disease. Prior to widespread implementation of genetic testing beyond the research setting, careful analysis is needed to assure that this is the best use of resources to improve population health40 and that appropriate, culturally tailored genetic counseling will be available, affordable, and acceptable to inform action.41 Finally, we cannot ignore the potential adverse social and psychological effects of challenging underlying beliefs about self-identity. These ethical issues should not be seen as obstacles that prevent doing research, but rather as questions that help ensure that the use of precision medicine is implemented in a way that benefits all concerned.
Conclusions
Increasingly, the genetic contributions to health and disease are being leveraged to advance clinical management of CVD as part of the precision medicine endeavor. It is unclear whether these advances will ameliorate or exacerbate current CVD disparities. The emerging consensus suggests that the lack of diversity in genomics studies risk exacerbating disparities. Strategies that directly address diversity and inclusion and go beyond just race and ethnicity to explore ancestry-matched controls, as well as cultural, social, and environmental determinants, are crucially needed. Early successes of TOPMed and the NIH All of Us Research Program are reassuring. There is also an emerging consensus that precision medicine will not be immune to the challenges of unequal healthcare access and care delivery challenges. Designing interventions for broad reach, affordability, sustainability, and cultural acceptability in racial and ethnic minority populations and tribal communities will also be crucial.
Supplementary Material
Acknowledgements
We thank our colleagues, Dr. David Goff, Jr. and Dr. Nakela Cook, who provided constructive comments on an earlier version of the manuscript.
Non-standard Abbreviations and Acronyms
- CVD
Cardiovascular diseases
- NHLBI
National Heart, Lung, and Blood Institute
- NIH
National Institutes of Health
- TOPMed
Trans-Omics for Precision Medicine
- GWAS
Genome-wide association studies
- H3Africa
Human Health and Heredity in Africa
- PAGE
Population Architecture using Genomics and Epidemiology
- SDOH
Social and environmental determinants of health
Footnotes
Disclosures and Disclaimer
The authors have no conflicts to disclose.
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services.
References
- 1.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. November 10 2018;392(10159):1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. November 10 2018;392(10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy SL, Xu J, Kochanek KD, Arias E. Mortality in the United States, 2017. NCHS Data Brief. November 2018(328):1–8. [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. February 2 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 6.Kathiresan S, Srivastava D. Genetics of human cardiovascular disease. Cell. March 16 2012;148(6):1242–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins FS, Varmus H. A new initiative on precision medicine. N Engl. J Med. 2/26/2015 2015;372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burchard EG, Ziv E, Coyle N, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. March 20 2003;348(12):1170–1175. [DOI] [PubMed] [Google Scholar]
- 9.Cooper RS, Kaufman JS, Ward R. Race and genomics. N. Engl. J. Med 3/2003 2003;348(12):1166–1170. [DOI] [PubMed] [Google Scholar]
- 10.Executive Office of the President, Office of Management and Budget. Standards for Maintaining, Collecting, and Presenting Federal Data on Race and Ethnicity Federal Register. 2016;81(190):67398–67401. [Google Scholar]
- 11.Divers J, Redden DT, Rice KM, et al. Comparing self-reported ethnicity to genetic background measures in the context of the Multi-Ethnic Study of Atherosclerosis (MESA). BMC genetics. March 4 2011;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. January 8 2015;96(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DR. Race/ethnicity and socioeconomic status: measurement and methodological issues. Int J Health Serv. 1996;26(3):483–505. [DOI] [PubMed] [Google Scholar]
- 14.LaVeist TA. Disentangling race and socioeconomic status: a key to understanding health inequalities. Journal of urban health : bulletin of the New York Academy of Medicine. June 2005;82(2 Suppl 3):iii26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. October 13 2016;538(7624):161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Heart, Lung, and Blood Institute. Trans-Omics for Precision Medicine (TOPMed) Program. NHLBI. 2016. https://www.nhlbi.nih.gov/research/resources/nhlbi-precision-medicine-initiative/topmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Human Heredity and health in Africa. H3Africa Vision. https://h3africa.org/index.php/about/vision/. Accessed December 8, 2018.
- 18.Matise TC, Ambite JL, Buyske S, et al. The Next PAGE in understanding complex traits: design for the analysis of Population Architecture Using Genetics and Epidemiology (PAGE) Study. Am J Epidemiol. October 1 2011;174(7):849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manrai AK, Funke BH, Rehm HL, et al. Genetic Misdiagnoses and the Potential for Health Disparities. N Engl J Med. August 18 2016;375(7):655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antman EM, Loscalzo J. Precision medicine in cardiology. Nat Rev Cardiol. October 2016;13(10):591–602. [DOI] [PubMed] [Google Scholar]
- 21.Zekavat SM, Ruotsalainen S, Handsaker RE, et al. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nature communications. July 4 2018;9(1):2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Harst P, de Windt LJ, Chambers JC. Translational Perspective on Epigenetics in Cardiovascular Disease. J Am Coll Cardiol. August 1 2017;70(5):590–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goff DC Jr., Buxton DB, Pearson GD, Wei GS, et al. Implementing the National Heart, Lung, and Blood Institute’s Strategic Vision in the Division of Cardiovascular Sciences. Circulation Research. 2019;124:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dishman E Letter from the Director: Guest Director’s Letter: Driving Toward Quadruple Diversity in the All of Us℠ Research Program; October 20, 2016. https://www.niams.nih.gov/about/about-the-director/letter/driving-toward-quadruple-diversity.
- 25.Laurie CC, Blackwell TW, Kang HM, Wong Q, Blangero J, et al. The NHLBI Trans-Omics for Precision Medicine (TOPMed) Program. Journal. 2019;(in review). [Google Scholar]
- 26.Mensah GA, Cooper RS, Siega-Riz AM, et al. Reducing Cardiovascular Disparities Through Community-Engaged Implementation Research: A National Heart, Lung, and Blood Institute Workshop Report. Circ Res. January 19 2018;122(2):213–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoury MJ, Coates RJ, Evans JP. Evidence-based classification of recommendations on use of genomic tests in clinical practice: dealing with insufficient evidence. Genetics in medicine : official journal of the American College of Medical Genetics. November 2010;12(11):680–683. [DOI] [PubMed] [Google Scholar]
- 28.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. March 15, 2005;111(10):1233–1241. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. [PubMed] [Google Scholar]
- 30.Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. May 17 2000;283(19):2579–2584. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Stable EJ, Collins FS. Science Visioning in Minority Health and Health Disparities. Am J Public Health. January 2019;109(S1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purnell TS, Calhoun EA, Golden SH, et al. Achieving Health Equity: Closing The Gaps In Health Care Disparities, Interventions, And Research. Health Aff (Millwood). August 1 2016;35(8):1410–1415. [DOI] [PubMed] [Google Scholar]
- 33.Walker ER, Nelson CR, Antoine-LaVigne D, et al. Research participants’ opinions on genetic research and reasons for participation: a Jackson Heart Study focus group analysis. Ethn Dis. Summer 2014;24(3):290–297. [PubMed] [Google Scholar]
- 34.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. November 26 2014;312(20):2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito K, Bick AG, Flannick J, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res. February 28 2014;114(5):845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crosby J, Peloso GM, Auer PL, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. July 3 2014;371(1):22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White RM. Misinformation and misbeliefs in the Tuskegee Study of Untreated Syphilis fuel mistrust in the healthcare system. J. Natl. Med. Assoc 11/2005 2005;97(11):1566–1573. [PMC free article] [PubMed] [Google Scholar]
- 38.Batten JN. How Stratification Unites Ethical Issues in Precision Health. AMA journal of ethics. 2018;20(9):E798–803. [Google Scholar]
- 39.Clayton EW. Ethical, legal, and social implications of genomic medicine. N Engl J Med. August 7 2003;349(6):562–569. [DOI] [PubMed] [Google Scholar]
- 40.Chowkwanyun M, Bayer R, Galea S. “Precision” Public Health - Between Novelty and Hype. N Engl J Med. October 11 2018;379(15):1398–1400. [DOI] [PubMed] [Google Scholar]
- 41.Halbert CH, Harrison BW. Genetic counseling among minority populations in the era of precision medicine. American journal of medical genetics. Part C, Seminars in medical genetics. March 2018;178(1):68–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.