Abstract
OBJECTIVES:
To assess the extent to which persons aged 70 and older undergoing hemodialysis (HD) had greater changes in health-related quality of life (HRQOL) over 3 years than younger patients undergoing HD.
DESIGN:
Longitudinal.
SETTING:
The Hemodialysis Study (HEMO Study) was a randomized, clinical trial of the effects of HD dose and membrane flux on mortality and morbidity in patients undergoing chronic dialysis.
PARTICIPANTS:
Secondary analysis of the HEMO Study.
MEASUREMENTS:
Participants completed the Index of Well-Being (IWB) and the Kidney Disease Quality of Life—Long Form (KDQOL-LF), which also includes the Medical Outcomes Study 36-item Short Form Questionnaire (SF-36) annually. Changes in subjects those aged 70 and older were compared with changes in subjects aged 55 to 69 and 18 to 54.
RESULTS:
At baseline, 1,813 (98%) of HEMO participants completed HRQOL surveys. Their mean age was 58, 56% were female, 64% were black, and mean duration of dialysis was 3.8 years. In subjects with HRQOL data at the first three annual assessments, there were no substantial mean declines in the SF-36 Physical or Mental Component Summary scales over 3 years. In models incorporating effects of attrition, the differences in average change over 3 years between patients undergoing HD aged 70 and older and the younger cohorts were small in magnitude. There were high rates of adverse HRQOL events in all age groups and significantly higher composite event rates of death or clinically significant decline in HRQOL over 3 years was found in subjects aged 70 and older.
CONCLUSION:
Although HRQOL was impaired in the population undergoing HD, HRQOL scores at baseline reflect a better-preserved multidimensional quality of life in respondents in the HEMO Study aged 70 and older than in younger patients undergoing HD. There was no substantial relationship between age and average decline in HRQOL score over 3 years in participants in the HEMO Study.
Keywords: hemodialysis, quality of life, aging, chronic health conditions
The proportion of older patients undergoing hemodialysis (HD) in the United States is rapidly increasing.2 This has been a worldwide phenomenon, with a marked increase in the rate of elderly patients undergoing incident dialysis over the past 2 decades in Canada3 and similar rates of increase of dialysis in elderly people in Europe and Japan.4,5 Greater access to kidney care for elderly people has driven part of this increased incidence of dialysis. Older and sicker patients have been referred for HD in Asia, Europe, and North America because of perceived improvements in quality of life on dialysis and cultural factors. Once referred for dialysis, older patients have been surviving longer with renal replacement therapy as dialysis adequacy has increased and kidney transplant outcomes have improved.
As a result of improvements in technology and greater access to dialysis, the increased prevalence of older adults undergoing renal replacement therapy generally mirrors the aging trend of the general population. Healthcare providers are increasingly called on to advise older patients on the prospect of life supported by renal replacement therapy and care for older patients supported by HD. Individual experience largely drives these judgments, because there is a paucity of evidence regarding the outcomes of older persons undergoing HD. Although improving health-related quality of life (HRQOL) may be the most important role of health care in elderly patients with chronic illness,6 long-term HRQOL data in elderly patients undergoing HD are lacking.
Despite the increasing numbers of older patients undergoing HD, information on HRQOL in the elderly population undergoing HD has been conflicting, with some studies relating impaired HRQOL and others failing to find impairment. Early studies of older patients undergoing dialysis have shown markedly lower functional status than in older community-dwelling adults without kidney disease,7 but the delivery of HD has improved, with advances in technology, treatment of comorbidities such as anemia8 and hyperparathyroidism,9 and quality improvement initiatives.10 In addition, patients undergoing HD are now more likely to be older, have limited functional status, and multiple comorbid illnesses.11 Other studies of older patients undergoing HD have demonstrated preserved HRQOL,12 particularly when compared with the magnitude of impairment found in younger patients with end-stage renal disease (ESRD).13,14 However, most studies have been limited to cross-sectional comparisons and have been unable to describe the patterns of change in HRQOL in older patients undergoing HD. The few contemporary longitudinal studies that have examined HRQOL and healthcare utilization in older patients undergoing dialysis have been limited in their scope of HRQOL assessment,15 sample size, and duration of follow-up.16 Moreover, many of these studies have relied on self-report rather than the use of an interviewer.17,18 Although using interviewers increases the costs of gathering HRQOL data, interviewing patients permits the acquisition of HRQOL information from older patients and patients with physical and visual disabilities.19 Hence, it remains unclear to what extent older age would be associated with declines in HRQOL in contemporary patients with multiple comorbidities undergoing thrice-weekly HD.
To address the gap in knowledge regarding the HRQOL of older persons undergoing HD, this report used data gathered by the HEMO Study, a multicenter, randomized trial of HD dose and membrane flux. The HEMO Study previously reported that higher-dose HD was associated with a significantly smaller decline in physical health and bodily pain than standard-dose treatment, but the treatment effects were small.20 There was no association between higher HD flux and better HRQOL.20 Because the HEMO Study recruited adults undergoing HD in multiple centers across the United States, a substantial number of participants were aged 70 and older. Therefore, this report assesses whether persons aged 70 and older undergoing HD had greater changes in HRQOL over 3 years than younger patients undergoing HD.
METHODS
Study Design
The HEMO Study was a 15-center randomized clinical trial of the effects of HD dose and membrane flux on mortality and morbidity in patients undergoing chronic dialysis.1 Patients in this study were randomized to standard- or high-dose (targeted eKt/V of 1.05 vs 1.45) and to high- or low-flux membranes (targeted beta-2 microglobulin clearance of <10 vs >20mL/min). Patient eligibility criteria have been described previously.21 The institutional review boards at the 15 institutions approved the study protocol, and written informed consent was obtained from all study participants. Enrollment in the HEMO Study began in March 1995 and ended in October 2000. At randomization and annually during follow-up, HEMO Study patients were administered the Campbell Index of Well Being (IWB) and the Kidney Disease Quality of Life-Long Form (KDQOL-LF) questionnaires.20
Data Collection
Study interventions and general data collection procedures have been described previously.1 Demographic information and clinical history were collected through review of medical records and self-reported questionnaires. Clinical data, including laboratory measurements, were obtained using standardized protocols. Comorbidity was assessed at baseline using the Index of Coexistent Disease (ICED),21 which aggregates the presence and severity of 19 medical conditions and 11 physical impairments into two summary indices: the Index of Disease Severity (IDS) and the Index of Physical Impairment (IPI). An algorithm combining peak scores for the IDS and IPI determines the final ICED score. ICED scores range from 0 to 3, with a higher score reflecting greater disease severity.
The HRQOL questionnaires were self- or interviewer-administered using a standard protocol for assessment of the IWB and KDQOL-LF.22 Research coordinators administered an interview version of the HRQOL survey when patients were unable to self-administer the form because of physical impairment or when they stated a strong preference for the interview format. Interviewers were directed to read the survey verbatim and not to rephrase items. The survey comprised the IWB and the KDQOL-LF.23 The IWB has been used extensively to assess psychological well-being in patients with ESRD. It consists of the Index of General Affect (IGA), which measures how a subject feels about his or her life (e.g., boring to interesting, enjoyable to miserable), and the Index of Life Satisfaction (ILS), which is a single question regarding how satisfied a subject is with his or her life. The scoring of the IWB combines these two instruments (IWB = [1.0 × IGA] + [1.1 × ILS]), to yield scores ranging from 2.1 to 14.7. The range for the IWB is 2.1 (low well-being) to 14.7 (high well-being). The IWB has been shown to be reliable and valid in populations with and without ESRD.24
The Medical Outcomes Study 36-item Short Form Health Survey (SF-36) is the generic core of the KDQOL-LF. It has been evaluated extensively in the general population and the population of people with ESRD.25–27 The SF-36 questions are grouped into eight scales: physical functioning (10 items), role-physical (4 items), bodily pain (2 items), general health (5 items), vitality (4 items), social functioning (2 items), role-emotional (3 items), and mental health (5 items).1 The range for all scales is from 0 to 100, with higher scores indicating better health. Two component summary scores are derived from the eight subscales. The Physical Component Summary Scale (PCS) aggregates items from physical functioning, role-physical, bodily pain, general health, vitality, and social functioning, and the Mental Component Summary Scale (MCS) aggregates items from role-emotional and mental health and also includes elements of General health, vitality, and social functioning. In the general population, the scores for the components are computed using an algorithm that standardizes the scores so that the mean for each summary scale is 50 points with a standard deviation of 10 points. The KDQOL-LF includes a symptoms and problems scale (34 items) that assesses the extent to which symptoms such as dry itchy skin, thirst and hunger, pain in the joints or back, muscle cramps or soreness, and clotting or other problems with the dialysis access site, bother the subject. The Effects of Kidney Disease scale (20 items) measures the effect of dialysis on daily life with questions about restrictions on fluid and dietary intake, work, travel, lifting, and personal appearance. Sleep quality measures the daytime symptoms of fatigue and sleepiness and perceived sleep adequacy. The Burden of Kidney Disease (4 items) considers the effect of kidney failure on a subject’s sense of accomplishment and achievement. Cognitive Function (6 items) assesses difficulty with memory and concentration. Social Support (4 items) measures satisfaction with family and social life. Dialysis Staff Encouragement (6 items) measures the extent to which dialysis staff encourage patients to be independent and to lead as normal a life as possible. Patient Satisfaction (2 items) assesses how well care meets expectations. The range of scores for the dialysis-targeted scales was 0 to 100, with higher scores reflecting better health. The internal consistency reliability for the IWB and KDQOL-LF are adequate for group-level comparisons, with all scales having an internal consistency reliability as measured according to Cronbach alphas ranging from 0.72 to 0.79.19
Selection of Covariates
Factors found to predict HRQOL and survival in the general population and cross-sectional studies of dialysis patients were used to guide the selection of potential confounders. These were demographics (sex, race, education), study factors (mode of survey administration, study site), and clinical HD factors (dose of dialysis, dialysis flux);1 laboratory factors (hematocrit,28 serum albumin,29,30 serum creatinine,29 serum phosphate31); and comorbid disease. Patients undergoing dialysis often have substantial comorbid diseases that are associated with poor HRQOL.32–34 Therefore, the models included the diagnosis of diabetes mellitus, the cause of kidney failure, and comorbidity as measured using the ICED.
Statistical Methods
Demographic and laboratory factors are described as means for continuous variables and frequencies for categorical variables. Differences between groups were assessed using analysis of variance for continuous or ratio-level variables (e.g., albumin). Cochran-Mantel-Haenszel tests were used for categorical variables (e.g., race). Descriptive statistics were calculated for each HRQOL scale (mean, standard deviation, response rate, and percentage of patients at the floor and ceiling). The scales’ internal consistency reliability was estimated using Cronbach coefficient alphas.
Attrition of patients over time often complicates analysis of changes in HRQOL.35–38 High rates of death and kidney transplantation make this concern particularly salient in dialysis studies. The overall mortality rate in the HEMO Study was 16.6% per year, and the combined attrition rate for mortality, transplantation, dialysis modality switches, and transfers to nonstudy dialysis units exceeded 23% per year.
Changes in each HRQOL scale according to age group were estimated from baseline to follow-up Years 1, 2, and 3 using a mixed-effects model for mean changes in all randomized patients, including those who died or otherwise dropped out.39,40
An unstructured covariance matrix was specified for each model. Each analysis controlled for prespecified baseline covariates: albumin, creatinine, phosphate, ICED, duration of dialysis, race, sex, diabetic status, dose of dialysis, dialysis flux, education level (college vs no college), study site, and mode of questionnaire administration. Because this statistical model incorporated patients who died during the defined follow-up, the resulting mean changes tended to show greater declines in HRQOL than would models that only incorporate patients who survived. Other censoring events included transplantations, transfers, and the close of the study.
To gauge the effects of informative censoring, each change-in-HRQOL analysis was repeated using an approach for mitigating attrition-related bias (mixture informative censoring method).41 The resulting subgroup comparisons were similar to those obtained using methods that did not adjust for attrition, so it was decided to report results from the more-standard model. To illustrate the influence of attrition on the mean changes, estimates were calculated only for patients who provided data at follow-up Years 1, 2, and 3, this time using generalized linear models.
In light of informative censoring, testing associations between the age groups and changing HRQOL was also approached by estimating Kaplan-Meier curves for the composite endpoints of time until death or declines in MCS or PCS. Clinically significant HRQOL declines were pre-defined as a 0.5 standard deviation (SD) drop (e.g., 5.1 points for MCS and 5.2 points for PCS) from each patient’s baseline score. Composite outcomes were based on approximately 5-point decreases in PCS and MCS scores, because a 5-point decrease represents 0.5 SDs in the general population and has been used previously as a minimal important difference threshold.42,43 These results were then compared informally with the Kaplan-Meier results for death alone.
The hypotheses of effects of age on different HRQOL dimensions were regarded as nonexchangeable and distinct. Therefore, to avoid the loss of statistical power associated with a multiple-comparisons adjustment, P-values for the effects of the interventions on the individual HRQOL scales were calculated on a comparison-wise basis for each scale. All analyses were performed in SAS v8.0 (SAS Institute, Inc., Cary, NC).
RESULTS
HEMO Study Patient Characteristics
A total of 2,677 patients undergoing HD were screened; 1,846 were randomized, and 1,813 (98 %) completed the HRQOL questionnaire at baseline. The 33 patients who did not respond to the survey did not speak English or Spanish. Among the 1,813 participants, the average age was 57.6, 56% were female, and nearly two-thirds were African-American. A majority of the patients had diabetes mellitus or hypertension as the cause of chronic kidney failure, and approximately one-third had the highest possible comorbidity index score. The average duration of dialysis was 3.75 years. Sixty percent were on high-flux dialysis membranes at baseline; the average Kt/V before randomization was 1.42. Table 1 shows relevant sociodemographic and clinical characteristics of the 1,813 respondents according to age group. Those age 70 and older were less likely to be black and more likely to have been undergoing HD for fewer years and to have lower serum creatinine and lower serum phosphate. Table 1 also shows the overall difference in the respective HRQOL scales at baseline without adjustment for baseline covariates. Subjects aged 18 to 55 had a significantly higher PCS (better physical well-being), worse effects of kidney disease, and poorer sleep quality than those aged 70 and older. Subjects aged 55 to 70 had a significantly higher index of well-being, lower effects of kidney disease, and poorer sleep quality than those aged 70 and older.
Table 1.
Baseline Distribution of 1,813 Hemodialysis Study Participant Characteristics and Health-Related Quality of Life According to Age Group
| Age | |||
|---|---|---|---|
| variable | 18–54 (n = 708) | 55–69 (n = 747) | ≥70 (n = 391) |
| Age, mean | 42.7 | 62.9 | 74.4 |
| Female, %** | 49.6% | 36.8% | 46.5% |
| Black, %** | 61.3 | 68.7 | 53.5 |
| Diabetes mellitus, %** | 30.5 | 55.0 | 50.1 |
| Severe comorbidity (Index of Coexistent Disease = 3)** | 29.0 | 36.7 | 35.0 |
| Self-administration of survey** | 24.7 | 46.5 | 50.1 |
| Years undergoing hemodialysis, mean ± SD | 4.66 ± 5.29# | 3.42 ± 3.79# | 2.73 ± 2.99 |
| Education, college or higher, % | 32.8 | 22.1 | 23.5 |
| Albumin, mg/dL, mean ± SD | 3.71 ± 0.43# | 3.58 ± 0.36 | 3.56 ± 0.36 |
| Creatinine, mg/dL, mean ± SD | 11.5 ± 3.07# | 9.77 ± 2.56# | 8.93 ± 2.27 |
| Phosphate, mg/dL, mean ± SD | 6.18 ± 2.02# | 5.68 ± 1.79# | 5.22 ± 1.57 |
| Index of Well-Being, mean ± SD | 9.8 ± 2.8 | 10.3 ± 3.0* | 9.8 ± 3.1 |
| SF-36 Physical Component Summary, mean ± SD | 37.7 ± 10.2∧ | 34.6 ± 10.0 | 34.4 ± 9.8 |
| SF-36 Mental Component Summary, mean ± SD | 48.8 ± 11.0 | 50.7 ± 10.8 | 50.2 ± 10.9 |
| Effects of Kidney Diseases, mean ± SD | 62.2 ± 20.9∧ | 67.0 ± 20.6∧ | 71.7 ± 20.1 |
| Symptoms of Kidney Diseases, mean ± SD | 74.8 ± 14.6 | 75.3 (13.9 | 76.0 ± 13.5 |
| Sleep Quality, mean ± SD | 57.3 ± 22.7∧ | 58.2 ± 22.4* | 62.4 ± 22.8 |
| Cognitive Function, mean ± SD | 76.3 ± 20.5 | 75.2 ± 20.9 | 74.3 ± 20.1 |
| Patient Satisfaction, mean ± SD | 68.7 ± 20.7 | 70.0 ± 19.6 | 68.5 ± 19.3 |
Global test of between-group differences: P<.001.
Compared with aged 70 and older:
P<.001;
P<.01;
P<.05. SD = standard deviation.
The adjusted mean physical and mental component summary scores for subjects who survived and completed the HRQOL questionnaires at the first three annual assessments were assessed and shown in Table 2. Among these subjects, there were no substantial mean declines in PCS or MCS over the 3-year period. The largest 3-year declines were in PCS levels for subjects aged 55 to 70 (1.2 points). In comparison, subjects aged 70 and older had a 0.7-point 3-year decline in PCS. Mean 3-year changes in MCS were negligible. The number of respondents was 27% lower at Year 1, 53% lower at Year 2, and 67% lower at Year 3 from 1,813 at baseline.
Table 2.
Adjusted Mental and Physical Well-Being According to Age Group in Survivors
| Mental Component Summary |
Physical Component Summary |
|||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 1 | Year 2 | Year 3 | |
| Age | Mean (95% Confidence Interval) | |||||
| 18–54 | 48.4 (47.4–49.5) | 48.7 (47.4–50.0) | 47.7 (46.1–49.3) | 36.0 (35.1–36.9) | 36.6 (35.4–37.8) | 36.1 (34.7–37.6) |
| 55–69 | 50.1 (49.1–51.1) | 49.2 (47.9–50.6) | 50.6 (49.0–52.2) | 35.1 (34.2–36.0) | 34.6 (33.4–35.8) | 33.9 (32.5–35.3) |
| ≥70 | 50.8 (49.4–52.3) | 51.5 (49.5–53.5) | 51.1 (48.6–53.6) | 35.4 (34.1–36.7) | 34.5 (32.7–36.3) | 34.7 (32.4–36.9) |
Note: Using a generalized linear model adjusting for baseline values for albumin, creatinine, phosphate, Index of Coexistent Disease, duration of dialysis, dose of dialysis, dialysis flux, race, sex, diabetic status, level of education, and mode of questionnaire administration.
The difference in mean changes incorporating effects of attrition between subjects aged 70 and the younger age groups in HRQOL over 3 years are shown in Table 3. In Table 3, a positive value demonstrates a better score in that particular quality-of-life domain. Overall, the differences in average change over 3 years between those aged 70 and older and the younger cohorts were small in magnitude. The older age group had a better IWB score (global quality of life) than those aged 55 to 70 and a trend toward a better IWB score than those 18 to 55. There were no significant differences in the average changes between those aged 70 and older and the younger age groups in PCS or MCS scores. There were also no significant differences in the average changes between those aged 70 and older and the younger age groups in any of the SF-36 subscale scores (data not shown). However, subjects aged 70 and older had significantly lower symptoms and problems scores over the 3-year period and a trend toward worsening sleep quality than the younger age groups. Subjects aged 70 and older demonstrated significantly worsening cognitive function scores over the 3-year period. In addition, subjects aged 70 and older had better patient satisfaction than those aged 55 to 70 but not different from that of those younger than 55. There were no significant differences in other domains of the KDQOL-LF, such as burden of kidney disease, social support, or staff encouragement (data not shown). In addition, whether there was a significant interaction of albumin and age in the models of longitudinal HRQOL was examined, although there was not a significant multiplicative interaction between albumin and age in the domains of HRQOL assessed in the HEMO Study
Table 3.
Adjusted Mean Changes in Health-Related Quality of Life over 3 Years Comparing Subjects Aged 70 and Older with Younger Subjects
| Year 1 to Baseline Effect |
Year 2 to Baseline Effect |
Year 3 to Baseline Effect |
Average Effect |
P-Value |
|
|---|---|---|---|---|---|
| Domain | Change (Standard Error) | ||||
| Index of Well-Being | |||||
| ≥70 vs < 18–54 | 0.51 (0.22) | 0.22 (0.29) | 0.41 (0.37) | 0.38 (0.23) | .10 |
| ≥70 vs 55–69 | 059 (0.23) | 0.33 (0.29) | 0.35 (0.37) | 0.42 (0.22) | .05 |
| Physical Component Summary score | |||||
| ≥70 vs < 18–54 | −0.42 (0.70) | −1.44 (0.91) | −0.78 (1.08) | −0.60 (0.72) | .41 |
| ≥70 vs 55–69 | 0.18 (0.70) | −0.71 (0.92) | 0.06 (1.06) | −0.16 (0.71) | .83 |
| Mental Component Summary score | |||||
| ≥70 vs o 18–54 | −0.38 (0.79) | −0.07 (1.04) | −0.77 (1.28) | −0.41 (0.82) | .62 |
| ≥70 vs 55–69 | 0.38 (0.79) | 1.06 (1.05) | −0.59 (1.26) | 0.28 (0.80) | .72 |
| Symptom and Problems | |||||
| ≥70 vs <18–54 | −0.80 (0.79) | −1.77 (0.98) | −3.03 (1.39) | −1.87 (0.84) | .03 |
| ≥70 vs 55–69 | −1.41 (0.79) | −0.85 (0.95) | −2.04 (1.38) | −1.43 (0.81) | .08 |
| Effects of Kidney Disease | |||||
| ≥70 vs <18–54 | −0.20 (1.32) | −1.37 (1.10) | 1.64 (1.97) | 0.03 (1.97) | .98 |
| ≥70 vs 55–69 | −0.64 (1.29) | −0.87 (1.60) | −0.29 (1.96) | −0.60 (1.29) | .64 |
| Sleep | |||||
| ≥70vs <18–54 | −4.53 (1.52) | −3.42 (2.13) | −0.71 (2.36) | −2.89 (1.59) | .07 |
| ≥70 vs 55–69 | −3.20 (1.49) | −2.10 (2.12) | −3.58 (2.34) | −2.96 (1.55) | .06 |
| Cognition Function | |||||
| ≥70 vs <18–54 | −2.59 (1.33) | −5.43 (1.78) | −4.77 (2.35) | −4.26 (1.44) | .003 |
| ≥70 vs 55–69 | −1.30 (1.33) | −3.80 (1.77) | −3.35 (2.38) | −2.82 (1.41) | .05 |
| Patient Satisfaction | |||||
| ≥70 vs <18–54 | −0.63 (1.53) | 0.17 (1.91) | 0.61 (2.35) | 0.05 (1.52) | .97 |
| ≥ 70 vs 55–69 | 0.98 (1.49) | 3.38 (1.83) | 4.56 (2.27) | 2.97 (1.43) | .04 |
Note: Using mixed-effects models adjusting for baseline values for albumin, creatinine, phosphate, Index of Coexistent Disease, duration of dialysis, dose of dialysis, dialysis flux, race, sex, diabetic status, level of education, and mode of questionnaire administration.
To further explore the relationship between longitudinal changes in HRQOL and age, composite end-points of significant declines in HRQOL or death were examined. The event rate of a clinically significant drop in HRQOL is also displayed for each domain of HRQOL in Figure 1A–F according to age group. In general, the composite event rate of a clinically significant decline in HRQOL or death was significantly higher in subjects aged 70 and older, whereas the event rate only for the lowered quality of life tended to be higher for those younger than 55.
Figure 1.
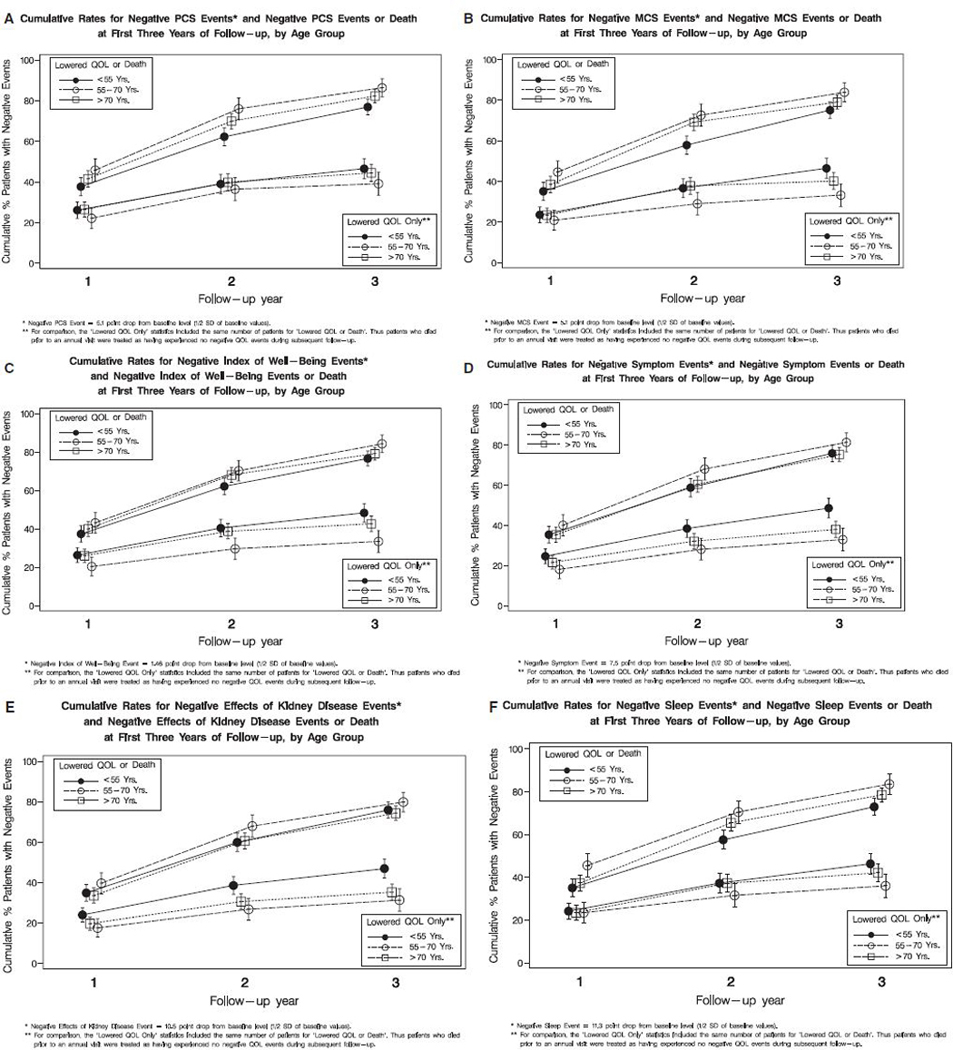
(A) Composite outcome of decline in Physical Component Summary (PCS) or death and outcome of decline in PCS according to age group. The 3-year event rate of decline in PCS was 40.3% for subjects younger than 55 (<55 vs > 70; P =.06), 41.6% for those aged 55 to 70 (55–70 vs >70; P =.14), and 33.5% for those aged 70 and older. The 3-year composite event rate of decline in PCS or death, was 73.0% for subjects younger than 55 (<55 vs > 70; P =.002), 79.1% for those aged 55 to 70 was (55–70 vs >70; P =.15), and 82.0% for those aged 70 and older. (B) Composite outcome of decline in Mental Component Summary (MCS) or death and outcome of decline in MCS according to age group. The 3-year event rate of decline in MCS for was 46.5% for subjects younger than 55 (<55 vs >70; P =.001), 40.2% for those aged 55 to 70 (55–70 vs >70; P =.05), and 33.3% for those aged 70 and older. The 3-year composite event rate of decline in MCS or death was 75.1% for subjects younger than 55 (<55 vs >70; P =.007), 79.1% for those aged 55 to 70 (55–70 vs >70; P =.10), and 83.9% for those aged 70 and older. (C) Composite outcome of decline in Index of Well-Being (IWB) or death and outcome of decline in IWB according to age group. The 3-year event rate of decline in IWB was 48.4% for subjects younger than 55 (<55 vs > 70; P <.001), 42.7% for those aged 55 to 70 (55–70 vs >70; P =.001), and 33.5% for those aged 70 and older. The 3-year composite event rate of decline in IWB or death was 76.7% for subjects younger than 55 (<55 vs >70; P =.02), 79.2% for those aged 55 to 70 (55–70 vs >70; P =.07), and 84.3% for those aged 70 and older. (D) Composite outcome of decline in Symptoms of Kidney Disease or death and outcome of decline in symptoms according to age group. The 3-year event rate of decline in Symptoms of Kidney Disease was 48.6% for subjects younger than 55 (<55 vs >70; P<.001), 38.0% for those aged 55 to 70 (55–70 vs >70; P =.15), and 33.0% for those aged 70 and older. The 3-year composite event rate of decline in symptoms of kidney disease or death was 75.9% for subjects younger than 55 (<55 vs >70; P =.10), 75.3% for those aged 55 to 70 (55–70 vs >70; P =.04), and 81.4 % for those aged 70 and older. (E) Composite outcome of decline in Effects of Kidney Disease or death and outcome of decline in effects according to age group. The 3-year event rate of decline in Effects of Kidney Disease was 46.9% for subjects younger than 55 (<55 vs >70; P <.001), 35.3% for those aged 55 to 70 (55–70 vs >70; P =.25), and 31.3% for those aged 70 and older. The 3-year composite event rate of decline in Effects of Kidney Disease or death was 75.9% for subjects younger than 55 (<55 vs >70; P =.24), 74.3% for those aged 55 to 70 (55–70 vs >70; P =.07), and 79.8 % for those aged 70 and older. (F) Composite outcome of decline in Sleep Quality or death and outcome of decline in Sleep Quality according to group. The 3-year event rate of decline in Sleep Quality was 46.3% for subjects younger than 55 (<55 vs >70; P <.008), 42.1% for those aged 55 to 70 (55–70 vs >70; P =.08), and 36.0% for those aged 70 and older. The 3-year composite event rate of decline in sleep quality or death was 72.9% for subjects younger than 55 (<55 vs >70; P =.008), 78.5% for those aged 55 to 70 (55–70 vs >70; P = 0.09), and 83.5% for those aged 70 and older.
Sensitivity Analyses
Variations of the informative censoring model added terms to distinguish between patients censored because of kidney transplantation and surviving patients who dropped out of the study for other reasons. The results were essentially the same as those of the main analysis presented, with estimated effects differing by no more than 0.05 units.
CONCLUSION
The burden of chronic kidney failure and HD treatment was shown in older and younger patients in the domains of general-well being, physical well-being, symptoms and effects of kidney disease, and sleep quality. Although HRQOL was impaired in the older adult population undergoing HD, the HRQOL scores at baseline reflected a relatively preserved multidimensional quality of life in respondents in the HEMO Study aged 70 than in younger patients undergoing HD. This in part reflects the poor HRQOL of younger patients. In persons in the HEMO Study surviving on HD for 3 years and completing the HRQOL surveys, multidimensional HRQOL did not substantially change in the three age groups. After accounting for attrition due to death, there were still only small differences in the average declines in HRQOL over time between patients undergoing HD who were aged 70 and older and the younger patients. Alternatively, the composite outcomes of clinically significant decline in HRQOL or death of subjects aged 70 and older over 3 years of follow-up was 70%.
This event rate largely reflects the higher risk of death in those older patients undergoing HD and indicates the difficulties in presenting longitudinal HRQOL when there are high rates of attrition due to death, because HRQOL scores are highly linked to the likelihood of survival. Although people of all ages undergoing HD would benefit from efforts to improve and maintain HRQOL, there was not a particular HRQOL burden on the older patients participating in the HEMO Study.
This report addresses a gap in the data on HRQOL and clinical outcomes in older people undergoing HD. The HEMO Study design overcame many of the limitations of prior reports regarding age and HRQOL; the study population was a large, multicenter HD cohort receiving an adequate dose of dialysis, and the HRQOL instrument measured a multidimensional concept of health. These findings also capture subjects who would otherwise be unable to respond to a self-report survey by providing interviewers when patients were unable to complete a survey.19 This was particularly important in older patients; nearly half of those who responded used an interviewer.19 The analysis incorporated extensive adjustment for demographic and socioeconomic factors, as well as a validated index of comorbidity.34 This analysis also accounts for treatment assignment to high-flux or high-dose HD and reports HRQOL findings on a population of patients with ESRD receiving an adequate HD dose. Analytical techniques designed to defend against potential bias from informative censoring aided the interpretation of longitudinal change in HRQOL.
This study extends the previous longitudinal studies of HRQOL in patients undergoing HD by examining a racially diverse cohort over long-term follow-up, using a multidimensional assessment, and having long-term follow up. The decline in scores on physical domains over time in the subjects aged 55 and older (Table 2) and stability of the mental domains is consistent with a previous study of incident patients remaining on HD over 18 months.18 A previous study also suggested that comorbid disease burden, rather than age, was chiefly associated with a composite outcome of hospitalization, decline in albumin, and an SF-36 MCS or PCS of 2 SDs below the general population mean score.32 The findings of preserved longitudinal HRQOL may be due to response shift,44 lower expectations of health,45 development of coping skills,46 treatment of anemia,47 and attention to symptom management48 by healthcare teams, but the HEMO Study high-dose and high-flux interventions did not substantially influence longitudinal HRQOL.20
Although markedly greater worsening of the functional status of elderly people undergoing HD than of elderly controls has been shown,49 the findings of the current study demonstrated comparable declines in multidimensional well-being in older and younger patients receiving HD. The longitudinal HRQOL data in the HEMO Study are also consistent with the North-of-Thames Study findings showing moderate use of resources and cross-sectional differences in quality of life in patients aged 65 and older undergoing HD.50 The HEMO Study population demonstrated only an incremental decrease of HRQOL in older adults on HD over 3 years in a racially diverse population. In cross-sectional studies of HRQOL in the population with ESRD, there were larger differences in HRQOL between younger patients undergoing HD and younger norms than between older patients undergoing HD and older norms.14 In another study, markedly greater decreases in functional status of older patients undergoing HD than of elderly controls have been shown.49 In the HEMO Study, overall physical well-being was lower, and the longitudinal effect of age was small when compared with the large differences suggested by cross-sectional studies using comparisons with general population norms of HRQOL.
Several limitations should be considered when interpreting the results of this study. First, the assessment of a prevalent population of patients undergoing HD may lead to a survivor bias, but an interaction between change in HRQOL and median years undergoing dialysis was tested for, and it was found to be nonsignificant. Although patients who had been undergoing HD for less than 3 months were excluded from enrollment in the HEMO Study, 490 patients were randomized within 1 year of starting dialysis.51 Second, attrition due to death was high in the HEMO Study. Hence, the method of accounting for deaths and dropouts could have influenced the assessment of mean changes in HRQOL. Therefore, it was felt that the findings required sensitivity analyses under different models for the missing data to determine the consistency of the age group comparisons. These alternative models did not yield materially different conclusions. Third, the distribution of patients in the HEMO Study differed from the U.S. population receiving HD because of a preponderance of urban centers; 63% of HEMO Study patients were African American, compared with 41% in the general U.S. dialysis population.51 Although the HEMO trial included a higher percentage of African Americans than in the U.S. population undergoing HD, non-African Americans were nonetheless well represented (n = 690); previous work has demonstrated that African Americans have slightly better HRQOL than non-African Americans in certain domains, and the larger proportion of African Americans in the younger age groups34 could have biased the findings toward larger differences between the younger and older age groups. Because this was not the case, it is unlikely that an increase in non-African-American enrollment in the study would have increased the likelihood of finding larger differences in HRQOL according to age group. Moreover, the multiple race-adjusted models in this report should provide accurate parameter estimates for the three age groups in this diverse patient population. Fourth, depression has been shown to have a significant influence on HRQOL of patients undergoing HD.52,53 The HEMO Study did not perform an assessment of clinical or subclinical depression such as the Beck Depression Index, although it did use the SF-36, and the MCS has been used as a proxy marker of depression.54 There were no differences in average MCS scores between the age groups, suggesting that the distribution of mood disorders would be similar across the age spectrum.
The HEMO Study provided a unique opportunity to examine change in HRQOL over time in a prevalent population of patients undergoing thrice-weekly HD. Although there was poor HRQOL in general well-being, physical well-being, symptoms and effects of kidney disease, and sleep quality, patients aged 70 and older had a similar decline in HRQOL over 3 years to that of younger patients. These findings may be informative to older patients and healthcare providers, but there remains a great need for study of the decisions facing older patients with chronic kidney disease, because HRQOL concerns shape the decision to initiate and withdraw from dialysis. These findings also underline the need to improve HRQOL in all patients undergoing thrice-weekly HD. Interventions aimed at preserving residual renal function,55 monitoring HRQOL,6 treatment of anemia,28 physical therapy and rehabilitation,56 measurement of symptoms and application of palliative care principles,53 and perhaps more-frequent and longer HD treatments57,58 may preserve HRQOL in patients undergoing HD. The pattern for the size and direction of the change in HRQOL score between baseline and Years 1 to 3 of follow-up were consistent over time (Table 3). This may suggest particular domains such as cognitive function, sleep, and physical well-being that could be addressed earlier in the course of treatment for ESRD in older patients. Studies focused on identifying patients at risk for decline in HRQOL and specific interventions to improve the HRQOL of older patients need to be undertaken.
ACKNOWLEDGMENTS
Dr. Unruh was supported by Fresenius Medical Care Young Investigator Grant of the National Kidney Foundation, Baxter Extramural Grant Program, DK077785 and DK66006.
The HEMO Study is supported by the National Institutes of Diabetes, Digestive and Kidney Diseases (NIDDK) via cooperative agreements: U01DK 46109, U01 DK 46114, U01DK 46126, U01DK 46143, U01DK 49240, U01DK 49241, UO1DK 49242, U01DK 49243, U01DK, 49244, U01DK 49249, U01DK 49252, U01DK 49254, U01DK 49259, U01DK 49261, U01DK 49264, U01DK 49271.
MLU: Grant support from Dialysis Clinics Incorporated, Baxter Extramural Grant Fund; Consultants Merck and Qualitymetric KBM: Salary support from Dialysis Clinic Inc.; honoraria from ESRD Network of New England, Inc., Amgen, National Kidney Foundation; member of Board of Directors of ESRD Network of New England and Forum of ESRD Networks; consulting for Merck, Gerson-Lehrman, Primary Insight; grant support from the NIDDK and Covidien. DCM: Salary support from Dialysis Clinic Inc. MVR: Grant support from NIDDK; consultant for Amgen, DaVita, Hoffman LaRoche, Rennaissance Health Care.
Sponsor’s Role: The funding sources had no role in the design, methodology, data analysis, or preparation of this manuscript.
Footnotes
A list of HEMO Study participating investigators and institutions has been previously described.1
Conflict of Interest:
REFERENCES
- 1.Eknoyan G, Beck GJ, Cheung AK et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. [comment]. N Engl J Med 2002;347:2010–2019. [DOI] [PubMed] [Google Scholar]
- 2.USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, 2006. [Google Scholar]
- 3.Krishnan M, Lok CE, Jassal SV. Epidemiology and demographic aspects of treated end-stage renal disease in the elderly. Semin Dial 2002;15:79–83. [DOI] [PubMed] [Google Scholar]
- 4.Iseki K, Tozawa M, Iseki C et al. Demographic trends in the Okinawa Dialysis Study (OKIDS) registry (1971–2000). Kidney Int 2002;61: 668–675. [DOI] [PubMed] [Google Scholar]
- 5.Schena F Epidemiology of end-stage renal disease: International comparisons of renal replacement therapy. Kidney Int 2000;74:S39–S45. [Google Scholar]
- 6.Unruh ML, Weisbord SD, Kimmel PL. Health-related quality of life in nephrology research and clinical practice. Semin Dial 2005;18:82–90. [DOI] [PubMed] [Google Scholar]
- 7.Kutner NG, Brogan DJ. Assisted survival, aging, and rehabilitation needs: Comparison of older dialysis patients and age-matched peers. Am J Phys Med Rehabil 1992;71:97–101. [DOI] [PubMed] [Google Scholar]
- 8.Levin NW, Lazarus JM, Nissenson AR. National Cooperative rHu Erythropoietin Study in patients with chronic renal failure—an interim report. The National Cooperative rHu Erythropoietin Study Group. Am J Kidney Dis 1993;22:3–12. [DOI] [PubMed] [Google Scholar]
- 9.Yudd M, Llach F. Current medical management of secondary hyperparathyroidism. Am J Med Sci 2000;320:100–106. [DOI] [PubMed] [Google Scholar]
- 10.Rocco MV, Frankenfield DL, Hopson SD et al. Relationship between clinical performance measures and outcomes among patients receiving long-term hemodialysis. Ann Intern Med 2006;145:512–519. [DOI] [PubMed] [Google Scholar]
- 11.System USRD. USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases, 2006. [Google Scholar]
- 12.Parsons DS, Harris DCH. A review of quality of life in chronic renal failure. Pharmacoeconomics 1997;12:140–160. [DOI] [PubMed] [Google Scholar]
- 13.Rebollo P, Gonzalez MP, Bobes J et al. In process citation. Nefrologia 2000;20:431–439. [PubMed] [Google Scholar]
- 14.Rebollo P, Ortega F, Baltar JM et al. Is the loss of health-related quality of life during renal replacement therapy lower in elderly patients than in younger patients? Nephrol Dial Transplant 2001;16:1675–1680. [DOI] [PubMed] [Google Scholar]
- 15.Kutner NG, Brogan D, Kutner MH. End-stage renal disease treatment modality and patients’ quality of life. Longitudinal assessment. Am J Nephrol 1986;6:396–402. [DOI] [PubMed] [Google Scholar]
- 16.Meers C, Singer MA, Toffelmire EB et al. Self-delivery of hemodialysis care: A therapy in itself. Am J Kidney Dis 1996;27:844–847. [DOI] [PubMed] [Google Scholar]
- 17.Wu AW, Fink NE, Marsh-Manzi JV et al. Changes in quality of life during hemodialysis and peritoneal dialysis treatment: Generic and disease specific measures. J Am Soc Nephrol 2004;15:743–753. [DOI] [PubMed] [Google Scholar]
- 18.Merkus MP, Jager KJ, Dekker FW et al. Quality of life over time in dialysis: The Netherlands Cooperative Study on the Adequacy of Dialysis. NECOSAD Study Group. Kidney Int 1999;56:720–728. [DOI] [PubMed] [Google Scholar]
- 19.Unruh M, Yan G, Radeva M et al. Bias in assessment of health-related quality of life in a hemodialysis population: A comparison of self-administered and interviewer-administered surveys in the HEMO study. J Am Soc Nephrol 2003;14:2132–2141. [DOI] [PubMed] [Google Scholar]
- 20.Unruh M, Benz R, Greene T et al. Effects of hemodialysis dose and membrane flux on health-related quality of life in the HEMO Study. Kidney Int 2004;66:355–366. [DOI] [PubMed] [Google Scholar]
- 21.Miskulin DC, Athienites NV, Yan G et al. Comorbidity assessment using the Index of Coexistent Diseases in a multicenter clinical trial. Kidney Int 2001;60:1498–1510. [DOI] [PubMed] [Google Scholar]
- 22.Unruh M YG, Radeva M, Hays RD et al. Bias in assessment of health-related quality of life in a hemodialysis population: A comparison of self-administered and interviewer administered surveys in the HEMO study. J Am Soc Nephrol 2003;14:2132–2141. [DOI] [PubMed] [Google Scholar]
- 23.Hays RD, Kallich JD, Mapes DL et al. Development of the Kidney Disease Quality of Life (KDQOL) instrument. Qual Life Res 1994;3: 329–338. [DOI] [PubMed] [Google Scholar]
- 24.Edgell ET, Coons SJ, Carter WB et al. A review of health-related quality-of-life measures used in end-stage renal disease. Clin Ther 1996;18: 887–938. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Unruh M. Health related quality of life in patients with chronic kidney disease. Int Urol Nephrol 2005;37:367–378. [DOI] [PubMed] [Google Scholar]
- 26.Merkus MP, Jager KJ, Dekker FW et al. Quality of life in patients on chronic dialysis: Self-assessment 3 months after the start of treatment. The Necosad Study Group. Am J Kidney Dis 1997;29:584–592. [DOI] [PubMed] [Google Scholar]
- 27.Wu AW, Fink NE, Cagney KA et al. Developing a health-related quality-of-life measure for end-stage renal disease: The CHOICE Health Experience Questionnaire. Am J Kidney Dis 2001;37:11–21. [DOI] [PubMed] [Google Scholar]
- 28.Beusterien KM, Nissenson AR, Port FK et al. The effects of recombinant human erythropoietin on functional health and well-being in chronic dialysis patients. J Am Soc Nephrol 1996;7:763–773. [DOI] [PubMed] [Google Scholar]
- 29.Allen KL, Miskulin D, Yan G et al. Association of nutritional markers with physical and mental health status in prevalent hemodialysis patients from the HEMO Study. J Renal Nutr 2002;12:160–169. [DOI] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Kopple JD, Block G et al. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol 2001;12:2797–2806. [DOI] [PubMed] [Google Scholar]
- 31.Unruh ML, Evans IV, Fink NE et al. Skipped treatments, markers of nutritional nonadherence, and survival among incident hemodialysis patients. Am J Kidney Dis 2005;46:1107–1116. [DOI] [PubMed] [Google Scholar]
- 32.Merkus MP, Jager KJ, Dekker FW et al. Predictors of poor outcome in chronic dialysis patients: The Netherlands Cooperative Study on the Adequacy of Dialysis. The NECOSAD Study Group. Am J Kidney Dis 2000; 35:69–79. [DOI] [PubMed] [Google Scholar]
- 33.Van Manen JG, Korevaar JC, Dekker FW et al. Adjustment for comorbidity in studies on health status in ESRD patients: Which comorbidity index to use? J Am Soc Nephrol 2003;14:478–485. [DOI] [PubMed] [Google Scholar]
- 34.Unruh M, Miskulin D, Yan G et al. Racial differences in health-related quality of life among hemodialysis patients. Kidney Int 2004;65:1482–1491. [DOI] [PubMed] [Google Scholar]
- 35.Curran D, Molenberghs G, Aaronson NK et al. Analyzing longitudinal continuous quality of life data with dropout. Stat Methods Med Res 2002; 11:5–23. [DOI] [PubMed] [Google Scholar]
- 36.Fairclough D Design and Analysis of Quality of Life Studies in Clinical Trials. New York: Chapman & Hall, 2002. [Google Scholar]
- 37.Billingham LJ, Abrams KR. Simultaneous analysis of quality of life and survival data. Stat Methods Med Res 2002;11:25–48. [DOI] [PubMed] [Google Scholar]
- 38.Ribaudo HJ, Thompson SG, Allen-Mersh TG. A joint analysis of quality of life and survival using a random effect selection model. Stat Med 2000;19:3237–3250. [DOI] [PubMed] [Google Scholar]
- 39.Vonesh E, Chinchilli V. Linear and Nonlinear Models for the Analysis of Repeated Measurements Linear Mixed-Effects Models for Repeated Measurements. New York: Marcel Dekker, 1997. [Google Scholar]
- 40.Verbeke G, Molenbeghs G. Linear Mixed Models for Longitudinal Data. New York: Springer, 2000. [Google Scholar]
- 41.Rocco MV, Dwyer JT, Larive B et al. The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: Results of the HEMO Study. Kidney Int 2004;65:2321–2334. [DOI] [PubMed] [Google Scholar]
- 42.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. [see comment]. Med Care 2003;41:582–592. [DOI] [PubMed] [Google Scholar]
- 43.Lash JP, Wang X, Greene T et al. Quality of life in the African American study of kidney disease and hypertension: Effects of blood pressure management. Am J Kidney Dis 2006;47:956–964. [DOI] [PubMed] [Google Scholar]
- 44.Sharpe L, Butow P, Smith C et al. Changes in quality of life in patients with advanced cancer: Evidence of response shift and response restriction. J Psychosom Res 2005;58:497–504. [DOI] [PubMed] [Google Scholar]
- 45.Carr AJ, Gibson B, Robinson PG. Measuring quality of life: Is quality of life determined by expectations or experience? BMJ 2001;322:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsay SL, Lee YC. Effects of an adaptation training programme for patients with end-stage renal disease. J Adv Nurs 2005;50:39–46. [DOI] [PubMed] [Google Scholar]
- 47.Besarab A, Bolton WK, Browne JK et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998;339:584–590. [DOI] [PubMed] [Google Scholar]
- 48.Kimmel PL, Emont SL, Newmann JM et al. ESRD patient quality of life: Symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am J Kidney Dis 2003;42:713–721. [DOI] [PubMed] [Google Scholar]
- 49.Brogan DJ, Haber M, Kutner NG. Functional decline among older adults: Comparing a chronic disease cohort and controls when mortality rates are markedly different. J Clin Epidemiol 2000;53:847–851. [DOI] [PubMed] [Google Scholar]
- 50.Lamping DL, Constantinovici N, Roderick P et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: A prospective cohort study. Lancet 2000;356:1543–1550. [DOI] [PubMed] [Google Scholar]
- 51.Rocco MV, Cheung AK, Greene T et al. The HEMO Study: Applicability and generalizability. Nephrol Dial Transplant 2005;20:278–284. [DOI] [PubMed] [Google Scholar]
- 52.Kimmel PL. Psychosocial factors in adult end-stage renal disease patients treated with hemodialysis: Correlates and outcomes. Am J Kidney Dis 2000;35:S132–S140. [DOI] [PubMed] [Google Scholar]
- 53.Weisbord SD, Fried LF, Arnold RM et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol 2005;16:2487–2494. [DOI] [PubMed] [Google Scholar]
- 54.DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis 1997;30:204–212. [DOI] [PubMed] [Google Scholar]
- 55.Termorshuizen F, Korevaar JC, Dekker FW et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis 2003;41: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 56.Tawney KW, Tawney PJ, Hladik G et al. The life readiness program: A physical rehabilitation program for patients on hemodialysis. Am J Kidney Dis 2000;36:581–591. [DOI] [PubMed] [Google Scholar]
- 57.Heidenheim AP, Muirhead N, Moist L et al. Patient quality of life on quotidian hemodialysis. Am J Kidney Dis 2003;42:36–41. [DOI] [PubMed] [Google Scholar]
- 58.Culleton BF, Walsh M, Klarenbach SW et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA 2007;298: 1291–1299. [DOI] [PubMed] [Google Scholar]


