Abstract
River and cave-adapted populations of Astyanax mexicanus show differences in morphology, physiology, and behavior. Research focused on comparing adult forms has revealed the genetic basis of some of these differences. Less is known about how the populations differ at post-larval stages (at the onset of feeding). Such studies may provide insight into how cavefish survive through adulthood in their natural environment. Methods for comparing post-larval development in the laboratory require standardized aquaculture and feeding regimes. Here we describe how to raise fish on a diet of nutrient-rich rotifers in non-recirculating water for up to two-weeks post fertilization. We demonstrate how to collect post-larval fish from this nursery system and perform whole-mount immunostaining. Immunostaining is an attractive alternative to transgene expression analysis for investigating development and gene function in A. mexicanus. The nursery method can also be used as a standard protocol for establishing density-matched populations for growth into adults.
Keywords: Genetics, Issue 142, Astyanax mexicanus, immunohistochemistry, fish husbandry, cavefish, aquaculture, whole-mount immunostaining
Introduction
The Mexican tetra, Astyanax mexicanus, is a single species of fish that exists as river-dwelling populations (surface fish) and a number of cave-dwelling populations (cavefish) named for the caves they inhabit (i.e., Tinaja, Molino, Pachón). A growing number of researchers are using A. mexicanus to investigate the genetic and developmental bases of behavioral1,2,3,4, metabolic5,6,7,8, and morphological evolution9,10,11. Available resources for studies of A. mexicanus include a sequenced and annotated genome12; transcriptome13; developmental staging table14; and methods for breeding15,16,17, creating transgenics18, and editing genes19. Dissemination of additional tools and updated standard protocols will accelerate growth of the cavefish research community (see this methods collection20).
Our goal is to add to the existing repertoire of tools by providing a robust method for assessing gene activity in situ in post-larval A. mexicanus, in a manner comparable between laboratories. There are two challenges to achieving this goal. First, there is a need for standardized regimes for hatching and raising the fish between laboratories, as differences in parameters such as feeding and density affect growth and maturation, thereby impacting gene activity. Second, there is a need for a standardized yet adaptable method for examining patterns of gene activity in the post-larval fish. We address these issues here, establishing standard practices for raising fish to post-larval stages and introducing a robust whole-mount immunohistochemistry (IHC) protocol for assessing gene expression in A. mexicanus.
We first demonstrate how to breed the fish through natural spawning and identify fertilized eggs. Described next is how to hatch fertilized eggs (larvae) and transfer them to nursery containers, where they are maintained at a density of 20 fish per container for two weeks without recirculating or changing the water. At 5-days post fertilization, the fish have developed to post-larval stages (no longer having a yolk supply) and are provided algae-fed Brachionus plicatilis (rotifers) as a nutrient-rich food source that do not require daily replenishment. This method provides consistent growth parameters for larval and post-larval development.
To assess gene function, we demonstrate how to remove the fish from nursery containers and perform whole-mount IHC. The IHC method presented is adapted from protocols developed for use with Danio rerio21 and is effective for examining antigens in all A. mexicanus tissues tested, including the brain, intestine, and pancreas. IHC is a faster alternative to generating transgenic animals for examination of gene expression and protein localization. This protocol will be useful for studies aimed at growing A. mexicanus and comparing the phenotypes of surface fish and cavefish at post-larval stages.
Protocol
The procedures described throughout this protocol have been approved by the Institutional Animal Care and Use Committee (IACUC) at Harvard Medical School.
1. Breeding
NOTE: There are several published methods for breeding15, 16, 17, 20 that could also be used at this step. Prior to breeding, adult fish are maintained on a 10:14 light:dark cycle at 23 °C and fed a pellet diet (see Table of Materials) once daily. Fish can be bred in a recirculating system with mechanical filtration and UV sterilization. Breeding can also be accomplished in static (non-recirculating) tanks, but fish should not be left in a static tank for more than 3 days, as water quality quickly degrades.
| Name | Company | Catalog Number | Comments |
|---|---|---|---|
| methylene blue | Kordon | B016CBHZUS | antifungal |
| heater | Finnex | 4711457836017 | 100W Digital Control Heater |
| airstone | Lee's Aquarium & Pet Products | 10838125202 | disposable air stone |
| salt | Instant Ocean | 51378014021 | Sea Salt |
| nursery container | IPC | 21545-002 | 40 oz or 1.5 L clear containers |
| transfer pipette | VWR | 414004-002 | plastic bulb pipettes |
| compact culture system(CCS) starter kit with Brachionus plicatilis (L-type) rotifers | Reed Mariculture | na | fish food |
| RGcomplete | APBreed | 817656016572 | 32 oz bottle of rotifer food |
| Programmable Auto Dosing Pump DP-4 | Jebao | DP-4 | automatic feeder for rotifers |
| Tricane-S | Western Chemical | MS 222 | fish anesthetic |
| sodium bicarbonate | Sigma-Aldrich | S5761-500G | for tricane solution |
| nylon mesh strainer | HIC (Harold Import Co.) | 735343476235 | 3-inch diameter |
| Formalin solution, neutral buffered, 10% | Sigma-Aldrich | HT501128-4L | fixative |
| 10X PBS | Invitrogen | AM9625 | buffer, dilute to 1X using distilled water |
| Triton-x 100 | Sigma-Aldrich | T8787-250ML | detergent |
| sodium azide | Sigma-Aldrich | S2002-25G | anti-bacterial |
| bovine serum albumin | Sigma-Aldrich | A9647-100G | blocking reagent |
| glass vial with screw-top cap 4mL | Wheaton | 224742 | staining vial |
| plastic mesh screen for breeding tank | Pentair | N1670 | Cut into a rectangle 6mm larger on all edges than the dimensions of the bottom of the breeding tank. Cut a 6mm square from each corner of the rectangle. Bend the edges of the screen down along all four edges.Place a pair of 6mm vinyl-coated disk magnets on either side (top and bottom) of the mesh on each corner. The screen should be as snug as possible to the sides of the tank. The screen can be removed from the tank with a metal fish net. |
| vinyl-coated disk magnets | Kjmagnets | D84PC-AST | |
| New Life Spectrum Thera-A pellet fish food | New Life International | na | Adult fish food. A list of retailers for this product is available on the company website |
| Antibodies | |||
| insulin antibody from guinea pig | Dako | A0564 | 1:200 |
| glucagon antibody from sheep | Abcam | ab36215 | 1:200 |
| acetylated tubulin antibody from mouse | Sigma | T6793 | 1:500 |
| HuD/HuC antibody from mouse | Life Technologies | A-21271 | 1:500 |
| nitric oxide synthase (nNOS) antibody from rabbit | Abcam | ab106417 | 5μg/mL |
| choline acetyltransferase (ChAT) from rabbit | Abcam | ab178850 | 1:2000 |
| seratonin (5HT) from rabbit | Immunostar | 20080 | 1:500 |
Fill a 5 gal tank with fish-ready water (dechlorinated water adjusted to: pH = 7.1 +/− 2, conductivity = 900 +/− 150 μS, temperature = 23 °C).
-
Place plastic mesh (see Materials) in the bottom of the tank. If breeding in static tanks, affix a water heater to the side of the tank. If breeding in a recirculating system, place a water heater in the system sump.
NOTE: The plastic mesh prevents adults from consuming eggs.
Place one female and two male A. mexicanus fish of greater than 1 year old into the tank. Allow the fish to acclimate for 30 min.
Set the temperature of the heater to 24 °C (or 1 °C warmer than the starting temperature).
After 24 h, increase the temperature by 1 °C.
After 24 h, increase the temperature by 1 °C. Check the tanks daily for eggs by shining a flashlight into the bottom of the tank. Withhold the fish from food during this 3-day period.
If spawning has not been induced after 3 days, turn off the heater and allow the water to return to room temperature (RT) before moving the fish to their original tank.
2. Hatching Fertilized Eggs
-
Once eggs are identified in a breeding tank, remove the adults and plastic mesh, and reduce the water to a depth of 10 cm using a beaker or cup.
NOTE: To estimate time of spawning, use a transfer pipette to place several eggs into a Petri dish and view them with a stereomicroscope to determine the stage14 and estimate the time of fertilization.
-
Move the tank to a convenient work surface and remove any opaque eggs or feces, leaving only the translucent, fertile eggs in the tank.
NOTE: The number of fertile eggs can be recorded at this time.
-
Fill the tank with fish-ready water (see step 1.1) and add 6-7 drops of methylene blue to the tank as the water fills.
NOTE: The final concentration of methylene blue is approximately 1.5 ppm.
Add a heater and aquarium bubbler (attached to an air pump, with a regulator) to the tank.
Set the heater to 24 °C and adjust the airstream regulator to produce a gentle stream of bubbles.
-
Place a cover on the tank to help maintain the water temperature.
NOTE: The eggs should begin hatching within 24 h of the spawning time.
3. Transfer of Hatched Larvae to Nursery Containers
Add 20 g of salt (see Materials) to 8 L of fish-ready water (see step 1.1) and stir until dissolved. Fill each 1.5 L nursery container (see Materials) with 1 L of the prepared water.
-
Use a transfer pipette to move hatched larvae into prepared nursery containers at a density of 20 fish per container.
NOTE: A headlamp can be useful to locate and transfer the hatched larvae.
-
After all the visible hatched larvae have been removed, agitate the water in the tank by stirring, and/or blowing water jets into the edges and corners of the tank with the pipette.
NOTE: This will help reveal larvae that were missed on the first pass.
Dispose of unused larvae at this stage using the guidelines of the Office of Laboratory Welfare (OLAW). Add sodium hypochlorite to the tank to achieve a final concentration of 6.15%. Wait at least 5 min before pouring down the sink.
Label each nursery container with the date and time of fertilization. View nursery containers daily and continue to remove any dead larvae.
4. Preparation of Rotifer-based Fish Food
See Table of Materials for information on obtaining rotifers. Follow the referenced protocol to set up, maintain, and harvest rotifers22.
Prepare fish food by adding 3 mL of algae mixture (see Table of Materials) to 1 L of harvested rotifers. This mixture will be added directly to the nursery containers as a food supply.
5. Feeding of Post-larval Fish
When the fish are 5 days post fertilization (dpf), add 3 mL of fish food (prepared in step 4.2) to each nursery container. At the optimal density, rotifers should be visible in dense groups at the corners of the nursery containers, and less apparent at the center of the container. Add additional rotifer mixture until the appropriate density has been reached.
Check the containers daily for the presence of rotifers and add more if the concentration gets depleted. Continue to remove any dead larvae.
-
When fish reach 14 dpf, move them to a tank fit with a recirculating system at a density of 5 fish/L of water.
NOTE: The number of surviving post-larval fish can be recorded at this time.
6. Whole-mount Immunohistochemistry of Post-larval Fish
-
Remove post-larval fish of the desired stage from food for 24 h by pouring the nursery container containing the fish through a nylon mesh strainer and placing the strainer into a container with clean fish-ready water (see step 1.1).
NOTE: It is essential to remove all of the food. Even small amounts of food in the gut are auto-fluorescent and will influence imaging.
-
Collect and euthanize the fish.
NOTE: The euthanasia protocol should follow OLAW guidelines and be approved by your Institutional Animal Care and Use Committee. We have noted that tricaine alone does not euthanize post-larval fish, and the fish begin to move when transferred from tricaine to fixative if the fish are not also kept on ice.- Prepare tricaine solution in a beaker by adding 0.4 g of tricaine-S and 0.8 g of sodium bicarbonate to 1 L of deionized water, and place it on ice.
- Pour the water containing the fish through a nylon mesh strainer to collect the fish. Gently submerge the strainer in ice-cold Tricaine solution and leave it on ice for 10 min.
- Fix the euthanized fish.
- Use a transfer pipette with a cut tip to transfer the fish to a conical tube.
-
Remove the Tricaine solution with a transfer pipette and replace with fixative. Incubate with rocking.NOTE: Fixative and fixation time must be determined based on the antibody used. 10% formalin solution (4% formaldehyde, see materials) overnight at 4 °C is adequate for the antibodies listed in the Table of Materials.CAUTION: Formalin is toxic and flammable. Wear personal protective equipment (gloves, lab coat, and splash goggles) and handle in a chemical hood. Solutions containing formalin should be disposed as hazardous waste.
- Use a transfer pipette to carefully remove the fixative without disturbing the fish. Add 3 mL of phosphate buffered saline-triton solution [PBS with 0.1% Triton (PBST), see Materials] and incubate for 15 min at RT with rocking.
-
Remove PBST and replace with fresh PBST and incubate for 15 min. Repeat this “washing” one additional time.NOTE: Fish can be stored in PBS containing 0.02% sodium azide at 4 °C for several weeks.
- Perform whole-mount immunostaining.
-
Prepare 50 mL of blocking solution [PB-0.5% Triton X, 0.2% bovine serum albumin (BSA), 1% dimethyl sulfoxide (DMSO), 0.02% sodium azide, 5% donkey serum].CAUTION: Sodium azide and DMSO are toxic. Personal protective equipment (gloves, lab coat, and splash goggles) should be used when handling. Any solutions should be disposed as hazardous waste.
- Use a transfer pipette to transfer the fish to a 4 mL glass vial with screw-top cap. Use a transfer pipette to remove the PBST and add 3 mL blocking solution. Incubate for 1 h at RT with rocking.
-
Use a transfer pipette to remove the blocking solution and add primary antibody diluted in blocking solution. Incubate overnight at room temperature with agitation.NOTE: For example, add 1:250 dilution of anti-HuC/HuD Neuronal Protein Mouse Monoclonal Antibody (see Table of Materials for a list of antibodies that have been successfully used in A. mexicanus). A set of fish with no primary antibody added should be included at this step. Volume of antibody should be enough to cover the fish and allow for agitation.
-
Wash the fish 3 times with PBST as described in step 6.3.4. Replace PBST with secondary antibody diluted in blocking solution and incubate overnight at RT with agitation.NOTE: Optimal primary and secondary antibody concentrations, incubation time, and incubation temperature should be determined for each antibody. Overnight at RT was effective for the antibodies listed in Table of Materials.
- Wash the fish 3 times with PBST, with each wash lasting 15 min as described in step 6.3.4.
- Transfer the fish to PBS for short-term storage before proceeding with dissecting, mounting, or sectioning the fish.
-
Representative Results
Table 1 shows success during one year of breeding surface fish and Tinaja, Molino, and Pachón cavefish in static breeding tanks. Surface and Pachon spawns with fertilized embryos always produced hatched larvae, while Molino and Tinaja were unsuccessful some of the time (2/6 and 2/18 spawning events did not produce hatched larvae, respectively). There is variation in clutch size that does not appear to be attributed to age of the parent fish. Table 2 shows the total number of hatched larvae resulting from some of the spawning events, and the age of the parent fish. In general, we found that surface fish produce the greatest number of larvae per spawn (average 1,550 ± 894, n = 5), followed by Pachon (average 879 ± 680, n = 6), Tinaja (average 570 ± 373, n = 11), and Molino (average 386 ± 276, n =3). The number of larvae produced is typically more than are needed per experiment or for growth into adults. We typically set up 6-18 nursery containers (120-360 larvae) and euthanize the remaining fish.
Table 1: Summary of data from one year of breeding A. mexicanus in static tanks.
Number of breeding attempts, resulting spawning events, and number of spawning events that produced hatched larvae.
| population | attempts | spawning events | clutches |
|---|---|---|---|
| surface | 94 | 23 | 23 |
| Molino | 110 | 6 | 4 |
| Pachón | 167 | 13 | 13 |
| Tinaja | 242 | 18 | 16 |
Table 2: Approximate female age and number of hatched larvae from individual spawning events from the indicated populations of A. mexicanus.
| population | parent age | hatched larvae |
|---|---|---|
| surface | 1 year | 989 |
| surface | 1 year | 1050 |
| surface | 3 years | 2214 |
| surface | 3 years | 432 |
| surface | 3 years | 1768 |
| surface | 4 years | 2852 |
| Pachón | 1 year | 1194 |
| Pachón | 1 year | 1933 |
| Pachón | 1.5 years | 371 |
| Pachón | 3 years | 480 |
| Pachón | 4 years | 1190 |
| Pachón | 4 years | 110 |
| Tinaja | 9 months | 259 |
| Tinaja | 9 months | 253 |
| Tinaja | 10 months | 1100 |
| Tinaja | 11 months | 857 |
| Tinaja | 1 year | 713 |
| Tinaja | 1 year | 853 |
| Tinaja | 1 year | 542 |
| Tinaja | 1.5 years | 360 |
| Tinaja | 1.5 years | 58 |
| Tinaja | 1.5 years | 1100 |
| Tinaja | 4 years | 185 |
| Molino | 2.5 years | 460 |
| Molino | 2.5 years | 619 |
| Molino | 3 years | 81 |
To measure the success of the nursery protocol we recorded the number of hatched larvae and surviving post-larval fish from successful spawning events. Table 3 shows data from 1 month of breeding in recirculating tanks and includes the number of larvae transferred to nursery containers that survived to 14 dpf. During this month, the survival rate ranged from 41-81 percent, resulting in 65-293 fish available per population for experiments or growth into adults.
Table 3: Summary of data from one month of breeding A. mexicanus in a recirculating system and raising the larvae.
Number of breeding attempts, resulting spawning events, clutches that produced hatched larvae, average number of larvae per clutch, larvae transferred to the nursery containers, and post-larval fish present in the nursery containers after 14 days.
| population | attempts | spawning events |
clutches | clutch size | larvae transferred to nursery cups |
post-larval fish at 14dpf |
survival (%) |
|---|---|---|---|---|---|---|---|
| surface | 4 | 3 | 2 | 576 & 1728 | 360 | 174 | 48 |
| Molino | 4 | 2 | 1 | 228 | 159 | 65 | 41 |
| Tinaja | 4 | 1 | 1 | 1952 | 175 | 93 | 53 |
| Pachón | 4 | 1 | 1 | 1696 | 360 | 293 | 81 |
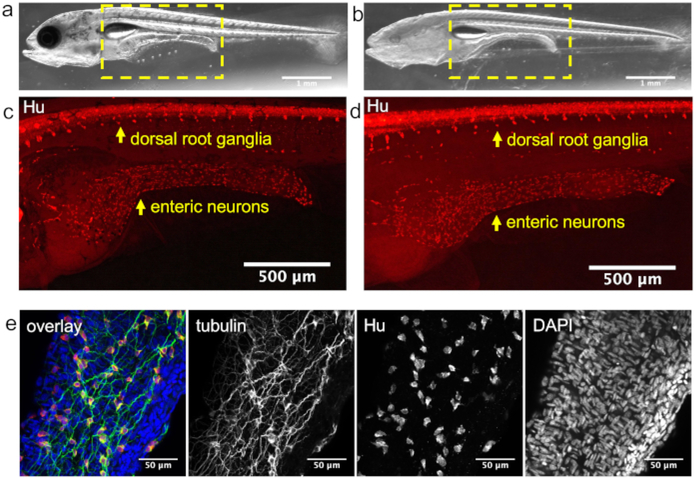
To determine if whole-mount immunostaining is successful, we compared the fluorescence of samples incubated with primary antibody to those incubated with secondary antibody only. The fluorescent signal is only visible in the fish incubated with primary antibody. We have used this protocol to successfully label neurons10 (Figure 1) and pancreatic cells6 at stages up to 12.5 dpf in both surface and cave morphotypes.
Figure 1: Neuron labeling.
Whole-mount immunostaining of A. mexicanus. Image of 12.5 dpf surface fish (a) and Pachón cavefish (b). Image of the mid-body region [hatched yellow outline shown in (a) and (b)] of surface fish (c) and Pachón cavefish (d) stained with pan-neuronal antibody (Hu), (e) Confocal image of a region of the surface fish intestine showing enteric neurons (Hu) and their projections (acetylated tubulin). For this image, the intestine was dissected out and mounted in medium containing DAPI to stain the nuclei.
Discussion
Comparing gene activity between surface and cave A. mexicanus requires carefully controlled environmental parameters and methods that can be replicated across laboratories. Our protocol for raising A. mexicanus provides consistent nutritional content during post-larval development. Following this feeding regime, gene function can be confidently compared between populations using the robust immunohistochemistry protocol we present. Here we discuss the significance of this method as well as its limitations and future applications.
To achieve density-matched growth, we found that post-larval fish can be raised without recirculating water for two weeks in 1.5 L containers on a diet of rotifers. This protocol can also be used to raise fish on a recirculating system; however, rotifers must be added daily to compensate for those lost through the tank outflow. Newly hatched Artemia nauplii are commonly used as a food source in aquaculture, but we found that using rotifers has considerable advantages, including: reduced price, improved biosecurity, consistent nutrition, and better water quality (see below).
First, the weekly cost in consumables is 4 dollars for rotifers, compared to 14 dollars for Artemia. Regarding biosecurity, rotifers are raised in the laboratory under controlled conditions, while Artemia are collected from the wild and subject to natural variation in microbial or pathogen contents23. Additionally, the nutrient composition of Artemia nauplii are environmentally determined and therefore inconsistent. Nauplii thrive on their own energy stores after hatching; they quickly loose nutritional value as they develop and should optimally be fed to the fish within several hours. Artemia begin feeding at 12 house post-hatching, representing the first time that they could be nutritionally enriched; however, at this stage they have become too large for 5 dpf fish to consume. In comparison, rotifers continuously feed on marine microalgae resulting in high nutrient content regardless of when the rotifers are harvested. Rotifers are much smaller than Artmeia nauplii (160 vs. 400 microns), making them easier for the fish to capture and swallow. Post-larval surface fish and cavefish consume rotifers in similar quantities suggesting no difference in preference or ability to capture the rotifers10.
Finally, Artemia nauplii begin dying in fresh water several hours after being introduced. Uneaten nauplii will decay, rapidly decreasing the water quality if they are not manually removed. Removing dead Artemia is time-consuming and dangerous for post-larval fish that are not much bigger than Artemia and may be accidently removed or injured. Rotifers can live indefinitely in the nursery containers and provide food to the fish at all times without significantly impacting water quality.
While using rotifers as a food source has considerable benefits, to maintain the rotifer stock, algae must be added to the culture system daily. This can be achieved with an automatic feeder that dispenses liquid algae into the rotifer culture container (see Table of Materials). Rotifers must also be harvested from this set-up every 24-48 hours to maintain the health of the culture. Researchers that breed fish very infrequently (once a year, for example) and are not concerned with making comparisons between the populations at post-larval stages may prefer Artemia as a food source, since the encysted embryos can be hatched at any time.
We recommend tracking the number of hatched and surviving larvae to monitor the success of hatching and growth. If most of the embryos or larvae die, it may be due to bacterial or fungal contamination. It is recommended to monitor the water quality of the fish-ready water and sterilize any equipment with 70% ethanol. Nursery containers can be re-used after they are cleaned and sterilized. To minimize risk of disease, it is also critical to remove any dead fish from the nursery containers and not add rotifers before 5 dpf, when the fish begin to eat.
A. mexicanus are sexually mature at approximately one year old. This is a limitation for generating transgenic A. mexicanus compared to Danio rerio (zebrafish) that are able to breed at 10-12 weeks24. Immunohistochemistry (IHC) is an alternative method to investigate gene expression and protein localization. The protocol described here can be adapted at each step and used to detect antigens in any tissue of interest. It is important to note, however, that some tissues may be more difficult to visualize in surface fish due to pigmentation (a barrier that does not exist in unpigmented cavefish), which may influence the interpretation of comparative studies. To address this potential problem, surface fish pigment can be bleached after fixation using 3% hydrogen peroxide.
IHC requires successful tissue fixation, blocking, and antibody penetration. Methods for each vary depending on the tissue and protein of interest. The fixative and fixation time must preserve cell architecture while maintaining the antigen epitope. For this protocol, we use a cross-linking fixative (paraformaldehyde) and omit a denaturing fixative (such as methanol or acetone). We found that incubation in acetone diminished antibody signal for neuronal and pancreatic markers. The blocking step is essential to prevent antibodies from binding to non-target proteins in the tissue. This method uses a combination of normal serum (5%) and BSA (0.2%) in the blocking solution. The blocking solution contains antibodies and proteins that bind to reactive sites on proteins in the tissue, diminishing non-specific binding of the primary and secondary antibodies.
To achieve antibody penetration, the tissue must be permeabilized. This can be achieved with detergents or denaturing solvents but must be optimized to preserve the antigen epitope. Our protocol uses a combination of Triton and dimethyl sulfoxide (DMSO). Triton and DMSO are included during the blocking and antibody incubation steps at concentrations of 0.5% and 1 %, respectively. Using this concentration, we have observed staining in the brain, pancreas, intestine, and muscle, suggesting that it is likely effective for penetration of all tissues. Fish size may also influence penetration. This protocol has not been tested on fish that are greater than 14 days old (approximately 7 mm in length). To troubleshoot staining, it is recommended to alter the fixation, blocking, and antibody penetration steps. It is also important to examine the sequence conservation of the immunogen with the A. mexicanus protein of interest using the available genome25.
A. mexicanus is an excellent model to investigate evolution as populations of the same species that have evolved in dramatically different environments can be directly compared in the laboratory. Standard husbandry protocols, both within and among laboratories, are essential to understanding the biological differences between surface fish and cavefish. Our article provides a method to examine development and gene activity in post-larval fish exposed to consistent growth parameters.
Materials
Acknowledgements
This work was supported by grants from the National Institutes of Health [HD089934, DK108495].
Footnotes
Video Link
The video component of this article can be found at https://www.jove.com/video/58972/
Disclosures
The authors have nothing to disclose.
References
- 1.Carlson BM, Klingler IB, Meyer BJ, Gross JB Genetic analysis reveals candidate genes for activity QTL in the blind Mexican tetra, Astyanax mexicanus. PeerJ. 6, e5189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin JSR, Gassant CE, et al. Convergence on reduced stress behavior in the Mexican blind cavefish. Developmental Biology. 441 (2), 319–327 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd E, Olive C, et al. Evolutionary shift towards lateral line dependent prey capture behavior in the blind Mexican cavefish. Developmental Biology. 441 (2), 328–337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabin JA, Aspiras A, et al. Temperature preference of cave and surface populations of Astyanax mexicanus. Developmental Biology. 441 (2), 338–344(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong S, Krishnan J, Peuß R, Rohner N Early adipogenesis contributes to excess fat accumulation in cave populations of Astyanax mexicanus. Developmental Biology. 441 (2) 297–304 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Riddle MR, Aspiras AC, et al. Insulin resistance in cavefish as an adaptation to a nutrient-limited environment. Nature. 555 (7698), 647–651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran D, Softley R, Warrant EJ Eyeless Mexican cavefish save energy by eliminating the circadian rhythm in metabolism. PloS One. 9 (9), e107877 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aspiras AC, Rohner N, Martineau B, Borowsky RL, Tabin CJ Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions. Proceedings of the National Academy of Sciences. 112 (31), 9668–9673 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang JLY, Guo Y, et al. The developmental origin of heart size and shape differences in Astyanax mexicanus populations. Developmental Biology. 441 (2), 272–284 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddle MR, Boesmans W, Caballero O, Kazwiny Y, Tabin CJ Morphogenesis and motility of the Astyanax mexicanus gastrointestinal tract. Developmental Biology. 441 (2), 285–296 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gore AV, Tomins KA, et al. An epigenetic mechanism for cavefish eye degeneration. Nature Ecology & Evolution. 2 (7), 1155–1160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGaugh SE, Gross JB, et al. The cavefish genome reveals candidate genes for eye loss. Nature Communications. 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross JB, Furterer A, Carlson BM, Stahl BA An Integrated Transcriptome-Wide Analysis of Cave and Surface Dwelling Astyanax mexicanus. PLoS One. 8 (2), e55659 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinaux H, Pottin K, et al. A Developmental Staging Table for Astyanax mexicanus Surface Fish and Pachon Cavefish. Zebrafish. 8 (4), 155–165 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Borowsky R Breeding Astyanax mexicanus through natural spawning. Cold Spring Harbor Protocols. 3 (11), (2008). [DOI] [PubMed] [Google Scholar]
- 16.Borowsky BR Handling Astyanax mexicanus Eggs and Fry. CSH Protocols. (2008). [DOI] [PubMed] [Google Scholar]
- 17.Stahl BA, Gross JB A Comparative Transcriptomic Analysis of Development in Two Astyanax Cavefish Populations. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 328 (6), 515–532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elipot Y, Legendre L, Père S, Sohm F, Rétaux S Astyanax Transgenesis and Husbandry: How Cavefish Enters the Laboratory. Zebrafish. 11 (4), 291–299 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Jeffery WR, Essner JJ, Kowalko JE Genome editing using TALENs in blind Mexican Cavefish, Astyanax mexicanus. PloS One. 10 (3), e0119370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.JoVE. Current methods in Astyanax mexicanus research, at <https://www.jove.com/methods-collections/16/current-methods-inastyanax-mexicanusresearch> (2018).
- 21.Heanue TA, et al. A Novel Zebrafish ret Heterozygous Model of Hirschsprung Disease Identifies a Functional Role for mapk10 as a Modifier of Enteric Nervous System Phenotype Severity. PLoS Genetics. 12 (11), (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed Mariculture Culturing Rotifers in Small Systems (Home or Lab), at <http://reedmariculture.com/support_rotifers_culturing.php> (2018).
- 23.Riddle MR, Baxter BK, Avery BJ Molecular identification of microorganisms associated with the brine shrimp Artemia franciscana. Aquatic Biosystems. 9 (1), (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang B, et al. Breeding Zebrafish: A Review of Different Methods and a Discussion on Standardization. Zebrafish. 14 (6), 561–573 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Something, B. Cave fish assembly and gene annotation, at <http://useast.ensembl.org/Astyanax_mexicanus/Info/Annotation> (2018).